Abstract
Automated continuous noninvasive ward monitoring may enable subtle changes in vital signs to be recognized. There is already some evidence that automated ward monitoring can improve patient outcome. Before automated continuous noninvasive ward monitoring can be implemented in clinical routine, several challenges and problems need to be considered and resolved; these include the meticulous validation of the monitoring systems with regard to their measurement performance, minimization of artifacts and false alarms, integration and combined analysis of massive amounts of data including various vital signs, and technical problems regarding the connectivity of the systems.
Keywords: Hemodynamic monitoring, Postoperative complications, Blood pressure, Hypotension, Peripheral oxygen saturation, Hypoxemia, Remote monitoring, Normal ward, Artifacts, False alarms
Patient monitoring by definition is the repeated or continuous observation of vital signs or physiologic functions to ensure patient safety and guide therapeutic interventions. Today, most advanced cardiorespiratory monitoring systems depend on invasive sensors, cables, and bulky monitors to recognize, transfer, process, and display the bio-signals to be monitored. Therefore, continuous advanced cardiorespiratory monitoring is mainly restricted to the intensive care unit, the operating room, and the post anesthesia care unit. Most other monitoring in the hospital continues to be basic and intermittent—including monitoring on medical and surgical general care wards. When at home, before-and-after hospital admission, patients are usually not monitored at all [1].
While most advanced monitoring is in place in intensive care units, nearly half of all adverse events in hospitalized patients occur on the general care ward [2–4]. Ironically, this area—also referred to as “the patient’s room”—is traditionally regarded as a place of recovery for the more stable medical or surgical patients, who will (in the absence of setbacks) transition to leave the hospital. In addition, the European Surgical Outcomes Study (EuSOS) [5] revealed that about three quarters of patients who died in the hospital after surgery were not admitted to an intensive care unit at any stage after surgery; this indicates that the general care ward plays a pivotal role in the care for patients in the postoperative period, a period in which patients are especially prone to developing clinical deterioration and life-threatening complications [5, 6]. Not only are catastrophic cardiorespiratory events common in general care ward environments, their outcomes are significantly worse compared with similar events in monitored intensive care units. For example, a large national registry identified 44,551 index events across more than 300 US hospitals [7]. More importantly these acute respiratory events on inpatient wards had an associated in-hospital mortality of approximately 40% [7].
Current ward monitoring protocols typically consist of intermittent spot checks by a nurse about every 4–8 h. This leaves patients unmonitored for most of the time during their hospital stay [8]. Alterations in vital signs as warning signs of clinical deterioration are frequently not or only belatedly recognized by the conventional spot check-based monitoring strategy. In hospitalized patients recovering from non-cardiac surgery, severe prolonged hypoxemia is common and unfortunately seriously underestimated using intermittent vital sign checks [9, 10]. Similar patterns regarding the rate of recognized abnormal vital signs were observed for tachycardia, bradycardia, tachypnea, and bradypnea [10]. In patients recovering from abdominal surgery on the general care ward, postoperative hypotension (mean arterial pressure < 65 mmHg for ≥ 15 min) has been shown to occur in about one fifth of patients and not to be recognized by routine vital sign assessments in about half of the cases [11]. In addition to missing critical changes in vital signs, the recognition of abnormal vital signs by a bedside nurse often triggers a long chain of commands resulting in delays until an intervention can be taken [12].
A closed claims analysis of opioid induced respiratory compromise on the general care ward identified nearly half of all these events occurred within 2 h of the last nursing check [13]. In addition, the authors concluded that nearly all of these events would have been prevented by better continuous monitoring and education [13]. Considering that most nursing spot checks of vital signs leave gaps of about 4 h in-between two consecutive assessments, this period is associated with the highest vulnerability.
