Abstract
Rationale: Growing evidence suggests that compromised lung health may be linked to dementia and worsening cognitive ability.
Objectives: To test the hypothesis that impaired lung function or lung disease in midlife is associated with greater risk of incident dementia and mild cognitive impairment (MCI) later in life.
Methods: A total of 14,184 Atherosclerosis Risk in Communities study participants who underwent spirometry and were asked about lung health (1987–1989) were followed. Dementia and MCI were defined by hospitalization diagnosis codes (1987–2013) in the whole cohort and with adjudication among 42% who attended a comprehensive neurocognitive examination (2011–2013).
Measurements and Main Results: In analysis using adjudicated outcomes, odds of dementia or MCI were higher among participants with restrictive (multivariable-adjusted odds ratio, 1.58; 95% confidence interval, 1.14–2.19) and obstructive lung disease (multivariable-adjusted odds ratio, 1.33; 95% confidence interval, 1.07–1.64), compared with those without disease or respiratory symptoms. Associations were similar in analyses restricted to nonsmokers, and present for both Alzheimer’s disease–related dementia and cerebrovascular etiologies. Low FEV1% predicted and FVC% predicted were also associated with increased dementia risk.
Conclusions: Midlife lung disease and reduced lung function were associated with modestly increased odds of dementia and MCI later in life. Magnitudes of association were more pronounced for restrictive impairment than for obstructive lung disease. These associations were present in smokers and nonsmokers. If the observed associations are causal, policy and public health efforts to reduce smoking and improve air quality may have the added benefit of preventing the development of dementia and MCI.
Keywords: restrictive impairment, chronic obstructive pulmonary disease, Alzheimer’s disease dementia, dementia
At a Glance Commentary
Scientific Knowledge on the Subject
Prior research suggests that lung disease and impaired lung function may be linked to dementia. However, few studies have been prospective, evaluated different types of lung disease, or considered lung health in midlife.
What This Study Adds to the Field
In a community-based cohort followed for 27 years, both restrictive and, to a lesser extent, obstructive lung disease were associated with greater risk of incident dementia and mild cognitive impairment. This pattern was present for both Alzheimer’s disease–related dementia and cerebrovascular disease etiologies, and persisted in analyses restricted to nonsmokers.
Identification of modifiable risk factors for dementia and mild cognitive impairment (MCI) is a research priority, because given the high prevalence of these conditions (1) even a modest reduction in risk factors could reduce the societal burden (2) of dementia and MCI. Lung disease and impaired lung function are preventable, and growing evidence suggests that compromised lung health may be linked to greater risk of dementia or worsening cognitive ability (3, 4). Evidence exists for lung impairment as assessed by objective measures, such as low FEV1, FVC, and the ratio of FEV1/FVC (5–8), and clinically recognized chronic obstructive pulmonary disease (COPD), asthma, or chronic bronchitis (9–11). Although these prior studies provide valuable information about the possible role of lung health in dementia risk, they often lacked comprehensive event adjudication or had relatively short follow-up. Importantly, for many dementia risk factors, stronger associations have been observed when the risk factors were measured at middle-age than when they were measured later in life (12–14).
Mechanistically, impaired lung function could influence dementia and MCI risk through several pathways, largely mediated through chronic hypoxemia (3, 15). These include systemic inflammation, oxidative stress, physiologic stress (e.g., sympathetic nervous system activation), and cerebral arterial stiffness and small-vessel damage (3, 15). Impaired lung function has also been linked to incident stroke, independent of smoking (16, 17). Hypoxemia within the context of obstructive sleep apnea has also been associated with greater risk of dementia (18).
Using data from the community-based ARIC (Atherosclerosis Risk in Communities) cohort, we tested the hypotheses that development of dementia and MCI over 27 years of follow-up would be more common among participants who at baseline had COPD or restrictive impairment or poorer lung function, as assessed by spirometry. Analyses were also conducted according to dementia or MCI primary etiology (i.e., Alzheimer’s disease [AD] or cerebrovascular disease). Furthermore, given the importance of smoking to lung health, additional analyses were conducted restricted to nonsmokers. Lastly, we explored interactions by race.
Methods
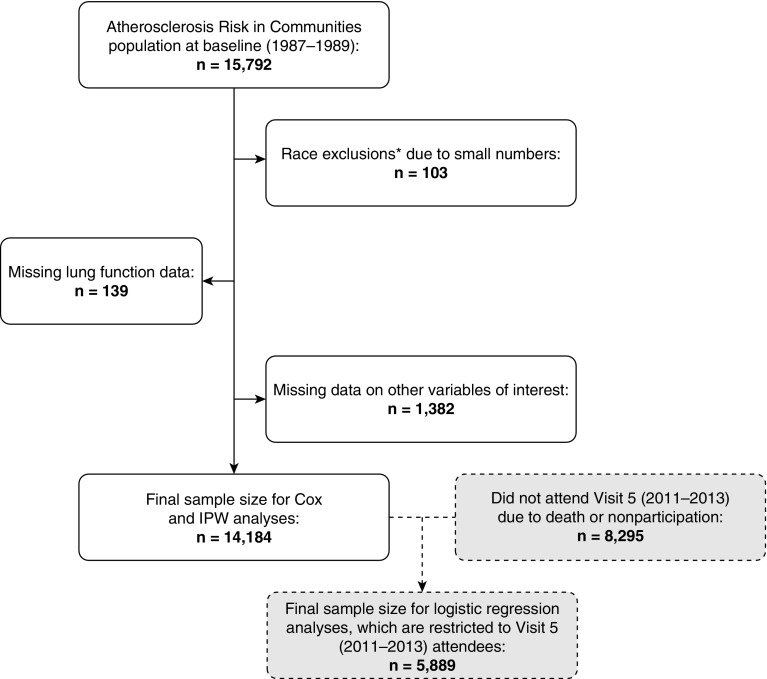
The ARIC study is a community-based prospective cohort of 15,792 participants who in 1987–1989 were recruited from four U.S. communities: 1) suburbs of Minneapolis, Minnesota; 2) Jackson, Mississippi; 3) Forsyth County, North Carolina; and 4) Washington County, Maryland (19). Participants were aged 45–64 at baseline. In the Minnesota, Maryland, and North Carolina sites, recruitment was representative of the racial/ethnic composition of the communities (i.e., mostly white in Minnesota and Maryland, 15% black and 85% white in North Carolina), whereas in Mississippi only black participants were recruited. Since cohort inception participants have been followed continuously for hospitalizations and have taken part in several follow-up clinic visits. The present manuscript uses data from baseline (visit 1: 1987–1989) and the ARIC Neurocognitive Study visit 5 (2011–2013). The final analytic sample for the incidence analysis comprised 14,184 individuals; exclusions are shown in Figure 1. All study protocols have been approved by local institutional review boards and participants provided written informed consent.
Figure 1.
Participant flow chart for incidence and inverse probability-weighted analyses. IPW = inverse probability weighting. *Not black or white, and black individuals from the Minnesota and Maryland centers.
Exposure Measurement
Pulmonary function was assessed by certified pulmonary technicians at baseline using a water-sealed Collins Survey II volume displacement spirometer (Collins Medical) and PULMO-SCREEN II software (PDS Healthcare Products), based on American Thoracic Society guidelines (20), as has been described previously in ARIC (21) and is detailed in the Methods section of the online supplement. Briefly, for each participant, at least three acceptable spirograms were sought from a minimum of five forced expirations, and a best reading was then selected. FEV1, FVC, and the FEV1/FVC ratio, as a percentage of age-, race-, and sex-specific predicted values and lower limit of normal (LLN) values, were calculated (22).
