Abstract
Rationale: Dyspnea is a common and distressing physical symptom among patients in the ICU and may be underdetected and undertreated.
Objectives: To determine the frequency of dyspnea relative to pain, the accuracy of nurses and personal caregiver dyspnea ratings relative to patient-reported dyspnea, and the relationship between nurse-detected dyspnea and treatment.
Methods: This was an observational study of patients (n = 138) hospitalized in a medical ICU (MICU). Nurses and patients’ personal caregivers at the bedside reported on their perception of patients’ symptoms.
Measurements and Main Results: Dyspnea was assessed by patients, caregivers, and nurses with a numerical rating scale. Across all three raters, the frequency of moderate to severe dyspnea was similar or greater than that of pain (P < 0.05 for caregiver and nurse ratings). Personal caregivers’ ratings of dyspnea had substantial agreement with patient ratings (κ = 0.65, P < 0.001), but nurses’ ratings were not significantly related to patient ratings (κ = 0.19, P = 0.39). Nurse detection of moderate to severe pain was significantly associated with opioid treatment (odds ratio, 2.70; 95% confidence interval, 1.10–6.60; P = 0.03); however, nurse detection of moderate to severe dyspnea was not significantly associated with any assessed treatment.
Conclusions: Dyspnea was reported at least as frequently as pain among the sampled MICU patients. Personal caregivers had good agreement with patient reports of moderate to severe dyspnea. However, even when detected by nurses, dyspnea appeared to be undertreated. These findings suggest the need for improved detection and treatment of dyspnea in the MICU.
Keywords: symptom assessment, dyspnea, critical care, palliative care, symptom management
At a Glance Commentary
Scientific Knowledge on the Subject
Dyspnea and pain are both common and distressing symptoms in critically ill patients; however, the detection and treatment of pain in this population has been the subject of more research than that of dyspnea.
What This Study Adds to the Field
Our study shows that personal caregivers have better sensitivity than nurses in the detection of patient-reported dyspnea in critically ill patients. When detected by clinicians, patient dyspnea is inconsistently treated as compared to pain.
Critically ill patients often suffer from pain and dyspnea (1–3). Effective and specific management guidelines are available for pain (4) but not for dyspnea. More information about the detection and treatment of dyspnea in ICUs is a first step toward better management of, and development of management guidelines for, this distressing symptom.
Dyspnea is defined by the American Thoracic Society as “a subjective experience of breathing discomfort” (5), which is distinct from physiological measurements of the adequacy of gas exchange (6). Patient self-report is the gold, or criterion, standard for detection of dyspnea. Although routine screening for dyspnea through self-report is not in widespread use, there has been recent evidence for the feasibility, acceptability, and benefit of doing so (7–9). However, screening patients by self-report excludes ICU patients who are unable to communicate. Multiple strategies have been developed to allow clinicians to assess dyspnea in such patients. However, the validity of proxy reports has been mixed in previous studies (10–12), and behavioral scales (13, 14) are not yet in widespread use and require further research into their clinical applicability.
Similar to its detection, the symptomatic treatment of dyspnea has proven difficult. Opioids are the mainstay of pharmacologic treatment for refractory dyspnea (2) and can effectively reduce breathlessness in patients with a variety of conditions (3, 15, 16). Other interventions, such as ventilator adjustments (17), noninvasive positive pressure ventilation (18), supplemental oxygen (19, 20), inhaled bronchodilators (21), and benzodiazepines (22), may be effective in certain populations (23).
The present study sought to fill some of the above-noted knowledge gaps by comparing the prevalence of dyspnea and pain in samples of both communicative and noncommunicative ICU patients. It also sought to examine the concordance of nurse and personal caregiver (e.g., family member) proxy report with patient self-report and to compare associations between the nurse detection of dyspnea and pain and treatments administered. Because of the previously described challenges in both evaluation and management of dyspnea in the ICU, we hypothesized that dyspnea in the ICU would be underdetected. Given lack of clear treatment guidelines for dyspnea, we hypothesized further that nurse-detected dyspnea, unlike pain, would not be associated with nurse intervention (e.g., opioid analgesics) for dyspnea.
Some of the results of this study have been previously reported in the form of an abstract (24).
Methods
Sample
This study derived data from a prospective cohort of patients hospitalized in the Medical ICU (MICU) at New York–Presbyterian Hospital/Weill Cornell Medical Center (New York, NY) recruited between June 2016 and April 2018, their nurses, and their personal caregivers at the bedside. The eligibility criteria included admission to the MICU during the study period, requirement for an ICU level of care (i.e., not scheduled for transfer to the general floor), fluency in English, and either the capacity to consent to the study as determined by the medical team or the presence of a personal caregiver at the bedside to serve as a proxy for consent. All such patients were approached if they were available on a day when recruitment occurred.