Automated continuous noninvasive ward monitoring is a promising approach to closely follow changes in vital signs over time and thus identify patients who are deteriorating in a timely fashion (Fig. 1). The rationale behind continuous ward monitoring is that most hospitalized patients do not deteriorate all of a sudden. Although complications often become clinically apparent as acute cardiocirculatory or respiratory failure and acute changes in consciousness, we know for a long time that subtle abnormalities in vital signs usually precede these life-threatening conditions, sometimes by 6–12 h [12, 14–16]. Subtle changes in blood pressure, heart rate, respiratory rate, or oxygen saturation are early signs of clinical deterioration eventually leading to adverse events [12, 16]. Automated continuous noninvasive ward monitoring may enable a patient’s clinical deterioration to be identified well before a serious adverse event occurs [16]. Further, novel monitoring technologies also may enable advanced hemodynamic variables such as stroke volume, cardiac output, and dynamic cardiac preload parameters to be monitored continuously in patients on the general care ward [17–19]; to date, these variables—in contrast to surgical or critically ill patients—play no role in the treatment of patients in this environment.
Fig. 1.
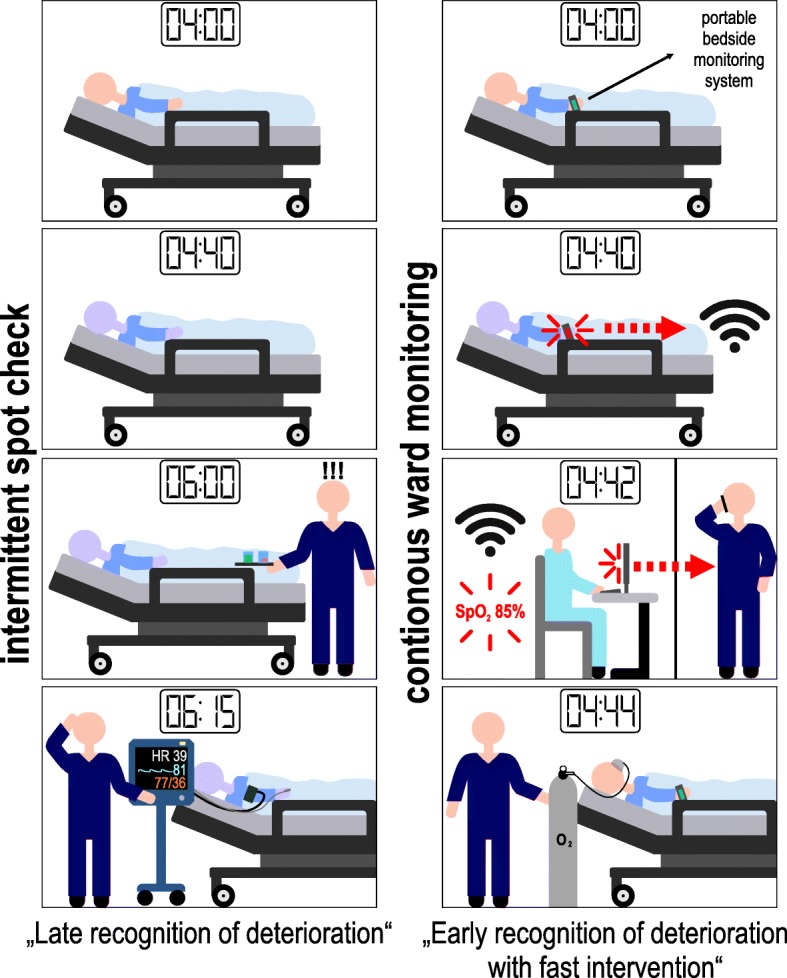
Automated continuous noninvasive ward monitoring allows the healthcare provider to closely follow changes in vital signs over time and identify patients who are deteriorating earlier than conventional intermittent spot check monitoring. Early recognition of clinical deterioration enables rapid therapeutic interventions which may be life saving in certain situations
There is already some evidence that intensified and automated ward monitoring of vital signs may improve patient outcome by a reduction of rescue events [1]. Before-and-after studies showed that the deployment of an electronic automated advisory vital signs monitoring and notification system is associated with significant improvements in key patient-centered clinical outcomes in patients treated on the normal ward [20, 21]. In an orthopedic ward, the implementation of a continuous pulse oximetry surveillance system linked to a nursing notification system reduced the number of rescue events from 3.4 to 1.2 per 1000 patient discharges and also reduced the rate of intensive care unit transfers (before-and-after study) [22]. In another study, the implementation of a system allowing for continuous monitoring of heart and respiratory rate in a medical-surgical unit was associated with lower “code blue” rates (6.3 before to 0.9 after implementation per 1000 patients) [23].