Participants also self-reported whether a doctor has ever told them they had asthma, chronic bronchitis, or emphysema. Participants were also classified into four mutually exclusive groups (23), on the basis of both spirometry results and self-reported information (24):
-
1.
COPD: FEV1/FVC < LLN
-
2.
Restrictive impairment: FEV1/FVC ≥ LLN and FVC < LLN (with or without self-reported respiratory symptoms)
-
3.
Respiratory symptoms with normal spirometric results (without COPD or restrictive impairment)
-
4.
Normal (without respiratory symptoms, COPD, or restrictive impairment)
Covariates and Potential Effect Modifiers
Covariate information was obtained at baseline, using standard ARIC procedures (see Methods section of the online supplement). Briefly, questionnaire data were obtained; height, weight, and sitting blood pressure were measured; a fasting blood draw was conducted; and information on participant medication bottles (which were brought to the visit) was recorded. Methods for the measurement and classification of the APOE (apolipoprotein E) ε4 risk allele have been described elsewhere (5).
Dementia and MCI Ascertainment
Several different approaches were used to ascertain dementia and MCI during follow-up (25). First, 6,471 of the 6,538 ARIC participants attending visit 5 (2011–2013) underwent a detailed neurocognitive assessment, and a selected subset (25) received a neurologic examination and brain magnetic resonance imaging. Second, a validated telephone-based cognitive assessment, the modified telephone interview for cognitive status (TICSm), was performed in 1,461 participants who at the time of visit 5 were alive but unable or unwilling to participate in an in-person examination. Informants provided additional information in some instances, when participants were deceased or unable to complete the TICSm assessment themselves (25). Lastly, in the full cohort, hospitalization diagnosis codes were used to identify incident dementia occurring from 1987 to 2013.
Outcomes of interest for the present analysis were defined according to methodology previously used in ARIC (25). Incident dementia was defined using data from all of the potential diagnostic sources described previously (i.e., visit 5 assessment, TICSm, and hospitalization codes). An expert panel adjudicated syndromic dementia, MCI, and etiology (AD or vascular), as detailed in the Methods section of the online supplement.
Statistical Analysis
Participant characteristics were described according to visit 5 participation status, lung function impairment categories, and quintiles of FVC% predicted. Figure 1 is a study flow chart, describing who was included in various analyses.
For the incidence analyses, Cox proportional hazards regression was used. Follow-up time began on the date of the baseline examination, and accrued until a dementia hospitalization International Classification of Diseases (ICD) code; loss to follow-up; death; December 31, 2013; or the visit 5 examination date. The proportional hazards assumption was checked by plotting of log(–log) survival curves and testing the interaction between the exposures and time.
For analyses of the association between baseline lung function and risk of the neurocognitive study adjudicated outcomes we used logistic regression. Five outcomes were considered: 1) dementia or MCI, 2) dementia, 3) MCI, 4) dementia or MCI caused by AD, and 5) dementia or MCI caused by cerebrovascular disease. For outcomes 2 through 5, we excluded from the analyses those with outcomes different from the outcome under study (e.g., for the dementia outcome, dementia was defined as yes or no, and participants with MCI were excluded). For these analyses selection bias may have occurred as a result of differential participation and survival to visit 5. As such, we used inverse probability weighting (IPW) (26, 27) to adjust for attrition caused by either death or failure to attend the follow-up neurocognitive examination (censoring) (see the Methods section of the online supplement).
A series of nested models was used for both the Cox and logistic regression analyses, with covariate information obtained from baseline. Model 1 adjusted for demographic characteristics. Race and center were combined into a five-level variable (i.e., white individuals/Minnesota, white individuals/Maryland, white individuals/North Carolina, black individuals/North Carolina, and black individuals/Mississippi) reflective of the race–center combinations in ARIC. Model 2 additionally adjusted for cigarette smoking and pack-years of smoking. Model 3 further adjusted for physical activity, body mass index, traditional cardiovascular risk factors, prevalent cardiovascular disease, and apolipoprotein E genotype. Model 4 additionally adjusted for fibrinogen, which is a marker of inflammation.
Multiplicative interactions by race were explored by including cross-product terms in the models. Additionally, because of the importance of smoking on lung health, we also conducted analyses restricted to nonsmokers. Statistical significance was defined as α of 0.05.
Results
At baseline, the 14,184 participants included in this analysis were on average 54.2 ± 5.8 years old; 55.3% were female, and 25.9% were African American. Table E1 in the online supplement provides baseline participant characteristics according to whether the participants took part in visit 5, were alive but did not take part, or died before visit 5. Those who participated in visit 5 were on average slightly younger, had higher educational attainment, were less likely to smoke, and overall had a slightly better health profile than those who did not take part or died.
At baseline, mean ± SD measured FEV1 was 2.82 ± 0.77 L (% predicted, 93.5% ± 17.0), measured FVC 3.80 ± 0.99 L (% predicted, 98.1% ± 14.6), and FEV1/FVC 74.4 ± 8.1% (% predicted, 94.5 ± 10.0%). Table 1 provides baseline participant characteristics according to lung disease categories; 17.6% were classified as having the COPD pattern, 5.9% restrictive impairment, 33.5% respiratory symptoms with normal spirometric results, and 43.1% as normal. Men, those with lower educational attainment, and those who were current smokers were classified less frequently as having normal lung function. Participant characteristics according to quintiles of FVC% predicted are provided in Table E2.
Table 1.
