Abstract
A genetic influence on spontaneous pneumothoraces—those occurring without a traumatic or iatrogenic cause—is supported by several lines of evidence: 1) pneumothorax can cluster in families (i.e., familial spontaneous pneumothorax), 2) mutations in the FLCN gene have been found in both familial and sporadic cases, and 3) pneumothorax is a known complication of several genetic syndromes. Herein, we review known genetic contributions to both sporadic and familial pneumothorax. We summarize the pneumothorax-associated genetic syndromes, including Birt-Hogg-Dubé syndrome, Marfan syndrome, vascular (type IV) Ehlers-Danlos syndrome, alpha-1 antitrypsin deficiency, tuberous sclerosis complex/lymphangioleiomyomatosis, Loeys-Dietz syndrome, cystic fibrosis, homocystinuria, and cutis laxa, among others. At times, pneumothorax is their herald manifestation. These syndromes have serious potential extrapulmonary complications (e.g., malignant renal tumors in Birt-Hogg-Dubé syndrome), and surveillance and/or treatment is available for most disorders; thus, establishing a diagnosis is critical. To facilitate this, we provide an algorithm to guide the clinician in discerning which cases of spontaneous pneumothorax may have a genetic or familial contribution, which cases warrant genetic testing, and which cases should prompt an evaluation by a geneticist.
Keywords: pneumothorax, genetics, familial spontaneous pneumothorax, FLCN gene, Birt-Hogg-Dubé syndrome
Spontaneous pneumothoraces are defined by air in the pleural space arising from neither trauma nor an iatrogenic cause. An anatomic or mechanical cause is identifiable in all cases of secondary spontaneous pneumothorax, which by definition arise from clinically recognizable underlying lung disease (e.g., tuberculosis). Even in primary spontaneous pneumothorax, defined by a lack of overt lung disease, emphysema-like anomalies (blebs, cysts, or bullae) are observed at surgery or on computed tomography (CT) imaging in most cases (1). Additional anatomic associations include being taller and thinner than average (2). Smoking is the primary environmental risk factor for primary spontaneous pneumothorax (3).
Several lines of evidence support genetic contributions to pneumothorax. Foremost are familial clustering, observed in 10% to 12% of cases, and the finding of gene mutations in both familial and sporadic cases. In addition, pneumothorax is a feature of several Mendelian disorders, for example Birt-Hogg-Dubé and Marfan syndromes.
In this review, we discuss known genetic contributions to both sporadic and familial pneumothorax and summarize the pneumothorax-associated genetic syndromes, all of which have serious potential complications and of which pneumothorax is occasionally the presenting feature. We provide an algorithm to guide the clinician in discerning which cases of spontaneous pneumothorax may have a genetic or familial contribution and which of these cases should prompt genetic testing and/or evaluation by a geneticist.
Sporadic Pneumothorax
Primary spontaneous pneumothoraces occur without a family history in the majority (88–90%) of cases (4, 5). We refer to these nonfamilial cases as sporadic pneumothorax.
Genetic studies of sporadic pneumothorax cohorts have focused on FLCN, the gene for Birt-Hogg-Dubé syndrome (BHDS). The BHDS phenotype includes lung cysts and pneumothorax in addition to renal cancer and skin findings, so investigators hypothesized that some variants in this gene might lead to a lung-only phenotype. No mutations were identified among 10 sporadic cases screened by FLCN sequencing (6). However, among 92 patients with sporadic pneumothorax screened for sequence errors and deletions, 5 (5%) had FLCN mutations (5). FLCN promoter methylation changes do not explain FLCN-negative sporadic pneumothorax (7).
Several additional studies of spontaneous pneumothorax have been reported that did not adequately enumerate family background. Nonetheless, if one were to assume that the majority of these cases are sporadic (supported by the higher prevalence of sporadic over familial pneumothorax), then these studies of all comers can provide insights regarding the role of genetics in sporadic pneumothorax.