Although continuous ward monitoring is not standard of care today, innovative monitoring systems—in theory—would already allow us to noninvasively and continuously monitor heart rate, blood pressure, respiratory rate, oxygen saturation, skin temperature, body posture, activity, and location within the hospital [24–28]. Battery powered, wearable or adhesive, wireless monitoring systems that communicate with mobile devices or patient monitors may—in the near future—give hospitalized patients the freedom to move within their rooms and the hospital while being monitored [25, 28]. While, the best-case scenario would be the implementation of universal continuous smart monitoring for all inpatients, there may also be value in attempting to identify the highest risk strata of those most likely to face sudden unprecedented episodes of cardiorespiratory compromise. Novel scores such as PRODIGY developed using continuous capnography and oximetry may help the perioperative clinician in early interventions using a combination of better monitoring and other proactive strategies to avert future problems [29].
Furthermore, before automated continuous noninvasive ward monitoring becomes a reality in routine clinical care outside of studies, several problems and limitations need to be considered. Most importantly, monitoring systems need to be reliable, accurate, and be able to provide readings of vital signs with a low rate of artifacts and false alarms. However, some of the currently available monitoring systems lack clinically acceptable accuracy and precision [30]. Therefore, meticulous validation needs to precede the use of novel cardiorespiratory monitoring systems in studies or clinical practice. Especially for blood pressure, a key hemodynamic variable, reliable continuous noninvasive monitoring is technically challenging and unavailable in most smart portable systems [27]. Some monitoring systems still suffer from high rates of artifactual readings and false alarms [31]. Not only is the problem of false alarms a nuisance, it will almost always lead to an increasing level of alarm fatigue within bedside providers, the so called first-responders to needless alarms. In this regard, some vital signs are more prone to artifacts and false alarms than others—for example, capnography as a measure of ventilation being one that has always been a prime suspect for this. Frequent and false alarms may be automatically identified and reduced by cross-checking and machine learning algorithms [32–34].
Automated continuous noninvasive ward monitoring of a variety of vital signs with one or more sensors will create a massive amount of data that need to be processed in real-time, stored, and secured. These data reflecting different bio-signals will need to be integrated and analyzed together to allow the identification of certain patterns of vital sign alterations instead of merely recognizing that single values of single variables are outside of their normal range. Several predictive statistical models have already been developed, validated, and embedded in electronic medical records as automated aggregated “early warning scores” that assign weights to altered vital signs proportionate to their deviation from normal ranges [35–37]. Identifying changes in physiologic variables over time and using machine and deep learning methods may improve the predictive capabilities of these risk stratification tools [38, 39].
Other challenges concern the technical connectivity between sensors and monitoring systems. While the “internet of things” (i.e., a network of devices, vehicles, and home appliances) became part of our daily life, wireless data transmission and processing are not yet well established in hospitals and other health care facilities. Problems for the implementation of wireless monitoring systems include—but are not limited to—range, power consumption, integration in electronic health records, and cybersecurity [1]. Legal issues regarding data protection and privacy rights are beyond the scope of this article but are essential topics that need to be taken care of before ward monitoring can be implemented in healthcare systems.
Finally, before automated continuous noninvasive ward monitoring can be recommended for routine clinical use, we need to await the results of adequately powered randomized controlled trials demonstrating its effectiveness in improving the quality of care and carefully chosen patient-centered outcomes. As a next step, research may then focus on investigating which patients benefit from expanding ward monitoring to home monitoring in the period after hospital discharge [1, 40].