Baseline Characteristics according to Lung Function Categories: ARIC Study, 1987–1989
| Lung Function Category |
P Value | ||||
|---|---|---|---|---|---|
| Normal | Respiratory Symptoms with Normal Spirometric Results | Restrictive Impairment Pattern | COPD Pattern | ||
| n (%) | 6,108 (43) | 4,754 (34) | 832 (6) | 2,490 (18) | |
| Demographics | |||||
| Age, yr | 53.9 (5.7) | 53.9 (5.7) | 54.5 (5.6) | 55.1 (5.8) | <0.001 |
| Female, % | 57.1 | 56.0 | 52.4 | 50.5 | <0.001 |
| African American, % | 27.1 | 27.4 | 20.3 | 22.1 | <0.001 |
| Education level, % | <0.001 | ||||
| <High school | 17.9 | 26.0 | 31.3 | 29.5 | |
| High school graduate | 40.8 | 41.8 | 39.5 | 40.2 | |
| College/graduate school | 41.3 | 32.2 | 29.2 | 30.3 | |
| Behaviors | |||||
| Smoking status, % | <0.001 | ||||
| Current | 12.3 | 29.2 | 35.1 | 49.7 | |
| Former | 33.7 | 30.4 | 29.6 | 30.1 | |
| Never | 54.0 | 40.4 | 35.3 | 20.2 | |
| Pack-years* | 12.5 (6.5–30.0) | 18.1 (11.2–37.0) | 22.0 (16.0–43.0) | 28.9 (21.0–48.0) | <0.001 |
| Physical activity† | 2.5 (0.8) | 2.4 (0.8) | 2.3 (0.8) | 2.4 (0.8) | <0.001 |
| Respiratory indicators | |||||
| FEV1% predicted | 101.0 (12.1) | 97.2 (12.0) | 72.6 (8.3) | 74.8 (18.4) | <0.001 |
| FVC% predicted | 102.3 (11.7) | 99.2 (11.4) | 72.6 (7.3) | 94.1 (18.0) | <0.001 |
| FEV1/FVC % predicted | 98.2 (5.7) | 97.4 (5.6) | 99.5 (7.5) | 78.4 (9.5) | <0.001 |
| FEV1, L | 3.03 (0.71) | 2.91 (0.71) | 2.23 (0.54) | 2.31 (0.74) | <0.001 |
| FVC, L | 3.93 (0.95) | 3.81 (0.95) | 2.87 (0.72) | 3.74 (1.06) | <0.001 |
| FEV1/FVC | 77.3 (4.7) | 76.7 (4.7) | 78.0 (6.2) | 61.4 (7.8) | <0.001 |
| Self-reported symptoms, % | |||||
| Cough | 0.0 | 20.0 | 17.9 | 26.4 | <0.001 |
| Phlegm | 0.0 | 15.0 | 13.2 | 21.8 | <0.001 |
| Dyspnea | 0.0 | 13.2 | 16.0 | 14.1 | <0.001 |
| Self-reported medical diagnosis, % | |||||
| Bronchitis | 2.5 | 11.4 | 10.9 | 15.9 | <0.001 |
| Emphysema | 0.3 | 1.2 | 1.4 | 6.2 | <0.001 |
| Asthma | 2.1 | 7.1 | 4.9 | 13.3 | <0.001 |
| Other physiologic characteristics | |||||
| Body mass index, kg/m2 | 27.3 (4.8) | 28.5 (5.7) | 30.3 (6.5) | 26.0 (4.9) | <0.001 |
| Systolic blood pressure, mm Hg | 120.4 (17.8) | 121.2 (18.7) | 124.8 (20.1) | 120.7 (19.2) | <0.001 |
| Antihypertensive medications, % | 22.4 | 27.3 | 36.2 | 22.2 | <0.001 |
| Prevalent diabetes, % | 9.4 | 13.0 | 22.4 | 9.1 | <0.001 |
| HDL cholesterol, mg/dl | 53.3 (17.0) | 50.8 (16.6) | 46.1 (15.0) | 52.3 (17.7) | <0.001 |
| LDL cholesterol, mg/dl | 137.3 (38.7) | 139.1 (39.6) | 140.3 (40.0) | 134.5 (39.4) | <0.001 |
| Lipid-lowering medication, % | 2.7 | 2.7 | 4.9 | 2.5 | 0.002 |
| Prevalent CHD, % | 3.3 | 4.4 | 10.3 | 6.3 | <0.001 |
| Prevalent heart failure, % | 0.8 | 7.3 | 5.9 | 17.6 | <0.001 |
| Prevalent stroke, % | 3.2 | 5.9 | 8.1 | 4.7 | <0.001 |
| APOE, % | 0.27 | ||||
| ε4/ε4 | 2.7 | 2.6 | 3.5 | 2.3 | |
| ε2/ε4 or ε3/ε4 | 27.4 | 28.0 | 27.9 | 29.5 | |
| Other | 69.9 | 69.5 | 68.6 | 68.3 | |
| Weights | |||||
| Unstabilized weights (all) | 3.1 | 3.8 | 5.7 | 5.8 | |
| Unstabilized weights (visit 5) | 2.0 | 2.3 | 3.2 | 2.9 | |
| Stabilized weights (visit 5) | 0.9 | 1.0 | 1.2 | 1.1 | |
Definition of abbreviations: APOE = apolipoprotein E; ARIC = Atherosclerosis Risk in Communities; CHD = coronary heart disease; COPD = chronic obstructive pulmonary disease; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Data are shown as mean (SD) or percentage unless otherwise indicated.
Among ever-smokers. Data are shown as geometric mean (25th percentile–75th percentile).
Score on the sport index of the Baecke physical activity questionnaire (41).
Lung Disease, Lung Function, and Incident Dementia
A total of 1,407 incident dementia events were identified among the full sample of 14,184 ARIC participants, over a median follow-up of 23.0 years (25th and 75th percentiles: 18.3–24.2; maximum, 27.1). As shown in Table 2, relative to participants classified as normal, risk of dementia was elevated among those with the COPD pattern (hazard ratio [HR], 1.23; 95% confidence interval [CI], 1.06–1.43) and those with the restrictive impairment (HR, 1.31; 95% CI, 1.03–1.66), after accounting for demographics (model 1). The associations were attenuated with additional covariate adjustment, and became nonsignificant. Participants in the lowest (vs. highest) quintiles of FEV1% predicted and FVC% predicted were at elevated risk of incident dementia after accounting for smoking (model 2), but estimates were attenuated and became nonsignificant with additional adjustment for cardiovascular risk factors (model 3). FEV1/FVC % predicted was not associated with dementia risk.
Table 2.
Lung Disease Categories, Objective Indices of Lung Function, and Risk of Incident Dementia: ARIC Study, 1996–2013
| Lung Disease Category |
||||||
|---|---|---|---|---|---|---|
| Normal | Respiratory Symptoms with Normal Spirometric Results | Restrictive Impairment Pattern | COPD Pattern | |||
| n | 6,108 | 4,754 | 832 | 2,490 |
||
| Dementia cases, n | 616 | 483 | 79 | 229 |
||
| Person-years | 130,103 | 96,713 | 15,485 | 46,012 |
||
| Incidence rate* | 4.7 | 5.0 | 5.1 | 5.0 |
||
| Hazard ratio (95% CI) | |
|||||
| Model 1 | 1 | 1.10 (0.97–1.24) | 1.31 (1.03–1.66) | 1.23 (1.06–1.43) |
||
| Model 2 | 1 | 1.06 (0.94–1.20) | 1.24 (0.97–1.57) | 1.11 (0.94–1.31) |
||
| Model 3 | 1 | 0.99 (0.88–1.12) | 0.99 (0.78–1.27) | 1.08 (0.92–1.28) |
||
| Model 4 | 1 | 0.99 (0.87–1.12) | 0.99 (0.78–1.27) | 1.08 (0.92–1.27) | ||
| FEV1% Predicted |
||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Trend (per 1 SD Decrease) | |
| n | 2,836 | 2,838 | 2,837 | 2,837 | 2,836 | |