To investigate whether mutations in the genes for additional pneumothorax-associated syndromes explain isolated pneumothorax, Zhang and colleagues screened for point mutations and deletions in FBN1, COL3A1, CBS, SERPINA1, TSC1 and TSC2, and FLCN (8). Three of 21 subjects had predicted pathogenic mutations: 2 (10%) in FLCN and 1 (5%) in FBN1. Three variants of uncertain significance (one in FBN1, TSC1, and FLCN) were also found. Although blebs and emphysema-like changes in spontaneous pneumothorax are typically apical (1), BHDS features predominately lower-lobe cysts (9, 10). To investigate whether nonapical lung cysts might herald FLCN mutations among patients with spontaneous pneumothorax, Johannesma and colleagues screened 40 patients with nonfamilial and familial spontaneous pneumothorax with chest CT imaging; indeed, all three subjects with cysts below the carina had FLCN mutations (11). To determine whether common genetic variants play a role in pneumothorax risk, Sousa and colleagues performed a genome-wide association study of spontaneous pneumothorax (12). No SNPs met the Bonferroni correction threshold in the replication dataset.
Familial Pneumothorax
Some 10% to 12% of patients with spontaneous pneumothorax have a family history, termed familial spontaneous pneumothorax (FSP) (4, 5). The male:female ratio in FSP is 1.7:1 (4), less skewed than for all spontaneous pneumothoraces (2.1:1 to 6.2:1) (13–16). The risk of recurrent pneumothorax may be higher in FSP (68–72%) (6, 17) than in sporadic pneumothorax (13–54%) (11–13; 18), although the studies arriving at these recurrence rates differ in methodology, making the comparison imperfect. A higher recurrence rate when a family history is known could argue for surgical intervention after the first pneumothorax (19, 20).
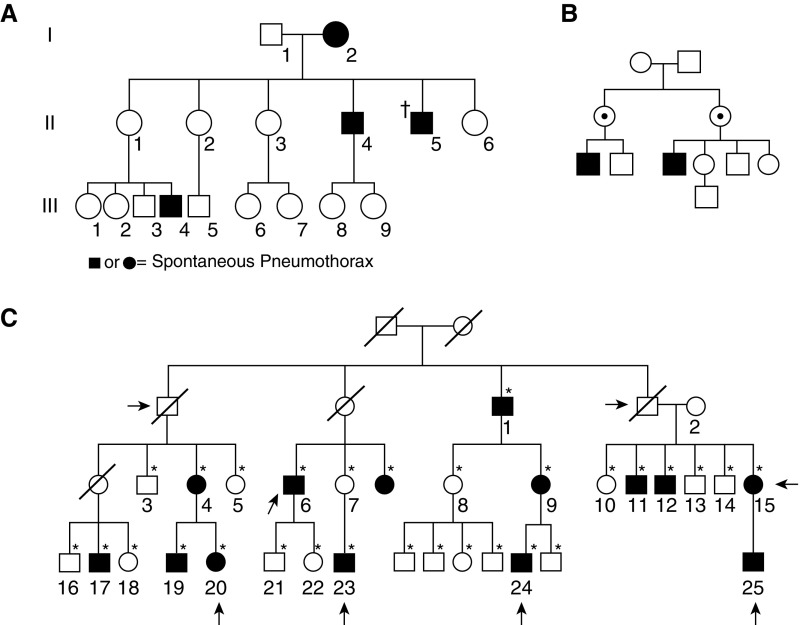
Although some FSP families are identifiably autosomal dominant (AD) (Figure 1A), in others the inheritance pattern is ambiguous (21). Indeed, among 29 FSP pedigrees, all were consistent with AD inheritance, with a penetrance of 21% in females and 50% in males, but many of the pedigrees could also follow an X-linked recessive model (Figure 1B) (4).
Figure 1.
Pedigrees demonstrating familial spontaneous pneumothorax. (A) Most pedigrees are consistent with autosomal dominant (AD) inheritance with incomplete penetrance. No causal gene reported for this family. Circles, females; squares, males; solid, pneumothorax; dagger, deceased. Reproduced by permission from Reference 21. (B) Some pedigrees are also consistent with X-linked recessive inheritance (versus AD with reduced penetrance in females). No causal gene reported for this family. Dot, obligate carrier. Reproduced by permission from Reference 4. (C) Family with known FLCN mutation. Computed tomography (CT) lung findings (black shading) are more clearly AD than pneumothorax (arrows). Individual 23 has a different bullae phenotype (apical instead of random distribution) and is mutation negative, likely explaining why his mother does not have bullae (different cause of pneumothorax in this branch of family). *CT of the lung performed; diagonal line, deceased. Reproduced by permission from Reference 26.
