Abstract
A quality improvement initiative was conducted to provide guidelines for opioid prescribing following mastectomy with immediate reconstruction. Patients undergoing mastectomy with concurrent tissue expander reconstruction were surveyed at their first postoperative visit to determine use of pain medication, satisfaction, and refill rates. Opioid prescriptions were converted to total oral morphine milligram equivalents (MMEs). Guidelines for postdischarge prescriptions were developed. During phase I, 16 patients were surveyed to determine baseline prescribed MMEs and rate of satisfaction. A guideline was subsequently developed to standardize postdischarge prescribing (550 MMEs prescribed average risk vs 900 MMEs high risk), and the survey was repeated (phase II). Median 210 MMEs were used. Of the 23 patients, 1 required a refill, 83% were highly satisfied, and 77% of opioids were unused. Guidelines were further revised to limit prescribed opioids (290 MME average risk vs 450 MME high risk), and the survey was repeated (phase III). A median of 118 MMEs was used. Of the 22 patients, 5 required refills, 73% were highly satisfied, and 53% of opioids were unused. Phase IV included 27 patients. A median of 98 MMEs was used. Two patients required refills, 93% were highly satisfied, and 58% of opioids were unused. Our finding showed that there is significant overprescription of opioids after elective breast surgery. Practice guidelines can reduce the amount of opioids prescribed. Reducing excess opioids available in the community is a noble goal; however, it must be done cautiously, as decreased patient satisfaction can be an unintended consequence.
Abbreviations and Acronyms: IQR, interquartile range; MME, oral morphine milligram equivalents
Opioid overdoses accounted for more than 42,000 deaths in 2016, and it is estimated that 40% of opioid overdose deaths involved prescription opioids.1 The US Department of Health and Human Services (HHS) declared this epidemic a public health emergency and announced a 5-point strategy to fight its devastating consequences.1, 2, 3 An important step in this strategy is developing safe opioid-prescribing practices.
Surgeons account for more than 10% of all opioid prescriptions written in the United States, and patients prescribed opioids after surgery are at risk of opioid dependence and abuse.4, 5 In an effort to develop safe opioid-prescribing practices for patients undergoing surgical procedures, it is critical to understand patients' needs and provide balanced guidelines without over- or underprescribing. Wide variations in opioid prescribing patterns exist, and prescribing guidelines need to be individualized based on procedures and patients' risk factors.5, 6
Our aim is to conduct a multiphase initiative to provide evidence-based practice-management guidelines for discharge-prescribing practices for patients undergoing mastectomy with immediate tissue-expander reconstruction.
Methods
A single-institution quality improvement project was conducted involving adult female patients undergoing mastectomies with concurrent tissue-expander placement from November 2015, to September 2016. Patients with chronic pain or preoperative opioid use were excluded. Patients undergoing both subpectoral and subcutaneous tissue-expander placement were initially included; however, as a result of differences in pain and the limited number of submuscular tissue-expander placement procedures performed, only subcutaneous tissue-expander placement procedures were included in this analysis.7 The initiative was conducted for quality improvement purposes and was exempted by our Institutional Review Board. Patients were surveyed at their first postoperative follow-up visit to determine use of pain medications. The aim was to include approximately 20 patients in each phase. The amount of opioid prescribed was abstracted from the medical record.
Survey
Patients were surveyed at their first postoperative follow-up visit, which is routinely scheduled between 1 and 2 weeks postoperatively. As part of the routine clinic visit, patients were asked to report opioid use for each of the prescriptions they were provided. All prescribed opioids were included in the analysis. Patients were also asked to report their satisfaction with their pain management after discharge using a 0 to 10 Likert scale. We defined “highly satisfied” as a score of ≥9. Finally, patients were asked if they had received any refills, regardless of providing prescriber.
Patient and Procedural Data
Operative factors, including unilateral vs bilateral mastectomy, procedure type (nipple sparing vs skin sparing), and axillary surgery (none, sentinel lymph node biopsy, axillary dissection) were abstracted from the medical record and grouped for analysis. Our standard practice is to premedicate patients undergoing mastectomy with acetaminophen, gabapentin, and celecoxib. Intraoperatively, a standard infiltrate of bupivacaine HCI and liposomal bupivacaine is performed in the surgical field.
A baseline audit of prescribing patterns was first obtained (phase I) followed by 2 additional phases (phases II and III) with the intent of developing opioid-prescribing guidelines. Because of unexpected decline in patient satisfaction and an increase in refill rates, a fourth phase was added before implementing the new guidelines.