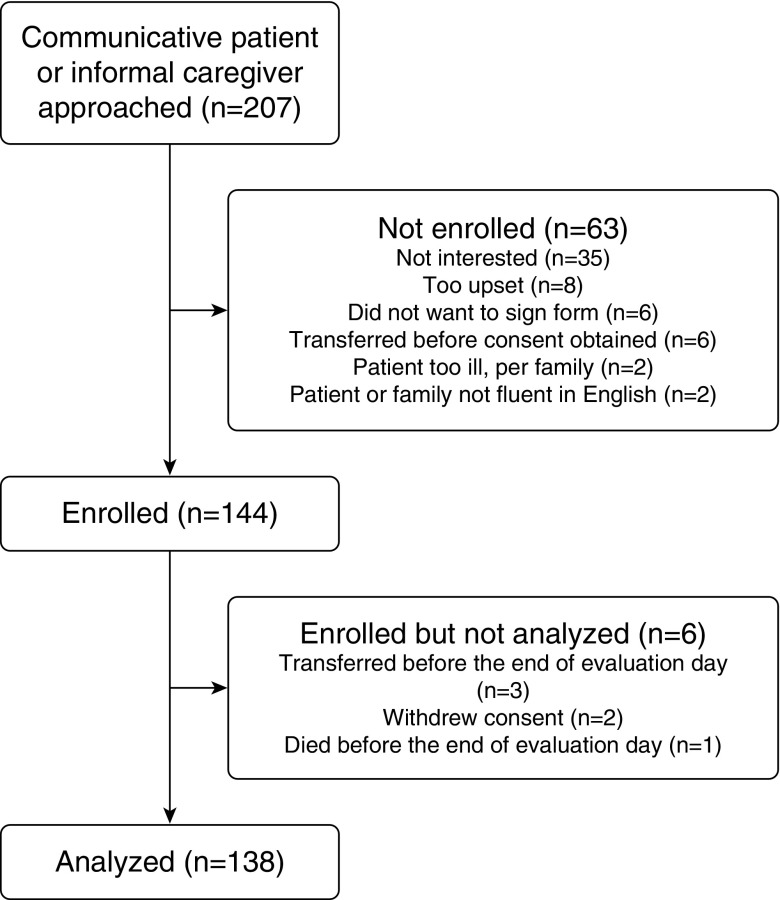
In total, 207 communicative patients or family members were approached. Of these, 144 patients were enrolled (69.6%). Reasons for nonenrollment and withdrawal are detailed in Figure 1. One hundred thirty-eight patients were included in the final sample, of whom 101 had a personal caregiver at the bedside who also enrolled in the study. The remaining 37 patients did not have a personal caregiver present at the time of evaluation and had capacity to consent for themselves.
Figure 1.
Enrollment flow chart.
Ninety-two percent of nurses who were approached agreed to participate, for a total enrollment of 46 nurses caring for 138 different patients. Nurses were chosen as the clinician evaluators, as they are most closely involved in the detection and management of acute symptoms. Each ICU nurse at our study site typically took care of two patients for a 12-hour shift.
Institutional review board approval was obtained from the institution. After eligible patients were identified, study subjects (patients, nurses, and personal caregivers) provided written consent. Personal caregivers consented by proxy for patients who did not have capacity to consent.
Measures
Sociodemographic characteristics and reason for ICU admission
Age, sex, race, and ethnicity for patient participants were obtained from the medical chart. The patients’ reasons for ICU admission were obtained from the medical chart, according to the initial attending or resident note on admission to the ICU. If both attending and resident cited reasons and they were discordant, the attending’s reason was selected.
Communication status
Trained research evaluators (e.g., medical student or M.D.-trained research staff) rated the patients’ ability to communicate at the beginning of the evaluation day according to the following scale: 1 = not at all, 2 = can answer yes or no questions, 3 = can use a 0 to 10 scale, 4 = can communicate clearly about symptoms, and 5 = can communicate elaborately about symptoms. Patients rated 1 or 2 were categorized as unable to rate their symptoms using a 0 to 10 numerical rating scale (NRS); patients rated 3 or above were considered able to rate their symptoms. Patients who were unable to speak because of mechanical ventilation, but were able to communicate using gestures, were rated 3 and pointed to numbers on an enlarged 0 to 10 NRS. In addition, for a more objective measure of a patient’s mental status, a Richmond Agitation-Sedation Scale (RASS) was performed by the patient’s nurse at the time of evaluation. This scale evaluates a patient’s degree of sedation or agitation from −5 (unarousable) to +4 (combative) (25). No patient outside the range of −1 to +1 was included in self-reports.
Of note, the decision of whether a patient was able to consent for him- or herself or required consent by proxy was made by the treating medical team and nursing staff and was separate from the researcher’s assessment of communication status.