Several attempts have been made to map the genetic cause(s) of FSP. In three FSP families, pneumothorax did not segregate with FBN1, the disease gene for Marfan syndrome (22). Linkage to the HLA-A2B40 haplotype exists in some families (23, 24) but not others (17, 25). In a large FSP family, CT scans revealed multiple asymptomatic family members with bullae randomly distributed throughout the lungs, transmitted in an AD pattern (Figure 1C) (26). A heterozygous, predicted-truncating mutation was found in FLCN, the disease gene for BHDS. The penetrance of bullae in mutation carriers was 100%; for pneumothorax it was ∼40%. The prevalence of FLCN mutations in FSP is 17% to 50% (5, 6).
Thus, a considerable proportion of FSP is attributable to mutations in FLCN. The remainder of FSP is likely caused by mutations in one or more yet-undescribed disease genes. By definition, FSP families do not manifest renal or dermatological findings of BHDS. It is unknown what contributes to the full BHDS versus restricted FSP phenotype.
Genetic Syndromes Predisposing to Pneumothorax
Pneumothorax is at times a feature of a multisystem genetic syndrome (Table 1 and Figure 2). These can be divided into three mechanistic classes (27): 1) those arising from mutations in tumor suppressor genes, 2) connective tissue disorders, and 3) those in which normal lung architecture is effaced. Given that these syndromes have serious, often preventable complications, it is essential to make the correct diagnosis when pneumothorax is the presenting manifestation.
Table 1.
Mendelian Diseases Associated with Spontaneous Pneumothorax
| Condition/Inheritance Pattern | Pulmonary Features (In Addition to Pneumothorax) | Extrapulmonary Features | Penetrance (PTX/Cysts) | Gene(s) | Diagnostic Criteria |
|---|---|---|---|---|---|
| Syndromes resulting from mutated tumor suppressor genes | |||||
| Birt-Hogg-Dubé syndrome, AD | Cysts: elliptical or lentiform, with predominantly basilar, medial, and subpleural distribution | Skin lesions: fibrofolliculomas, trichodiscomas, acrochordons (skin tags) | P: 22–41% | FLCN | 29 |
| Renal cancer: renal cell carcinomas, oncocytomas, and others | C: 83–100% | ||||
| Tuberous sclerosis complex, AD | LAM: cysts, bullae, reticulonodular infiltrates, pleural effusions, obstructive physiology; female predominance | Brain: subependymal nodules, cortical dysplasia (tubers), cerebral white matter migration lines, subependymal giant cell astrocytomas, seizures or infantile spasms, developmental delay/intellectual disability, autism, ADHD | In LAM: | TSC2 | 168 |
| Micronodular pneumocyte hyperplasia | Skin: hypopigmented macules (ash leaf spots), Shagreen patches, confetti lesions, facial angiofibromas, fibrous cephalic plaques, ungual fibromas | P: 56–66% | TSC1 | ||
| Kidney: angiomyolipomas (renal or extrarenal), renal cysts, renal cell carcinomas, oncocytomas | C: ∼100% | ||||
| Heart: rhabdomyomas, arrhythmias | |||||
| Eye: retinal nodular hamartomas, achromic retinal patches | |||||
| Mouth: dental pits, intraoral fibromas | |||||
| Syndromes of disordered connective tissue | |||||
| Marfan syndrome, AD | Lungs usually normal; rare features include: | Skeletal: tall, thin habitus, decreased upper:lower segment ratio, reduced elbow extension, arachnodactyly, hand/wrist signs, pectus excavatum/carinatum, scoliosis/kyphosis, hindfoot deformity, flat feet | P: 4–11% | FBN1 | 170 |
| Cysts | Facial features: dolichocephaly, down-slanting palpebral fissures, enophthalmos, retrognathia, malar hypoplasia | C: 10% (bullae/blebs) | |||
| Emphysema | Eye: lens dislocation, severe myopia | ||||
| Congenital lung malformations | Skin: striae | ||||
| Increased TLC and RV | Cardiac: aortic dilation/dissection/aneurysm/rupture, mitral valve prolapse | ||||
| Vascular (type IV) Ehlers-Danlos syndrome, AD | Cavitary lesions | Vascular: arterial aneurysm, dissection, rupture, carotid–cavernous sinus fistula | P: 16–80% | COL3A1 | 94 |