Conclusions
Automated continuous noninvasive ward monitoring seems to be an intriguing opportunity to timely detect clinical problems by recognizing subtle changes in vital signs and improve patient outcomes on the general care ward. There is already some evidence—mainly from before-and-after studies—that automated ward monitoring can improve patient outcome. From a technical point of view, monitoring systems for automated continuous noninvasive ward monitoring are already available and will be further refined during the next years, probably resulting in small, wireless, and wearable sensors. However, before automated continuous noninvasive ward monitoring can be implemented in clinical routine, several challenges and problems need to be considered and resolved; these include the meticulous validation of the monitoring systems with regard to their measurement performance, minimization of artifacts and false alarms, integration and combined analysis of massive amounts of data including various vital signs, and technical problems regarding the connectivity of the systems. The primary scientific aim, though fairly simple, needs some thought and well-planned trial design, and would look to evaluate in robust and adequately powered trials whether automated continuous noninvasive ward monitoring can improve patient outcome compared with current standard spot-check monitoring. Till such time, it seems rather inappropriate to leave our patients under-monitored and unprotected for large periods of time as they recover from illness on our general care hospital wards.
Acknowledgements
Not applicable
Authors’ contributions
AKK, PH, and BS performed the literature search, drafted the manuscript, created the figure, and approved the final version of the manuscript to be published.
Funding
Not applicable
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
AKK collaborates with Medtronic (Boulder, CO, USA) as a member of the executive advisory board on respiratory monitoring and steering committee member of the PRODIGY trial and receives honoraria for these services including giving lectures and refunds of travels expenses. AKK serves on the clinical advisory board for Retia Medical (Valhalla, NY, USA), Linshom Medical (Ellicott City, MD, USA), and also serves as a consultant for La Jolla pharmaceuticals (San Diego, CA, USA) and as a subject matter expert for the development of the Anesthesia SimStat system for CAE healthcare (Sarasota, FL, USA). BS collaborates with Pulsion Medical Systems (Feldkirchen, Germany) as a member of the medical advisory board and received honoraria for giving lectures and refunds of travel expenses from Pulsion Medical Systems. BS received research support and honoraria for giving lectures from Edwards Lifesciences (Irvine, CA, USA). BS received institutional restricted research grants, honoraria for consulting, and refunds of travel expenses from Tensys Medical (San Diego, CA, USA). BS received honoraria for giving lectures and refunds of travel expenses from CNSystems Medizintechnik (Graz, Austria). BS received institutional restricted research grants from Retia Medical. BS received honoraria for giving lectures from Philips Medizin Systeme Böblingen (Böblingen, Germany). PH declared that he has no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ashish K. Khanna, Email: ashish@or.org
Phillip Hoppe, Email: p.hoppe@uke.de.
Bernd Saugel, Phone: 0049 40 7410 18866, Email: bernd.saugel@gmx.de.
References
- 1.McGillion MH, Duceppe E, Allan K, Marcucci M, Yang S, Johnson AP, Ross-Howe S, Peter E, Scott T, Ouellette C, et al. Postoperative remote automated monitoring: need for and state of the science. Can J Cardiol. 2018;34(7):850–862. doi: 10.1016/j.cjca.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 2.de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Qual Saf Health Care. 2008;17(3):216–223. doi: 10.1136/qshc.2007.023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perman SM, Stanton E, Soar J, Berg RA, Donnino MW, Mikkelsen ME, Edelson DP, Churpek MM, Yang L, Merchant RM, et al. Location of in-hospital cardiac arrest in the United States-variability in event rate and outcomes. J Am Heart Assoc. 2016;5(10):e003638. [DOI] [PMC free article] [PubMed]
