Abstract
Polyhydroxyalkanoate (PHA) is a thermoplastic polymer with several advantageous properties, including biomass origin, biocompatibility, and biodegradability. PHA is synthesized in transgenic plants harboring 3 enzymatic genes: phaA, phaB, and phaC (collectively referred to as phaABC). PHA-producing plants exhibit severe growth inhibition that leads to extremely low PHA accumulation when these enzymes are localized in the cytosol. This growth inhibition could be attributed to the deleterious effects of the PHA biosynthetic pathway on endogenous essential metabolites or to PHA cytotoxicity itself. We performed precise morphological observations of phaABC-overexpressing Arabidopsis (ABC-ox), which displayed typical growth inhibition. On growth medium without sucrose, ABC-ox exhibited a pale green phenotype, dwarfism, including small cotyledons and true leaves, and short roots. ABC-ox partially recovered from this growth inhibition when the growth medium was supplemented with 1% sucrose. This recovery was reversed after ABC-ox grown on 1% sucrose medium was transferred to soil. ABC-ox grown on 1% sucrose medium not only demonstrated recovery from growth inhibition but were also the only examined plants with PHA accumulation, suggesting that growth inhibition was not caused by PHA cytotoxicity but rather by a lack of essential metabolites.
Keywords: biosynthesis, cytotoxicity, metabolic pathway, polyhydroxyalkanoate
Polyhydroxyalkanoates (PHAs) are thermoplastic polymers with several favorable properties, such as biomass origin, biocompatibility, and biodegradability (Numata et al. 2009; Sudesh et al. 2000). PHA is a carbon storage molecule that is accumulated in a wide variety of bacteria, including Ralstonia eutropha (Re). Supplementation with glucose or other carbon sources is necessary for bacterial fermentation to produce PHA; thus, in industrial processes, PHA production via bacteria requires two steps, in other words, addition of carbon source. By contrast, photosynthesis can produce carbohydrates that are used for PHA biosynthesis in plants (Somleva et al. 2013). This phenomenon enables plants to biosynthesize PHA from CO2 in a single step. Therefore, PHA production in plants could have economic and environmental advantages relative to PHA production by bacteria. Three enzymes, including 3-ketothiolase (PhaA), acetoacetyl-CoA reductase (PhaB), and PHA synthase (PhaC), are essential for synthesizing PHA from acetyl-CoA (Snell and Peoples 2002). Previous studies have indicated that phaC is a key enzyme for modifying the chemical structure of PHA (Chuah et al. 2013; Taguchi et al. 2008). PHA accumulates in transgenic plants at relatively low levels compared with the quantities generated when enzymes synthesize PHA in the cytosol of bacteria (Nawrath et al. 1994; Poirier et al. 1992). The reason for this low PHA accumulation is the severe growth inhibition observed in PHA-producing plants (Bohmert et al. 2002; Nawrath et al. 1994; Poirier et al. 1992). To avoid growth inhibition, compartmental production of PHA has been achieved via the subcellular localization of PHA-synthesizing enzymes in organelles, including chloroplasts and peroxisomes (Matsumoto et al. 2009; Nawrath et al. 1994; Tilbrook et al. 2011). Transformation of tobacco chloroplasts with genes for PHA synthesis increased PHA accumulation to as much as 11 wt% dry weight (dw) without any growth inhibition or deleterious effects; thus, this type of compartmental biosynthesis appears to be the preferred approach for achieving PHA accumulation in plants (Bohmert-Tatarev et al. 2011). This compartmental method would also be effective for separating toxic compounds or preventing a deleterious metabolic pathway from targeting molecules. Researchers have suggested that a deficiency in essential metabolites due to the expression of genes for PHA synthesis induces growth inhibition (Bohmert et al. 2002; Poirier et al. 1992; Ruiz and Daniell 2005). Although several reports have addressed growth inhibition in PHA-producing plants (Somleva et al. 2013), a comprehensive analysis that focuses on this growth inhibition has not been performed; therefore, the possibility that PHA cytotoxicity inhibits plant growth cannot be excluded.