| Cysts, bullae | Organ rupture: of colon or gravid uterus | ||||
| Fibrous nodules with osseous metaplasia | Facial appearance: thin lips and nose, micrognathia, prominent eyes | ||||
| Hemopneumothorax or pulmonary hemorrhage | Skin: translucent skin with visible veins, easy bruising | ||||
| Orthopedic: clubfoot, congenital hip dislocation, lax small joints, muscle/tendon rupture | |||||
| Loeys-Dietz syndrome, AD | N/A | Vascular: arterial aneurysms, dissection, tortuosity | Unknown | TGFBR1 | None |
| Skeletal: pectus excavatum or carinatum, joint laxity or contractures, cervical spine instability, scoliosis, arachnodactyly, club foot | TGFBR2 | ||||
| Craniofacial: bifid uvula or cleft palate, hypertelorism, retrognathia | SMAD3 | ||||
| Cutaneous: translucent or dystrophic skin, easy bruising | TGFB2 | ||||
| Uterine rupture | TGFB3 | ||||
| SMAD2 | |||||
| Homocystinuria, AR | N/A | Skeletal: Marfanoid habitus | Unknown | CBS | 171 |
| Eye: dislocated lens, myopia | |||||
| Neurologic: intellectual disability, seizures | |||||
| Vascular: thrombosis | |||||
| Cutis laxa,* AD/AR | Bronchiectasis | Cutaneous: redundant, loose, hypoelastic skin | Unknown | ELN | N/A |
| Emphysema | Facial: aged appearance | FBLN4 | |||
| Vascular: aortic aneurysms, tortuosity | FBLN5 | ||||
| Musculoskeletal: joint laxity, scoliosis | LTBP4 | ||||
| Other: hernias | |||||
| Syndromes that disrupt lung architecture | |||||
| Alpha-1 antitrypsin deficiency, AR | Panacinar emphysema | Liver: cirrhosis, hepatocellular carcinoma, neonatal cholestatic jaundice | Unknown | SERPINA1 | 172 |
| Bullae | Skin: panniculitis | ||||
| Bronchiectasis | Inflammatory: vasculitis | ||||
| Obstruction | |||||
| Cystic fibrosis, AR | Bronchiectasis | Gastrointestinal: meconium ileus, pancreatic insufficiency, pancreatitis, malabsorption, failure to thrive | P: 8% | CFTR | 173 |
| Bacterial colonization/infection | Male infertility | ||||
| Cysts/cavitations obstruction respiratory failure | Ear/nose/throat: nasal polyps, sinus disease |
Definition of abbreviations: AD = autosomal dominant; ADHD = attention-deficit/hyperactivity disorder; AR = autosomal recessive; C = cysts; LAM = lymphangioleiomyomatosis; N/A = not applicable; P or PTX = pneumothorax; RV = residual volume.
Note that features vary by subtype.
Figure 2.
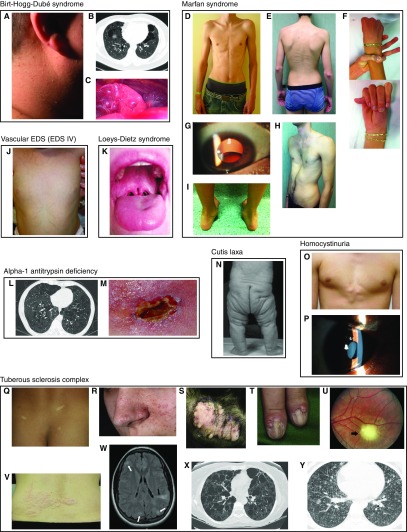
Physical examination findings of pneumothorax-associated syndromes. (A–C) Birt-Hogg-Dubé syndrome. (A) Fibrofolliculomas of the neck (159). (B) Lung cysts and bullae; extra-apical location is characteristic (159). (C) Pleural blebs on the surface of the left lower lobe; these can be missed on computed tomography but seen during thoracoscopy/video-assisted thorascopic surgery (159). (D–J) Marfan syndrome. (D) Marfanoid body habitus (90). (E) Scoliosis, striae, reduced elbow extension (160). (F) Positive thumb and wrist signs indicating arachnodactyly (160). (G) Lens dislocation (161). (H) Pectus excavatum (160). (I) Hindfoot deformity (160). (J) Vascular (type IV) Ehlers-Danlos syndrome: translucent skin on the torso of an infant (162). (K) Loeys-Dietz syndrome: bifid uvula (163) (L and M) Alpha-1 antitrypsin deficiency. (L) Panlobular emphysema (case courtesy of Dr. Jeremy Jones, Radiopaedia.org, rID:13441). (M) Panniculitis (164). (N) Cutis laxa: autosomal recessive cutis laxa IIa (165). (O and P) Homocystinuria. (O) Chest wall deformity (166). (P) Lens dislocation (167). (Q–Y) Tuberous sclerosis complex. (Q) Hypopigmented macules (ash leaf spots) (168). (R) Angiofibromas (168). (S) Fibrous plaques (168). (T) Ungual fibromas (168). (U) Retinal hamartoma (168). (V) Shagreen patch (168). (W) Cortical dysplasia (tubers, white arrows; radial migration lines, black arrow) (168). (X) Lymphangioleiomyomatosis (168). (Y) Multifocal micronodular pneumocyte hyperplasia (169). Images are reproduced by permission from the sources cited above.
