Abstract
Background
Genitourinary infections (GUIs) are common among sexually active women. Yet, little is known about the risk of birth defects associated with GUIs.
Methods
Using data from the National Birth Defects Prevention Study, a multisite, population-based, case-control study, we assessed self-reported maternal GUIs in the month before through the third month of pregnancy (periconception) from 29,316 birth defect cases and 11,545 unaffected controls. We calculated odds ratios (ORs) and 95% confidence intervals to estimate the risk of 52 major structural birth defects associated with GUIs. We also calculated risk of birth defects associated with each type of GUI: urinary tract infection (UTI) and sexually transmitted infection (STI).
Results
In our analysis, 10% (n = 2,972) of case and 9% (n = 1,014) of control mothers reported a periconceptional GUI. A GUI was significantly associated with 11 of the 52 birth defects examined (ORs ranging from 1.19 to 2.26): encephalocele, cataracts, cleft lip, esophageal atresia, duodenal atresia/stenosis, small intestinal atresia/stenosis, colonic atresia/stenosis, transverse limb deficiency, conoventricular septal defect, atrioventricular septal defect, and secundum atrial septal defect. A periconceptional UTI was significantly associated with nine birth defects (ORs from 1.21 to 2.48), and periconceptional STI was significantly associated with four birth defects (ORs ranging from 1.63 to 3.72).
Conclusions
While misclassification of GUIs in our analysis is likely, our findings suggest GUIs during the periconceptional period may increase the risk for specific birth defects.
Keywords: birth defects, congenital malformations, genitourinary infections, infections, sexually transmitted infections, urinary tract infection
1 |. INTRODUCTION
Genitourinary infections (GUIs) include both sexually transmitted infections (STIs) and urinary tract infections (UTIs). These infections are common among sexually active women (Centers for Disease Control and Prevention [CDC], 2017; Sheffield & Cunningham, 2005). Several epidemiological studies reported an association between GUIs and gastroschisis, (Baer et al., 2015; Draper et al., 2008; Elliott et al., 2009; Feldkamp et al., 2008; Feldkamp et al., 2015; Yazdy, Mitchell, & Werler, 2014) including one using data from the National Birth Defects Prevention Study ([NBDPS]; Feldkamp et al., 2008). Few studies have assessed the association of maternal GUIs with other birth defects and those that have done so often present conflicting results. Comparing the existing findings is challenging, because studies have different exposure definitions and include different birth defect phenotypes or groupings.
Prior analyses of NBDPS data examined the association between specific GUIs and birth defects. Analyzing births from 1997 to 2003, Cleves, Malik, Yang, Carter, and Hobbs (2008) identified associations between maternal UTIs and two congenital heart defects (CHDs): left ventricular outflow tract obstructive defects and atrioventricular septal defects. Carter et al. (2011) examined GUIs (defined as STIs, pelvic inflammatory disease [PID], and group B streptococcus), finding associations with three birth defects among NBDPS births from 1997 to 2004: renal agenesis/hypoplasia, cleft lip, and transverse limb deficiency. Both analyses were limited by few numbers of exposed mothers and did not explore all birth defects collected in the NBDPS. We conducted a detailed analysis of the association between GUIs and the risk of major birth defects (excluding gastroschisis) using NBDPS data on births from 1997 to 2011. We sought to use the findings to generate hypotheses for future research.
2 |. METHODS
The NBDPS was a large, multisite, population-based, case-control study of birth defects that included pregnancies with estimated delivery dates from October 1997 through December 2011 (Reefhuis et al., 2015). Pregnancies affected by one or more of 30 categories of major structural birth defects (cases), excluding those attributed to a known chromosomal or single-gene abnormality, were ascertained through birth defects surveillance programs in 10 states (Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah). Control infants were live born without major birth defects randomly selected from hospital records or birth certificates in the same time period and geographic area as the cases. Each study site and the Centers for Disease Control and Prevention obtained Institutional Review Board approval for the NBDPS, and participants provided informed consent.
Case inclusion criteria have been described previously (Reefhuis et al., 2015). Briefly, case information (abstracted from medical records) was obtained from birth defects surveillance programs. Clinical geneticists reviewed each case to determine eligibility and to classify cases as having isolated (only one defect), multiple (major birth defects in more than one organ system), or complex birth defects (Rasmussen et al., 2003). CHD cases were further classified according to a structured protocol that took into account cardiac phenotype, complexity, and presence of non-cardiac defects (Botto, Lin, Riehle-Colarusso, Malik, & Correa, 2007). Birth defects that were known or strongly suspected to be caused by single-gene disorder or chromosomal anomaly were excluded from the NBDPS.
Trained interviewers conducted computer-assisted telephone interviews in English or Spanish with mothers of case and control infants. Interviews occurred between 6 weeks and 24 months after the estimated date of delivery. Mothers reported demographics, pregnancy history, health conditions, medication use, and other exposures before and during pregnancy. Overall, 66.7% of eligible case and 63.7% of eligible control mothers participated in the interview. The average time between the estimated delivery date and interview was 11 months among case mothers and 9 months among control mothers. We considered mothers exposed if they reported a GUI at any time in the month before conception through the third month of pregnancy (periconceptional period). The first 3 months of pregnancy include the critical period in embryonic development associated with most structural birth defects. We included the month prior to conception in the exposure window as it is often difficult to pinpoint the exact date of conception. Mothers reported GUIs in response to the following three questions: (a) “Did you have any of the following illnesses: a kidney, bladder, or urinary tract infection?”, (b) “Did you have pelvic inflammatory disease or PID?”, and (c) “Did you have any other disease or illnesses that we have not already talked about such as infectious diseases including sexually transmitted diseases or chickenpox?”. Two investigators (MLF, KEA), blinded to case/control status, reviewed all free-text responses to the latter question to identify any mention of a GUI. Mothers were classified into four exposure groups: (a) any GUI (either UTI or STI), (b) UTI only (including bladder and kidney infections), (c) STI only (including Chlamydia trachomatis, gonorrhea, genital herpes, human papillomavirus, syphilis, trichomoniasis, bacterial vaginosis, PID, or mention of an unspecified STI), or (d) both a UTI and STI. The unexposed group included infants of mothers who did not report a periconceptional GUI.
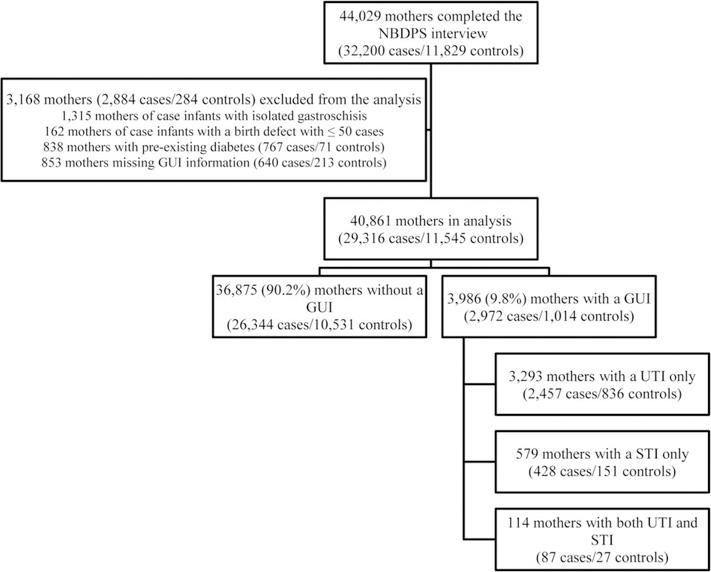
We excluded 3,168 infants (2,883 cases/285 controls) from analysis (Figure 1). We excluded mothers with missing information on GUIs and those reporting pre-gestational diabetes. As Feldkamp et al. (2008) published results from NBDPS for the association between maternal GUIs and gastroschisis, we excluded infants with isolated gastroschisis from the current analysis. Lastly, we excluded mothers of infants with isolated birth defects from case groups with ≤ 50 cases. Some birth defects (oral clefts, glaucoma, cataracts, ventricular septal defects [VSDs], and pulmonary valve stenosis) were not ascertained by all sites for all years of the NBDPS (Rasmussen et al., 2003; Reefhuis et al., 2015); when analyzing these birth defects, we excluded cases and controls for the sites and years with incomplete data.
FIGURE 1.
Study population, exclusions, and genitourinary infection status in the month before pregnancy through the third month of pregnancy among women in the National Birth Defects Prevention Study, 1997–2011
We analyzed second- and third-degree hypospadias (i.e., subcoronal/penile, scrotal, or perineal meatal opening) and restricted the control group to males.