| Dementia cases, n | 275 | 282 | 246 | 290 | 314 | |
| Person-years | 50,632 | 57,102 | 59,400 | 60,115 | 61,066 | |
| Incidence rate* | 5.4 | 4.9 | 4.1 | 4.8 | 5.1 | |
| Hazard ratio (95% CI) | ||||||
| Model 1 | 1.36 (1.15–1.60) | 1.12 (0.95–1.32) | 0.92 (0.77–1.08) | 1.08 (0.92–1.26) | 1 | 1.13 (1.07–1.19) |
| Model 2 | 1.23 (1.04–1.47) | 1.07 (0.91–1.26) | 0.89 (0.75–1.06) | 1.07 (0.91–1.25) | 1 | 1.09 (1.03–1.15) |
| Model 3 | 1.10 (0.93–1.32) | 0.98 (0.83–1.16) | 0.83 (0.70–0.99) | 1.02 (0.86–1.19) | 1 | 1.05 (0.98–1.11) |
| Model 4 | 1.11 (0.93–1.32) | 0.99 (0.83–1.16) | 0.84 (0.71–0.99) | 1.02 (0.87–1.20) | 1 | 1.05 (0.98–1.11) |
| FVC% Predicted |
||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Trend (per 1 SD Decrease) | |
| n | 2,836 | 2,838 | 2,835 | 2,839 | 2,836 | |
| Dementia cases, n | 283 | 292 | 257 | 297 | 278 | |
| Person-years | 52,072 | 56,824 | 58,743 | 59,860 | 60,815 | |
| Incidence rate* | 5.4 | 5.1 | 4.4 | 5.0 | 4.6 | |
| Hazard ratio (95% CI) | ||||||
| Model 1 | 1.44 (1.22–1.70) | 1.29 (1.09–1.52) | 1.10 (0.93–1.30) | 1.24 (1.06–1.47) | 1 | 1.12 (1.08–1.19) |
| Model 2 | 1.36 (1.14–1.61) | 1.25 (1.06–1.47) | 1.08 (0.91–1.28) | 1.24 (1.05–1.46) | 1 | 1.11 (1.04–1.17) |
| Model 3 | 1.14 (0.96–1.36) | 1.19 (1.00–1.40) | 0.99 (0.83–1.17) | 1.17 (0.99–1.38) | 1 | 1.06 (1.00–1.11) |
| Model 4 | 1.15 (0.96–1.37) | 1.20 (1.01–1.42) | 1.00 (0.84–1.19) | 1.18 (1.00–1.39) | 1 | 1.06 (1.00–1.11) |
| FEV1/FVC% Predicted |
||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Trend (per 1 SD Decrease) | |
| n | 2,836 | 2,838 | 2,837 | 2,835 | 2,838 | |
| Dementia cases, n | 257 | 247 | 266 | 287 | 350 | |
| Person-years | 52,834 | 58,333 | 59,125 | 59,380 | 58,652 | |
| Incidence rate* | 4.9 | 4.2 | 4.5 | 4.8 | 6.0 | |
| Hazard ratio (95% CI) | ||||||
| Model 1 | 0.97 (0.83–1.15) | 0.83 (0.70–0.98) | 0.84 (0.72–0.99) | 0.91 (0.78–1.06) | 1 | 1.03 (0.97–1.08) |
| Model 2 | 0.87 (0.73–1.03) | 0.79 (0.67–0.93) | 0.82 (0.70–0.97) | 0.90 (0.77–1.05) | 1 | 0.99 (0.92–1.05) |
| Model 3 | 0.93 (0.78–1.10) | 0.85 (0.72–1.00) | 0.87 (0.74–1.03) | 0.94 (0.80–1.10) | 1 | 1.00 (0.94–1.06) |
| Model 4 | 0.93 (0.78–1.10) | 0.85 (0.72–1.00) | 0.88 (0.75–1.04) | 0.94 (0.80–1.10) | 1 | 1.00 (0.94–1.06) |
Definition of abbreviations: ARIC = Atherosclerosis Risk in Communities; CI = confidence interval; COPD = chronic obstructive pulmonary disease.
Model 1: Cox regression adjusted for age, sex, center, education level, and race–center (five-level variable). Model 2: model 1 + additional adjustment for cigarette smoking and pack-years of smoking. Model 3: model 2 + additional adjustment for physical activity, body mass index, systolic blood pressure, blood pressure medication use, diabetes, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, lipid-lowering medications, prevalent coronary heart disease, heart failure, stroke, and apolipoprotein E genotype. Model 4: model 3 + fibrinogen.
Per 1,000 person-years.
In analyses restricted to never-smokers (n = 6,018; see Table E3), results were generally similar to those of the full analyses, although CIs were less precise. In model 3, the HRs for COPD and restrictive impairment, versus being classified as normal, were 1.31 (0.99–1.72) and 1.12 (0.78–1.62), respectively. Although interactions by race were not statistically significant at P = 0.05, associations were generally stronger in black individuals than in white individuals (see Table E4). Among black individuals, the model 3 HRs for having COPD and restrictive impairment patterns (vs. normal) were 1.31 (95% CI, 0.98–1.76) and 1.23 (95% CI, 0.76–1.98), respectively, whereas in white individuals the HRs were 0.99 (95% CI, 0.82–1.21) and 0.93 (95% CI, 0.70–1.23). Also, for FEV1% predicted and FVC% predicted there was some evidence that the proportional hazards assumption was violated, whereby associations were stronger earlier in follow-up than later in follow-up (see Table E5).
Lung Disease, Lung Function, and Neurocognitive Study–adjudicated Dementia
Among the 5,889 participants who had lung function data and cognitive assessments as part of the neurocognitive examination, we also evaluated the association between baseline lung disease category and risk of dementia or MCI, dementia, MCI, and MCI or dementia caused by AD, or caused by cerebrovascular disease (Table 3). After model 3 adjustments, odds ratios (ORs) of associations between participants with restrictive impairment and those who were normal were 1.58 (95% CI, 1.14–2.19) for dementia or MCI, 1.16 (95% CI, 0.56–2.40) for dementia, 1.71 (95% CI, 1.23–2.38) for MCI, 1.79 (95% CI, 1.24–2.58) for dementia or MCI caused by AD, and 1.60 (95% CI, 0.78–3.31) for dementia or MCI caused by cerebrovascular disease. Presence of the COPD pattern, versus normal, was after model 3 adjustments associated with ORs of 1.33 (95% CI, 1.07–1.64) for dementia or MCI, 1.16 (95% CI, 0.74–1.82) for dementia, 1.40 (95% CI, 1.12–1.76) for MCI, 1.24 (95% CI, 0.97–1.60) for AD-type dementia or MCI, and 1.33 (95% CI, 0.79–2.23) for dementia or MCI caused by cerebrovascular disease. Magnitudes of association were smaller for comparisons of participants categorized as having respiratory symptoms with normal spirometric results to those classified as normal. The previously mentioned results were similar in analyses restricted to nonsmokers (see Table E6). For instance, after model 3 adjustments, the restrictive impairment and COPD patterns were associated with ORs for dementia or MCI of 1.69 (95% CI, 1.04–2.76) and 1.72 (95% CI, 1.23–2.40), respectively.
Table 3.