Syndromes Related to Tumor Suppressor Genes
Birt-Hogg-Dubé syndrome
BHDS is an AD condition caused by heterozygous mutations in FLCN, encoding folliculin (28). The full BHDS phenotype includes skin lesions, kidney cancer, lung cysts, and pneumothorax (Figures 2A–2C) (29). Incomplete and age-dependent penetrance of features is characteristic (30). Skin findings include fibrofolliculomas (hamartomas of hair follicles) and trichodiscomas (tumors of the hair disk) (Figure 2A) (29). These lesions are clinically identical, appearing as small, dome-shaped papules typically on the face, neck, chest, back, and arms. Skin tags are also observed frequently. Renal cancer subtypes include hybrid oncocytic/chromophobe tumors, chromophobe carcinomas, clear cell carcinomas, oncocytomas, and papillary renal cell carcinomas (31); their combined prevalence was estimated at 6.5% in a review of the literature (29), whereas a higher rate (∼20%) was found in a selected set of NIH cases (30). Screening for renal tumors via imaging is the key intervention for BHDS; however, there is to date no consensus regarding the optimal modality (e.g., magnetic resonance imaging, contrast CT, ultrasound) or the timing of imaging (i.e., earliest age, interval between tests), although algorithms have been suggested (29, 32). Although some authors have suggested colorectal cancer as a feature of BHDS (33), it is not an accepted part of the phenotype (29, 32, 34).
The lung cysts of BHDS tend to be basal (below the carina) and subpleural (Figures 2B and 2C) (9, 34). They vary in number and size, and most are nonspherical (9). Pulmonary cysts in BHDS have a penetrance of 83% to 100% (30; 34–37), and they are detected in 10% of unaffected control family members (34). BHDS cysts are usually asymptomatic, and spirometry is typically normal.
The prevalence of pneumothorax in BHDS is 22% to 41% (30; 34–38). Pneumothoraces in BHDS tend to arise in early to midadulthood (37) but can affect children (39). They can be the presenting sign of BHDS (40). They are often recurrent (40–75% [35, 37]). Resection or pleurodesis have been suggested after first-time pneumothorax in BHDS (41, 42).
Among patients with BHDS, risk factors for pneumothorax include family history of pneumothorax (36), larger cyst size and number (37), extent of lower lung disease (35), flying (42, 43), and scuba diving (43). Curiously, smoking is not a risk factor (32, 37). There is no genotype–phenotype correlation or modifier gene known to affect pneumothorax risk in BHDS (36).
How FLCN mutations lead to cyst formation is unknown. One proposal is based on the observation that folliculin is involved in cell–cell adhesion via the desmosomal protein PKP4/p0071 (44, 45); this suggests that poor stretch tolerance to lung pressure may allow cyst formation (46).
Tuberous sclerosis and pulmonary lymphangioleiomyomatosis
Pulmonary lymphangioleiomyomatosis (LAM) is a progressive lung disease involving infiltration of the alveolar septa with smooth muscle–like LAM cells and the development of cysts that compromise normal lung parenchyma (47). LAM is typically diagnosed in young adulthood (48) and affects almost exclusively females—a presumed effect of estrogen (49–52). LAM occurs both sporadically and in association with tuberous sclerosis complex (TSC), an AD genetic syndrome caused by germline loss-of-function mutations in TSC1 (encoding hamartin) or TSC2 (encoding tuberin) (53, 54). Multitudinous clinical findings are possible in TSC (Table 1 and Figures 2Q–2Y), affecting the brain, skin, abdominal viscera, heart, eyes, mouth, and lung. The lung phenotypes include LAM and micronodular pneumocyte hyperplasia. Among patients with LAM, patients with TSC (TSC-LAM) make up 15%, whereas the remaining 85% are sporadic (48). A very small number of men have LAM, all of whom have TSC-LAM (55).
