Abstract
Background
Bordetella trematum is an infrequent Gram-negative coccobacillus, with a reservoir, pathogenesis, a life cycle and a virulence level which has been poorly elucidated and understood. Related information is scarce due to the low frequency of isolates, so it is important to add data to the literature about this microorganism.
Case presentation
We report a case of a 74-year-old female, who was referred to the hospital, presenting with ulcer and necrosis in both legs. Therapy with piperacillin-tazobactam was started and peripheral artery revascularization was performed. During the surgery, a tissue fragment was collected, where Bordetella trematum, Stenotrophomonas maltophilia, and Enterococcus faecalis were isolated. After surgery, the intubated patient was transferred to the intensive care unit (ICU), using vasoactive drugs through a central venous catheter. Piperacillin-tazobactam was replaced by meropenem, with vancomycin prescribed for 14 days. Four days later, levofloxacin was added for 24 days, aiming at the isolation of S. maltophilia from the ulcer tissue. The necrotic ulcers evolved without further complications, and the patient’s clinical condition improved, leading to temporary withdrawal of vasoactive drugs and extubation. Ultimately, however, the patient’s general condition worsened, and she died 58 days after hospital admission.
Conclusions
Despite being a rare finding, B. trematum is typically associated with the clinical manifestation of disorders that predispose to ulcer development, which can be infected by microorganisms. The combination of antibiotic therapy and surgical debridement plays a key role in preventing systemic infections. Monitoring the appearance of new cases of B. trematum is essential, since it can be an emerging microorganism. Isolating and defining the clinical relevance of unusual bacteria yields a more accurate perspective in the development of new diagnostic tools and allows for assessment of proper antimicrobial therapy.
Keywords: Foot ulcer, diabetic; Bordetella infection; Antimicrobial; Susceptibility breakpoint determination
Background
Bordetella trematum is an infrequent gram-negative coccobacillus [1], typically related to tissue infections. Related information is scarce due to the low frequency of isolates [2]. As laboratories gain greater access to technologies for accurate and specific bacterial identification, rare microorganisms can arise. It is essential to understand the clinical significance of these unusual findings and the need for treatment. We reviewed the published case reports and will be discussing a new one here.
Case presentation
A 74-year-old female patient attended the vascular surgery outpatient clinic and was referred to the hospital for revascularization of the distal arteries. She had necrotic ulcers in both legs, worse in the right. She reported pain, signs of local infection and myiasis on the lateral side of the ankle, tendon exposure, edema, and dry skin, but no signs of acute ischemia. Her underlying diseases were difficult to control: systemic arterial hypertension for 20 years; type II diabetes mellitus (DM) for 13 years; hypothyroidism; a stroke 6 years ago, chronic renal failure class IV; peripheral arterial occlusive disease, and postmenopausal osteoporosis. The patient referred to previous angioplasty performed 1 year earlier on the lower right leg due to peripheral arterial occlusive disease. Upon hospital admission, several sites of infection other than skin and soft tissue were discarded. Laboratory tests showed a normal leukocyte count and reactive C protein of 3.98 mg/dL (reference value: < 0.30 mg/dL). Empiric treatment with piperacillin-tazobactam (4.5 g IV 6/6 h) was initiated, which was prescribed for 5 days.
Two days after admission, surgical debridement was performed. Limb amputation was discussed, but rejected by the patient and family members. During the surgery, a fragment of the ulcer tissue was collected and sent to the hospital’s microbiology laboratory. In the staining procedure, a few gram-positive cocci and gram-negative bacilli were observed. The specimen was submitted for enrichment in the brain-heart infusion broth for 24 h/37 °C and later seeded in 5% sheep blood agar and MacConkey agar, incubated for 37 °C, and presented growth after 24 h. VITEK 2 system (bioMérieux, Marcy l’Etoile, France) identified Enterococcus faecalis, Stenotrophomonas maltophilia, and B. trematum. The isolate was subsequently identified as B. trematum, using VITEK MS (bioMérieux, Marcy l’Etoile, France) and confirmed by 16S rRNA gene sequencing with Illumina MiSeq (Illumina, San Diego, CA, USA). The oxidase test was negative. MICs were determined by Sensititre gram-negative MIC plate (Thermo Scientific, Waltham, MA, USA) (Table 1).