In this study, gas chromatography/mass spectrometry (GC/MS) was used to quantitatively analyze growth inhibition and PHA content in Arabidopsis plants overexpressing phaA, phaB, and phaC (collectively referred to as phaABC). We demonstrate that sucrose supplementation enhanced PHA accumulation and suppressed growth inhibition in these plants, indicating that PHA cytotoxicity could be excluded as the reason for growth inhibition and low PHA accumulation. Finally, we discuss the potential factor of growth inhibition with respect to PHA accumulation in plants.
We created transgenic Arabidopsis that produced PHA by overexpressing phaA, phaB, and/or phaC genes from R. eutropha under the control of CaMV 35S promoters (Figure 1). pCAM-ephaCB-wtphaA (phaABCox) and pCAM-ephaB-wtphaA (phaABox) has phaAB genes along with and without phaC, respectively, from R. eutropha. The preferred codon usage in tobacco was chosen for phaB and phaC based on Codon Usage Database supplied by Kazusa DNA Research Institute, Japan (http://www.kazusa.or.jp/codon/), as reported previously (Matsumoto et al. 2011). The HindIII/EcoRI fragments of pBIeBeC, which was constructed and contained the codon optimized phaB and phaC (Matsumoto et al. 2009), was inserted into pCAMBIA (Cambia) to pCAM-ephaCB and pCAM-ephaB. The HindIII/SacI fragments of pBI221E, which contained phaA as reported previously (Matsumoto et al. 2011), were inserted into pCAM-ephaCB and pCAM-ephaCB to yield pCAM-ephaCB-wtphaA and pCAM-ephaB-wtphaA, respectively (Figure 1). Arabidopsis thaliana (accession number: Col-0) was grown under long-day conditions (16 h of light and 8 h of dark) at 22°C in a greenhouse or in growth chambers. Arabidopsis plants were transformed using the floral dipping method (Clough and Bent 1998). T1 seedlings were selected on 0.5×MS (Wako Pure Chemical Industries, Ltd., Osaka, Japan) agar without sucrose supplemented with 25 mg/L of hygromycin B (Roche Diagnostics GmbH, Mannheim, Germany). The existence of phaC in T1 ABC-ox was confirmed by PCR.
Figure 1. Constructs for the overexpression of PHA genes. (A) Structure of phaABCox. (B) Structure of phaABox. 35S and NosT indicate the CaMV 35S promoter and the nopaline synthase terminator, respectively.
Abnormalities exhibited by T1 plants were thoroughly observed to quantify growth inhibition. In transgenic plants harboring phaABC (ABC-ox), 5 plants displayed abnormal growth, with eventual lethality observed for 2 of these plants from 20 tested plants (Table 1). By contrast, abnormalities were not observed among plants that overexpressed phaA and phaB (AB-ox). In the adult stage, ABC-ox exhibited tiny leaves, short heights, and short siliques; these traits were identical to those of typical phenotypes reported in a previous study (Bohmert et al. 2002) (Figure 2A).
Table 1. Frequencies of abnormalities in T1 plants.