Rating of dyspnea and pain
The NRS was selected because it is simple to use, reliable and sensitive for both dyspnea and pain (26–28), and preferred by patients to a visual analog scale for dyspnea (28). To administer the NRS, the patients, nurses, and personal caregivers were asked the following questions at the beginning of the evaluation day: “At this time, on a scale from 0 to 10, how much pain are you experiencing/do you believe the patient is experiencing?” and “At this time, on a scale from 0 to 10, how much difficulty breathing are you experiencing/do you believe the patient is experiencing?” NRS scales were bounded at 0 by the phrase “no pain/difficulty breathing” and at 10 by the phrase “worst pain/difficulty breathing ever.”
Each assessment was conducted privately, such that patients, caregivers, and nurses were blinded to each other’s ratings. Assessments were conducted on the same patient sequentially, such that no more than half an hour elapsed between ratings that were compared in this study.
Difficulty in management of dyspnea and pain
Nurses were asked the following question at the end of the evaluation day: “On a scale from 0 to 10, where 0 represents ‘no difficulty’ and 10 represents ‘extreme difficulty,’ how much did this patient’s (pain/dyspnea) present a challenge to effective symptom management?”
Medication treatment
Data on whether or not the following medications were administered during the 12-hour follow-up period were abstracted from the medical chart: opioids (fentanyl, hydromorphone, morphine, oxycodone, codeine), benzodiazepines (alprazolam, lorazepam, midazolam, diazepam, clonazepam), and those inhaled bronchodilators available in this ICU (ipratropium, tiotropium, albuterol, formoterol). Dichotomous variables (e.g., received opioid vs. did not receive opioid) were then coded for each category.
Oxygen delivery devices
Oxygen delivery device and/or ventilator settings (if applicable) were observed at the patient’s bedside by the study assessor and confirmed by review of the patient chart. From these data, variables were coded to indicate changes in respiratory support throughout the day by examining oxygen delivery, device adjustments, and ventilator settings. The following changes were examined: 1) increase in oxygen delivery (vs. no change or decrease) among patients using a respiratory device, 2) change in type of respiratory device (vs. no change), and 3) adjustment in ventilator settings (vs. no adjustment) among patients ventilated for the entire day. Adjustments documented included the ventilator mode, positive end-expiratory pressure, tidal volume in volume-control modes, and pressure support in pressure-control modes.
Data Analysis
All patients with a completed self and/or proxy dyspnea rating at the morning baseline assessment were included in analyses (N = 138).
For analyses involving detection of clinically significant pain or dyspnea, we used a widely used and validated cut-off for clinically significant symptoms (NRS ≥ 4) (29–31). NRS scores of 4 or greater indicated the presence of moderate to severe dyspnea, and scores below 4 indicated its absence. Nurse ratings of difficulty managing dyspnea and pain were dichotomized into “no difficulty” (score = 0, the most common rating) or “any difficulty” (scores > 0) in managing symptoms, for ease of analysis and interpretation.
McNemar’s tests for marginal homogeneity were used to determine whether the proportion of the sample with clinically notable dyspnea scores was statistically significantly different from the proportion of the sample with clinically notable pain scores. χ2 tests were used to determine whether the frequency of clinically notable dyspnea and pain as reported by caregivers and nurses differed between patients who could communicate their symptoms versus those who could not. Logistic regression models tested whether nurse detection of moderate to severe dyspnea predicted dichotomously coded treatment outcome variables (e.g., opioid medication, ventilator adjustment), with “dyspnea absent” (NRS < 4) as the reference category. Separate models used the same treatment outcomes, with nurse-detected moderate to severe pain as the predictor of interest. A two-tailed α level of P < 0.05 was considered statistically significant for all tests.
Among the subsample with complete ratings across patients, caregivers, and nurses (n = 29), the relationship between patient and proxy dyspnea ratings was examined. κ coefficients were used to evaluate pairwise agreement between proxy and patient ratings of moderate to severe dyspnea. Sensitivity and specificity were calculated, with patient-rated presence versus absence of moderate to severe dyspnea as the gold, or criterion, standard.
Results
The overall sample consisted of 138 patients (Table 1), of whom 51 self-reported symptoms on a scale from 0 to 10; RASS scores ranged from −1 to +1 for the 51 patients who self-reported. Of the total sample of 138 patients, 101 patients received personal caregiver evaluations, and 137 received nurse evaluations (by 46 distinct nurses). Sixty-two percent of patients were men and 67% were white, with a mean age of 64.51 years (SD = 18.44 yr). They had been in the ICU for a median of 4 days (interquartile range, 6 d) before evaluation. Eighty-nine patients (65%) were receiving mechanical ventilation, either by endotracheal tube or tracheostomy; of these, 19% rated their symptoms. The most common reason for ICU admission was respiratory failure (n = 87, 63%), followed by hypotension (n = 21, 15%), sepsis or septic shock (n = 19, 14%), and cardiac arrest (n = 7, 5%).
