Pulmonary arterial hypertension (PAH) is a debilitating disease associated with increased pulmonary artery pressures, reduced lung function and exercise ability, and progressive right heart failure (1). Endothelial dysfunction, vasoconstriction, pulmonary vascular remodeling secondary to smooth muscle cell proliferation and hypertrophy, muscularization of precapillary arterioles, and distal vessel loss are among the key pathophysiological processes in PAH (2). Present pharmacologic interventions target primarily vasoconstriction and are, as such, able to slow down, but not reverse, disease progression. Hence, therapies that could reverse the proliferative phenotype of pulmonary vascular cells and, thus, improve RV function without causing adverse effects are highly desirable (3).
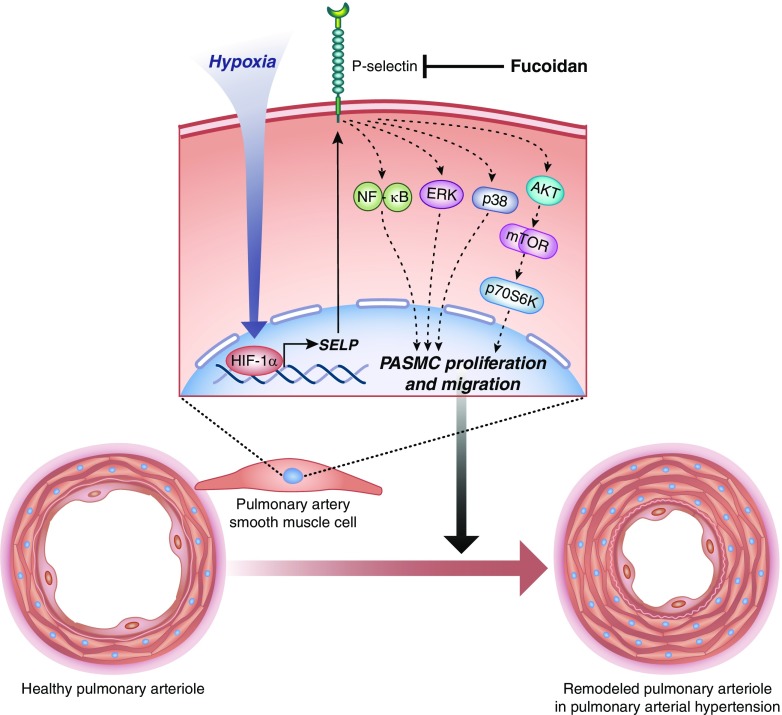
P-selectin expressed on activated endothelium and platelets promotes inflammation by serving as a ligand for PSGL-1 (P-selectin glycoprotein ligand-1) on leukocytes to mediate leukocyte rolling and leukocyte–platelet aggregation, respectively (4, 5). P-selectin has been shown to be upregulated on circulating platelets and pulmonary endothelium of patients with PAH and corresponding rat models (6, 7). Although these findings suggest a role for P-selectin in promoting inflammation in PAH, a direct role for P-selectin in vascular remodeling or RV dysfunction has remained elusive. Using pulmonary vascular tissue from patients with PAH and the mouse model of chronic hypoxic pulmonary hypertension (PH), the study published in this issue of the Journal by Novoyatleva and colleagues (pp. 1407–1420) reports the paradigm-shifting discovery (Figure 1) that P-selectin is also expressed in pulmonary artery smooth muscle cells (PASMCs) and upregulated in PAH (8). P-selectin in PASMCs was found to be a direct transcriptional target of HIF-1α (hypoxia-induced factor-1α). Remarkably, P-selectin inhibition or genetic deletion led to reversal of pulmonary vascular remodeling and improved RV function in PH mice, highlighting the therapeutic potential of anti–P-selectin therapy. Consistent with this notion, the brown algae–derived P-selectin inhibitor fucoidan improved RV function, ameliorated inflammation, and reduced pulmonary vascular remodeling in PH mice in vivo and proliferation/migration of PASMCs in cell culture in vitro by suppressing NF-κB (nuclear factor-κB), Akt–mTOR (mammalian target of rapamycin)–p70S6K (p70 ribosomal S6 kinase), ERK (extracellular signal–regulated kinase), and p38 signaling pathways in PASMCs. Taken together, Novoyatleva and colleagues (8) establish an important role for P-selectin in the pathogenesis of PAH. In addition to P-selectin’s well-established function for immune cell–cell interaction, this new role is dependent on P-selectin expression on PASMCs and P-selectin–mediated signaling driving PASMCs proliferation.