To assess potential confounding, we used chi-square tests to compare characteristics among control infants whose mothers reported GUIs and those who did not. We used logistic regression to estimate crude odds ratios (cORs) and adjusted odds ratios (aORs) with 95% confidence intervals (CI) for any GUI, UTI only, STI only, both UTI and STI, and specific STI pathogens and the risk of birth defects. We calculated aORs and 95% CIs for birth defects with five or more exposed cases, controlling for the following a priori set of covariates: maternal age at delivery (a continuous variable), race/ethnicity (non-Hispanic white, Non-Hispanic black, Hispanic, other), education (high school or less, more than high school), pre-pregnancy body mass index (BMI; weight in kilograms/height in meters2), periconceptional cigarette smoking, folic acid-containing supplement use in the month before through the first month of pregnancy, and state of residence at the time of the infant’s birth. For birth defects with three or four exposed cases, we calculated cORs and Fisher’s exact CIs. We did not calculate estimates for birth defects with fewer than three exposed cases. We examined whether the associations between any GUI and birth defects varied by maternal age or smoking by evaluating additive interaction. We calculated the relative excess risk due to interaction along with the 95% CIs for each birth defect using a logistic regression model adjusted for the covariates listed above (Hosmer & Lemeshow, 1992).
We conducted three sub-analyses to determine whether specific changes in the analysis would affect the results. To reduce heterogeneity, we restricted the analysis of noncardiac defects to isolated cases. Similarly, we restricted the analysis of CHDs to cases with only one CHD or a well-recognized combination of defects that are considered a single CHD, referred to as “simple isolated” cases. Lastly, as GUIs can cause inflammation that may persist longer than the underlying infection and could impact embryonic development, we examined the association between reported GUIs and the risk of birth defects in a longer exposure window, the 3 months before conception through the third month of pregnancy. For each of these, we implemented the change and recalculated all ORs and 95% CIs as described above. No adjustment was made for the multiple comparisons performed. We conducted analyses in SAS (9.3; SAS Corporation, Cary, NC).
3 |. RESULTS
After exclusions, 29,316 case and 11,545 control infants were included in the analysis (Figure 1). Overall, 2,972 (10.1%) case and 1,014 (8.8%) control mothers reported a GUI in the periconceptional period. By type of GUI, 2,457 (8.4%) case and 836 (7.2%) control mothers reported having only a UTI, 428 (1.5%) case and 151 (1.3%) control mothers reported having only an STI, and 87 (0.3%) case and 27 (0.2%) control mothers reported having both a UTI and STI (Figure 1).
The distributions of selected characteristics stratified by the presence or absence of a GUI among control mothers are shown in Table 1. Mothers of control infants who reported a GUI differed from those who did not report a GUI in terms of age, race/ethnicity, education, pre-pregnancy BMI, smoking status, folic acid-containing supplement use, and study center. The percentage of mothers reporting a GUI decreased with increasing age, and this pattern was consistent across the type of GUI (Supporting Information Figure S1). The proportion of reported GUIs was consistently lower in control mothers compared to case mothers, regardless of maternal age or type of GUI.
TABLE 1.
Selected characteristics of mothers of controls by reported periconceptional genitourinary infection, National Birth Defects Prevention Study, 1997–2011a
| Maternal characteristic | Genitourinary infection (n = 1,014) n (%)b | No genitourinary infection (n = 10,531) n (%)b | p valuec |
|---|---|---|---|
| Age (years) | <.001 | ||
| <24 | 489 (48.2) | 3,251 (30.9) | |
| 25–29 | 250 (24.7) | 2,940 (27.9) | |
| 30 + | 275 (27.1) | 4,340 (41.2) | |
| Race/ethnicity | |||
| Non-Hispanic white | 527 (52.0) | 6,160 (58.5) | <.001 |
| Non-Hispanic black | 130(12.8) | 1,138 (10.8) | |
| Hispanic | 291 (28.7) | 2,541 (24.1) | |
| Other | 66 (6.5) | 686 (6.5) | |
| Education | |||
| High school or less | 503 (51.0) | 4,012 (39.0) | <.001 |
| More than high school | 484 (49.0) | 6,281 (61.0) | |
| Pre-pregnancy BMI | |||
| < 18.5 | 73 (7.5) | 515 (5.1) | <.001 |
| 18.5-<25.0 | 472 (48.5) | 5,483 (54.4) | |
| 25-<30 | 223 (22.9) | 2,284 (22.7) | |
| ≥ 30 | 205 (21.1) | 1,804 (17.9) | |
| Periconceptional smokinga | |||
| Yes | 263 (26.6) | 1,764(17.1) | <.001 |
| No | 726 (73.4) | 8,573 (82.9) | |
| Periconceptional alcohol usea | |||
| Yes | 375 (38.0) | 3,840 (37.3) | .655 |
| No | 612 (62.0) | 6,462 (62.7) | |
| Gestational diabetes | |||
| Yes | 54 (5.5) | 474 (4.6) | .224 |
| No | 931 (94.5) | 9,776 (95.4) | |
| Folic acid-containing supplement used | |||
| Yes | 489 (48.8) | 5,579 (53.3) | .006 |
| No | 514(51.3) | 4,883 (46.7) | |
| Study Center | |||
| Arkansas | 188 (18.5) | 1,248 (11.9) | <.001 |
| California | 111 (11.0) | 1,131 (10.7) | |
| Georgia | 92 (9.1) | 1,148 (10.9) | |
| Iowa | 92 (9.1) | 1,171 (11.1) | |
| Massachusetts | 79 (7.8) | 1,303 (12.4) | |
| New Jersey | 40 (3.9) | 534 (5.1) | |
| New York | 74 (7.3) | 886 (8.4) | |
| North Carolina | 84 (8.3) | 908 (8.6) | |
| Texas | 165 (16.3) | 1,199 (11.4) | |
| Utah | 89 (8.8) | 1,003 (9.5) | |
Note. BMI = body mass index (weight in kilograms/height in meters2).
Periconceptional defined as the month before through the third month of pregnancy.
Totals vary because of missing values.
Chi-square test for difference in the distribution within each covariate.
In the month before pregnancy through the first month of pregnancy.
The percentage of mothers who reported a periconceptional GUI varied slightly across the 52 birth defects examined, ranging from 5.8% of mothers who reported a GUI for total anomalous pulmonary venous return (TAPVR) to 20.7% for colonic atresia/stenosis (Tables 2 and 3). Any GUI was associated with statistically significant elevated aORs ranging from 1.19 to 2.26 for 11 of the 52 birth defects were studied: encephalocele, congenital cataracts, cleft lip only, esophageal atresia, duodenal atresia/stenosis, small intestinal atresia/stenosis, colonic atresia/stenosis, transverse limb deficiency, conoventricular VSD, atrioventricular septal defect (AVSD), and secundum atrial septal defect (secundum ASD). UTI only was associated with statistically significant elevated aORs ranging from 1.21 to 2.48 for 9 of the 52 birth defects we examined: congenital cataracts, cleft lip only, duodenal atresia/stenosis, small intestinal atresia/steno-sis, colonic atresia/stenosis, conoventricular VSD, AVSD, hypoplastic left heart syndrome, and secundum ASD. STI only was associated with statistically significant aORs ranging from 1.63 to 3.72 for 4 of 52 birth defects in the study: holoprosencephaly, cleft lip, transverse limb deficiency, and Ebstein anomaly. While the analysis of mothers reporting both UTI and STI was limited by the small number of exposed cases, reporting both periconceptional STI and UTI was associated with statistically significant elevated cORs for encephalocele and renal agenesis/hypoplasia.
TABLE 2.