Weighted* Odds Ratios and 95% Confidence Intervals of Lung Disease Categories with Dementia, MCI, Alzheimer’s Disease–Type Dementia or MCI, and Dementia or MCI Caused by Cerebrovascular Disease: ARIC Study, 1987–2013
| Lung Disease Category |
||||
|---|---|---|---|---|
| Normal | Respiratory Symptoms with Normal Spirometric Results | Restrictive Impairment Pattern | COPD Pattern | |
| n | 2,953 | 1,967 | 239 | 730 |
| Dementia or MCI, n | 721 | 518 | 87 | 212 |
| Model 1 | 1 | 1.15 (1.00–1.33) | 1.92 (1.40–2.63) | 1.30 (1.07–1.60) |
| Model 2 | 1 | 1.15 (0.99–1.33) | 1.89 (1.37–2.59) | 1.28 (1.03–1.58) |
| Model 3 | 1 | 1.10 (0.95–1.28) | 1.58 (1.14–2.19) | 1.33 (1.07–1.64) |
| Model 4 | 1 | 1.09 (0.94–1.27) | 1.56 (1.12–2.16) | 1.31 (1.06–1.62) |
| Dementia, n | 147 | 94 | 15 | 42 |
| Model 1 | 1 | 1.00 (0.74–1.37) | 1.67 (0.86–3.26) | 1.20 (0.79–1.82) |
| Model 2 | 1 | 0.98 (0.71–1.34) | 1.56 (0.78–3.12) | 1.10 (0.71–1.69) |
| Model 3 | 1 | 0.94 (0.68–1.32) | 1.16 (0.56–2.40) | 1.16 (0.74–1.82) |
| MCI, n | 574 | 424 | 72 | 170 |
| Model 1 | 1 | 1.21 (1.04–1.40) | 1.97 (1.42–2.74) | 1.35 (1.10–1.68) |
| Model 2 | 1 | 1.21 (1.04–1.42) | 1.98 (1.42–2.76) | 1.36 (1.08–1.71) |
| Model 3 | 1 | 1.15 (0.99–1.35) | 1.71 (1.23–2.38) | 1.40 (1.12–1.76) |
| AD-type dementia or MCI, n | 474 | 344 | 59 | 127 |
| Model 1 | 1 | 1.18 (1.00–1.40) | 1.97 (1.38–2.82) | 1.14 (0.90–1.45) |
| Model 2 | 1 | 1.20 (1.02–1.43) | 2.02 (1.41–2.90) | 1.18 (0.92–1.52) |
| Model 3 | 1 | 1.16 (0.98–1.38) | 1.79 (1.24–2.58) | 1.24 (0.97–1.60) |
| Cerebrovascular dementia or MCI, n | 88 | 62 | 12 | 26 |
| Model 1 | 1 | 1.04 (0.72–1.51) | 2.39 (1.15–4.97) | 1.46 (0.89–2.39) |
| Model 2 | 1 | 0.98 (0.67–1.44) | 2.10 (1.00–4.38) | 1.19 (0.71–2.00) |
| Model 3 | 1 | 0.92 (0.62–1.36) | 1.60 (0.78–3.31) | 1.33 (0.79–2.23) |
Definition of abbreviations: AD = Alzheimer’s disease; ARIC = Atherosclerosis Risk in Communities; COPD = chronic obstructive pulmonary disease; MCI = mild cognitive impairment.
Model 1: Logistic regression adjusted for age, sex, education level, and race–center (five-level variable). Model 2: model 1 + additional adjustment for cigarette smoking and pack-years of smoking. Model 3: model 2 + additional adjustment for physical activity, body mass index, systolic blood pressure, blood pressure medication use, diabetes, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, lipid-lowering medications, prevalent coronary heart disease, heart failure, stroke, and apolipoprotein E genotype. Model 4: model 3 + fibrinogen.
Inverse-probability weighting was used.
There was some evidence of effect modification by race, as shown in Table E7. Among black individuals, the COPD pattern was most associated with elevated odds of the outcomes (e.g., model 3 OR [95% CI] of dementia/MCI for COPD pattern vs. normal: 2.13 [95% CI, 1.34–3.40]), whereas in white individuals, there was no association. Among white individuals, the restrictive impairment pattern was most strongly associated with increased odds (e.g., model 3 OR of dementia/MCI vs. normal: 1.79 [95% CI, 1.27–2.54]), whereas in black individuals, there was no association.
ORs for the associations of FEV1% predicted and odds of outcomes are shown in Table 4. The lowest (vs. highest) quartile of FEV1% predicted was associated with an OR of 1.27 (95% CI, 1.05–1.54) for dementia or MCI, after model 3 adjustments. The ORs were 1.23 (95% CI, 0.98–1.54) for dementia or MCI caused by AD, and 1.43 (95% CI, 0.91–2.24) for dementia or MCI caused by cerebrovascular disease. The associations between FEV1% predicted and the dementia outcomes did not differ significantly by race; however, in general, the magnitudes of association were larger in black individuals than in white individuals (see Table E7).
Table 4.
Weighted* Odds Ratios and 95% Confidence Intervals of FEV1% Predicted Quartile with Dementia, MCI, Alzheimer’s Disease–Type Dementia or MCI, and Dementia or MCI Caused by Cerebrovascular Disease: ARIC Study, 1987–2013
| FEV1% Predicted |
|||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Trend (per 1 SD Decrease) | |
| n | 1,473 | 1,471 | 1,472 | 1,473 | |
| Dementia or MCI, n | 450 | 364 | 346 | 378 | |
| Model 1 | 1.38 (1.15–1.65) | 1.02 (0.85–1.23) | 0.92 (0.77–1.11) | 1 | 1.14 (1.07–1.22) |
| Model 2 | 1.35 (1.12–1.63) | 1.01 (0.84–1.22) | 0.92 (0.77–1.11) | 1 | 1.13 (1.06–1.21) |
| Model 3 | 1.27 (1.05–1.54) | 0.97 (0.80–1.17) | 0.89 (0.73–1.07) | 1 | 1.11 (1.04–1.20) |
| Model 4 | 1.26 (1.04–1.53) | 0.96 (0.79–1.16) | 0.88 (0.73–1.06) | 1 | 1.11 (1.03–1.19) |
| Dementia, n | 87 | 59 | 69 | 83 | |
| Model 1 | 1.33 (0.91–1.93) | 0.88 (0.59–1.30) | 0.95 (0.65–1.40) | 1 | 1.10 (0.97–1.26) |
| Model 2 | 1.23 (0.83–1.81) | 0.85 (0.57–1.27) | 0.95 (0.65–1.39) | 1 | 1.06 (0.93–1.22) |
| Model 3 | 1.09 (0.73–1.65) | 0.77 (0.50–1.18) | 0.90 (0.61–1.33) | 1 | 1.03 (0.89–1.20) |
| MCI, n | 363 | 305 | 277 | 295 | |
| Model 1 | 1.41 (1.16–1.71) | 1.08 (0.89–1.31) | 0.93 (0.77–1.14) | 1 | 1.16 (1.08–1.24) |
| Model 2 | 1.41 (1.16–1.72) | 1.08 (0.89–1.32) | 0.94 (0.77–1.14) | 1 | 1.16 (1.08–1.25) |
| Model 3 | 1.34 (1.10–1.64) | 1.04 (0.85–1.27) | 0.90 (0.74–1.10) | 1 | 1.14 (1.06–1.23) |
| AD-type dementia or MCI, n | 284 | 223 | 237 | 260 | |
| Model 1 | 1.23 (1.00–1.52) | 0.91 (0.73–1.13) | 0.91 (0.74–1.13) | 1 | 1.09 (1.01–1.18) |
| Model 2 | 1.26 (1.01–1.56) | 0.92 (0.74–1.15) | 0.92 (0.74–1.13) | 1 | 1.10 (1.01–1.19) |
| Model 3 | 1.23 (0.98–1.54) | 0.90 (0.72–1.13) | 0.89 (0.72–1.10) | 1 | 1.10 (1.01–1.19) |
| Cerebrovascular dementia or MCI, n | 57 | 53 | 35 | 43 | |
| Model 1 | 1.84 (1.18–2.88) | 1.33 (0.85–2.07) | 0.97 (0.58–1.60) | 1 | 1.33 (1.14–1.55) |
| Model 2 | 1.58 (1.02–2.46) | 1.26 (0.80–1.97) | 0.95 (0.57–1.57) | 1 | 1.25 (1.08–1.44) |
| Model 3 | 1.43 (0.91–2.24) | 1.15 (0.72–1.83) | 0.90 (0.54–1.49) | 1 | 1.23 (1.05–1.43) |
Definition of abbreviations: AD = Alzheimer’s disease; ARIC = Atherosclerosis Risk in Communities; MCI = mild cognitive impairment.