TSC is a syndrome of hamartomas, explained by the role of hamartin and tuberin in regulating cell growth and division via the mTORC1 (mammalian target of rapamycin complex 1) and other pathways (56). Although women with sporadic LAM lack germline mutations in TSC1/2, mosaic inactivating mutations in these genes are found in the pulmonary LAM cells of most patients (57–60). Nearly 30% of patients with sporadic LAM have renal angiomyolipomas (48), a feature of TSC, which contain TSC2 mutations (57). The same TSC2 mutation is found in the angiomyolipoma and the LAM cells (57). In addition, LAM can recur after lung transplantation, and the recurrent LAM cells carry the original TSC2 mutation (61). These findings suggest that LAM is a low-grade, metastatic neoplasm (62).
Pneumothorax is the most common presenting sign of LAM (∼one-third of cases) (48). Other presenting features include shortness of breath, wheezing, and abnormal imaging including diffuse reticular pattern on X-ray and diffuse interstitial changes with infiltrates and cysts on CT scan (48). Cysts are generally less than 2 cm, round, and diffuse, with no relationship to pleura or vessels (41). CT imaging reveals cystic changes consistent with LAM in 34% to 81% of women and 0% to 10% of men with TSC (63–65). With progression, LAM can cause chest pain, cough, hemoptysis, pleural effusions including chylous effusions, airway obstruction demonstrated via spirometry, and respiratory failure (48). Beyond imaging, the diagnosis of LAM is aided by lung biopsy, lymph node biopsy, and the serum biomarker VEGF-D (66–70).
The lifetime incidence of pneumothorax in LAM is 56% to 66% (48, 71, 72). Pneumothoraces are recurrent in 73% to 77% of patients (72, 73), with a mean number of 4.4 ± 0.5 (48). Risk factors for pneumothorax in LAM are prior pneumothorax (72), faster rate of FEV1 decline (71), and larger cyst size (71). Individuals with a prior pneumothorax had lower VEGF-D (vascular endothelial growth factor D) levels (74). Chronic loculated pneumothoraces are not worsened by flight, leading to the recommendation to avoid flight only in patients with LAM with recent pneumothorax or current symptoms of pneumothorax (75). Pregnancy exacerbates respiratory symptoms in some women with LAM (48) and appears to be a risk factor for pneumothorax (76); however, a lack of prospective studies makes the risk difficult to quantify. Polymorphisms in extracellular matrix proteins (collagen, elastin, matrix metalloproteinase-1) were not associated with pneumothorax in one study (71).
The mTORC1 inhibitor sirolimus (rapamycin) lowers the rate of decline of FEV1 in women with LAM (77). This intervention is based on the discovery that TSC2 mutations result in mTORC1 hyperactivation. Treatment with sirolimus is usually continued indefinitely, because lung function was proven to decline after discontinuation. The impact of sirolimus on pneumothorax risk is unknown. Other treatments for LAM include oxygen, lung transplantation, and avoiding both smoking and estrogen supplementation (49, 66). Progesterone derivatives have been used therapeutically (78) but without definitive benefit (48, 79). Pleurodesis is recommended after the first pneumothorax (67, 72) because of the likelihood of recurrence.
Syndromes of Disordered Connective Tissue
Marfan syndrome
Marfan syndrome is an AD condition caused by heterozygous mutations in FBN1, encoding fibrillin 1. Marfan syndrome features include tall stature, thin body habitus, long limbs resulting in decreased upper segment to lower segment ratio, arachnodactyly, chest wall deformity, scoliosis, lens dislocation, and dilation/dissection/aneurysm/rupture of the aorta (Figures 2D–2I) (80). Screening and treatment for cardiovascular complications is the primary intervention in the disorder. Pulmonary features of Marfan syndrome can include congenital malformations (e.g., rudimentary middle lobe), cysts, emphysema, and pneumothorax (81). Large total and residual lung volumes may be present (82).