| Genotype | Lethal | Growth inhibition | n | |
|---|---|---|---|---|
| ABC-ox | 35S:phaA | 2 | 3 | 20 |
| 35S:phaB | ||||
| 35S:phaC | ||||
| AB-ox | 35S:phaA | 0 | 0 | 40 |
| 35S:phaB |
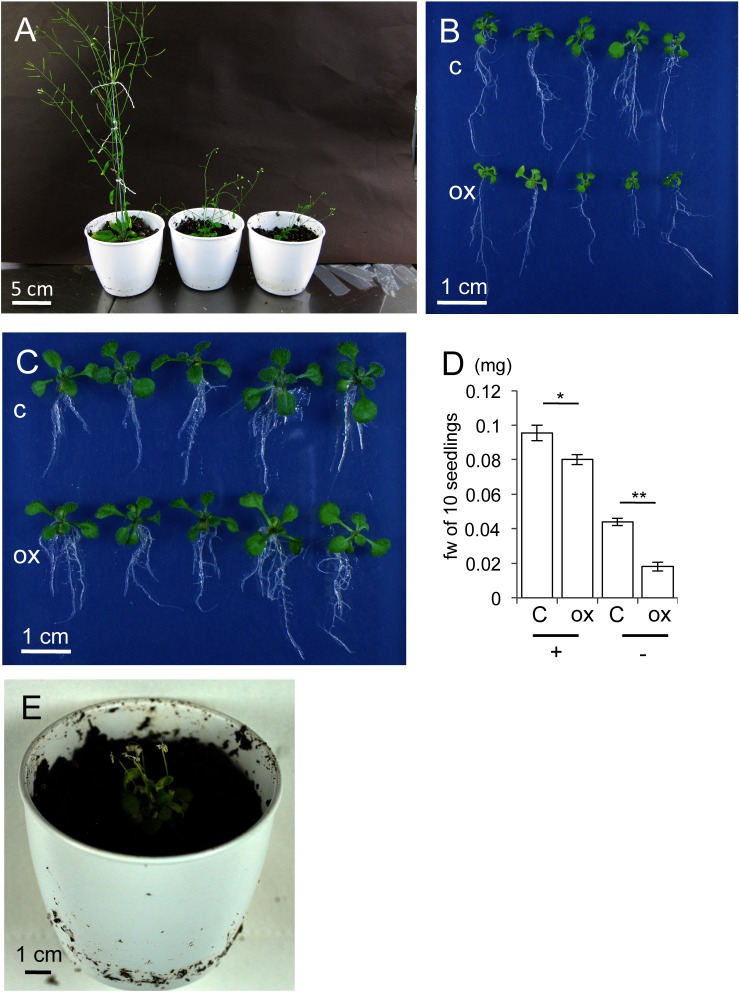
Figure 2. ABC-ox exhibited growth inhibition in the T1 and T3 generations. (A) Morphologies of T1 plants. The left plant is AB-ox, and the two plants to the right are ABC-ox, with typical growth inhibition. (B) Morphologies of 10 days-old T3 seedlings of ABC-ox growing on 0.5×MS without 1% sucrose. (C) Morphologies of 10 days-old T3 seedlings of ABC-ox growing on 0.5×MS with 1% sucrose. (D) Fw of 10 seedlings. Error bars represent standard deviation. + and − indicate supplementation of 1% sucrose and no sucrose, respectively. (E) Severe growth inhibition of T3 plant appeared after transfer from 1% sucrose medium to soil. c and ox in (B) to (D) denote vector control and the ABC-ox T3 line, respectively. Student t test: * 0.05>p and ** 0.01>p in (D).
Detailed morphological observations of 10-day-old ABC-ox seedlings that exhibited growth inhibition were performed (line no. #j). From counting antibiotic resistant seedlings at T2 generation of #j, 84% resistant plants appeared, suggesting this line had single insertion of T-DNA. Although this line showed severe growth inhibition representatively and reproductively at T2 and T3 generation, homozygous plants were not obtained because of their lethality by growing on soil. Therefore, we used heterozygous population of #j lines for following experiments. ABC-ox growing on 0.5×MS medium without sucrose displayed typical severe growth inhibition, including tiny true leaves and fewer lateral roots compared with control plants (Bohmert et al. 2002; Nawrath et al. 1994) (Figure 2B). In addition to this inhibition, a subset of ABC-ox seedlings had small cotyledons and true leaves with a pale green phenotype.
Supplementation with an extra carbon source would provide substrates such as acetyl-CoA for the PHA biosynthetic pathway, leading to enhanced PHA accumulation in ABC-ox. Supplementation of the medium with 1% sucrose was tested for these plants. Morphologically, this supplementation resulted in the nearly complete recovery of ABC-ox from growth inhibition and the pale green phenotype (Figure 2C). To evaluate this recovery, we measured fresh weights (fw) of ABC-ox growing on media with and without 1% sucrose. In terms of fw relative to fw of control plants, values for ABC-ox grown on 1% sucrose medium and on medium without sucrose were 83% and only 41%, respectively (Figure 2D). When ABC-ox on 1% sucrose medium exhibited partial recovery of growth, they were transferred to soil; severe growth inhibition was immediately observed (Figure 2E). Moreover, these plants had colorless, small flowers, which would lead to lower fertility.