Table 1.
Baseline Characteristics for Overall Sample (N = 138)
| Patient Characteristic | |
|---|---|
| Age, yr, mean (SD) | 64.51 (18.44) |
| Sex | |
| Male | 85 (62) |
| Female | 53 (38) |
| Race | |
| White | 92 (67) |
| Asian/Pacific Islander | 25 (18) |
| Black | 13 (9) |
| Biracial or multiracial | 1 (1) |
| Ethnicity | |
| Hispanic or Latino | 17 (12) |
| Non-Hispanic/Latino | 114 (83) |
| Reason(s) for ICU admission | |
| Respiratory failure | 87 (63) |
| Sepsis or septic shock | 19 (14) |
| Hypotension | 21 (15) |
| Cardiac arrest | 7 (5) |
| Other | 28 (20) |
| Communication status | |
| Able to communicate (i.e., rate symptoms) | 56 (41) |
| Unable to communicate | 82 (59) |
| RASS score | |
| −5 | 10 (7) |
| −4 | 15 (11) |
| −3 | 15 (11) |
| −2 | 9 (7) |
| −1 | 23 (17) |
| 0 | 53 (38) |
| 1 | 11 (8) |
| 2 | 2 (1) |
| Respiratory device | |
| None | 10 (7) |
| Ventilator | 89 (65) |
| Other* | 39 (28) |
Definition of abbreviation: RASS = Richmond Agitation-Sedation Scale.
Data are presented as n (%) unless otherwise noted. Data were missing for several participants on race (n = 7) and ethnicity (n = 7). Patients with multiple reasons for medical ICU admission were represented in each category, thus adding to greater than 100%.
Other respiratory devices included tracheostomy mist collar, nasal cannula, high-flow nasal cannula, non-rebreather mask, noninvasive ventilation, or humidified face mask.
Table 2 displays the frequencies of moderate to severe dyspnea and pain (NRS ≥ 4) as rated by patients, personal caregivers, and nurses, as well as the results of McNemar’s tests comparing the proportion of moderate to severe (NRS ≥ 4) dyspnea versus pain in the sample. Among patients who were able to rate their symptoms, 47% reported moderate to severe dyspnea (NRS ≥ 4) and 41% reported moderate to severe pain; the frequency of these symptoms was not statistically significantly different (P = 0.68). According to caregiver proxy ratings, the proportion of patients with moderate to severe dyspnea (61%) was significantly greater than the proportion with moderate to severe pain (46%; P = 0.01). Similarly, nurse ratings also showed a significantly greater proportion of patients with moderate to severe dyspnea (34%) than moderate to severe pain (22%; P = 0.02). As rated by either personal caregivers or nurses, there were no significant differences in the frequency of proxy-reported moderate to severe dyspnea or pain for patients who could versus could not communicate their symptoms, according to χ2 tests (P values > 0.29).
Table 2.
Frequency and Comparison of Clinically Significant Pain and Dyspnea Numerical Rating Scale Ratings by Patients and Proxy Raters
| Dyspnea Present | Dyspnea Absent | Row Total | χ2, McNemar’s Test | P Value | |
|---|---|---|---|---|---|
| Patient ratings (n = 51) | |||||
| Pain present | 11 | 10 | 21 (41%) | ||
| Pain absent | 13 | 17 | 30 (59%) | ||
| Column total | 24 (47%) | 27 (53%) | 0.17 | 0.68 | |
| Caregiver ratings (n = 101) | |||||
| Pain present | 36 | 10 | 46 (46%) | ||
| Pain absent | 26 | 29 | 55 (54%) | ||
| Column total | 62 (61%) | 39 (39%) | 6.25 | 0.01 | |
| Nurse ratings (n = 137) | |||||
| Pain present | 17 | 13 | 30 (22%) | ||
| Pain absent | 29 | 78 | 107 (78%) | ||
| Column total | 46 (34%) | 91 (66%) | 5.36 | 0.02 |
Using McNemar’s test, P < 0.05 indicates that the proportion of moderate to severe dyspnea is significantly different than the proportion of moderate to severe pain in the sample, according to each rater.