Model 1: Logistic regression adjusted for age, sex, center, education level, and race–center (five-level variable). Model 2: model 1 + additional adjustment for cigarette smoking and pack-years of smoking. Model 3: model 2 + additional adjustment for physical activity, body mass index, systolic blood pressure, blood pressure medication use, diabetes, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, lipid-lowering medications, prevalent coronary heart disease, heart failure, stroke, and apolipoprotein E genotype. Model 4: model 3 + fibrinogen.
Inverse-probability weighting was used.
Associations between FVC% predicted and dementia are presented in Table 5. The model 3 OR for the lowest versus highest quartile of FVC% predicted was 1.25 (95% CI, 1.04–1.51) for dementia or MCI, 1.30 (95% CI, 1.04–1.62) for dementia or MCI caused by AD, and 1.51 (95% CI, 0.95–2.39) for dementia or MCI caused by cerebrovascular disease. No statistical interaction by race was present, although magnitudes of effect tended to be larger in black individuals than in white individuals (see Table E7).
Table 5.
Weighted* Odds Ratios and 95% Confidence Intervals of FVC% Predicted Quartile with Dementia, MCI, Alzheimer’s Disease–Type Dementia or MCI, and Dementia or MCI Caused by Cerebrovascular Disease: ARIC Study, 1987–2013
| FVC% Predicted |
|||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Trend (per 1 SD Decrease) | |
| n | 1,472 | 1,473 | 1,471 | 1,473 | |
| Dementia or MCI, n | 434 | 381 | 361 | 362 | |
| Model 1 | 1.40 (1.16–1.68) | 1.15 (0.96–1.38) | 1.08 (0.89–1.29) | 1 | 1.17 (1.10–1.25) |
| Model 2 | 1.37 (1.14–1.64) | 1.14 (0.95–1.37) | 1.07 (0.89–1.29) | 1 | 1.16 (1.09–1.24) |
| Model 3 | 1.25 (1.04–1.51) | 1.06 (0.88–1.28) | 1.04 (0.86–1.25) | 1 | 1.12 (1.05–1.20) |
| Model 4 | 1.25 (1.04–1.51) | 1.06 (0.88–1.28) | 1.04 (0.86–1.25) | 1 | 1.12 (1.05–1.20) |
| Dementia, n | 80 | 69 | 71 | 78 | |
| Model 1 | 1.29 (0.88–1.89) | 1.07 (0.73–1.57) | 1.19 (0.82–1.74) | 1 | 1.17 (1.02–1.33) |
| Model 2 | 1.20 (0.82–1.77) | 1.03 (0.70–1.52) | 1.18 (0.81–1.72) | 1 | 1.14 (0.99–1.30) |
| Model 3 | 1.06 (0.71–1.59) | 0.92 (0.62–1.37) | 1.15 (0.78–1.70) | 1 | 1.08 (0.93–1.25) |
| MCI, n | 354 | 312 | 290 | 284 | |
| Model 1 | 1.43 (1.17–1.73) | 1.17 (0.96–1.42) | 1.04 (0.86–1.27) | 1 | 1.18 (1.09–1.27) |
| Model 2 | 1.41 (1.16–1.72) | 1.17 (0.96–1.42) | 1.04 (0.85–1.26) | 1 | 1.17 (1.09–1.27) |
| Model 3 | 1.32 (1.08–1.60) | 1.11 (0.91–1.35) | 1.02 (0.84–1.24) | 1 | 1.14 (1.06–1.23) |
| AD-type dementia or MCI, n | 284 | 237 | 238 | 245 | |
| Model 1 | 1.34 (1.09–1.66) | 1.02 (0.82–1.26) | 1.07 (0.86–1.32) | 1 | 1.15 (1.06–1.24) |
| Model 2 | 1.35 (1.09–1.68) | 1.01 (0.82–1.26) | 1.07 (0.86–1.32) | 1 | 1.15 (1.06–1.25) |
| Model 3 | 1.30 (1.04–1.62) | 0.99 (0.79–1.23) | 1.04 (0.84–1.29) | 1 | 1.13 (1.04–1.23) |
| Cerebrovascular dementia or MCI, n | 57 | 52 | 43 | 36 | |
| Model 1 | 2.02 (1.26–3.23) | 1.68 (1.05–2.68) | 1.31 (0.80–2.14) | 1 | 1.37 (1.16–1.61) |
| Model 2 | 1.80 (1.14–2.84) | 1.61 (1.00–2.58) | 1.29 (0.79–2.12) | 1 | 1.31 (1.11–1.53) |
| Model 3 | 1.51 (0.95–2.39) | 1.40 (0.87–2.26) | 1.27 (0.78–2.08) | 1 | 1.22 (1.04–1.43) |
For definition of abbreviations, see Table 4.
Model 1: Logistic regression adjusted for age, sex, center, education level, and race–center (five-level variable). Model 2: model 1 + additional adjustment for cigarette smoking and pack-years of smoking. Model 3: model 2 + additional adjustment for physical activity, body mass index, systolic blood pressure, blood pressure medication use, diabetes, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, lipid-lowering medications, prevalent coronary heart disease, heart failure, stroke, and apolipoprotein E genotype. Model 4: model 3 + fibrinogen.
Inverse-probability weighting was used.
FEV1/FVC% predicted was not associated with risk of any of the outcomes, regardless of degree of adjustment (see Table E8).
For the main results of analyses of adjudicated dementia outcomes, we also conducted sensitivity analyses without IPW. Results of these sensitivity analyses are presented in Tables E9 (exposure lung disease category), E10 (exposure FEV1), and E11 (exposure FVC), respectively. Effect estimates were similar to those of the primary IPW-weighted analysis.
Discussion
Lung disease and impaired lung function were associated with greater risk of dementia and MCI in this community-based sample of more than 14,000 individuals followed for more than 23 years. Several important patterns emerged, particularly in analyses using adjudicated neurocognitive outcomes, although associations were at most of moderate strength and results were not always statistically significant after multivariable adjustment for a broad array of dementia risk factors. First, although both the COPD and restrictive impairment patterns tended to be associated with greater dementia and MCI risk, the magnitude of association was generally stronger for the restrictive impairment pattern. Second, there was evidence that suboptimal lung health may be related to dementia or MCI risk through both AD and cerebrovascular etiologies. Third, patterns were similar among nonsmokers, as in the overall population. Fourth, when evaluating spirometric measures and dementia risk, inverse associations were present for FEV1% predicted and FVC% predicted, but not for the ratio FEV1/FVC % predicted. These results provide novel information about the potential influence of lung disease and impaired lung function on future risk of dementia and MCI caused by both AD and cerebrovascular disease. An important strength of this study is the prospective evaluation of midlife lung health and dementia risk more than 20 years later, because for many dementia risk factors, stronger associations have been observed when the risk factors were measured at middle-age than when they were measured later in life (12–14).
Comparison with Prior Studies
Relatively little is known about the relationship between restrictive impairment and risk of dementia and MCI. In the present analysis, after extensive covariate adjustment, participants with the restrictive impairment pattern were at 58% greater risk of developing dementia or MCI over 27 years of follow-up. There was evidence this pattern was present for dementia and MCI of both AD etiology (79% increased risk) and cerebrovascular disease etiology (60% increased risk). The association for dementia of cerebrovascular etiology was not significant in the fully adjusted model, but notably precision was poor. A prior ARIC publication reported that the restrictive pattern was associated with 60% (0–160%) increased risk of hospitalized dementia after adjusting for demographics (HR, 1.6; 95% CI, 1.0–2.6), although the association was attenuated with additional adjustment (HR, 1.4; 95% CI, 0.9–2.3) (5). Diseases that result in restrictive impairment are characterized by reduced lung volumes, consequent to alteration in lung parenchyma or caused by a disease of the pleura, chest wall, or neuromuscular apparatus (28).