Apical blebs/bullae were found in patients with Marfan syndrome at a rate of 8.9% with X-ray and 10% by CT scan (83). As 0% to 15% of healthy individuals have emphysema-like lesions including bullae by CT scan (1, 84) and 6% have blebs at surgery (85), it is unclear if these lung lesions are enriched in Marfan syndrome.
Pneumothorax is enriched among patients with Marfan syndrome, estimated at 4% to 11% (83, 86, 87). Pneumothorax is one of the criteria of the Marfan systemic score (80). Among eight patients, the median number of pneumothoraces was one (range, one to three) (83). Blebs or bullae are a risk factor for pneumothorax; pectus excavatum and smoking are not (83). Despite some familial clustering (88, 89), no genotype–phenotype correlation for pneumothorax is known (83).
At times, pneumothorax is the presenting feature of Marfan syndrome (90). Among 10 patients with sporadic pneumothorax screened for the syndrome via hand X-rays (for arachnodactyly) and clinical examination, four had “possible” Marfan syndrome and one had “full-fledged” disease (91). One of 21 (5%) patients with sporadic pneumothorax had a stop-gain mutation in FBN1 (8).
In the lung, fibrillin-containing microfibrils associate with elastin to help form elastic fibers. In a Marfan syndrome mouse model, age-dependent alveolar destruction occurs and appears to involve aberrant TGF-β (transforming growth factor-β) signaling (92, 93). It is unclear in patients whether skeletal shape, lack of fibrillin, altered elastin, altered TGF-β signaling, or a combination leads to blebs/bullae and/or pneumothorax.
Vascular Ehlers-Danlos syndrome
Ehlers-Danlos syndrome (EDS) is a family of connective disorders mostly resulting from mutant collagens or related proteins (94). Of these subtypes, pneumothorax is a feature only of vascular EDS (type IV; vEDS).
vEDS is AD, caused by heterozygous mutations in COL3A1, encoding the sole subunit of the homotrimeric type III collagen (95). vEDS can have fatal complications, including rupture of arteries, the uterus, and intestines (96). Arterial rupture may be preceded by aneurysms or dissection (96). Other features include thin, translucent skin (Figure 2J), characteristic facial features, easy bruising, clubfoot, congenital hip dislocation, lax small joints, carotid–cavernous sinus fistula, and pulmonary features (96). Screening and prevention include periodic imaging of the heart and vessels, blood pressure control, counseling regarding the risks of uterine rupture in pregnancy, and avoidance of colonoscopy and elective invasive arteriography (97).
Pulmonary complications of vEDS include cavitary lesions, cysts, bullae, fibrous nodules, pneumothorax, hemopneumothorax, and pulmonary hemorrhage (98, 99). Type III collagen in the lung is expressed in vessels and parenchymal fibroblasts (100). Thus, pulmonary complications of vEDS are proposed to result from poor tissue integrity (101, 102). The fibrous nodules, or fibrous pseudotumors, feature osseous metaplasia and may result from inefficient repair after injury to the pulmonary vessels or interstitium, with type I collagen favored over the absent type III collagen (99; 103–105).
Pneumothorax (99, 106, 107) or hemopneumothorax (108) can be the presenting symptom of vEDS. The penetrance of either pneumothorax or hemothorax in vEDS was 16% in a series diagnosed clinically (97); of individuals diagnosed molecularly, pneumothorax prevalence was 80% (109). There is no known genotype–phenotype correlation for vEDS. Standard treatment of pneumothorax has worked (98).
Loeys-Dietz syndrome
Loeys-Dietz syndrome is an AD genetic disorder caused by mutations in TGFBR2, TGFBR1, SMAD3, TGFB2, and TGFB3. SMAD2 is a provisional gene. These genes encode components of the TFG-β signaling pathway. Pneumothorax is an occasional feature of Loeys-Dietz syndrome (110). It has been described once as the presenting feature (111). Other features are vascular (arterial aneurysms, dissections, and tortuosity), skeletal (pectus excavatum or carinatum, joint laxity or contractures, cervical spine instability, scoliosis, arachnodactyly, club foot), craniofacial (bifid uvula [Figure 2K] or cleft palate, hypertelorism, craniosynostosis), cutaneous (translucent skin, dystrophic scars, easy bruising), and uterine (rupture during pregnancy) (112). Vascular features lead to premature death (112).