Of the 51 patients who self-reported their symptoms, 22 did not have a caregiver proxy rating. Among the 29 cases with a complete set of patient, nurse, and caregiver ratings (detailed in Table E1 in the online supplement), Cohen’s κ coefficient between personal caregivers and patients for ratings of presence versus absence of moderate to severe dyspnea was 0.65 (95% confidence interval [CI], 0.40–0.90; P < 0.001), indicating a substantial degree of agreement. Cohen’s κ coefficient between nurse and patient ratings was 0.19 (95% CI, −0.10 to 0.48; P = 0.39), and these two ratings were not statistically significantly associated with one another (32). Table 3 displays personal caregivers’ and nurses’ sensitivity for detecting the presence of patient-rated moderate to severe dyspnea and their specificity for indicating the absence of patient-rated dyspnea.
Table 3.
Caregiver and Nurse Agreement, Sensitivity, and Specificity for Identifying Presence (n = 15) and Absence (n = 14) of Patient-rated Dyspnea
| Rater | κ Coefficient, Agreement with Patient | Sensitivity, Dyspnea Present; NRS ≥ 4 (%) | Specificity, Dyspnea Absent; NRS < 4 (%) |
|---|---|---|---|
| Caregiver | 0.65 (0.40 to 0.90) | 100 (78 to 100) | 64 (35 to 72) |
| Nurse | 0.19 (−0.10 to 0.48) | 33 (12 to 62) | 86 (57 to 98) |
Definition of abbreviation: NRS = numerical rating scale.
Data are displayed for 29 complete sets of patient, caregiver, and nurse ratings; 95% confidence intervals are included in parentheses.
Among the overall sample of 138 patients, 77 (56%) received opioids, 30 (22%) received benzodiazepines, and 42 (30%) received inhaled bronchodilators during the evaluated nurse’s shift. Of patients who received opioids, 13 patients received opioids for pain, 16 patients received opioids for dyspnea, 27 patients received opioids for both, and 21 patients received opioids but the nurse declined to specify the reason why. Respiratory device was changed for 27 (20%) patients. Of the 111 patients who remained on the same respiratory device throughout the shift, oxygen delivery was increased for 7 (6%). Of the 74 patients who were using ventilators throughout the entire shift, 23 (31%) had an adjustment in ventilator settings during the nurses’ shift. As shown in Table 4, patients whose nurses detected moderate to severe pain were significantly more likely to receive opioid medications during the evaluated nurse’s shift than those whose nurses did not detect moderate to severe pain (odds ratio, 2.70; 95% CI, 1.10–6.60; P = 0.03). However, detection of dyspnea did not increase the likelihood of receiving opioid medication (odds ratio, 0.72; 95% CI, 0.35–1.46; P = 0.36; Table 5). Logistic regression models using nurse detection of dyspnea or pain to predict the administration of benzodiazepines, inhaled bronchodilators, ventilator adjustments, supplemental oxygen, or respiratory device changes were also tested and were not significant (P values > 0.16). Last, the proportion (74%) of patients for whom nurses reported any difficulty managing dyspnea was greater than the proportion (61%) for whom nurses reported any difficulty managing pain (McNemar’s test, χ2 = 6.28, P = 0.01).
Table 4.
Nurse Detection of Moderate to Severe Pain (NRS ≥ 4) as Individual Predictors of Treatment in Separate Logistic Regression Models
| Predictor | Outcome: Medication administration |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Opioids |
Benzodiazepines |
Inhaled Bronchodilators |
|||||||
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Nurse-detected moderate to severe pain | 2.70 | 1.10–6.60 | 0.03 | 0.69 | 0.24–2.00 | 0.50 | 0.96 | 0.40–2.32 | 0.93 |
| Predictor | Outcome: Respiratory Support Administration |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Supplemental Oxygen |
Ventilator Adjustment |
Device Change |
|||||||
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Nurse-detected moderate to severe pain | 3.11 | 0.65–15.03 | 0.16 | 0.67 | 0.19–2.35 | 0.53 | 1.32 | 0.50–3.51 | 0.57 |
Definition of abbreviations: CI = confidence interval; NRS = numerical rating scale; OR = odds ratio.
Note: n = 137 for all models except those testing oxygen increase (n = 110) and ventilator adjustment (n = 73), because only a subset of patients remained on the same respiratory device or ventilator respectively throughout the shift. Bold indicates statistically significant effects.
Table 5.
Nurse Detection of Moderate to Severe Dyspnea (NRS ≥ 4) as Individual Predictors of Treatment in Separate Logistic Regression Models
| Predictor | Outcome: Medication Administration |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Opioids |
Benzodiazepines |
Inhaled Bronchodilators |
|||||||
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Nurse-detected moderate to severe dyspnea | 0.72 | 0.35–1.46 | 0.36 | 0.70 | 0.28–1.74 | 0.44 | 1.55 | 0.73–3.29 | 0.26 |
| Predictor | Outcome: Respiratory support administration |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Supplemental oxygen |
Ventilator adjustment |
Device change |
|||||||
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Nurse-detected moderate to severe dyspnea | 0.30 | 0.03–2.57 | 0.27 | 0.69 | 0.23–2.06 | 0.50 | 0.80 | 0.32–1.99 | 0.63 |
For definition of abbreviations, see Table 4.