Although symptoms of restrictive impairment are specific to the underlying condition, in addition to reduced lung volumes, patients tend to have ventilation–perfusion mismatch and hypoxemia. Overnight polysomnography data from the Study of Osteoporotic Fractures demonstrated that two indicators of hypoxemia (elevated oxygen desaturation and a high percentage of sleep time in apnea or hypopnea) were associated with elevated risk of developing MCI or dementia over a mean follow-up of 4.7 years (18). In a recent ARIC publication based on a smaller sample than the present analysis, there was modest evidence that obstructive sleep apnea was associated with greater dementia and MCI risk (29). Extensive work in experimental rodent models of sleep apnea has suggested that intermittent hypoxia and asphyxia lead to neuronal damage and adverse behavioral consequences (30, 31). Less research has evaluated the impact of a constant state of hypoxemia, as may be expected in the context of restrictive impairment, on neurologic structure and function.
Our finding that COPD was linked to greater risk of dementia and MCI when using the adjudicated outcome definition is consistent with prior literature. Two studies have reported that diagnosis with COPD is associated with an approximately 80% higher risk of developing MCI over 5 years (9), and MCI or dementia over 25 years (10), respectively. Furthermore, in the shorter study a dose–response relationship was observed according to COPD duration and risk of MCI (9). Clinical history of COPD has also been associated with decreasing cognitive performance over time (11). Notably, in a prior analysis of the ARIC data, which followed participants through 2005, presence of an obstructive ventilator function pattern was not associated with greater risk of dementia hospitalization (5). Unique aspects of the present analysis include the objective ascertainment of COPD in a community-based sample (as opposed to COPD diagnosed via clinical diagnosis codes) and evaluation of the association in analyses restricted to nonsmokers. Patients with COPD suffer from systemic manifestations of the disease (32), and growing evidence suggests that these comorbidities are independent of smoking and traditional risk factors (33–35).
In the present analysis, spirometry-assessed impaired lung function, as quantified by being in the lowest versus highest quartile of % predicted FEV1 and FVC, was associated with greater risk of MCI and dementia overall and caused by AD and cerebrovascular disease. Several other studies (6–8), although not all (3), have also shown impaired lung function to be associated with worsening cognitive ability. Some of the most important previous work exploring the relationship between objectively measured impaired lung function and cognitive status comes from a prior ARIC analysis. In this publication, impaired lung function was associated cross-sectionally with poorer performance in baseline cognitive assessments, and with increased risk of dementia hospitalization (5). However, no association was found between lung function and cognitive decline over approximately 6 years of follow-up (between ARIC visits 2 and 4). Limitations of this previous analysis include short intervals between cognitive assessments in the cohort and insensitivity of the dementia definition used.
In the present analysis, associations between lung disease and function persisted even in analyses restricted to nonsmokers. This enhances etiologic understanding because it suggests that impaired lung function is linked to dementia and MCI risk independent of smoking and smoking-related confounders.
An unexpected finding from the present analysis was the suggestive (but nonsignificant) difference in associations by race, whereby among black individuals the COPD pattern was most strongly associated with dementia and MCI risk, whereas in white individuals the restrictive impairment pattern was most strongly associated. Importantly, both restrictive impairment and COPD are heterogeneous classifications, and the prevalence of specific pathologies is known to vary by race (36–39). It is possible that these underlying pathologies differ in their association with dementia and MCI risk, which could explain the observed race differences. Other possible explanations for the interaction are poor precision (e.g., there were only 11 black individuals with restrictive impairment and MCI), selection bias that is differential by race, or chance. Future studies should aim to replicate these observations.
Strengths and Limitations
The 23-year time span between assessment of lung health and the neurocognitive examination is an important strength of our study, because all-cause and AD-type dementia have a long natural history. However, this timespan also complicates the interpretation of our results, because we undoubtedly missed numerous cases of dementia that occurred among individuals who did not attend the neurocognitive examination because they had died (36.7%) or did not participate for other reasons (21.8%). Although for these participants we do not have information from the full neurocognitive battery, we do have some information about their cognitive status via dementia hospitalization ICD codes and in some instances TICSm and informant interviews. Sensitivity of dementia hospitalization ICD codes is, however, poor (25, 40). A prior ARIC Neurocognitive Study publication reported that hospital and death diagnostic codes for dementia had a sensitivity of 25% and a specificity of 99% (25). This may explain why in the present analysis, as in a prior ARIC analysis (29), associations were stronger when adjudicated outcomes were used than when hospitalization ICD codes were also used to define dementia.
In the present analysis we used IPW to attempt to correct for selection bias resulting from differential outcome ascertainment between participants and nonparticipants of the neurocognitive examination. The true cognitive status of nonattenders is, however, unknown and it is possible that some bias remained. Nonattendees were also more likely to be smokers, have greater pack-years, and more respiratory impairment by both spirometry and self-report. Although we attempted to correct for this selection bias through IPW, the fact that participation at visit 5 was differential by smoking and lung function status is noteworthy.
Additional limitations are the single assessment of lung function, lack of biomarkers to verify AD-type dementia, residual confounding, and poor precision for some comparisons despite the relatively large sample size. Additionally, bronchodilation was not used when assessing baseline lung function, and total lung capacity was not quantified. Furthermore, also absent are details about symptoms, such as the nature of dyspnea, chronic cough, chronic sputum production, or history of recurrent lower respiratory tract infections. Despite these limitations our study had important strengths, including the large community-based sample, objective ascertainment of lung function in using standardized protocols, comprehensive neurocognitive assessment, and representation of men and women and black and white individuals.
Conclusions
In this large prospective community-based cohort, both lung disease and impaired lung function were associated with greater risk of dementia and MCI over 23 years of follow-up, with evidence that this increased risk of dementia was due to both AD and vascular etiologies. Although COPD and restrictive impairment were associated with increased risk of the dementia phenotypes, magnitudes of association were most pronounced for restrictive impairment. These associations were present in smokers and nonsmokers. If the observed associations are causal, policy and public health efforts to reduce smoking and improve air quality may have the added benefit of preventing the development of dementia and MCI.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions.
Footnotes
The Atherosclerosis Risk in Communities study is a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data were collected with support by U01 HL096812, HL096814, HL096899, HL096902, and HL096917 from the NHLBI and the National Institute of Neurological Disorders and Stroke and with previous brain magnetic resonance imaging examinations funded by R01-HL70825 from the NHLBI.