Associations between periconceptional genitourinary infections and noncardiac birth defects, by type of infection, National Birth Defects Prevention Study 1997–2011
| Any GUI |
UTI Only |
STI Only |
Both STI & UTI |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Birth defect | % with GUI | Unexp | Exp | OR (95% CI)a | Exp | OR (95% CI)a | Exp | OR (95% CI)a | Exp | OR (95% CI)a |
| Controlsb | 8.8 | 10,531 | 1,014 | 836 | 151 | 27 | ||||
| Amniotic band sequence | 11.4 | 289 | 37 | 1.16 (0.81, 1.66) | 32 | 1.23 (0.84, 1.81) | 2 | NC | 3 | 4.05 (0.78, 13.3) |
| Anencephaly | 12.3 | 548 | 77 | 1.31 (1.00, 1.72) | 65 | 1.32 (0.99, 1.77) | 9 | 1.23 (0.62, 2.44) | 3 | 2.14 (0.41, 6.97) |
| Spina bifida | 10.0 | 1,134 | 126 | 1.07 (0.86, 1.32) | 112 | 1.18 (0.95, 1.48) | 11 | 0.55 (0.28, 1.10) | 3 | 1.03 (0.20, 3.36) |
| Encephalocele | 13.2 | 190 | 29 | 1.53 (1.01, 2.32) | 22 | 1.41 (0.88, 2.26) | 4 | 1.47 (0.39, 3.90) | 3 | 6.16 (1.19, 20.3) |
| Holoprosencephaly | 13.1 | 133 | 20 | 1.55 (0.95, 2.51) | 14 | 1.32 (0.75, 2.32) | 5 | 2.66 (1.06 6.70) | 1 | NC |
| Dandy-Walker malformation | 9.0 | 162 | 16 | 1.06 (0.62, 1.79) | 12 | 0.99 (0.54, 1.80) | 4 | 1.72(0.46, 4.59) | 0 | NC |
| Hydrocephaly | 11.0 | 430 | 53 | 1.14 (0.83, 1.56) | 41 | 1.07 (0.75, 1.52) | 11 | 1.56(0.81, 3.01) | 1 | NC |
| Cerebellar hypoplasiac | 11.5 | 54 | 7 | 1.35 (0.52, 2.98) | 7 | 1.63 (0.62, 3.61) | 0 | NC | 0 | NC |
| Anophthalmia/microphthalmia | 12.7 | 192 | 28 | 1.42 (0.94, 2.16) | 25 | 1.55 (1.00, 2.41) | 2 | NC | 1 | NC |
| Congenital cataracts | 11.9 | 305 | 41 | 1.53 (1.09, 2.15) | 33 | 1.48 (1.02, 2.16) | 6 | 1.48 (0.64, 3.42) | 2 | NC |
| Glaucoma | 11.0 | 162 | 20 | 1.34 (0.81, 2.22) | 17 | 1.35 (0.78, 2.32) | 3 | 1.37 (0.28, 4.18) | 0 | NC |
| Anotia/microtia | 10.6 | 592 | 70 | 1.15 (0.87, 1.52) | 59 | 1.15 (0.85, 1.56) | 11 | 1.41 (0.75, 2.66) | 0 | NC |
| Choanal atresia | 10.2 | 141 | 16 | 1.32 (0.75, 2.32) | 12 | 1.14 (0.59, 2.20) | 4 | 1.98 (0.52, 5.28) | 0 | NC |
| Cleft palate only | 8.6 | 1,424 | 134 | 1.01 (0.83, 1.23) | 112 | 1.05 (0.85, 1.30) | 18 | 0.85 (0.51, 1.41) | 4 | 1.08 (0.27, 3.11) |
| Cleft lip only | 11.2 | 955 | 120 | 1.36 (1.10, 1.67) | 96 | 1.32 (1.05, 1.66) | 22 | 1.63 (1.02, 2.60) | 2 | NC |
| Cleft lip with cleft palate | 10.7 | 1,771 | 211 | 1.15 (0.98, 1.36) | 168 | 1.11 (0.93, 1.34) | 33 | 1.24(0.84, 1.83) | 10 | 1.80 (0.83, 3.90) |
| Esophageal atresia | 10.3 | 665 | 76 | 1.31 (1.01, 1.69) | 62 | 1.28 (0.97, 1.70) | 10 | 1.19 (0.62, 2.29) | 4 | 2.35 (0.59, 6.76) |
| Duodenal atresia/stenosis | 14.8 | 201 | 35 | 1.89 (1.29, 2.75) | 27 | 1.80 (1.18, 2.75) | 6 | 2.02 (0.88, 4.67) | 2 | NC |
| Small intestinal atresia/stenosis | 12.7 | 414 | 60 | 1.51 (1.12, 2.01) | 52 | 1.61 (1.18, 2.20) | 7 | 1.11 (0.51, 2.40) | 1 | NC |
| Colonic atresia/stenosis | 20.7 | 46 | 12 | 2.17 (1.10, 4.29) | 11 | 2.48 (1.22, 5.03) | 1 | NC | 0 | NC |
| Anorectal atresia/stenosis | 10.5 | 928 | 109 | 1.10 (0.87, 1.37) | 89 | 1.16 (0.91, 1.48) | 15 | 0.74(0.39, 1.41) | 5 | 1.11 (0.33, 3.69) |
| Biliary atresia | 7.3 | 179 | 14 | 0.83 (0.47, 1.48) | 11 | 0.87 (0.47, 1.61) | 2 | NC | 1 | NC |
| Hypospadias | 8.4 | 2,320 | 213 | 1.07 (0.89, 1.28) | 184 | 1.10 (0.91, 1.34) | 25 | 0.81 (0.49, 1.34) | 4 | 0.93 (0.21, 3.21) |
| Renal agenesis/hypoplasia | 13.9 | 155 | 25 | 1.31 (0.82, 2.10) | 18 | 1.23 (0.72, 2.08) | 4 | 1.80(0.48, 4.80) | 3 | 7.55 (1.45, 24.9) |
| Bladder exstrophy | 6.9 | 67 | 5 | 0.82 (0.33, 2.06) | 4 | 0.75 (0.20, 2.02) | 0 | NC | 1 | NC |
| Cloacal exstrophy | 11.1 | 88 | 11 | 1.40 (0.74, 2.66) | 10 | 1.57 (0.80, 3.06) | 1 | NC | 0 | NC |
| Longitudinal limb deficiency | 11.4 | 443 | 57 | 1.20 (0.89, 1.62) | 45 | 1.17 (0.84, 1.63) | 11 | 1.48 (0.77, 2.84) | 1 | NC |
| Transverse limb deficiency | 11.5 | 623 | 81 | 1.33 (1.03, 1.72) | 58 | 1.17 (0.87, 1.57) | 20 | 2.06 (1.24, 3.40) | 3 | 1.88 (0.36, 6.13) |
| Craniosynostosis | 8.5 | 1,440 | 133 | 1.03 (0.85, 1.26) | 120 | 1.12 (0.91, 1.39) | 12 | 0.59 (0.31, 1.14) | 1 | NC |
| Diaphragmatic hernia | 10.7 | 763 | 91 | 1.23 (0.97, 1.56) | 73 | 1.20 (0.92, 1.56) | 15 | 1.32(0.75, 2.30) | 3 | 1.53 (0.30, 5.00) |
| Omphalocele | 8.4 | 393 | 36 | 0.94 (0.65, 1.34) | 33 | 1.05 (0.72, 1.53) | 3 | 0.53 (0.11, 1.60) | 0 | NC |
| Sacral agenesis | 14.7 | 64 | 11 | 1.82 (0.94, 3.53) | 9 | 1.78 (0.87, 3.66) | 1 | NC | 1 | NC |
Note. GUI = genitourinary infection, UTI = urinary tract infection, STI = sexually transmitted infection, Unexp = unexposed, Exp = exposed, OR = odds ratio, CI = confidence interval, NC = not calculated. Bold font indicates a statistically significant finding.
For defects with 5+ exposed cases, estimates were adjusted for maternal age (continuous), race/ethnicity, education, BMI, smoking, folic acid supplement use, and state of residence at the time of birth. Counts in the adjusted analysis were slightly lower than presented due to missing values for some covariates. Crude ORs and exact 95% CIs are presented for defects groups with 3–4 exposed cases. Estimates are not presented for analyses based on <3 exposed cases.
The number of controls differed for the following birth defect analyses: congenital cataracts and glaucoma (865 exposed and 8,971 unexposed controls), clefts (1,001 exposed and 10,409 unexposed controls), and hypospadias (521 exposed and 5,366 unexposed male controls).
Adjusted logistic model did not converge; estimates presented are crude OR and exact 95% CIs.
TABLE 3.