Note: n = 137 for all models except those testing oxygen increase (n = 110) and ventilator adjustment (n = 73), because only a subset of patients remained on the same respiratory device or ventilator respectively throughout the shift.
Discussion
In this observational study of ICU patients, we found that personal caregivers’ ratings of clinically significant dyspnea had substantial agreement with the patients’ own ratings and had high sensitivity for patient-reported dyspnea. By contrast, nurses’ ratings were not significantly associated with patient ratings of dyspnea. Although nurse-detected pain was associated with treatment with opioid analgesics, nurse-detected dyspnea was not associated with any of the examined treatments.
Nearly half of communicative patients in our sample reported moderate to severe dyspnea, consistent with the results of other studies (1, 33, 34) indicating that dyspnea is a significant problem in the ICU. According to patient ratings, there was no statistically significant difference in frequency between moderate to severe dyspnea and moderate to severe pain. However, according to nurse and caregiver ratings, the proportion of patients with moderate to severe dyspnea was greater than the proportion with moderate to severe pain. Furthermore, we found no statistically significant differences in nurse and caregiver ratings of dyspnea between communicative and noncommunicative patients.
It is possible that patients experiencing sedation or an altered mental status may have either a diminished or increased sensation of dyspnea. Although we limited self-report to patients with RASS scores between +1 and −1, and we did not survey any patient who appeared agitated, we cannot rule out that some patients may have been experiencing unobserved delirium affecting their symptomatology.
Previous studies have shown that clinicians tend to underestimate symptoms (10, 11), but this study is the first we have encountered to directly compare clinician and personal caregiver proxy ratings of dyspnea with patient ratings. Although we found a tendency of personal caregivers to overestimate patient-reported dyspnea, personal caregivers did not miss a case of patient-reported dyspnea, and their ratings significantly agreed with patients’ self-report. In contrast, nurse ratings were not statistically significantly associated with patient-reported dyspnea, and nurses’ sensitivity for patient-reported dyspnea was significantly lower than that of personal caregivers. Nurses’ and caregivers’ specificity did not differ significantly in our small sample, although this result should be interpreted with caution, as a larger sample might reveal a statistically significant difference in specificity. Our results on the accuracy of caregivers suggest that personal caregivers might serve as useful informants for clinicians in calling attention to patients who can communicate for whom further symptom assessment and treatment could be beneficial. These results also highlight the need for standardized guidelines for clinicians to detect dyspnea in their critically ill patients, as personal caregivers are not always available and can overestimate symptoms.
Furthermore, even when dyspnea was detected by nurses, our results did not show an association between that detection and any of the examined changes in management. Nurse-detected pain was significantly associated with the administration of opioid analgesics, whereas nurse-detected dyspnea was not significantly associated with any treatment we examined. No statistically significant association was found between nurse ratings of dyspnea and adjustments of oxygen delivery devices, suggesting that these adjustments may not be perceived as a treatment for dyspnea; however, oxygen delivery devices and levels were likely adjusted for other reasons, such as hypoxemia, during the course of the day, and our results cannot distinguish among these reasons. In addition, we cannot rule out that treatments were used for dyspnea that were neither documented in the medical chart nor reported by nurses, such as uncharted ventilator adjustments or physical interventions such as bed adjustment or bringing a fan into the room. Nevertheless, the lack of association between dyspnea scores and any examined intervention is concerning. These results suggest the need for guidelines for the detection and treatment of dyspnea, much like those already in place for pain. Finally, nurses in our sample reported that dyspnea presented a greater challenge to symptom management than pain, potentially due to the lack of clarity surrounding clinical guidelines for the management of dyspnea.
Another limitation is the restricted number (n = 29) of patients who had data available for all three (nurse, personal caregiver, and self) ratings. This restricted sample size limited our ability to detect a significant association between nurse and patient ratings and also limited the precision of our estimates of nurse and caregiver sensitivity and specificity for patient-reported dyspnea. However, the close association between caregiver and patient reports was statistically significant even in this subsample, suggesting the robustness of this association.
Personal caregivers were not always available to give proxy consent at the time when patient recruitment occurred. This means that we were not able to study a significant number of patients who were unable to communicate and did not have personal caregivers available for proxy consent, and we do not know the exact number of patients who were ineligible for the study for this reason. Because of this limitation, it is possible that we enrolled a population of patients with more attentive family members or a greater ability to communicate than a fully representative sample of ICU patients would have and that our sample may have had different symptomatology than a less selected sample. Specifically, it is likely that mechanically ventilated patients are underrepresented in our sample, as the majority of such patients in the ICU are unable to communicate and thus require proxy consent.