Phase I
Sixteen patients were surveyed between November 2015, and December 2015. The MME prescribed at discharge, refill rates, and follow-up patient satisfaction were collected; however, individual patient use after discharge was not obtained during this phase. Thereafter, guidelines for postdischarge acetaminophen, tramadol, and oxycodone prescriptions were developed for average- and high-risk patients. Patients were considered at high risk for postmastectomy pain if they were 65 years of age or less, hospitalization opioid consumption was ≥6, 5-mg oxycodone pills, and if their pain scores were frequently >7.6 These criteria for high-risk patients were followed across all phases. Patients older than 65 years of age were excluded from the high-risk category owing to concern for adverse effects associated with increased use of pain medications. The guidelines were developed after using input from a multidisciplinary team of breast surgeons, plastic surgeons, residents, nurse practitioners/physicians assistants, and pharmacists. Guidelines were approved and disseminated.
Phase II
The survey was repeated after initiating the new standardized guidelines between March and May 2016. During phase II, which included 23 patients, patients were surveyed, and the prescribing guidelines were modified to decrease opioid prescribing further.
Phase III
This phase was conducted between July and September 2016, after implanting the modified guidelines. Twenty-two patients were included. Because of the higher-than-expected refill rates and lower patient-experience scores, an additional modification to the guidelines was required, and a fourth phase was conducted.
Phase IV
The final survey was repeated between December 2016, and February 2017, including 27 patients, and the final opioid-prescribing guidelines were adopted.
Analysis
Opioid prescriptions and use were converted to total oral MMEs. Data from each phase were reviewed individually; comparisons between phases and aggregate analysis were performed.
χ2 tests, Students' t-tests, and Mann–Whitney U tests were used. Analyses were performed using JMP Pro Version 13.0.0 (SAS Institute Inc., Cary NC), and P values <0.05 were considered to be statistically significant.
Guideline Development
A multidisciplinary team, including surgeons, pharmacist, and advanced practitioners, reviewed the data to inform the development of guidelines for opioid prescribing at discharge. Final guidelines were developed, approved, and disseminated for use across the breast-surgery practice.
Results
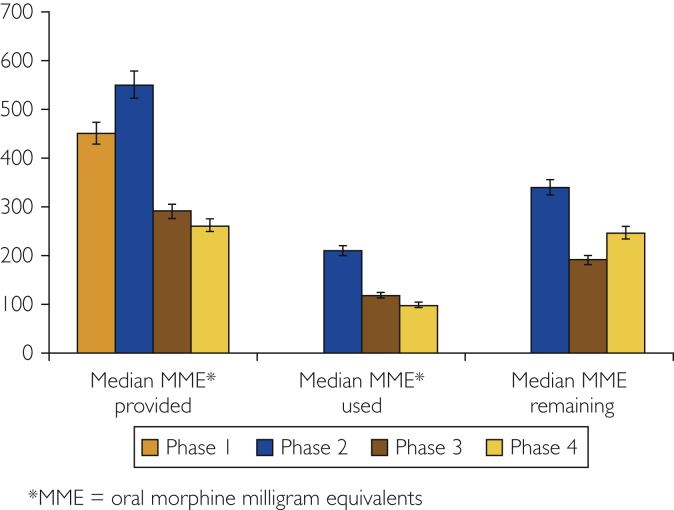
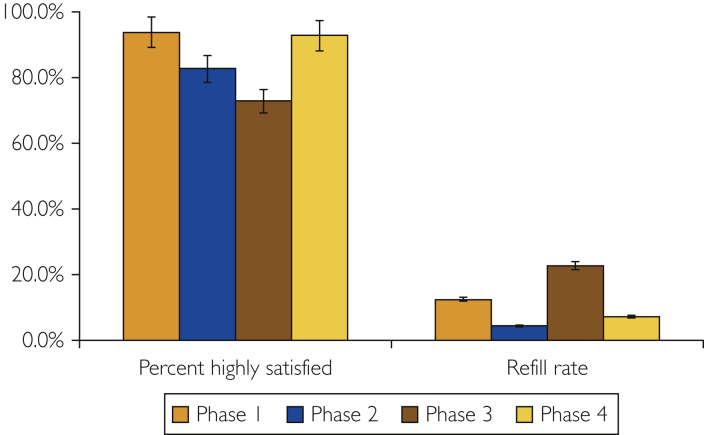
Across all 4 phases, 88 patients who underwent mastectomy with concurrent immediate reconstruction with subcutaneous tissue-expander placement were surveyed. The average age was 50 years, range 27 to 72. Overall, 85% of patients were highly satisfied with their pain control, with a refill rate of 11%. The median prescribed MME was 363, whereas the consumed MME was 123. Unconsumed opioids remaining at the time of survey averaged an MME of 263, and 14 patients (16%) did not take any opioids.