Author Contributions: P.L.L., N.C., and A.A. developed the research idea. D.S.K., R.F.G., and T.H.M. were involved with outcome ascertainment. N.C. and A.A. conducted the analysis. P.L.L. drafted the manuscript. P.L.L., N.C., M.C.M., K.L., D.S.K., K.A.V., R.F.G., T.H.M., and A.A. critically reviewed the manuscript and edited the manuscript for intellectual content.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201807-1220OC on November 15, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Dementia fact sheet. 2016 Apr [accessed 2017 Jan 27]. Available from: http://www.who.int/mediacentre/factsheets/fs362/en/
- 2.Rose G.Sick individuals and sick populations Int J Epidemiol 200130427–432.discussion 433–434 [DOI] [PubMed] [Google Scholar]
- 3.Dodd JW. Lung disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther. 2015;7:32. doi: 10.1186/s13195-015-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahousse L, Tiemeier H, Ikram MA, Brusselle GG. Chronic obstructive pulmonary disease and cerebrovascular disease: a comprehensive review. Respir Med. 2015;109:1371–1380. doi: 10.1016/j.rmed.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Pathan SS, Gottesman RF, Mosley TH, Knopman DS, Sharrett AR, Alonso A. Association of lung function with cognitive decline and dementia: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Neurol. 2011;18:888–898. doi: 10.1111/j.1468-1331.2010.03340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidal JS, Aspelund T, Jonsdottir MK, Jonsson PV, Harris TB, Lopez OL, et al. Pulmonary function impairment may be an early risk factor for late-life cognitive impairment. J Am Geriatr Soc. 2013;61:79–83. doi: 10.1111/jgs.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards M, Strachan D, Hardy R, Kuh D, Wadsworth M. Lung function and cognitive ability in a longitudinal birth cohort study. Psychosom Med. 2005;67:602–608. doi: 10.1097/01.psy.0000170337.51848.68. [DOI] [PubMed] [Google Scholar]
- 8.Chyou PH, White LR, Yano K, Sharp DS, Burchfiel CM, Chen R, et al. Pulmonary function measures as predictors and correlates of cognitive functioning in later life. Am J Epidemiol. 1996;143:750–756. doi: 10.1093/oxfordjournals.aje.a008812. [DOI] [PubMed] [Google Scholar]
- 9.Singh B, Mielke MM, Parsaik AK, Cha RH, Roberts RO, Scanlon PD, et al. A prospective study of chronic obstructive pulmonary disease and the risk for mild cognitive impairment. JAMA Neurol. 2014;71:581–588. doi: 10.1001/jamaneurol.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rusanen M, Ngandu T, Laatikainen T, Tuomilehto J, Soininen H, Kivipelto M. Chronic obstructive pulmonary disease and asthma and the risk of mild cognitive impairment and dementia: a population based CAIDE study. Curr Alzheimer Res. 2013;10:549–555. doi: 10.2174/1567205011310050011. [DOI] [PubMed] [Google Scholar]
- 11.Hung WW, Wisnivesky JP, Siu AL, Ross JS. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:134–137. doi: 10.1164/rccm.200902-0276OC. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the atherosclerosis risk in communities (aric) cohort. JAMA Neurol. 2017;74:1246–1254. doi: 10.1001/jamaneurol.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317:1443–1450. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70-81 years. BMJ. 2004;328:548. doi: 10.1136/bmj.37977.495729.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maclay JD, MacNee W. Cardiovascular disease in COPD: mechanisms. Chest. 2013;143:798–807. doi: 10.1378/chest.12-0938. [DOI] [PubMed] [Google Scholar]
- 16.Truelsen T, Prescott E, Lange P, Schnohr P, Boysen G. Lung function and risk of fatal and non-fatal stroke. The Copenhagen City Heart Study. Int J Epidemiol. 2001;30:145–151. doi: 10.1093/ije/30.1.145. [DOI] [PubMed] [Google Scholar]
- 17.Hozawa A, Billings JL, Shahar E, Ohira T, Rosamond WD, Folsom AR. Lung function and ischemic stroke incidence: the Atherosclerosis Risk in Communities study. Chest. 2006;130:1642–1649. doi: 10.1378/chest.130.6.1642. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 20.American Thoracic Society. Standardization of spirometry: 1987 update. Am Rev Respir Dis. 1987;136:1285–1298. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 21.The ARIC Investigators. Atherosclerosis Risk in Communities Study Manual 4: pulmonary function. Chapel Hill, NC: NHLBI of the NIH, Collaborative Studies Coordinating Center; 1987. [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.Vaz Fragoso CA, McAvay G, Van Ness PH, Casaburi R, Jensen RL, MacIntyre N, et al. Phenotype of spirometric impairment in an aging population. Am J Respir Crit Care Med. 2016;193:727–735. doi: 10.1164/rccm.201508-1603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubota Y, London SJ, Cushman M, Chamberlain AM, Rosamond WD, Heckbert SR, et al. Lung function, respiratory symptoms and venous thromboembolism risk: the Atherosclerosis Risk in Communities Study. J Thromb Haemost. 2016;14:2394–2401. doi: 10.1111/jth.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) Alzheimers Dement (Amst) 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weuve J, Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–128. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen-Roche K, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179:956–966. doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naureckas ET, Solway J. Disturbances of respiratory function. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, editors. Harrison's principles of internal medicine, 19e. New York: McGraw-Hill Education; 2015. p. Chap. 306e. [Google Scholar]
- 29.Lutsey PL, Misialek JR, Mosley T, Gottesman RF, Punjabi NM, Shahar E, et al. Sleep characteristics and risk of incident mild cognitive impairment and dementia: The Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2018;14:157–166. doi: 10.1016/j.jalz.2017.06.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Row BW. Intermittent hypoxia and cognitive function: implications from chronic animal models. Adv Exp Med Biol. 2007;618:51–67. doi: 10.1007/978-0-387-75434-5_5. [DOI] [PubMed] [Google Scholar]
- 31.Zhang SXL, Wang Y, Gozal D.Pathological consequences of intermittent hypoxia in the central nervous system Compr Physiol 201221767–1777 [DOI] [PubMed] [Google Scholar]
- 32.Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128:2099–2107. doi: 10.1378/chest.128.4.2099. [DOI] [PubMed] [Google Scholar]
- 33.Van Eeden S, Leipsic J, Paul Man SF, Sin DD. The relationship between lung inflammation and cardiovascular disease. Am J Respir Crit Care Med. 2012;186:11–16. doi: 10.1164/rccm.201203-0455PP. [DOI] [PubMed] [Google Scholar]
- 34.Stone IS, Barnes NC, Petersen SE. Chronic obstructive pulmonary disease: a modifiable risk factor for cardiovascular disease? Heart. 2012;98:1055–1062. doi: 10.1136/heartjnl-2012-301759. [DOI] [PubMed] [Google Scholar]
- 35.Barnes PJ. Chronic obstructive pulmonary disease: effects beyond the lungs. PLoS Med. 2010;7:e1000220. doi: 10.1371/journal.pmed.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamil F, Pinzon I, Foreman MG. Sex and race factors in early-onset COPD. Curr Opin Pulm Med. 2013;19:140–144. doi: 10.1097/MCP.0b013e32835d903b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akinbami LJ, Liu X. Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998-2009. NCHS Data Brief. 2011;((63)):1–8. [PubMed] [Google Scholar]
- 38.Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D, et al. Ethnic and racial differences in the presence of idiopathic pulmonary fibrosis at death. Respir Med. 2012;106:588–593. doi: 10.1016/j.rmed.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenblatt R, Mansour O, Zhao E, Ross M, Himes BE. Gender-specific determinants of asthma among U.S. adults. Asthma Res Pract. 2017;3:2. doi: 10.1186/s40733-017-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin YP, Gatz M, Johansson B, Pedersen NL. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology. 2004;63:739–741. doi: 10.1212/01.wnl.0000134604.48018.97. [DOI] [PubMed] [Google Scholar]
- 41.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.