Because of the discussed limitations, we encourage future studies of the prevalence of dyspnea that are able to obtain a more representative sample of ICU patients. In addition, further studies are needed to test the feasibility of widespread implementation of personal caregiver proxy reports or behavioral scales such as the Respiratory Distress Observation Scale (13) and Intensive Care Respiratory Distress Observation Scale (14), scales that have shown significant promise in detecting dyspnea in patients who cannot communicate their symptoms. We also encourage the development of new tools to assess dyspnea in noncommunicative mechanically ventilated patients, in which the etiology of dyspnea may be different than those patients not receiving mechanical ventilation. In terms of treatment, further research is necessary into the efficacy of treatments to develop more specific and practical guidelines, as current guidelines (5, 35) are limited by the mixed results of treatment studies and are often based on the palliative care population, not the entire ICU population (2, 23).
In conclusion, the results of this study suggest that moderate to severe dyspnea is highly prevalent in the studied ICU. In addition, dyspnea is inconsistently detected and inconsistently treated by clinicians. Further research and guideline development are needed for routine assessment of dyspnea in both communicative and noncommunicative patients and for treatment of dyspnea in ICU patients, similar to already existing guidelines for the detection and treatment of pain.
Supplementary Material
Footnotes
Supported by National Cancer Institute grant CA197730 (H.G.P.), National Institute on Aging postdoctoral fellowship grant T32 AG0496666, and Medical Student Training in Aging Research program grant (E.R.G.).
Author Contributions: E.R.G. and H.G.P.: substantial contribution to acquisition, analysis, and interpretation of data and drafted and revised the manuscript for important intellectual content. H.D.: substantial contribution to analysis and interpretation of data and drafted and revised the manuscript for important intellectual content. D.J.O., L.L., and D.A.B.: substantial contribution to acquisition and interpretation of data and revised the manuscript for important intellectual content. C.J.X. and P.K.M.: substantial contribution to analysis and interpretation of data and revised the manuscript for important intellectual content. Each author approved the final version to be published and agreed to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201805-0996OC on November 28, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Campbell ML. Caring for dying patients in the intensive care unit: managing pain, dyspnea, anxiety, delirium, and death rattle. AACN Adv Crit Care. 2015;26:110–120. doi: 10.1097/NCI.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 2.Puntillo K, Nelson JE, Weissman D, Curtis R, Weiss S, Frontera J, et al. Advisory Board of the Improving Palliative Care in the ICU (IPAL-ICU) Project. Palliative care in the ICU: relief of pain, dyspnea, and thirst–a report from the IPAL-ICU Advisory Board. Intensive Care Med. 2014;40:235–248. doi: 10.1007/s00134-013-3153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qaseem A, Snow V, Shekelle P, Casey DE, Jr, Cross JT, Jr, Owens DK, et al. Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:141–146. doi: 10.7326/0003-4819-148-2-200801150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Chanques G, Jaber S, Barbotte E, Violet S, Sebbane M, Perrigault PF, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34:1691–1699. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- 5.Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burki NK, Lee LY. Mechanisms of dyspnea. Chest. 2010;138:1196–1201. doi: 10.1378/chest.10-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker KM, DeSanto-Madeya S, Banzett RB. Routine dyspnea assessment and documentation: nurses’ experience yields wide acceptance. BMC Nurs. 2017;16:3. doi: 10.1186/s12912-016-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker K, Barsamian J, Leone D, Donovan BC, Williams D, Carnevale K, et al. Routine dyspnea assessment on unit admission Am J Nurs 201311342–49.[Quiz, p. 50.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mularski RA, Campbell ML, Asch SM, Reeve BB, Basch E, Maxwell TL, et al. A review of quality of care evaluation for the palliation of dyspnea. Am J Respir Crit Care Med. 2010;181:534–538. doi: 10.1164/rccm.200903-0462PP. [DOI] [PubMed] [Google Scholar]
- 10.Haugdahl HS, Storli SL, Meland B, Dybwik K, Romild U, Klepstad P. Underestimation of patient breathlessness by nurses and physicians during a spontaneous breathing trial. Am J Respir Crit Care Med. 2015;192:1440–1448. doi: 10.1164/rccm.201503-0419OC. [DOI] [PubMed] [Google Scholar]
- 11.Binks AP, Desjardin S, Riker R. ICU clinicians underestimate breathing discomfort in ventilated subjects. Respir Care. 2017;62:150–155. doi: 10.4187/respcare.04927. [DOI] [PubMed] [Google Scholar]