During the baseline assessment, all 16 patients received opioids at discharge. Opioid prescriptions ranged from 225 to 925 MMEs, with patients receiving a median of 450 (interquartile range [IQR] 347-551). Two patients required refills (12.5%), and 93% were highly satisfied with their pain management. Patients who required refills were prescribed 300 and 450 MMEs, respectively.
A guideline of prescribing 550 MMEs for average-risk and 900 MMEs for high-risk patients was established, and the survey was repeated (phase II). During phase II, patients consumed a median of 210 (IQR 0-303) MMEs (n=5 missing); 1 patient required a refill, and 83% were highly satisfied. Six patients did not use any opioids after discharge, and 94% had unconsumed opioids remaining, resulting in 77% of opioids being unused.
Guidelines were revised to further limit prescribed opioids (290 MMEs for average risk vs 450 MMEs for high risk), and the survey was repeated (phase III). During phase III, a median of 118 (IQR 10-290) MMEs were used (n=5 missing). Five patients required refills, and 73% were highly satisfied. Three patients did not use any opioids, and 71% had unconsumed opioids remaining, resulting in 53% of opioids being unused. Given lower patient-reported satisfaction, the guidelines were modified again to prescribe 263 MMEs for average-risk and 425 MMEs for high-risk patients (Table 1).
Table 1.
Number of Prescribed Opioid Tablets for the High- and Low-Risk Groups
| Phase | Average prescribed number of tablets (5-mg oxycodone or 50-mg tramadol) |
|
|---|---|---|
| High-risk group | Low-risk group | |
| I | 42 oxycodone, 12 tramadol | 42 oxycodone, 12 tramadol |
| II | 40 oxycodone, 60 tramadol | 20 oxycodone, 40 tramadol |
| III | 20 oxycodone, 30 tramadol | 12 oxycodone, 20 tramadol |
| IV | 23 oxycodone, 34 tramadol | 18 oxycodone, 32 tramadol |
During the final phase, a median of 98 (IQR 0-250) MME was consumed (n=13 missing). Two patients required refills, and 93% were highly satisfied (Table 2). Five patients did not use any opioids after discharge, and 79% had unconsumed opioids remaining, resulting in 58% of opioids being unused. Comparison between phases is shown in Figures 1 and 2.
Table 2.
Phases Summary
| Phase | Number of patients | Patients in the high-risk group, n (%) | Median MME prescribed | Median MME consumed | MME unused (%) | Number of patients requiring Refills | Satisfaction (%) |
|---|---|---|---|---|---|---|---|
| I | 16 | NA | 450 MME (range, 225-925) | N/A | N/A | 2 | 93% |
| II | 23 | 2 (9%) | 550 MME (average risk), 900 MME (high risk) | 210 MME | 77% | 1 | 83% |
| III | 22 | 2 (9%) | 290 MME (average risk), 450 MME (high risk) | 118 MME | 53% | 5 | 73% |
| IV | 27 | 6 (22%) | 263 MME (average risk), 425 MME (high risk) | 98 MME | 58% | 2 | 93% |
Abbreviation: MME = oral morphine milligram equivalents.
Figure 1.
Prescribed, used, and remaining opioids across all phases. MME = oral morphine milligram equivalents.
Figure 2.
Highly satisfied and refill rates across all phases.
Following phase IV, a final version of the prescribing guidelines was adopted (see Supplemental Appendix, available online at http://mcpiqojournal.org).
Discussion
Our findings have demonstrated that our opioid prescribing practice was extremely varied, based on each individual provider. Through a practice assessment followed by a prospective, evidence-based patient assessment and iterative learning, we were able to establish guidelines that maintained high patient satisfaction while decreasing our opioid prescribing by 58%, without an unintended consequence of increased refills. Our guidelines were not only standardized across the practice but, importantly, followed with high adherence.