- 4.Andersen LW, Berg KM, Chase M, Cocchi MN, Massaro J, Donnino MW. American Heart Association’s Get With The Guidelines-Resuscitation I: acute respiratory compromise on inpatient wards in the United States: incidence, outcomes, and factors associated with in-hospital mortality. Resuscitation. 2016;105:123–129. doi: 10.1016/j.resuscitation.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, Vallet B, Vincent JL, Hoeft A, Rhodes A, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380(9847):1059–1065. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G, Warner M, Lang BH, Huang L, Sun LS. Epidemiology of anesthesia-related mortality in the United States, 1999-2005. Anesthesiology. 2009;110(4):759–765. doi: 10.1097/ALN.0b013e31819b5bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison LJ, Neumar RW, Zimmerman JL, Link MS, Newby LK, McMullan PW, Jr, Hoek TV, Halverson CC, Doering L, Peberdy MA, et al. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations: a consensus statement from the American Heart Association. Circulation. 2013;127(14):1538–1563. doi: 10.1161/CIR.0b013e31828b2770. [DOI] [PubMed] [Google Scholar]
- 8.Leuvan CH, Mitchell I. Missed opportunities? An observational study of vital sign measurements. Crit Care Resusc. 2008;10(2):111–115. [PubMed] [Google Scholar]
- 9.Sun Z, Sessler DI, Dalton JE, Devereaux PJ, Shahinyan A, Naylor AJ, Hutcherson MT, Finnegan PS, Tandon V, Darvish-Kazem S, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg. 2015;121(3):709–715. doi: 10.1213/ANE.0000000000000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duus CL, Aasvang EK, Olsen RM, Sorensen HBD, Jorgensen LN, Achiam MP, Meyhoff CS. Continuous vital sign monitoring after major abdominal surgery-quantification of micro events. Acta Anaesthesiol Scand. 2018;62(9):1200–1208. doi: 10.1111/aas.13173. [DOI] [PubMed] [Google Scholar]
- 11.Turan A, Chang C, Cohen B, Saasouh W, Essber H, Yang D, Ma C, Hovsepyan K, Khanna AK, Vitale J, et al. Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology. 2019;130(4):550–559. doi: 10.1097/ALN.0000000000002626. [DOI] [PubMed] [Google Scholar]
- 12.Jones D, Mitchell I, Hillman K, Story D. Defining clinical deterioration. Resuscitation. 2013;84(8):1029–1034. doi: 10.1016/j.resuscitation.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Lee LA, Caplan RA, Stephens LS, Posner KL, Terman GW, Voepel-Lewis T, Domino KB. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122(3):659–665. doi: 10.1097/ALN.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 14.Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388–1392. doi: 10.1378/chest.98.6.1388. [DOI] [PubMed] [Google Scholar]
- 15.Franklin C, Mathew J. Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Crit Care Med. 1994;22(2):244–247. doi: 10.1097/00003246-199402000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Ogundele O, Clermont G, Hravnak M, Pinsky MR, Dubrawski AW. Dynamic and personalized risk forecast in step-down units. Implications for monitoring paradigms. Ann Am Thorac Soc. 2017;14(3):384–391. doi: 10.1513/AnnalsATS.201611-905OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saugel B, Cecconi M, Hajjar LA. Noninvasive cardiac output monitoring in cardiothoracic surgery patients: available methods and future directions. J Cardiothorac Vasc Anesth. 2019;33(6):1742–52. doi: 10.1053/j.jvca.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Saugel B, Cecconi M, Wagner JY, Reuter DA. Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine. Br J Anaesth. 2015;114(4):562–575. doi: 10.1093/bja/aeu447. [DOI] [PubMed] [Google Scholar]
- 19.Saugel B, Khanna AK. Managing hemodynamic instability - if you want to know cardiac output, you need to measure it! J Crit Care. 2019;49:185–186. doi: 10.1016/j.jcrc.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Bellomo R, Ackerman M, Bailey M, Beale R, Clancy G, Danesh V, Hvarfner A, Jimenez E, Konrad D, Lecardo M, et al. A controlled trial of electronic automated advisory vital signs monitoring in general hospital wards. Crit Care Med. 2012;40(8):2349–2361. doi: 10.1097/CCM.0b013e318255d9a0. [DOI] [PubMed] [Google Scholar]
- 21.Subbe CP, Duller B, Bellomo R. Effect of an automated notification system for deteriorating ward patients on clinical outcomes. Crit Care. 2017;21(1):52. doi: 10.1186/s13054-017-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010;112(2):282–287. doi: 10.1097/ALN.0b013e3181ca7a9b. [DOI] [PubMed] [Google Scholar]