Table 1.
Case reports associated with Bordetella trematum
| Case report | Clinical information | Infection isolate(s) | Susceptibilty profile of B. trematuma,b | Therapya | Outcome |
|---|---|---|---|---|---|
| Daxboeck et al. (2004) [3] | Male, 82-year-old, with type 2 diabetes mellitus (DM), and infected ulcer on left foot. | Bordetella trematum and Pseudomonas aeruginosa |
Susceptible to AMI, AMC, CTX, CAZ, GEN, IMI, TZP. Resistant to CXM and CIP. |
10-day treatment of AMC and CIP; without clinical improvement; the antimicrobial treatment was discontinued. | Favorable |
| Hernández-Porto et al. (2013) [2] | Female, 76-year-old, with DM, renal failure, peripheral vascular disease, and ulcers in lower extremities | B. trematum and Achromobacter xylosoxidans |
Susceptible to AMI (16 μg/mL), AMC (2 μg/mL), CAZ (8 μg/mL), GEN (4 μg/mL), IMI (0,5 μg/mL), MEM (0.125 μg/mL), TZP (1 μg/mL) and SXT (0.5 μg/mL) Resistant to ATM (> 32 μg/mL), CTX (> 32 μg/mL), CXM (4 μg/mL) and CIP (4 μg/mL). |
21 days with SXT and 14 days with CAZ (initiated 2 weeks after positive culture for B. trematum). | Favorable |
| Halim et al. (2014) [4] | Male, 60-year-old, no history of pathologies, presenting thorax burns by butane gas | B. trematum and Enterobacter cloacae in blood culture |
Susceptible to CIP, CLA, CL, DOX, IMI, MIN and NET. Resistant to AMI, AMC, CTX, CAZ, CF, GEN, and TOB. |
IMI, NET, and CL. | Death |
| Almagro-Molto, Eder, Schubert (Case 1) (2015) [5] | Male, 65-year-old, with DM, peripheral vascular disease and foot ulcer | B. trematum, Staphylococcus aureus, Proteus vulgaris, Alcaligenes faecalis and Morganella morganii |
Susceptible to AMI, AMC, AMP, CPM, GEN, IMI, LVX, MEM, MIN, PIP, TZP, SXT, TGC, and TOB. Resistant to CTX, FOX, CAZ, CXM, CIP, ETP, and FOS. |
Debridement and 7-day course of CIP | Persistence of infection |
| Almagro-Molto, Eder, Schubert (Case 2) (2015) [5] | Female, 72-year-old, suspected of osteomyelitis, bone defects in the feet and ankles, venous disorder, impaired renal function, ulcer in both feet | B. trematum, P. vulgaris and A. faecalis in both feet ulcer exsudate samples/B. trematum, MRSA, A. faecalis and S. maltophilia in the surgical sample of ulcer of both feet |
Susceptible to AMI, AMC, AMP, CPM, GENc, IMI, LVXc, MEM, MIN, PIP, TZP, SXT, TGC, and TOB. Intermediate to ETP and LVXc Resistant to CTX, FOX, CAZ, CXM, CIP, FOS, and GENc. |
Compression therapy and 14-day course of CIP; despite clinical improvement, both limbs were amputated after 3 weeks; antimicrobial therapy with TZP (7 days) followed by MEM (7 days) | Favorable |
| Saksena, Manchanda, Mittal (2015) [6] | Young girl, 7-month-old, febrile, presenting vomiting; provisional diagnosis of infantile tremor syndrome with protein energy malnutrition and developmental delay | B. trematum in blood culture |
Susceptible to AMS (8 μg/mL), CIP (1 μg/mL), IMI (1 μg/mL), TZP (8. μg/mL) and SXT (20 μg/mL) Intermediate to CPM (16 μg/mL), CRO (32 μg/mL), LVX (4 μg/mL), PIP (64 μg/mL) and TOB (8 μg/mL) Resistant to AMI (64 μg/mL), CAZ (64 μg/mL), CL (16 μg/mL), GEN (16 μg/mL), MEM (16 μg/mL) |
Empirical therapy with CRO for 5 days; then the treatment switched to TZP and AMI; on the 12th day, therapy was modified to CIP and AZM (5 days) | Favorable |
| Almuzara et al. (2015) [7] | Male, 14-year-old, febrile and hemodynamically unstable, presenting left hip septic arthritis. Diagnosis of chronic osteomyelitis (S. aureus) | Escherichia coli and B. trematum in bone biopsy |