We measured PHA accumulation in ABC-ox by GC/MS analysis to evaluate the effects of sucrose supplementation on PHA biosynthesis. For PHA extraction, seedlings were grown on 0.5×MS with or without 1% sucrose for 10 days. The method for extracting PHA was modified from a previous study (Higuchi-Takeuchi et al. 2016). Approximately 100 mg dw of 10-day-old seedlings were ground into fine powder with a mortar and pestle and mixed with 9 ml of hexane. The samples were incubated at 50°C for 24 h. During this incubation, the hexane was exchanged twice. The hexane was then removed, 9 ml of methanol was added, and the samples were incubated at 50°C for 24 h. The methanol was exchanged twice. After the methanol was removed, 6 ml of chloroform was added, and the samples were incubated at 65°C for 48 h. The supernatant was filtered through a 0.2 µm PTFE filter (Toyo Roshi Kaisha, Ltd. Tokyo, Japan), and the flow-through was completely dried. For ethanolysis, dry PHA was dissolved in 250 µl of chloroform at 65°C; subsequently, 850 µl of ethanol and 100 µl of HCl were added, and the mixture was incubated at 100°C for 4 h. The pH of this mixture was adjusted to approximately 7.0, and the chloroform layer was filtered through silica wool and anhydrous sodium sulfate in a Pasteur pipette. The flow-through was dehydrated using molecular sieve 4A. To measure ethyl-3-hydroxybutyrate (ethyl-3HB) as a monomer unit of PHA, 100 µl of the dehydrated sample was assessed by GC/MS (QP2010 Plus, Shimadzu, Kyoto, Japan) with an HP-5MS column and He gas. GC/MS analysis was performed according to previous work and purified 3HB was used as a standard (Higuchi-Takeuchi et al. 2016).
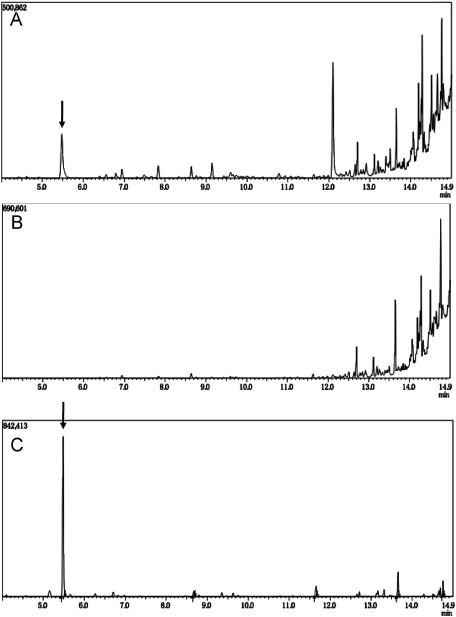
To extract PHA, 10-day-old seedlings (approximately 100 mg dw) was used. The ethyl-3HB peak generated by ABC-ox grown on 1% sucrose medium was determined (Figure 3A). GC/MS analysis revealed that this peak was identical to ethyl-3HB. Triplicate experiments for PHA measurement indicated that all ABC-ox accumulated PHA, although extremely small quantities were found with first, second, and third measurements of 0.0079 wt% dw, 0.0005 wt% dw, and 0.0050 wt% dw, respectively. By contrast, no PHA peaks were detected in ABC-ox grown on medium without sucrose (Figure 3B). In addition to the ethyl-3HB peak, ABC-ox grown on 1% sucrose had a peak at 12 min, which did not appear in both ABC-ox grown on medium without sucrose and the control sample (Figure 3). This might be a by-product of PHA biosynthesis in plants.
Figure 3. Ethyl-3HB was detected only in the ABC-ox grown on 1% sucrose medium. (A) Gas chromatography chart of ABC-ox grown on medium with 1% sucrose. (B) Gas chromatography chart of ABC-ox grown on medium without sucrose. (C) Gas chromatography chart of the purified 3HB (0.01 mg/ml) used as a standard. The arrow indicates an ethyl-3HB peak.