Associations between periconceptional genitourinary infections and congenital heart defects, by type of infection, National Birth Defects Prevention Study 1997–2011
| Any GUI |
UTI Only |
STI Only |
Both STI & UTI |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Birth Defect | % with GUI | Unexp | Exp | OR (95% CI)a | Exp | OR (95% CI)a | Exp | OR (95% CI)a | Exp | OR (95% CI)a |
| Controlsb | 8.8 | 10,531 | 1,014 | 836 | 151 | 27 | ||||
| Conotruncal defects | ||||||||||
| Truncus arteriosus | 13.8 | 106 | 17 | 1.63 (0.95, 2.79) | 14 | 1.62 (0.90, 2.93) | 3 | 1.97 (0.40, 6.04) | 0 | NC |
| Tetralogy of Fallot | 9.0 | 1,057 | 105 | 1.09 (0.88, 1.36) | 77 | 1.00 (0.78, 1.28) | 24 | 1.53 (0.97, 2.42) | 4 | 1.48(0.37, 4.25) |
| D-TGA | 9.1 | 683 | 68 | 0.99 (0.75, 1.31) | 61 | 1.09 (0.82, 1.47) | 5 | 0.43 (0.16, 1.16) | 2 | NC |
| DORV-TGA | 9.8 | 166 | 18 | 1.17 (0.71, 1.93) | 16 | 1.28 (0.75, 2.16) | 2 | NC | 0 | NC |
| Other DORV | 12.7 | 103 | 15 | 1.34 (0.74, 2.42) | 13 | 1.42 (0.75, 2.67) | 2 | NC | 0 | NC |
| Conoventricular VSD | 15.5 | 93 | 17 | 2.26 (1.32, 3.89) | 14 | 2.48 (1.38, 4.45) | 3 | 1.72 (0.34, 5.31) | 0 | NC |
| Atrioventricular septal defect | 14.8 | 299 | 52 | 1.82 (1.33, 2.49) | 43 | 1.86 (1.33, 2.61) | 7 | 1.64 (0.76, 3.55) | 2 | NC |
| TAPVR | 5.8 | 277 | 17 | 0.62 (0.37, 1.04) | 17 | 0.76 (0.46, 1.28) | 0 | NC | 0 | NC |
| Left ventricular outflow tract obstruction defects | ||||||||||
| Hypoplastic left heart syndrome | 10.7 | 568 | 68 | 1.26 (0.96, 1.65) | 61 | 1.37 (1.03, 1.82) | 5 | 0.67 (0.27, 1.65) | 2 | NC |
| Coarctation of the aorta | 8.9 | 1,036 | 101 | 1.08 (0.86, 1.35) | 89 | 1.13 (0.89, 1.44) | 10 | 0.78 (0.41, 1.50) | 2 | NC |
| Aortic valve stenosis | 7.2 | 463 | 36 | 0.79 (0.54, 1.14) | 32 | 0.83 (0.56, 1.23) | 4 | 0.60 (0.16, 1.59) | 0 | NC |
| Right ventricular outflow tract obstruction defects | ||||||||||
| Pulmonary atresia | 9.0 | 232 | 23 | 0.97 (0.62, 1.54) | 18 | 0.91 (0.54, 1.53) | 4 | 1.20 (0.32, 3.18) | 1 | NC |
| Pulmonary valve stenosis | 9.9 | 1,375 | 151 | 1.13 (0.93, 1.37) | 122 | 1.09 (0.88, 1.35) | 26 | 1.37 (0.88, 2.13) | 3 | 0.88 (0.17, 2.90) |
| Tricuspid atresia | 7.7 | 156 | 13 | 0.79 (0.42, 1.47) | 12 | 0.89 (0.46, 1.71) | 1 | NC | 0 | NC |
| Ebstein anomaly | 10.6 | 160 | 19 | 1.23 (0.75, 2.02) | 10 | 0.75 (0.38, 1.47) | 8 | 3.72 (1.77, 7.81) | 1 | NC |
| Septal defects | ||||||||||
| Perimembranous VSD | 8.2 | 1,233 | 110 | 0.94 (0.75, 1.18) | 78 | 0.87 (0.67, 1.13) | 26 | 1.13 (0.73, 1.75) | 6 | 1.52(0.59, 3.92) |
| Muscular VSD | 8.7 | 167 | 16 | 1.01 (0.54, 1.86) | 12 | 0.90 (0.45, 1.80) | 3 | 0.97 (0.17, 3.65) | 1 | NC |
| Secundum atrial septal defect | 11.3 | 2,611 | 332 | 1.19 (1.04, 1.37) | 274 | 1.21 (1.04, 1.41) | 47 | 1.06 (0.74, 1.51) | 11 | 1.36(0.65, 2.82) |
| Single ventricle defects | 11.5 | 139 | 18 | 1.39 (0.83, 2.34) | 16 | 1.48 (0.86, 2.56) | 2 | NC | 0 | NC |
| Heterotaxy | 12.5 | 281 | 40 | 1.35 (0.94, 1.93) | 30 | 1.25 (0.83, 1.88) | 7 | 1.40 (0.61, 3.22) | 3 | 4.16 (0.80, 13.65) |
Note. GUI = genitourinary infection, UTI = urinary tract infection, STI = sexually transmitted infection, Unexp = unexposed, Exp = exposed, OR = odds ratio, CI = confidence interval, NC = not calculated, TGA = transposition of the great arteries, DORV = double outlet right ventricle, VSD = ventricular septal defect, TAPVR = total anomalous pulmonary venous return. Bold font indicates a statistically significant finding.
For defects with 5+ exposed cases, estimates were adjusted for maternal age (continuous), race/ethnicity, education, BMI, smoking, folic acid supplement use, and state of residence at the time of birth. Counts in the adjusted analysis were slightly lower than presented due to missing values for some covariates. Crude ORs and exact 95% CIs are presented for defects groups with 3–4 exposed cases. Estimates are not presented for analyses based on < 3 exposed cases.
The number of controls differed for the following birth defects analyses: pulmonary valve stenosis (964 exposed and 10,116 unexposed controls) conoventricular and perimembranous VSDs (568 exposed and 6,130 unexposed controls), and muscular VSDs (63 exposed and 648 unexposed controls).
Among mothers who reported any STI, 434 (84%) case and 148 (83%) control mothers provided the specific pathogen; Chlamydia was most common (Table 4). Among the mothers who only reported an STI, Chlamydia was associated with statistically significant elevated aOR for cleft lip only and statistically significant elevated cOR for conoventricular VSD (Table 5). Additionally, we found elevated statistically significant aORs for human papillomavirus and transverse limb deficiency and for trichomoniasis and cleft lip with cleft palate. In crude analyses, gonorrhea was significantly associated with anotia/microtia.
TABLE 4.
Sexually transmitted infection pathogen reported by mothers of cases and controls, National Birth Defects Prevention Study 1997–2011a
| Among mothers reporting only STI (n = 579)a |
Among mothers reporting UTI and STI (n = 114)c |
|||
|---|---|---|---|---|
| Case mothers (n = 428) | Control mothers (n = 151) | Case mothers (n = 87) | Control mothers (n = 27) | |
| Type of STI | n (%)b | n (%)b | n (%)b | n (%)b |
| Chlamydia trachomatis | 132 (31) | 47 (31) | 28 (32) | 7 (26) |
| Bacterial vaginosis | 87 (20) | 38 (25) | 16 (18) | 5(19) |
| Human papillomavirus | 74 (17) | 23 (15) | 14 (16) | 3(11) |
| Herpes simplex virus | 42 (10) | 14(9) | 3(3) | 1 (4) |
| Trichomoniasis | 23 (5) | 7 (5) | 7 (8) | 3(11) |
| Gonorrhea | 20(5) | 6 (4) | 2(2) | 2(7) |
| Syphilis | 5(1) | 0 | 1(1) | 0 |
| Unknown pathogen | 61 (14) | 23 (15) | 20 (23) | 7(26) |
Note. UTI = urinary tract infection, STI = sexually transmitted infection.
Twenty-one mothers reported two STIs (14 case/7control), and one case mother reported three STIs.
Percentages do not total 100.
Five mothers reported two STIs (4 case mothers/1 control mother).
TABLE 5.