Susceptible to AMI (16 μg/mL), CPM (4 μg/mL), CAZ (4 μg/mL), CF (8 μg/mL), CL (≤ 5 μg/mL), GEN (4 μg/mL), IMI (≤ 1 μg/mL), MEM (≤ 0.25 μg/mL) and SXT (≤ 2 μg/mL) Intermediate to AMP (16 μg/mL), AMS (16 μg/mL), CTX (32 μg/mL) and CIP (2 μg/mL) |
Multiple surgical debridement, antimicrobial treatment with MEM and SXT for 6 months; MIN later | Favorable |
| Majewski et al. (2016) [8] | Transgender male, 61-year-old, with below-knee amputations in both lower limbs, DM, stage IV chronic kidney disease, and coronary artery disease, with a right femur fracture after a fall, respiratory distress, septic shock and worsening of left leg wound after hospitalization | B. trematum in blood culture |
Susceptible to AMI, AMC, CAZ (8 μg/mL), CIP (0,008 μg/mL), IMI (0.25 μg/mL), LVX (0,03 μg/mL), TZP (2 μg/mL) and TOB Intermediate to CTX (16 μg/mL) Resistant to CXM and GEN |
Empirical treatment with TZP and VAN; the left lower limb infection worsened, treatment switch to CIP; due to necrotizing fasciitis probability CLI was added; persistence of septic shock, TOB added | Death |
| Current report | Female, 74-year-old, with necrotic ulcers in both legs, presenting signs of local infection, systemic arterial hypertension; DM; hypothyroidism; peripheral arterial occlusive disease; stage IV chronic kidney disease | B. trematum, E. faecalis and S. maltophilia in a tissue fragment | MIC ≤025 μg/mL to TGC, CLI and PB; MIC 0.5 μg/mL to DOR; MIC 1 μg/mL to CIP, LVX, IMI and MEM; MIC 2 μg/mL to DOX, GEN, MIN, TOB and CPM; MIC 4 μg/mL to AMI and CAZ; MIC 8 μg/mL to CTX; MIC 4/2 μg/mL to AMS; MIC 8/4 μg/mL to TZP; MIC 9.5/4.5 μg/mL to SXT MIC 16/2 μg/mL to TIM; MIC > 16 μg/mL to ATM | Empirical treatment with TZP and surgical debridement; treatment switch to VAN and MEM due to septic shock; LVX added aiming S. maltophilia | Death |
aAMI amikacin, AMC amoxicillin-clavulanic acid, AMP ampicillin, AMS ampicillin-sulbactam, AZM azithromycin, ATM aztreonam, CPM cefepime, CTX cefotaxime, FOX cefoxitin, CAZ ceftazidime, CRO ceftriaxone, CXM cefuroxime, CIP ciprofloxacin, CLA clarithromycin, CLI clindamycin, CF cephalothin, CL colistin, DOR doripenem, DOX doxycycline, ETP ertapenem, FOS fosfomycin, GEN gentamicin, IMI imipenem, LVX levofloxacin, MEM meropenem, MIN minocycline, NET netilmicin, PB polymyxin B, PIP piperacillin, TIM ticarcillin-clavulanate, TZP piperacillin-tazobactam, SXT sulfamethoxazole-trimethoprim, TGC tigecycline, TOB tobramycin, VAN vancomycin, MIC minimum inhibitory concentration, MRSA methicillin-resistant Staphylococcus aureus
bMethodology for susceptibility testing was the E-test [2, 3, 5, 6]; disk-diffusion [2, 4, 8]; broth microdilution [3, 6]; VITEK 2 [5, 7]
cAfter amputation of lower limbs, change was seen in susceptibility profile in the second isolate
After surgery, the intubated patient was transferred to the ICU, using vasoactive drugs through a central venous catheter. Three days later, she presented a worsening clinical condition. Oxacillin-resistant Staphylococcus hominis was isolated from a blood culture drawn through a peripheral vein. Piperacillin-tazoctam was replaced by meropenem (500 mg IV 24/24 h) and vancomycin (1 g IV 24/24 h), prescribed for 14 days. Four days later, levofloxacin (750 mg IV 24/24 h) was added for 24 days aiming at S. maltophilia isolated from the ulcer tissue.