We investigated the growth inhibition of PHA-producing transgenic plants and evaluated potential causes of this growth inhibition via a sucrose supplementation test. Previous studies have noted that severe growth inhibition in such plants was caused by a lack of essential metabolites such as acetyl-CoA and acetoacetyl-CoA, given that the expression of a single enzymatic gene, e.g., phaA or phaB alone, induced growth inhibition (Nawrath et al. 1994; Poirier et al. 1992; Ruiz and Daniell 2005). These metabolites are important compounds that are involved in essential metabolic pathways. Additionally, the co-expression of phaB and phaC enhanced inhibitory phenotypes in transgenic plants; therefore, PHA cytotoxicity remained a viable possibility (Poirier et al. 1992). Our supplementation test revealed that growth inhibition in PHA-producing plants was not correlated with PHA accumulation, given that sucrose supplementation restored growth and enhanced PHA accumulation in ABC-ox. We therefore concluded that the PHA synthetic pathway markedly affected the essential metabolites acetyl-CoA and acetoacetyl-CoA, causing growth inhibition and/or lethality.
In this study, AB-ox exhibited no abnormalities in the T1 generation; this result was inconsistent with the findings of previous studies (Bohmert et al. 2002; Poirier et al. 1992; Ruiz and Daniell 2005). It is difficult to compare various studies because different expression systems were used in each investigation. PhaC is involved in a reaction that produces the end product PHA, and substantial metabolic flow is supplied to this end product. In this study, PhaA and PhaB in combination with PhaC could strongly influence endogenous metabolic pathways and cause severe growth inhibition of ABC-ox.
Acetyl-CoA is a substrate of PHA and is used for many biochemical reactions, including malonyl-CoA biosynthesis. Mutations in the Arabidopsis malonyl-CoA synthase gene give rise to severe growth inhibition and low fertility (Chen et al. 2011); similar effects are observed in ABC-ox, suggesting that growth inhibition in ABC-ox is attributable to low malonyl-CoA. To achieve high PHA yield, we must organize the PHA biosynthetic pathway and endogenous metabolic pathways to avoid interference.
An approach used in previous research is to increase PHA accumulation by targeting the biosynthesis of PHA to plant cellular compartments, such as peroxisomes and chloroplasts (Somleva et al. 2013). Chloroplast transformation with genes for PHA-synthesizing enzymes was performed and successfully enhanced PHA accumulation (Bohmert-Tatarev et al. 2011; Nawrath et al. 1994). Plant mitochondria have the potential to biosynthesize PHA because beta-oxidation occurs frequently in mitochondria (Baker et al. 2006) and provides large quantities of PHA substrates. Currently, exogenous genes can be delivered to plant mitochondria using peptide-based DNA carriers (Chuah et al. 2015; Lakshmanan et al. 2013), allowing the compartmental PHA production technique to be utilized for these organelles (Numata 2015). To overcome low PHA accumulation in plants, nuclear and chloroplast genomes as well as mitochondrial genomes should all be genetically modified at the same time.
Maximal PHA accumulation in plants is not comparable to PHA accumulation in bacteria due to many potential issues. Based on our ongoing study, a possible mitochondrial metabolite could be a key metabolite for avoiding plant growth inhibition in the context of PHA accumulation. By using engineered organelles and nuclei, we can open new avenues for conquering the field of plant bioproduction and bioengineering.
Acknowledgments
This study was financially supported by New Energy and Industrial Technology Development Organization (NEDO) (T.Y. and K.N), The Exploratory Research for Advanced Technology (ERATO), Japan Science and Technology Agency (JST) (K.N.), and Grants-in-Aid for Scientific Research (M.H).