Initial survey suggested that patients had overall good experiences with their pain control and low refill rates. However, there was significant overprescription of opioids, as all patients received more than the maximal amount of opioids recommended by state and federal guidelines, which resulted in excessive unused opioids.8, 9 Therefore a multiphase quality improvement initiative was conducted to determine the optimal number of opioids to prescribe. These findings should guide providers in their prescribing practices, and similar methodology should be used for all common surgical procedures. Although initially planned as a 3-phase study, a fourth phase was added because of the higher-than-expected refill rates and lower patient-experience scores seen in phase III. With an additional slight adjustment to the prescribing guidelines, we were able to maintain a low number of opioids prescribed while ensuring that patient experience and refill rates were back at baseline. There was not a statistical significant difference in satisfaction rates between phases II and III, because of the small sample sizes. However, our results can provide useful guidance to others interested in conducting iterative guideline evaluations.
Previous studies aimed at reducing opioid prescribing did not see a similar decline in refill rates.10, 11 However, they also were not able to assess patient satisfaction, and no other studies have included a multiphase reduction with associated quality improvement initiative design as ours did.
Several guidelines are being adopted to limit the amount of unnecessary prescribed opioids.5, 6 The Centers for Disease Control (CDC) recommend that opioid treatment of acute pain should be limited to less than 7 days.12 Many state and federal guidelines have followed suit, limiting the length of opioid prescriptions to 7 days.8, 9 Although treating postsurgical pain for less than 1 week might be enough for most surgical procedures, finding a balanced treatment without under- or overtreating remains a challenge.13 In an effort to find the optimal balance, several studies were conducted to identify the needs of patients undergoing different surgical procedures. The amount of opioid required varies based on many patient-related factors and the surgical procedure.5, 14 Understanding that 1 size will not fit all, we categorized patients into 2 groups—low risk vs high risk—for postoperative pain, based on in-hospital pain scores and opioid consumption before discharge. In the final guidelines, the high-risk group was prescribed 30 tablets of 5-mg oxycodone and 30 tablets of 50-mg tramadol; the low-risk group received 15 tablets of 5-mg oxycodone and 20 tablets of 50-mg tramadol. Acetaminophen is prescribed as a first-line agent for pain control, followed by tramadol as a second-line agent, to minimize the amount of oxycodone required. We provided patients with oxycodone as a third-line agent for breakthrough pain that is not controlled by acetaminophen and tramadol.
Many studies showed a strong association between opioid-prescribing patterns and devastating consequences.2, 3 The majority of abused opioids are obtained either from direct prescriptions or through diverted ways of unused prescribed opioids.15 A substantial number of opioid overdoses were related to prescriptions provided by surgeons, and approximately 77% of patients undergoing surgical procedures report having leftover opioids.6 This large proportion of unused opioids is at significant risk of diversion and misuse. Thus, limiting the amount of excess prescribed opioids based on individualized regimens is one of the cornerstones of fighting the opioid epidemic. In our study, the final phase resulted in 58% of opioids being unused while maintaining excellent patient-satisfaction rates. Although this resulted in a reduction of unused opioids compared with baseline, we still need more studies, including patient-reported surveys, to control the amount of leftover opioids.
Although overprescribing opioids is associated with devastating effects, a reduction in opioids prescribed should be pursued cautiously, as it can lead to unintended consequences. Decreasing the prescribed opioids in phase III of our quality initiative was associated with lower patient satisfaction along with higher refill rates. A high request for refills may appear easy for patients to address, as suggested by other studies; however, it does require use of additional institutional personnel resources.6 Acute postsurgical pain is the most common reported concern, and it is reported by 59% of patients.16 The incidence of pain occurring at least 1 year after surgery is higher in patients undergoing mastectomy with immediate reconstruction (49%) compared with patients undergoing mastectomy alone (31%), and women who had reconstruction with breast implants reported higher pain than those without.7 This is very important, as pain after breast surgery can affect quality of life.17 Aside from the ethical importance of adequate pain control, poorly controlled pain can lead to complications and prolonged recovery.18 As many nonopioid alternatives are proving beneficial control of pain, opioid analgesics may play a decreased role in controlling postsurgery pain. Patient satisfaction is closely monitored as a quality measurement and, in some cases, publicly reported, which highlights the complex situation providers can find themselves in trying to balance patients' desires and being globally conscious of the consequences of excess unused opioids in our communities.
Limitations
Our study has multiple limitations, including the single-center design and the small number of participants in each iterative phase. We did not use any pill-counting techniques, and thus our guidelines were developed based on patient-reported results, which is prone to response bias. Given the increased awareness of the opioid epidemic, patients may tend to report more socially desirable responses. Patients who underwent submuscular breast reconstruction were excluded, owing to a different pain pattern and too few patients included in our sample to allow meaningful recommendations. Our standard practice is to premedicate patients with acetaminophen, gabapentin, and celecoxib, as well as to infiltrate a mixture of bupivacaine HCI and liposomal bupivacaine in the surgical wound; thus, our results may not be translatable to practices that do not use these methods. Further studies are needed for ongoing monitoring and optimization of best practices.