The necrotic ulcers evolved without further complication and the patient’s clinical condition improved, leading to temporary withdrawal of the vasoactive drugs and extubation. However, the patient’s general condition and kidney function worsened, probably due to the severity of her underlying diseases, and she died from sepsis of cutaneous origin 58 days after hospital admission. Autopsy was not performed. Figure 1 shows a timeline of the events.
Fig. 1.
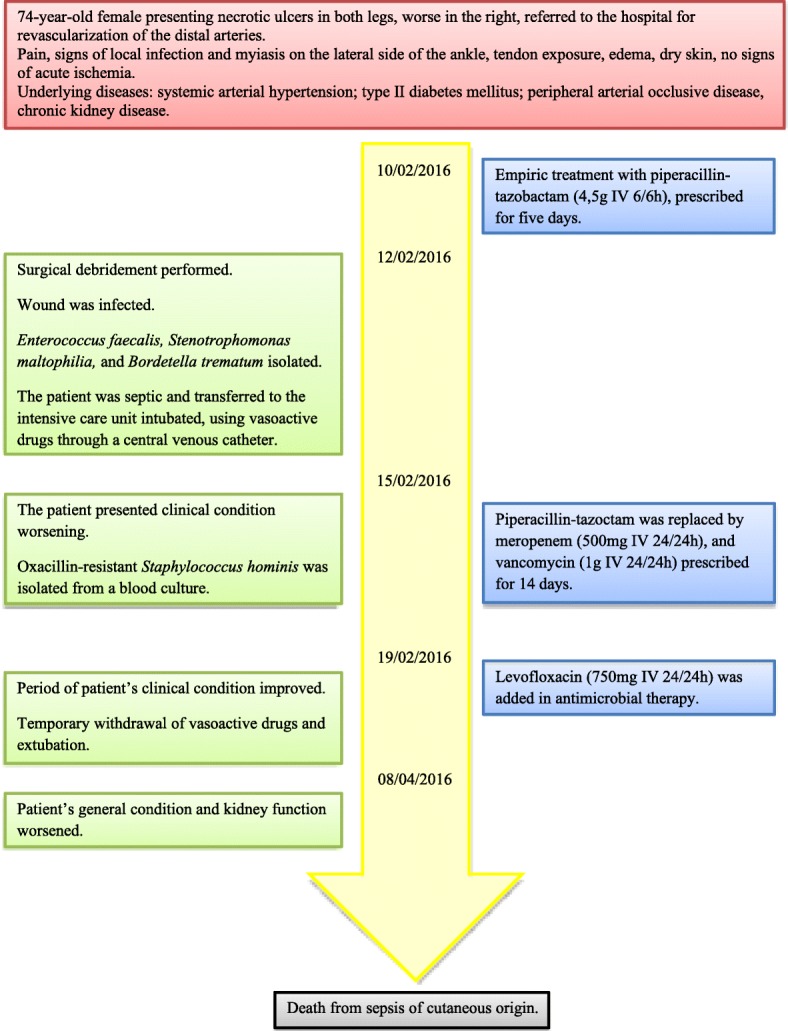
A timeline of the main events of the patient’s illness
Discussion and conclusions
B. trematum was described in 1996, isolated from human wounds and ear infections [1]. Information about its reservoir, life cycle and pathogenesis remain unknown. Regarding virulence, little is known [5]. Typically associated with tissue infections in diabetic patients, and generally occurs in polymicrobial infections, which further complicates its clinical interpretation [2]. In our case, where E. faecalis and S. maltophilia were isolated with B. trematum in the ulcer, the role of B. trematum in the patient’s prognosis became unclear. However, as this microorganism was previously reported as a causative agent of bloodstream infection [4, 6, 8], its interpretation and implication in disease was challenging and required integration of clinical, epidemiological, and microbiological issues.