- 23.Brown H, Terrence J, Vasquez P, Bates DW, Zimlichman E. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med. 2014;127(3):226–232. doi: 10.1016/j.amjmed.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Michard F, Pinsky MR, Vincent JL. Intensive care medicine in 2050: NEWS for hemodynamic monitoring. Intensive Care Med. 2017;43(3):440–442. doi: 10.1007/s00134-016-4674-z. [DOI] [PubMed] [Google Scholar]
- 25.Michard F. A sneak peek into digital innovations and wearable sensors for cardiac monitoring. J Clin Monit Comput. 2017;31(2):253–259. doi: 10.1007/s10877-016-9925-6. [DOI] [PubMed] [Google Scholar]
- 26.Michard F. Hemodynamic monitoring in the era of digital health. Ann Intensive Care. 2016;6(1):15. doi: 10.1186/s13613-016-0119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michard F, Sessler DI, Saugel B. Non-invasive arterial pressure monitoring revisited. Intensive Care Med. 2018;44(12):2213–2215. doi: 10.1007/s00134-018-5108-x. [DOI] [PubMed] [Google Scholar]
- 28.Michard F, Sessler DI. Ward monitoring 3.0. Br J Anaesth. 2018;121(5):999–1001. doi: 10.1016/j.bja.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 29.Khanna AK, Overdyk FJ, Greening C, Di Stefano P, Buhre WF. Respiratory depression in low acuity hospital settings-seeking answers from the PRODIGY trial. J Crit Care. 2018;47:80–87. doi: 10.1016/j.jcrc.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 30.van Loon K, Peelen LM, van de Vlasakker EC, Kalkman CJ, van Wolfswinkel L, van Zaane B. Accuracy of remote continuous respiratory rate monitoring technologies intended for low care clinical settings: a prospective observational study. Can J Anaesth. 2018;65(12):1324–1332. doi: 10.1007/s12630-018-1214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weenk M, van Goor H, Frietman B, Engelen LJ, van Laarhoven CJ, Smit J, Bredie SJ, van de Belt TH. Continuous monitoring of vital signs using wearable devices on the general ward: pilot study. JMIR Mhealth Uhealth. 2017;5(7):e91. doi: 10.2196/mhealth.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michard F, Bellomo R, Taenzer A. The rise of ward monitoring: opportunities and challenges for critical care specialists. Intensive Care Med. 2018;45(5):671–73. doi: 10.1007/s00134-018-5384-5. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Dunn J, Salins D, Zhou G, Zhou W, Schussler-Fiorenza Rose SM, Perelman D, Colbert E, Runge R, Rego S, et al. Digital health: tracking physiomes and activity using wearable biosensors reveals useful health-related information. PLoS Biol. 2017;15(1):e2001402. doi: 10.1371/journal.pbio.2001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Dubrawski A, Wang D, Fiterau M, Guillame-Bert M, Bose E, Kaynar AM, Wallace DJ, Guttendorf J, Clermont G, et al. Using supervised machine learning to classify real alerts and artifact in online multisignal vital sign monitoring data. Crit Care Med. 2016;44(7):e456–e463. doi: 10.1097/CCM.0000000000001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang MA, Churpek MM, Zadravecz FJ, Adhikari R, Twu NM, Edelson DP. Real-time risk prediction on the wards: a feasibility study. Crit Care Med. 2016;44(8):1468–1473. doi: 10.1097/CCM.0000000000001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovacs C, Jarvis SW, Prytherch DR, Meredith P, Schmidt PE, Briggs JS, Smith GB. Comparison of the National Early Warning Score in non-elective medical and surgical patients. Br J Surg. 2016;103(10):1385–1393. doi: 10.1002/bjs.10267. [DOI] [PubMed] [Google Scholar]
- 37.Smith ME, Chiovaro JC, O'Neil M, Kansagara D, Quinones AR, Freeman M, Motu'apuaka ML, Slatore CG. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann Am Thorac Soc. 2014;11(9):1454–1465. doi: 10.1513/AnnalsATS.201403-102OC. [DOI] [PubMed] [Google Scholar]
- 38.Churpek MM, Yuen TC, Winslow C, Meltzer DO, Kattan MW, Edelson DP. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. 2016;44(2):368–374. doi: 10.1097/CCM.0000000000001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 40.McGillion M, Yost J, Turner A, Bender D, Scott T, Carroll S, Ritvo P, Peter E, Lamy A, Furze G, et al. Technology-enabled remote monitoring and self-management - vision for patient empowerment following cardiac and vascular surgery: user testing and randomized controlled trial protocol. JMIR Res Protoc. 2016;5(3):e149. doi: 10.2196/resprot.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