- 12.Janssen DJA, Spruit MA, Wouters EFM, Schols JMGA. Symptom distress in advanced chronic organ failure: disagreement among patients and family caregivers. J Palliat Med. 2012;15:447–456. doi: 10.1089/jpm.2011.0394. [DOI] [PubMed] [Google Scholar]
- 13.Campbell ML, Templin T, Walch J. A Respiratory Distress Observation Scale for patients unable to self-report dyspnea. J Palliat Med. 2010;13:285–290. doi: 10.1089/jpm.2009.0229. [DOI] [PubMed] [Google Scholar]
- 14.Persichini R, Gay F, Schmidt M, Mayaux J, Demoule A, Morélot-Panzini C, et al. Diagnostic accuracy of respiratory distress observation scales as surrogates of dyspnea self-report in intensive care unit patients. Anesthesiology. 2015;123:830–837. doi: 10.1097/ALN.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 15.Smallwood N, Le B, Currow D, Irving L, Philip J. Management of refractory breathlessness with morphine in patients with chronic obstructive pulmonary disease. Intern Med J. 2015;45:898–904. doi: 10.1111/imj.12857. [DOI] [PubMed] [Google Scholar]
- 16.Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327:523–528. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt M, Demoule A, Polito A, Porchet R, Aboab J, Siami S, et al. Dyspnea in mechanically ventilated critically ill patients. Crit Care Med. 2011;39:2059–2065. doi: 10.1097/CCM.0b013e31821e8779. [DOI] [PubMed] [Google Scholar]
- 18.Nava S, Ferrer M, Esquinas A, Scala R, Groff P, Cosentini R, et al. Palliative use of non-invasive ventilation in end-of-life patients with solid tumours: a randomised feasibility trial. Lancet Oncol. 2013;14:219–227. doi: 10.1016/S1470-2045(13)70009-3. [DOI] [PubMed] [Google Scholar]
- 19.Davidson PM, Johnson MJ. Update on the role of palliative oxygen. Curr Opin Support Palliat Care. 2011;5:87–91. doi: 10.1097/SPC.0b013e3283463cd3. [DOI] [PubMed] [Google Scholar]
- 20.Abernethy AP, McDonald CF, Frith PA, Clark K, Herndon JE, II, Marcello J, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet. 2010;376:784–793. doi: 10.1016/S0140-6736(10)61115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Congleton J, Muers MF. The incidence of airflow obstruction in bronchial carcinoma, its relation to breathlessness, and response to bronchodilator therapy. Respir Med. 1995;89:291–296. doi: 10.1016/0954-6111(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 22.Simon ST, Higginson IJ, Booth S, Harding R, Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev. 2010:CD007354. doi: 10.1002/14651858.CD007354.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, et al. ATS End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177:912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 24.Gentzler E, Ouyang D, Lief L, Berlin D, Maciejewski R, Prigerson HG. Dyspnea in the intensive care unit: symptoms and outcomes; Presented at the Annual Scientific Meeting of the American Geriatrics Society. May 18–20, 2017, Houston, TX. [Google Scholar]
- 25.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 26.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 27.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. European Palliative Care Research Collaborative (EPCRC) Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41:1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Morris NR, Sabapathy S, Adams L, Kingsley RA, Schneider DA, Stulbarg MS. Verbal numerical scales are as reliable and sensitive as visual analog scales for rating dyspnea in young and older subjects. Respir Physiol Neurobiol. 2007;157:360–365. doi: 10.1016/j.resp.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Oldenmenger WH, de Raaf PJ, de Klerk C, van der Rijt CCD. Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manage. 2013;45:1083–1093. doi: 10.1016/j.jpainsymman.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Kako J, Kobayashi M, Kanno Y, Ogawa A, Miura T, Matsumoto Y. The optimal cutoff point for expressing revised Edmonton Symptom Assessment System scores as binary data indicating the presence or absence of symptoms. Am J Hosp Palliat Care. 2018;35:1390–1393. doi: 10.1177/1049909118775660. [DOI] [PubMed] [Google Scholar]
- 31.Stevens JP, Dechen T, Schwartzstein R, O’Donnell C, Baker K, Howell MD, et al. Prevalence of dyspnea among hospitalized patients at the time of admission. J Pain Symptom Manage. 2018;56:15–22, e2. doi: 10.1016/j.jpainsymman.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 33.Su A, Lief L, Berlin D, Cooper Z, Ouyang D, Holmes J, et al. Beyond pain: nurses’ assessment of patient suffering, dignity, and dying in the intensive care unit. J Pain Symptom Manage. 2018;55:1591–1598, e1. doi: 10.1016/j.jpainsymman.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt M, Banzett RB, Raux M, Morélot-Panzini C, Dangers L, Similowski T, et al. Unrecognized suffering in the ICU: addressing dyspnea in mechanically ventilated patients. Intensive Care Med. 2014;40:1–10. doi: 10.1007/s00134-013-3117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.