When first described, B. trematum presented the following phenotypic characteristics: non-glucose metabolizer, grown on MacConkey agar, motile, with variable nitrate reduction, catalase and citrate positive, urease, oxidase and lysine decarboxylase negative [1]. Methodologies such as MALDI-TOF MS and VITEK 2 system were efficient in identifying B. trematum. However, there are reports that demonstrate problems regarding microorganism misidentification by API 20 NE (bioMérieux, Marcy l’Etoile, France), due to the absence of B. trematum in its identification database [2]. In another case, also using API 20 NE, B. trematum was misidentified as Achromobacter denitrificans/Bordetella bronchiseptica. This may have occurred because the nitrate reduction test was variable, and the oxidase test reagent was different from the one used by other researchers [7]. In some cases, the confirmation of the microorganism identification was carried out by 16S rRNA gene sequencing [3–5, 7, 8]. In our case, the isolate was correctly identified by the VITEK 2 system and MALDI-TOF MS and confirmed by 16S rRNA gene sequencing. Even with credible identification by using routine laboratory instruments, the available literature only reports a few cases, as summarized in Table 1.
There is no standardized methodology by the Clinical Laboratory Standard Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing that performs an antimicrobial susceptibility test, specifically for B. trematum. Some authors have used the CLSI manual as an interbreeding source [2, 5–8], cited as the MIC interpretative standards for other Non-Enterobacteriaceae and MIC interpretative standards for Enterobacteriaceae, along with the use of antibiotics such as ampicillin and cephalothin.
Analyzing antimicrobial susceptibility tests performed by other authors (Table 1), B. trematum has always shown sensitivity to piperacillin-tazobactam, which was initially used as an empirical therapy. Indeed, for this antimicrobial, we obtained the same MIC (≤ 8/4 μg/mL) as reported by Saksena et al. (2015), which may have been a contributing factor to the favorable outcome of the surgical wound. Cefotaxime showed MIC 32 μg/mL in other studies [2, 7], as it is considered to be an intermediate resistant. The MIC in our study (8 μg/mL) was lower than that described by other authors. However, it is not possible to define whether this MIC represents susceptibility. The problems with MIC interpretation are probably due to lack of in vitro/in vivo susceptibility correlation or breakpoints [6].
Since B. trematum infection/colonization occurs primarily in wounds, debridement is essential for treatment, because it removes pathogens from nonviable tissues and reduces recurrence of the infection. Debridement associated with the correct use of antibiotics can lead to a favorable outcome [5]. However, there were no further cultures to determine whether B. trematum was actually eradicated.
The pathogenicity of this microorganism is not well-established, so it is unclear what the contribution of B. trematum was to the patient’s infection and outcome. Despite being a rare finding, B. trematum is typically associated with clinical manifestations of disorders that predispose to the development of ulcers that can become infected by microorganisms. The combination of antibiotic therapy and surgical debridement plays a key role in the cure, preventing systemic infections. Monitoring the appearance of new cases of B. trematum is essential, as it could be an emerging microorganism. Isolating and defining the clinical relevance of unusual bacteria facilitates an overall perspective towards the development of new diagnostic tools and allows for assessment of proper antimicrobial therapy.
Acknowledgments
We are grateful to the Microbiology Section of the Laboratório de Análises Clínicas do Hospital Universitário de Santa Maria for the isolation and initial identification of Bordetella trematum. We also thank the Microbiology Laboratory of the Central Laboratory Division at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo and the Laboratory of Bacteriology LIM-54 linked to the Departamento de Doenças Infecciosas e Parasitárias da Faculdade de Medicina da Universidade de São Paulo for confirming the microorganism by MALDI-TOF MS and sequencing the 16S rRNA gene, respectively.
Funding
Thaís R. y Castro received a Master’s degree grant provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Proccess Number 1740547. CAPES is a Governmental Agency that provides grants for graduate students.
Availability of data and materials
Data sharing is not applicable to this article, since no datasets were generated or analyzed during the current study.