Associations between specific periconceptional sexually transmitted infection pathogens and birth defects, National Birth Defects Prevention Study 1997–2011a
| Birth defect |
Chlamydia |
Bacterial vaginosis |
HPV |
Herpes virus |
Trichomoniasis |
Gonorrhea |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp | OR (95% CI) | Exp | OR (95% CI) | Exp | OR (95% CI) | Exp | OR (95% CI) | Exp | OR (95% CI) | Exp | OR (95% CI) | |
| Controlsb | 47 | 38 | 23 | 14 | 7 | 6 | ||||||
| Anencephaly | 6 | 2.39 (0.99, 5.72) | 2 | NC | 0 | NC | 0 | NC | 0 | NC | 1 | NC |
| Spina bifida | 4 | 0.79 (0.21, 2.17) | 4 | 0.98 (0.25, 2.72) | 0 | NC | 0 | NC | 1 | NC | 0 | NC |
| Holoprosencephaly | 3 | 5.05 (0.99, 16.0) | 1 | NC | 1 | NC | 1 | NC | 0 | NC | 1 | NC |
| Hydrocephaly | 3 | 1.56 (0.31,4.89) | 1 | NC | 2 | NC | 1 | NC | 0 | NC | 2 | NC |
| Anotia/microtia | 2 | NC | 2 | NC | 2 | NC | 2 | NC | 0 | NC | 4 | 11.9 (2.45, 50.1) |
| Cleft palate only | 5 | 0.87 (0.34, 2.21) | 4 | 0.77 (0.20, 2.14) | 0 | NC | 1 | NC | 2 | NC | 0 | NC |
| Cleft lip only | 11 | 2.76 (1.36, 5.60) | 4 | 1.15 (0.30, 3.20) | 4 | 1.98 (0.50, 5.85) | 2 | NC | 0 | NC | 0 | NC |
| Cleft lip with cleft palate | 14 | 1.63 (0.88, 3.01) | 4 | 0.62 (0.16, 1.72) | 5 | 1.30 (0.49, 3.50) | 1 | NC | 5 | 4.73 (1.46, 15.3) | 3 | 2.94 (0.48, 13.8) |
| Esophageal atresia | 2 | NC | 1 | NC | 3 | 2.07 (0.40, 6.86) | 1 | NC | 2 | NC | 1 | NC |
| Duodenal atresia/stenosis | 3 | 3.34 (0.66, 10.5) | 2 | NC | 0 | NC | 0 | NC | 1 | NC | 0 | NC |
| Small intestinal atresia/stenosis | 1 | NC | 3 | 2.01 (0.39, 6.37) | 0 | NC | 2 | NC | 0 | NC | 0 | NC |
| Anorectal atresia/stenosis | 4 | 0.97 (0.25, 2.65) | 5 | 0.65 (0.15, 2.69) | 0 | NC | 1 | NC | 0 | NC | 0 | NC |
| Hypospadias | 8 | 1.11 (0.46, 2.71) | 4 | 0.51 (0.13, 1.56) | 5 | 1.07 (0.27, 4.20) | 5 | 1.84 (0.50, 6.76) | 0 | NC | 1 | NC |
| Longitudinal limb deficiency | 3 | 1.52(0.30, 4.74) | 5 | 1.33 (0.41, 4.35) | 1 | NC | 0 | NC | 0 | NC | 2 | NC |
| Transverse limb deficiency | 4 | 1.44 (0.38, 3.95) | 5 | 2.23 (0.87, 5.74) | 6 | 4.78 (1.90, 12.0) | 1 | NC | 0 | NC | 1 | NC |
| Craniosynostosis | 2 | NC | 2 | NC | 6 | 1.27 (0.43, 3.74) | 0 | NC | 0 | NC | 0 | NC |
| Diaphragmatic hernia | 4 | 1.17 (0.31, 3.23) | 4 | 1.45 (0.38, 4.05) | 3 | 1.80 (0.35, 5.98) | 3 | 2.96 (0.54, 10.6) | 0 | NC | 0 | NC |
| Tetralogy of Fallot | 8 | 1.59 (0.71, 3.58) | 5 | 1.12(0.40,3.15) | 2 | NC | 4 | 2.85 (0.68, 9.08) | 3 | 4.27 (0.71, 18.7) | 1 | NC |
| D-TGA | 0 | NC | 4 | 1.62 (0.42, 4.53) | 0 | NC | 0 | NC | 0 | NC | 0 | NC |
| Conoventricular VSD | 3 | 5.65 (1.09, 18.4) | 0 | NC | 0 | NC | 0 | NC | 0 | NC | 0 | NC |
| Pulmonary valve stenosis | 7 | 1.16 (0.51, 2.64) | 8 | 1.78 (0.77, 4.11) | 5 | 1.51 (0.56, 4.10) | 1 | NC | 1 | NC | 0 | NC |
| Perimembranous VSD | 9 | 1.35 (0.64, 2.87) | 2 | NC | 6 | 1.48 (0.59, 3.72) | 4 | 1.81 (0.42, 6.11) | 2 | NC | 2 | NC |
| Secundum atrial septal defect | 18 | 1.20 (0.67, 2.13) | 8 | 0.66(0.28, 1.59) | 6 | 0.99 (0.39, 2.52) | 4 | 1.15 (0.28, 3.67) | 3 | 1.73 (0.29, 7.58) | 0 | NC |
| Heterotaxy | 4 | 3.19 (0.83, 8.81) | 0 | NC | 1 | NC | 1 | NC | 0 | NC | 0 | NC |
Note. STI = sexually transmitted infection, HPV = human papillomavirus, Exp = exposed, OR = odds ratio, CI = confidence interval, NC = not calculated, TGA = transposition of the great arteries, VSD = ventricular septal defect. Bold font indicates a statistically significant finding.
Not presented in table are birth defects for which there were less than three exposed cases for each STI pathogen: amniotic band sequence, encephalocele, Dandy-Walker malformation, cerebellar hypoplasia, anophthalmia/microphthalmia, congenital cataracts, glaucoma, choanal atresia, colonic atresia/stenosis, biliary atresia, renal agenesis/hypoplasia, bladder exstrophy, cloacal exstrophy, omphalocele, sacral agenesis, truncus arteriosus, double outlet right ventricle-TGA, other double outlet right ventricle, atrioventricular septal defect, total anomalous pulmonary venous return, hypoplastic left heart syndrome, coarctation of the aorta, aortic valve stenosis, pulmonary atresia, tricuspid atresia, Ebstein anomaly, muscular VSD, and single ventricle defects. For defects with 5+ exposed cases, estimates were adjusted for maternal age (continuous), race/ethnicity, education, BMI, smoking, folic acid supplement use, and state of residence at the time of birth. Counts in the adjusted analysis were slightly lower than presented due to missing values for some covariates. Crude ORs and exact 95% CIs are presented for defects groups with 3–4 exposed cases. Estimates are not presented for analyses based on <3 exposed cases.
As in Tables 2 and 3, 10,531unexposed controls were included in all sub-analyses except: clefts (46 exposed and 10,409 unexposed controls), hypospadias (19 exposed and 5,366 unexposed male controls), pulmonary valve stenosis (44 exposed and 10,116 unexposed controls), and conoventricular/perimembranous VSDs (35 exposed and 6,130 unexposed controls).
We examined whether the associations between any GUI and each birth defect differed across maternal age and smoking status (data not shown). We did not find patterns that suggest additive interaction by either maternal age or smoking status for any of the birth defects examined. When we restricted to isolated noncardiac birth defects and simple isolated CHDs, the point estimates were similar to the estimates in the main analysis for most birth defect phenotypes, with the exception being the estimate for isolated cloacal exstrophy which became elevated and statistically significant (Supporting Information Table S1). When we expanded the exposure window to include the 3 months before conception through the third month of pregnancy, we found point estimates that were similar to our main findings (Supporting Information Table S2).
4 |. DISCUSSION
We assessed associations between GUIs and 52 birth defects in the NBDPS. Self-reported periconceptional GUIs were common, and slightly higher in case mothers compared to control mothers. We observed that maternal GUI was significantly associated with increased risk of 11 of the 52 individual birth defects examined, although the magnitude of these associations varied.
The published literature on this topic mostly comes from three important epidemiologic studies that are large enough to examine GUIs and individual birth defects: the Hungarian Case-Control Surveillance of Congenital Abnormalities (HCCSCA) (Acs, Banhidy, Puho, & Czeizel, 2008a, 2008b, 2008c; Banhidy, Acs, Puho, & Czeizel, 2006, 2010; Metneki, Puho, & Czeizel, 2005; Norgard, Norgaard, Czeizel, Puho, & Sorensen, 2006), the Baltimore-Washington Infant Study (Ferencz, Loffredo, Correa-Villasenor, & Wilson, 1997; Wilson, Loffredo, Correa-Villasenor, & Ferencz, 1998), and the NBDPS (Ailes et al., 2016; Carter et al., 2011; Cleves et al., 2008). Comparing the existing findings is challenging (Table 6). Variations in exposure definitions (in terms of both the infections and exposure windows examined) as well as different outcome measures (individual or combinations of birth defects) included in these studies prevent a clear picture from emerging.
TABLE 6.