Figure 1.
P-selectin in pulmonary artery smooth muscle cells (PASMCs) contributes to the pathophysiology of pulmonary hypertension. Novoyatleva and colleagues (8) show that hypoxia upregulates P-selectin in PASMCs in a HIF-1α (hypoxia-induced factor-1α)-dependent manner. P-selectin–dependent activation of NF-κB (nuclear factor-κB), Akt–mTOR (mammalian target of rapamycin)–p70S6K (p70 ribosomal S6 kinase), ERK (extracellular signal–regulated kinase), and p38 signaling pathways in PASMCs contribute to PASMC proliferation and vascular remodeling, which is reversed by the P-selectin inhibitor fucoidan. Illustration by Jacqueline Schaffer.
The findings of Novoyatleva and colleagues open up exciting new avenues for mechanistic speculation, experimental interrogation, and potentially clinical application. First, what is the physiological function of P-selectin in PASMCs? Immunohistochemistry, Western blot analysis, and biotin pull-down show mild expression of P-selectin in PASMCs under basal, normoxic conditions and marked upregulation and surface expression in response to hypoxia. Because of its abluminal localization, this expression does apparently not serve the classic P-selectin–mediated interaction with circulating immune cells, and so its physiological role remains elusive. It also remains to be shown whether signaling via P-selectin in PASMCs is mediated by its cognate ligand PSGL-1 expressed on infiltrating leukocytes, or whether it occurs via an endogenous ligand expressed in PASMCs or via proteoglycans or glycoconjugates in the extracellular matrix. Second, is P-selectin expression in smooth muscle cells a unique feature of the pulmonary circulation, or could it also contribute to P-selectin–mediated pathologies in the systemic circulation? For example, P-selectin–deficient mice are largely protected from the formation of atherosclerotic lesions (9), a process that is intricately linked to the dysfunction of vascular smooth muscle cells (10). Third, the mechanism by which P-selectin expressed on PASMCs promotes signaling pathways responsible for PASMC proliferation remains unanswered. Unlike L-selectin, P-selectin has a cytoplasmic domain that regulates its sorting into storage granules, but presumably also mediates outside-in-signaling such as P-selectin–mediated Ca2+ signaling in endothelial cells (11); yet how such signals relate to the stimulation of the proliferative pathways reported by Novoyatleva and colleagues remains to be elucidated. Fourth, P-selectin expression on the cell surface is commonly regulated by vesicular trafficking. Endothelial cells and platelets store P-selectin in Weibel-Palade bodies and α-granules, respectively, from where P-selectin is rapidly mobilized to the cell surface in a Ca2+-dependent manner (12). PASMCs lack both Weibel-Palade bodies and α-granules, raising the question of whether P-selectin is equally stored in these cells in alternative vesicular compartments, or whether its surface expression is solely regulated by transcriptional activity and reinternalization. Fifth, is P-selectin blockade by, for example, fucoidan a feasible therapeutic strategy? PAH is a chronic disease, and therefore, reversal of PAH symptoms may require chronic therapy with anti–P-selectin agents. By blocking selectins, fucoidan effectively precludes selectin-mediated leukocyte-endothelial interaction in both the pulmonary and the systemic circulation (13). Chronic inhibition of P-selectin might hence compromise innate and adaptive immune responses leading to recurrent bacterial infections as seen, for example, in patients with leukocyte adhesion deficiency syndrome type II. Last but not least, the actual pathophysiological relevance of PASMC P-selectin in the context of PAH remains to be shown. Notably, all interventions tested by Novoyatleva and colleagues (fucoidan, blocking anti–P-selectin antibodies, or the use of P-selectin–deficient mice) will not only target PASMC P-selectin but, in parallel, block the classic P-selectin–mediated leukocyte-platelet-endothelium interaction. In light of the emerging relevance of both the immune and coagulation system in PH (14), it remains to be shown (e.g., by use of cell-specific knockout models) to what extent the observed protection is attributable to a direct role of PASMC P-selectin.