Abbreviations
- CLSI
Clinical Laboratory Standards Institute
- MIC
Minimal Inhibitory Concentration
Authors’ contributions
TC, PAT and AVS were major contributors in writing the manuscript and analyzing the patient records; RCRM and SFC performed 16 s rRNA sequencing, NLFDF, LS, and FR performed laboratory phenotypic identification of the isolate. All authors have approved the revisions and the submitted version. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Ethics approval and consent to participate
The study was reviewed and approved by the National Ethical Committee in Human Research, Brazil (Deliberation number 59563316.6.0000.5346).
Consent for publication
As the patient had already died, written consent to publish was obtained from her son.
Competing interests
Dr. Schwarzbold reports grants from Merck Co., Astellas, Gilead, outside of the submitted work. All other authors have no conflict of interest to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thaís Regina y Castro, Email: thais.y.c@hotmail.com.
Roberta Cristina Ruedas Martins, Email: betacristina@gmail.com.
Nara Lúcia Frasson Dal Forno, Email: naralfdf@gmail.com.
Luciana Santana, Email: luciana.santana@hc.fm.usp.br.
Flávia Rossi, Email: f.rossi@hc.fm.usp.br.
Alexandre Vargas Schwarzbold, Email: alexvspoa@gmail.com.
Silvia Figueiredo Costa, Email: silviacosta@usp.br.
Priscila de Arruda Trindade, Phone: +555532208464, Email: priscila.trindade@gmail.com.
References
- 1.Vandamme P, Heyndrickx M, Vancanneyt M, Hoste B, De Vos P, Falsen E, et al. Bordetella trematum sp. nov., isolated from wounds and ear infections in humans, and reassessment of Alcaligenes denitrificans Rüger and tan 1983. Int J Syst Bacteriol. 1996;46:849–858. doi: 10.1099/00207713-46-4-849. [DOI] [PubMed] [Google Scholar]
- 2.Hernández-Porto M, Cuervo M, Miguel-Gómez MA, Delgado T, Lecuona M. Diabetic leg ulcer colonized by Bordetella trematum. Rev Esp Quimioter. 2013;26:72–73. [PubMed] [Google Scholar]
- 3.Daxboeck F, Goerzer E, Apfalter P, Nehrt M, Krause R. Isolation of Bordetella trematum from a diabetic leg ulcer. Diabet Med. 2004;21:1247–1248. doi: 10.1111/j.1464-5491.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- 4.Halim I, Ihbibane F, Belabbes H, Zerouali K, El Mdaghri N. Isolement de Bordetella trematum au décours d’une bactériémie. Ann Biol Clin (Paris). 2014;72:612–4. Available from: https://www.jle.com/fr/revues/abc/e-docs/isolement_de_bordetella_trematum_au_decours_dune_bacteriemie_302630/article.phtml. [DOI] [PubMed]
- 5.Almagro-Molto M, Eder W, Schubert S. Bordetella trematum in chronic ulcers: report on two cases and review of the literature. Infection. 2015;43:489–494. doi: 10.1007/s15010-014-0717-y. [DOI] [PubMed] [Google Scholar]
- 6.Manchanda V, Mittal M, Saksena R. Bordetella trematum bacteremia in an infant: A cause to look for. Indian Journal of Medical Microbiology. 2015;33(2):305. doi: 10.4103/0255-0857.154891. [DOI] [PubMed] [Google Scholar]
- 7.Almuzara M, Barberis C, Traglia G, Sly G, Procopio A, Vilches V, et al. Isolation of Bordetella species from unusual infection sites. JMM case rep. 2015:1–7. Available from: https://jmmcr.microbiologyresearch.org/content/journal/jmmcr/10.1099/jmmcr.0.000029.
- 8.Majewski Lorrance L., Nogi Masayuki, Bankowski Matthew J., Chung Heath H. Bordetella trematum sepsis with shock in a diabetic patient with rapidly developing soft tissue infection. Diagnostic Microbiology and Infectious Disease. 2016;86(1):112–114. doi: 10.1016/j.diagmicrobio.2016.05.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, since no datasets were generated or analyzed during the current study.