Epidemiologic studies of genitourinary infections and birth defects (except gastroschisis)
| Author, year | Study design, setting | Exposure | Outcomes | Main findings |
|---|---|---|---|---|
| Carter et al., 2011 | NBDPS; case-control study of births from 1997 to 2004 from 10 states | Self-reported STIs, PED, or group B streptococcus infection in the month before through the third month of pregnancy; 290 case mothers and 139 control mothers were exposed | Individual birth defects and larger birth defect groupings (e.g., NTDs.) | OR (95% CI) for (a) bilateral renal agenesis/ hypoplasia: 2.89 (1.11, 7.50); CL ± CP: 1.46 (1.03, 2.06); transverse limb deficiency: 1.84 (1.04, 3.26) |
| Cleves et al., 2008 | NBDPS; case-control study of births from 1997 to 2003 from 10 states | Self-reported UTI in the first trimester; 247 case mothers and 290 control mothers were exposed | Individual CHDs, larger CHD groupings, and any CHD | OR (95% CI) for (a) LVOTO: 1.41 (1.04, 1.93); (b) HLHS: 1.61 (1.01, 2.59); (c)AVSD: 2.29 (1.11, 4.73) |
| Ailes et al., 2016 | NBDPS; case-control study of births from 1997 to 2016 from 10 states | Self-reported use of nitrofurantoin, trimethoprim-sulfamethoxazole, or cephalosporine to treat fever-free, doctor-diagnosed UTIs in the month before through the third month of pregnancy compared to penicillin; 608 case mothers and 231 control mothers were exposed | Individual birth defects and larger birth defect groupings (e.g., NTDs) | OR (95% CI) for nitrofurantoin and clefts: 1.97 (1.10,3.53. OR (95% CI) for trimethoprim-sulfamethoxazole for esophageal atresia: 5.31 (1.39, 20.24); diaphragmatic hernia: 5.09 (1.20, 21.69) OR (95% CI) for cephalosporine and anorectal atresia/stenosis: 5.01 (1.34, 18.76) |
| Ferencz et al., 1997 | BWIS; case-control study of births from 1981 to 1989 in the Baltimore-Washington area | Self-reported UTIs in the 3 months before through the third month of pregnancy; 38 ASD case mothers and 467 control mothers were exposed | Individual CHDs, larger CHD groupings, and any CHD | OR (95% CI) for ASD: 1.6 (1.1, 2.3). |
| Wilson et al., 1998 | BWIS; case-control study of births from 1981 to 1989 in the Baltimore-Washington area | Self-reported UTIs in the 3 months before through the third month of pregnancy; 38 ASD case mothers and 467 control mothers were exposed | Individual CHDs, larger CHD groupings, and any CHD | Attributable fraction for UTIs and ASD (95% CI): 6.4 (2.2, 10.7). |
| Acs et al., 2008a | HCCSCA; case-control study births from 1980 to 1996 in Hungary | Recurrent genital herpes in the first trimester and any time during pregnancy collected from antenatal care logbook, medical records, and self-report; 160 case mothers and 228 control mothers were exposed | Individual birth defects, larger birth defect groupings, and any birth defect | No significant ORs |
| Acs et al., 2008b | HCCSCA; case-control study births from 1980 to 1996 in Hungary | Acute PED during the second and third month of pregnancy that was prospectively collected and recorded in antenatal care logbook or medical record; 67 case mothers and 128 control mothers were exposed | Individual birth defects, larger birth defect groupings, and any birth defect | OR (95% CI) for any CHD: 2.6 (1.2, 5.4) |
| Acs et al., 2008c | HCCSCA; case-control study births from 1980 to 1996 in Hungary | Vulvovaginitis and bacterial vaginosis during the second and third month of pregnancy that was prospectively collected and recorded in antenatal care logbook or medical record; 1,536 case mothers and 2,698 control mothers were exposed | Individual birth defects, larger birth defect groupings, and any birth defect | No significant elevated ORs Protective ORs (95% CI) for (a) limb deficiencies: 0.6 (0.4, 0.9); (b) musculo-skeletal birth defects: 0.4 (0.2, 0.9) |
| Banhidy et al., 2006 | HCCSCA; case-control study births from 1980 to 1996 in Hungary | UTI during the second and third months of pregnancy was collected from antenatal care logbook, medical records, and self-report; 1,542 case mothers and 2,188 control mothers were exposed | Individual birth defects, larger birth defect groupings, and any birth defect | No significant ORs |
| Banhidy et al., 2010 | HCCSCA; case-control study of birth from 1980 to 1996 Hungary | Genital warts (caused by HPV) during the second and third months of pregnancy was collected from antenatal care logbook, medical records, and self-report; 17 case mothers and 25 control mothers were exposed | 17 case infants: 4 CHDs, 7 NTDs, 2 hypospadias, 2 syndactyly, and 7 with other birth defects | No significant ORs |
| Metneki et al., 2005 | HCCSCA; case-control study births from 1980 to 1996 in Hungary | UTIs and STIs during the second, third, and fourth month of pregnancy was collected from antenatal care logbook, medical records, and self-report; 14 NTD case mothers and 182 non-NTD case mothers (the comparison group) were exposed | Isolated CL ± CP and PCP | OR (95% CI) for UTI and (a) CL ± CP: 1.7 (1.1, 2.5); (b) PCP: 2.1 (1.2, 3.5) No significant ORs for STI and CL ± CP or PCP |
| Norgard et ah, 2006 | HCCSCA; case-control study births from 1980 to 1996 in Hungary | Herpes labialis during the first trimester and any time during pregnancy was collected from antenatal care logbook, medical records, and self-report; 14 NTD case mothers and 182 non-NTD case mothers (the comparison group) were exposed | Isolated NTDs | No significant ORs for NTDs |
| Dong, Binongo, & Kancherla, 2016 | Case-control study of live singleton births in the United States during 2012 | Chlamydia any time during pregnancy as reported on the birth certificate; 43 (1.7%) case mothers and 55,834 (1.7%) control mothers were exposed | Any cyanotic CHD as reported on the birth certificate | OR (95% CI) for cyanotic CHDs: 1.39 (1.02, 1.90) |
Note. NBDPS = National Birth Defects Prevention Study, BWIS = Baltimore-Washington Infant Study, HCCSCA = Hungarian Case-Control Surveillance of Congenital Abnormalities, STI = sexually transmitted infection., PID = pelvic inflammatory disease, UTI = urinary tract infection, HPV = human papillomavirus, NTDs = neural tube defects, CHD = congenital heart defect, ASD = atrial septal defect, CL ± CP = cleft lip with or without cleft palate, PCP = posterior cleft palate, LVOTO = left ventricular outflow tract obstruction defects; HLHS = hypoplastic left heart syndrome; AVSD = atrioventricular septal defect, OR = odds ratio, CI = confidence interval.
Of the 11 birth defects that were significantly associated with maternal GUI, the estimate for one birth defect, cleft lip, remained elevated and statistically significant among both subgroups of GUIs: mothers who reported UTI only (aOR 1.32) and STI only (aOR 1.63). We found the magnitude of the cleft lip estimate was even higher among infants whose mothers reported Chlamydia (aOR 2.76). These findings for cleft lip are consistent with two earlier NBDPS reports (Table 6), one that explored a sub-group of STIs (Chlamydia, gonorrhea, and PID) and another that examined maternal nitrofurantoin use for UTI (Ailes et al., 2016; Carter et al., 2011), which is unsurprising given the overlap in study participants. The HCCSCA explored maternal UTIs and cleft lip +/− cleft palate (and not cleft lip only) in two reports: one found an elevated (OR 1.7), but not statistically significant, association with cleft lip +/− palate, while the second observed a significant increased OR for cleft lip +/− palate as well as posterior cleft palate when compared to non-malformed control infants (Banhidy et al., 2006; Metneki et al., 2005). No associations with STIs were identified (Metneki et al., 2005).
Our estimates for 7 of the 11 birth defects significantly associated with maternal GUI remained elevated and statistically significant when we restricted to infants whose mothers reported only a UTI, and UTI only was also associated with an increased risk for hypoplastic left heart syndrome. Our findings regarding UTI and congenital cataracts, AVSD, and hypoplastic left heart syndrome expand on the previous NBDPS findings (Cleves et al., 2008; Prakalapakorn, Rasmussen, Lambert, & Honein, 2010). While the HCCSCA explored a combined outcome of all CHDs and did not identify any association with UTIs, the Baltimore-Washington Infant Study reported an association between UTI and secundum ASD (Banhidy et al., 2006; Ferencz et al., 1997; Wilson et al., 1998). The association between GUI and conoventricular VSDs has not been previously reported.
Three of the birth defects we observed to be associated with GUI, and more specifically with UTI, were related to the gastrointestinal tract: duodenal, small intestinal, and colonic atresia/stenosis. The previous NBDPS analysis that explored antibiotic use for UTIs observed an association with esophageal atresia, but not with other gastrointestinal birth defects (Ailes et al., 2016). The HCCSCA did not find any significant associations between UTI and gastrointestinal defects, but a nonsignificant elevated risk of diaphragmatic birth defects (congenital diaphragmatic hernia and eventration of the diaphragm) was reported with a wide confidence interval (Banhidy et al., 2006).