Conclusions
Routine postdischarge prescribing practices are associated with wide variability and lead to significant overprescription of opioids after elective breast surgery. Our experience suggests that evidence-based guidelines can reduce the amount of opioids prescribed at discharge. Although reducing excess opioids available in the community is a noble goal, it must be done cautiously, as the increased need for medication refills and decreased patient satisfaction can be unintended consequences.
Footnotes
Grant Support: No financial support or fund was provided for this project.
Potential Competing Interests: The authors have no conflicts of interest to disclose related to the content of this manuscript.
Supplemental Online Material
Supplemental material can be found online at http://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for all data.
References
- 1.U.S. Department of Health and Human Services. https://www.hhs.gov/opioids/about-the-epidemic/index.html Available at: [DOI] [PubMed]
- 2.Bohnert A.B., Valenstein M., Bair M.J., et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell J.C. The prescription drug epidemic in the United States: a perfect storm. Drug Alcohol Rev. 2011;30(3):264–270. doi: 10.1111/j.1465-3362.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- 4.Hooten W.M., St Sauver J.L., McGree M.E., Jacobson D.J., Warner D.O. Incidence and risk factors for progression from short-term to episodic or long-term opioid prescribing: a population-based study. Mayo Clin Proc. 2015;90(7):850–856. doi: 10.1016/j.mayocp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiels C.A., Anderson S.S., Ubl D.S., et al. Wide variation and overprescription of opioids after elective surgery. Ann Surg. 2017;266(4):564–573. doi: 10.1097/SLA.0000000000002365. [DOI] [PubMed] [Google Scholar]
- 6.Thiels C.A., Ubl D.S., Yost K.J., et al. Results of a prospective, multicenter initiative aimed at developing opioid-prescribing guidelines after surgery. Ann Surg. 2018;268(3):457–468. doi: 10.1097/SLA.0000000000002919. [DOI] [PubMed] [Google Scholar]
- 7.Wallace M.S., Wallace A.M., Lee J., Dobke M.K. Pain after breast surgery: a survey of 282 women. PAIN. 1996;66(2-3):195–205. doi: 10.1016/0304-3959(96)03064-3. [DOI] [PubMed] [Google Scholar]
- 8.Liepert A., Ackerman T. 2016 state legislative year in review and a look ahead. Bull Am Coll Surg. 2016;101(1):35–39. [Google Scholar]
- 9.Johnson C., Johnson C.J. 2017 state legislative year in review and a look toward 2018. Bull Am Coll Surg. 2018;103(5):68–72. [Google Scholar]
- 10.Hill M.V., McMahon M.L., Stucke R.S., Barth R.J.J. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709–714. doi: 10.1097/SLA.0000000000001993. [DOI] [PubMed] [Google Scholar]
- 11.Howard R., Waljee J., Brummett C., Englesbe M., Lee J. Reduction in opioid prescribing through evidence-based prescribing guidelines. JAMA Surg. 2018;153(3):285–287. doi: 10.1001/jamasurg.2017.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowell D., Haegerich T.M., Chou R. CDC guideline for prescribing opioids for chronic pain: United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lev R., Lee O., Petro S., et al. Who is prescribing controlled medications to patients who die of prescription drug abuse? Am J Emerg Med. 2016;34(1):30–35. doi: 10.1016/j.ajem.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Marcusa D.P., Mann R.A., Cron D.C., et al. Prescription opioid use among opioid-naive women undergoing immediate breast reconstruction. Plast Reconstr Surg. 2017;140(6):1081–1090. doi: 10.1097/PRS.0000000000003832. [DOI] [PubMed] [Google Scholar]
- 15.Manchikanti L., Fellows B., Janata J.W., Pampati V., Grider J.S., Boswell M.V. Opioid epidemic in the United States. Pain Physician. 2012;15(3):ES9–ES38. [PubMed] [Google Scholar]
- 16.Apfelbaum J.L., Chen C., Mehta S.S., Gan T.J. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 17.Vadivelu N., Schreck M., Lopez J., Kodumudi G., Narayan D. Pain after mastectomy and breast reconstruction. Am Surg. 2008;74(4):285–296. [PubMed] [Google Scholar]
- 18.Power I., Barratt S. Analgesic agents for the postoperative period: nonopioids. Surg Clin North Am. 1999;79(2):275–295. doi: 10.1016/s0039-6109(05)70383-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.