As so often after an important paradigm shift, we are left with an abundance of new questions. These should in no way diminish, but rather stress, the conceptual advancement by Novoyatleva and colleagues (8). The authors have identified a previously unrecognized site of P-selectin expression and a P-selectin dependent mechanism contributing to the progression of PAH. The findings that anti–P-selectin therapeutics such as fucoidan could be beneficial in reducing the PAH morbidity is particularly relevant in light of the fact that several P-selectin inhibitors are currently in clinical trials for treatment of auto-inflammatory diseases other than PAH (15, 16). The potential benefit of P-selectin inhibition demonstrated here may thus warrant the need for repurposing these inhibitors for treatment of patients with PAH.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201812-2242ED on December 28, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Pullamsetti SS, Schermuly R, Ghofrani A, Weissmann N, Grimminger F, Seeger W. Novel and emerging therapies for pulmonary hypertension. Am J Respir Crit Care Med. 2014;189:394–400. doi: 10.1164/rccm.201308-1543PP. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62(Suppl):D4–D12. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duarte JD, Hanson RL, Machado RF. Pharmacologic treatments for pulmonary hypertension: exploring pharmacogenomics. Future Cardiol. 2013;9:335–349. doi: 10.2217/fca.13.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundd P, Pospieszalska MK, Ley K. Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings. Mol Immunol. 2013;55:59–69. doi: 10.1016/j.molimm.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107:331–339. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hironaka E, Hongo M, Sakai A, Mawatari E, Terasawa F, Okumura N, et al. Serotonin receptor antagonist inhibits monocrotaline-induced pulmonary hypertension and prolongs survival in rats. Cardiovasc Res. 2003;60:692–699. doi: 10.1016/j.cardiores.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Yaoita N, Shirakawa R, Fukumoto Y, Sugimura K, Miyata S, Miura Y, et al. Platelets are highly activated in patients of chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2014;34:2486–2494. doi: 10.1161/ATVBAHA.114.304404. [DOI] [PubMed] [Google Scholar]
- 8.Novoyatleva T, Kojonazarov B, Owczarek A, Veeroju S, Rai N, Henneke I, et al. Evidence for the fucoidan/P-selectin axis as a therapeutic target in hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med. 2019;199:1407–1420. doi: 10.1164/rccm.201806-1170OC. [DOI] [PubMed] [Google Scholar]
- 9.Dong ZM, Brown AA, Wagner DD. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation. 2000;101:2290–2295. doi: 10.1161/01.cir.101.19.2290. [DOI] [PubMed] [Google Scholar]
- 10.Krohn R, Raffetseder U, Bot I, Zernecke A, Shagdarsuren E, Liehn EA, et al. Y-box binding protein-1 controls CC chemokine ligand-5 (CCL5) expression in smooth muscle cells and contributes to neointima formation in atherosclerosis-prone mice. Circulation. 2007;116:1812–1820. doi: 10.1161/CIRCULATIONAHA.107.708016. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzon P, Vecile E, Nardon E, Ferrero E, Harlan JM, Tedesco F, et al. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J Cell Biol. 1998;142:1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuebler WM, Ying X, Singh B, Issekutz AC, Bhattacharya J. Pressure is proinflammatory in lung venular capillaries. J Clin Invest. 1999;104:495–502. doi: 10.1172/JCI6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuebler WM, Kuhnle GE, Groh J, Goetz AE. Contribution of selectins to leucocyte sequestration in pulmonary microvessels by intravital microscopy in rabbits. J Physiol. 1997;501:375–386. doi: 10.1111/j.1469-7793.1997.375bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuebler WM, Bonnet S, Tabuchi A. Inflammation and autoimmunity in pulmonary hypertension: is there a role for endothelial adhesion molecules? (2017 Grover Conference Series) Pulm Circ. 2018;8:2045893218757596. doi: 10.1177/2045893218757596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429–439. doi: 10.1056/NEJMoa1611770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telen MJ, Wun T, McCavit TL, De Castro LM, Krishnamurti L, Lanzkron S, et al. Randomized phase 2 study of GMI-1070 in SCD: reduction in time to resolution of vaso-occlusive events and decreased opioid use. Blood. 2015;125:2656–2664. doi: 10.1182/blood-2014-06-583351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.