Among mothers who reported only an STI, we observed significant associations with three birth defects: holoprosencephaly, transverse limb deficiency, and Ebstein anomaly. The increased risk of holoprosencephaly among mothers who reported only an STI (n = 5) was largely driven by three cases exposed to Chlamydia (cOR 5.05 [0.99, 16.0]). This finding has not been observed in other studies, although brain anomalies, including holoprosencephaly, have been recently associated with other maternal infections including Zika virus (Hall, Broussard, Evert, & Canfield, 2017; Honein et al., 2017). The magnitude of the significant estimates for transverse limb deficiency increased among mothers who reported only an STI (aOR = 2.06), and increased further among those with maternal reports of HPV (aOR = 4.78). A previous NBDPS report identified an association with STI, however, their STI definition included group B streptococcal infections whereas ours did not (Carter et al., 2011). Using NBDPS data, Ailes et al. (2016) also observed elevated but not significant associations between women who used trimethoprim-sulfamethoxazole for UTI treatment and the risk of transverse limb deficiency in their offspring. Lastly, Ebstein anomaly was significantly associated with mothers reporting only an STI, but we were unable to explore associations by specific STI pathogens given the small number of exposed case mothers. While no other estimate for CHDs reached statistical significance among mothers who reported only an STI, there were elevated crude ORs for STIs and several CHDs, including truncus arteriosus, conoventricular VSDs, atrioventricular VSDs, and secundum ASDs. When we restricted to those reporting Chlamydia in the periconceptional period, we found a crude significant association between Chlamydia and conoventricular VSDs (cOR 5.65); all three conoventricular VSD case mothers who reported an STI reported having a chlamydial infection. A previous study identified a significant, but weak, association between Chlamydia and the grouped outcome of cyanotic CHDs, which includes both Ebstein anomaly and truncus arteriosus (Dong et al., 2016). We were unable to explore the relationship between Chlamydia and these two CHDs in more detail, given the lack of exposed infants.
Lastly, we found an elevated significant association between GUI and encephalocele, but the ORs among those exposed to UTI only and STI only were not elevated or statistically significant. Like holoprosencephaly, encephalocele has been with linked with maternal infections including Zika virus (Hall et al., 2017; Honein et al., 2017). However, encephalocele has not been linked to GUIs in previous studies.
The mechanism by which GUIs act to increase the risk of birth defects is unknown, but considerations may include pathogen-mediated damage, the resulting inflammatory response, or treatment-related effects. We explored associations by different STI-related pathogens, but were hindered by small numbers, resulting in crude estimates with wide confidence intervals for several sub-analyses. Additionally, roughly 20% of cases and controls did not report a specific pathogen. Another potential mechanism is through a maternal inflammatory response to a pathogen. Immune response, and the subsequent change in the expression of immune mediators and cytokines in the female reproductive tract after infection, can result in cell death and changes in gene expression, which could impair embryonic development. (Robertson, Chin, Femia, & Brown, 2018; Robertson, Chin, Schjenken, & Thompson, 2015). If inflammation were an important mediator of birth defects, we would expect that a GUI occurring immediately before conception might also contribute to increased risk of birth defects given that the inflammation from the infection may persist for some time. Finally, GUIs may increase the risk of birth defects through medications used to treat GUIs. The treatments for GUIs vary widely, so it seems unlikely that one medication is responsible for the range of significant associations observed. Studies exploring risk of medications used to treat GUIs, including antibiotics and antiherpetic medications, have been inconsistent (Ahrens et al., 2013; Ailes et al., 2016; Andersen et al., 2013; Hansen et al., 2016; Pasternak & Hviid, 2010; Reiff-Eldridge et al., 2000). We did not explore medications in this analysis.
There are many strengths of the NBDPS, including the use of a multisite, population-based design that used strict inclusion criteria and classification of cases by clinical geneticists (Rasmussen et al., 2003; Reefhuis et al., 2015). Given the large number study population in the NBDPS, we were able to evaluate the associations between GUIs and individual birth defects. Our analysis of NBDPS data, which included births from 1997 to 2011, benefited from the large number of NBDPS participants and included cases and controls analyzed in previous NBDPS publications on GUIs. It is important to acknowledge some of the study limitations. Misclassification of GUIs, and specific types of GUIs, is likely. Maternal self-reported infection during pregnancy is subject to both recognition of the infection and recall of the infection and its timing. GUIs, particularly STIs, are commonly asymptomatic, meaning that a mother may not have symptoms or may be unaware of the infection (Farley, Cohen, & Elkins, 2003; Korenromp et al., 2002). Mothers with an asymptomatic infection would have been classified as unexposed, potentially leading us to underestimate any true associations. The prevalence of GUI in the current analysis (9% of controls and 10% of cases) is lower than expected given the reported prevalence of GUIs during pregnancy and among women of reproductive age in the literature (CDC, 2017; Sheffield & Cunningham, 2005). Mothers were interviewed for NBDPS 6 weeks to 24 months after the estimated date of delivery; relying on retrospective self-reports of infection makes recall bias a concern. Mothers might have misreported or doctors may have misdiagnosed the type of GUI, given the similar symptoms. This would not have impacted our main analysis of GUI, but may have led to misclassification of specific GUI exposure type. Of the 3,407 mothers in our analysis who reported a UTI, 3,221 (95%) reported that the UTI was diagnosed by a doctor. While we do not have similar data from the interview for those mothers reporting an STI, we would expect a large proportion of positive STI reports to have been based on clinical diagnosis.
The number of exposed cases for some birth defects was small, limiting our ability to assess risk, especially in subanalyses by type of infection. Additionally, there may be some uncontrolled confounding by factors not measured, or residual confounding by measured factors. Finally, we conducted many statistical tests, and some of our findings may be due to chance. In our main analysis of 52 birth defects, we would expect to observe approximately two birth defects with a statistically significantly aOR (52 × 0.05 = 2.6) by chance alone. We observed 11 statistically significant associations, and all 11 were elevated aORs. While some of these associations were reported in other studies (e.g., secundum ASD), the majority of our significant findings have only been identified in our study and therefore should be interpreted cautiously until confirmed in other studies. Probable misclassification and underreporting of exposure lead us to be cautious when interpreting our findings. If GUIs do increase the risk of birth defects, this highlights a potential opportunity for pre-pregnancy prevention, given that GUIs are preventable and treatable.
We sought to identify associations between GUIs and birth defects that may generate future hypotheses. Our findings suggest that maternal GUIs in the month before through the third month of pregnancy may increase the risk of several different types of birth defects. Future research could focus on the larger birth defect phenotypes with more robust results, including cleft lip, or on the findings of associations between GUIs and three gastrointestinal birth defects (duodenal, small intestinal, and colonic atresia/stenosis). Given the limitations of the current analysis, other studies better able to identify infections, and pathogens or measure markers of inflammatory response to infections may help to confirm our findings and improve our understanding of the underlying mechanisms. Based on the findings of this research, women who are planning to or who have recently become pregnant should consider talking to their doctors about GUI prevention and treatment.