Abbreviations
- PHA
polyhydroxyalkanoate
- ethyl-3HB
ethyl-3-hydroxybutyrate
- fw
fresh weight
- dw
dry weight
- GC/MS
gas chromatography-mass spectrometry
References
- Baker A, Graham IA, Holdsworth M, Smith SM, Theodoulou FL (2006) Chewing the fat: β-oxidation in signalling and development. Trends Plant Sci 11: 124–132 [DOI] [PubMed] [Google Scholar]
- Bohmert K, Balbo I, Steinbuchel A, Tischendorf G, Willmitzer L (2002) Constitutive expression of the β-ketothiolase gene in transgenic plants. A major obstacle for obtaining polyhydroxybutyrate-producing plants. Plant Physiol 128: 1282–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmert-Tatarev K, McAvoy S, Daughtry S, Peoples OP, Snell KD (2011) High levels of bioplastic are produced in fertile transplastomic tobacco plants engineered with a synthetic operon for the production of polyhydroxybutyrate. Plant Physiol 155: 1690–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kim HU, Weng H, Browse J (2011) Malonyl-CoA synthetase, encoded by ACYL ACTIVATING ENZYME13, is essential for growth and development of Arabidopsis. Plant Cell 23: 2247–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah JA, Yamada M, Taguchi S, Sudesh K, Doi Y, Numata K (2013) Biosynthesis and characterization of polyhydroxyalkanoate containing 5-hydroxyvalerate units: Effects of 5HV units on biodegradability, cytotoxicity, mechanical and thermal properties. Polym Degrad Stab 98: 331–338 [Google Scholar]
- Chuah JA, Yoshizumi T, Kodama Y, Numata K (2015) Gene introduction into the mitochondria of Arabidopsis thaliana via peptide-based carriers. Sci Rep 5: 7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Higuchi-Takeuchi M, Morisaki K, Toyooka K, Numata K (2016) Synthesis of high-molecular-weight polyhydroxyalkanoates by marine photosynthetic purple bacteria. PLoS One 11: e0160981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan M, Kodama Y, Yoshizumi T, Sudesh K, Numata K (2013) Rapid and efficient gene delivery into plant cells using designed peptide carriers. Biomacromolecules 14: 10–16 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Morimoto K, Gohda A, Shimada H, Taguchi S (2011) Improved polyhydroxybutyrate (PHB) production in transgenic tobacco by enhancing translation efficiency of bacterial PHB biosynthetic genes. J Biosci Bioeng 111: 485–488 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Murata T, Nagao R, Nomura CT, Arai S, Arai Y, Takase K, Nakashita H, Taguchi S, Shimada H (2009) Production of short-chain-length/medium-chain-length polyhydroxyalkanoate (PHA) copolymer in the plastid of Arabidopsis thaliana using an engineered 3-ketoacyl-acyl carrier protein synthase III. Biomacromolecules 10: 686–690 [DOI] [PubMed] [Google Scholar]
- Nawrath C, Poirier Y, Somerville C (1994) Targeting of the polyhydroxybutyrate biosynthetic pathway to the plastids of Arabidopsis thaliana results in high levels of polymer accumulation. Proc Natl Acad Sci USA 91: 12760–12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata K (2015) Poly(amino acid)s/polypeptides as potential functional and structural materials. Polym J 47: 537–545 [Google Scholar]
- Numata K, Abe H, Iwata T (2009) Biodegradability of poly(hydroxyalkanoate) materials. Materials 2: 1104–1126 [Google Scholar]
- Poirier Y, Dennis DE, Klomparens K, Somerville C (1992) Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science 256: 520–523 [DOI] [PubMed] [Google Scholar]
- Ruiz ON, Daniell H (2005) Engineering cytoplasmic male sterility via the chloroplast genome by expression of β-ketothiolase. Plant Physiol 138: 1232–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell KD, Peoples OP (2002) Polyhydroxyalkanoate polymers and their production in transgenic plants. Metab Eng 4: 29–40 [DOI] [PubMed] [Google Scholar]
- Somleva MN, Peoples OP, Snell KD (2013) PHA bioplastics, biochemicals, and energy from crops. Plant Biotechnol J 11: 233–252 [DOI] [PubMed] [Google Scholar]
- Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog Polym Sci 25: 1503–1555 [Google Scholar]
- Taguchi S, Yamada M, Matsumoto K, Tajima K, Satoh Y, Munekata M, Ohno K, Kohda K, Shimamura T, Kambe H, et al. (2008) A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc Natl Acad Sci USA 105: 17323–17327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbrook K, Gebbie L, Schenk PM, Poirier Y, Brumbley SM (2011) Peroxisomal polyhydroxyalkanoate biosynthesis is a promising strategy for bioplastic production in high biomass crops. Plant Biotechnol J 9: 958–969 [DOI] [PubMed] [Google Scholar]