Supplementary Material
ACKNOWLEDGMENTS
We thank the participating families, scientists, and staff from the NBDPS sites. The manuscript reports findings from a study funded through a cooperative agreement from the Centers for Disease Control and Prevention (U01DD001032). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding information
National Center on Birth Defects and Developmental Disabilities, Grant/Award Number: U01DD001032; Centers for Disease Control and Prevention, Grant/Award Number: U01DD001032
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest or financial disclosures relevant to this manuscript.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Acs N, Banhidy F, Puho E, & Czeizel AE (2008a). No association between maternal recurrent genital herpes in pregnancy and higher risk for congenital abnormalities. Acta Obstetricia et Gynecalagica Scandinavica, 87, 292–299. 10.1080/00016340801898943 [DOI] [PubMed] [Google Scholar]
- Acs N, Banhidy F, Puho EH, & Czeizel AE (2008b). Possible association between acute pelvic inflammatory disease in pregnant women and congenital abnormalities in their offspring: A population-based case-control study. Birth Defects Research Part A, 82, 563–570. 10.1002/bdra.20480 [DOI] [PubMed] [Google Scholar]
- Acs N, Banhidy F, Puho EH, & Czeizel AE (2008c). No association between vulvovaginitis-bacterial vaginosis, related drug treatments of pregnant women, and congenital abnormalities in their offspring - A population-based case-control study. Central European Journal of Medicine, 3, 332–340. 10.2478/s11536-008-0027-9 [DOI] [Google Scholar]
- Ahrens KA, Anderka MT, Feldkamp ML, Canfield MA, Mitchell AA, & Werler MM (2013). Antiherpetic medication use and the risk of gastroschisis: Findings from the National Birth Defects Prevention Study, 1997–2007. Paediatric and Perinatal Epidemiology, 27, 340–345. 10.1111/ppe.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailes EC, Gilboa SM, Gill SK, Broussard CS, Crider KS, Berry RJ, … Reefhuis J (2016). Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, National Birth Defects Prevention Study 1997 to 2011. Birth Defects Research Part A, 106, 940–949. 10.1002/bdra.23570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JT, Petersen M, Jimenez-Solem E, Rasmussen JN, Andersen NL, Afzal S, … Poulsen HE (2013). Trimethoprim Use prior to Pregnancy and the Risk of Congenital Malformation: A Register-Based Nationwide Cohort Study. Obstetrics and Gynecology International, 364526, 1–8. 10.1155/2013/364526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RJ, Chambers CD, Jones KL, Shew SB, MacKenzie TC, Shaw GM, & Jelliffe-Pawlowski LL (2015). Maternal factors associated with the occurrence of gastroschisis. American Journal of Medical Genetics Part A, 167, 1534–1541. 10.1002/ajmg.a.37016 [DOI] [PubMed] [Google Scholar]
- Banhidy F, Acs N, Puho EH, & Czeizel AE (2006). Maternal urinary tract infection and related drug treatments during pregnancy and risk of congenital abnormalities in the offspring. BJOG An International Journal of Obstetrics & Gynaecology, 113, 1465–1471. 10.1111/j.1471-0528.2006.01110.x [DOI] [PubMed] [Google Scholar]
- Banhidy F, Acs N, Puho EH, & Czeizel AE (2010). Birth outcomes among pregnant women with genital warts. International Journal of Gynaecology and Obstetrics, 108, 153–154. 10.1016/j.ijgo.2009.09.010 [DOI] [PubMed] [Google Scholar]
- Botto LD, Lin AE, Riehle-Colarusso T, Malik S, & Correa A (2007). Seeking causes: Classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Research Part A, 79, 714–727. 10.1002/bdra.20403 [DOI] [PubMed] [Google Scholar]
- Carter TC, Olney RS, Mitchell AA, Romitti PA, Bell EM, & Druschel CM (2011). Maternal self-reported genital tract infections during pregnancy and the risk of selected birth defects. Birth Defects Research Part A, 91, 108–116. 10.1002/bdra.20749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017). Sexually transmitted disease surveillance 2016. Atlanta: U.S. Department of Health and Human Services; Retrieved from https://www.cdc.gov/std/stats16/default.htm [Google Scholar]
- Cleves MA, Malik S, Yang S, Carter TC, & Hobbs CA (2008). Maternal urinary tract infections and selected cardiovascular malformations. Birth Defects Research Part A, 82, 464–473. 10.1002/bdra.20460 [DOI] [PubMed] [Google Scholar]
- Dong DY, Binongo JN, & Kancherla V (2016). Maternal chlamydia infection during pregnancy and risk of cyanotic congenital heart defects in the offspring. Maternal and Child Health Journal, 20, 66–76. 10.1007/s10995-015-1804-0 [DOI] [PubMed] [Google Scholar]
- Draper ES, Rankin J, Tonks AM, Abrams KR, Field DJ, Clarke M, & Kurinczuk JJ (2008). Recreational drug use: A major risk factor for gastroschisis? American Journal of Epidemiology, 167, 485–491. 10.1093/aje/kwm335 [DOI] [PubMed] [Google Scholar]
- Elliott L, Loomis D, Lottritz L, Slotnick RN, Oki E, & Todd R (2009). Case-control study of a gastroschisis cluster in Nevada. Archives of Pediatrics & Adolescent Medicine, 163, 1000–1006. 10.1001/archpediatrics.2009.186 [DOI] [PubMed] [Google Scholar]
- Farley TA, Cohen DA, & Elkins W (2003). Asymptomatic sexually transmitted diseases: The case for screening. Preventive Medicine, 36, 502–509. [DOI] [PubMed] [Google Scholar]
- Feldkamp ML, Enioutina EY, Botto LD, Krikov S, Byrne JL, & Geisler WM (2015). Chlamydia trachomatis IgG3 seropositivity is associated with gastroschisis. Journal of Perinatology, 35, 930–934. 10.1038/jp.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldkamp ML, Reefhuis J, Kucik J, Krikov S, Wilson A, Moore CA, … Botto LD (2008). Case-control study of self reported genitourinary infections and risk of gastroschisis: Findings from the national birth defects prevention study, 1997–2003. BMJ, 336, 1420–1423. 10.1136/bmj.39567.509074.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferencz C, Loffredo CA, Correa-Villasenor A, & Wilson PD (1997). Genetic and environmental risk factors of major cardiovascular malformations: the Baltimore-Washington Infant Study: 1981–1989 (Vol. 5). Armonk, NY: Futura Publishing Co, Inc. [Google Scholar]
- Hall NB, Broussard K, Evert N, & Canfield M (2017). Notes from the field: Zika virus-associated neonatal birth defects surveillance - Texas, January 2016-July 2017. Morbidity and Mortality Weekly Report, 66, 835–836. 10.15585/mmwr.mm6631a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Andrade SE, Freiman H, Dublin S, Haffenreffer K, Cooper WO, … Davis R (2016). Trimethoprim-sulfonamide use during the first trimester of pregnancy and the risk of congenital anomalies. Pharmacoepidemiology and Drug Safety, 25, 170–178. 10.1002/pds.3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, … Jamieson DJ (2017). Birth defects among fetuses and infants of US women with evidence of possible zika virus infection during pregnancy. JAMA, 317, 59–68. 10.1001/jama.2016.19006 [DOI] [PubMed] [Google Scholar]
- Hosmer DW, & Lemeshow S (1992). Confidence interval estimation of interaction. Epidemiology, 3, 452–456. [DOI] [PubMed] [Google Scholar]
- Korenromp EL, Sudaryo MK, de Vlas SJ, Gray RH, Sewankambo NK, Serwadda D, … Habbema JD (2002). What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? International Journal of STD & AIDS, 13, 91–101. 10.1258/0956462021924712 [DOI] [PubMed] [Google Scholar]
- Metneki J, Puho E, & Czeizel AE (2005). Maternal diseases and isolated orofacial clefts in Hungary. Birth Defects Research Part A, 73, 617–623. 10.1002/bdra.20177 [DOI] [PubMed] [Google Scholar]
- Norgard B, Norgaard M, Czeizel AE, Puho E, & Sorensen HT (2006). Maternal herpes labialis in pregnancy and neural tube defects. Developmental Medicine and Child Neurology, 48, 674–676. 10.1017/s0012162206001411 [DOI] [PubMed] [Google Scholar]
- Pasternak B, & Hviid A (2010). Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA, 304, 859–866. 10.1001/jama.2010.1206 [DOI] [PubMed] [Google Scholar]
- Prakalapakorn SG, Rasmussen SA, Lambert SR, & Honein MA (2010). Assessment of risk factors for infantile cataracts using a case-control study: National Birth Defects Prevention Study, 2000–2004. Ophthalmology, 117, 1500–1505. 10.1016/j.ophtha.2009.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, & Moore CA (2003). Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Research Part A, 67, 193–201. 10.1002/bdra.10012 [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Gilboa SM, Anderka M, Browne ML, Feldkamp ML, Hobbs CA, … Honein MA (2015). The National Birth Defects Prevention Study: A review of the methods. Birth Defects Research Part A, 103, 656–669. 10.1002/bdra.23384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff-Eldridge R, Heffner CR, Ephross SA, Tennis PS, White AD, & Andrews EB (2000). Monitoring pregnancy outcomes after prenatal drug exposure through prospective pregnancy registries: A pharmaceutical company commitment. American Journal of Obstetrics and Gynecology, 182, 159–163. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Chin PY, Femia JG, & Brown HM (2018). Embryotoxic cytokines-Potential roles in embryo loss and fetal programming. Journal of Reproductive Immunology, 125, 80–88. 10.1016/j.jri.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Robertson SA, Chin PY, Schjenken JE, & Thompson JG (2015). Female tract cytokines and developmental programming in embryos. Advances in Experimental Medicine and Biology, 843, 173–213. 10.1007/978-1-4939-2480-6_7 [DOI] [PubMed] [Google Scholar]
- Sheffield JS, & Cunningham FG (2005). Urinary tract infection in women. Obstetrics and Gynecology, 106, 1085–1092. 10.1097/01.AOG.0000185257.52328.a2 [DOI] [PubMed] [Google Scholar]
- Wilson PD, Loffredo CA, Correa-Villasenor A, & Ferencz C (1998). Attributable fraction for cardiac malformations. American Journal of Epidemiology, 148, 414–423. [DOI] [PubMed] [Google Scholar]
- Yazdy MM, Mitchell AA, & Werler MM (2014). Maternal genitourinary infections and the risk of gastroschisis. American Journal of Epidemiology, 180, 518–525. 10.1093/aje/kwu157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.