Homocystinuria
Homocystinuria is an autosomal recessive metabolic disorder caused by biallelic mutations in CBS, which encodes cystathionine β-synthase (113). Features are skeletal (tall stature with long limbs, scoliosis, pectus excavatum), ocular (lens dislocation, myopia), vascular (blood clots), central nervous system (intellectual disability, seizures), and cutaneous (hypopigmentation, livedo reticularis, malar flush) (Figures 2O and 2P) (113). Plasma homocysteine and methionine are markedly elevated, and the condition can be diagnosed via elevated methionine on the newborn screen (113). Therapy exists in the form of a methionine-restricted diet, supplementation with folate, B12, B6 (if responsive), betaine, and, in certain circumstances, anticoagulation (113). Pneumothorax has been reported (114); the incidence is unknown. It has once been the presenting feature (115).
Cutis laxa
Cutis laxa describes loose, redundant, hypoelastic skin, particularly over the neck, hands, groin, face, and trunk (Figure 2N). It is a feature of at least 10 genetic syndromes (116). Several of the cutis laxa syndromes display early-onset emphysema, including AD cutis laxa (ELN gene), autosomal recessive cutis laxa Ia/Ib (FBLN4/FBLN5 genes), and Urban-Rifkin-Davis syndrome (LTBP4 gene). Pneumothorax has been reported occasionally in patients with cutis laxa syndromes (117), more frequently among the subtypes that feature emphysema (118, 119). The incidence of pneumothorax is unknown, and pneumothorax has never been reported as the presenting symptom of a cutis laxa syndrome.
Syndromes That Efface Normal Lung Architecture
Alpha-1 antitrypsin deficiency
A1AT (alpha-1 antitrypsin) deficiency is an autosomal recessive disease caused by mutations in SERPINA1 (120). Its protein product, alpha-1 antitrypsin, inhibits the protease neutrophil elastase in the lungs. Individuals with biallelic mutations that in combination reduce the circulating levels or activity of A1AT to less than ∼35% of normal can have early-onset chronic obstructive pulmonary disease (Figure 2L) (120). The typical pathology is a progressive, panacinar, predominantly lower-lobe emphysema presenting in early to midadulthood (121). Other lung findings can include chronic bronchitis and bronchiectasis (122), apical bullae (123), and pneumothorax.
Extrapulmonary complications include neonatal cholestatic jaundice, cirrhosis, and risk of hepatocellular carcinoma, resulting from polymerization of certain mutant forms of A1AT in the endoplasmic reticulum of hepatocytes (124). Panniculitis—painful inflammatory skin nodules/weeping lesions (Figure 2M)—and vasculitis have also been reported (120).
The incidence of pneumothorax in A1AT deficiency is unknown (125, 126); however, of patients homozygous for the most common mutant allele, Pi*Z, 2% to 3% die of pneumothorax (126, 127). Because pneumothorax can occasionally be the presenting symptom of A1AT deficiency (128, 129), several authors have screened for A1AT deficiency among patients with spontaneous pneumothorax: estimates of A1AT range from 0% to 8% (1; 130–133). Although smoking is an important risk factor for lung disease progression (134) and death (127) in A1AT deficiency, its effect on pneumothorax risk is unknown. Also unknown is whether genotype or augmentation therapy (infusions of A1AT) can impact pneumothorax incidence or recurrence.
Cystic fibrosis
Cystic fibrosis (CF) is caused by biallelic mutations in CFTR. Complications are gastrointestinal (meconium ileus, malabsorption, failure to thrive, pancreatic insufficiency and pancreatitis), reproductive (male infertility), and respiratory. Lung findings derive from thickened mucus affecting the mucociliary elevator and include recurrent infections, inflammation, bronchiectasis, cysts/cavitations, air trapping, hemoptysis, and pneumothorax (135). Pneumothorax as a herald sign of CF is probably rare because: 1) it is a late feature of the disease (136), and 2) CF is screened for in newborns in countries where it is prevalent, so the diagnosis is often made at birth.
Incidence of pneumothorax among patients with CF was 2% in children over a 15-year interval (137) and 3% in all ages over a 10-year period (136). Lifetime prevalence was estimated at 8% during the 1950s to 1980s (138). Specific risk factors for pneumothorax in CF include: older age; FEV1 less than 30% predicted; infection with Pseudomonas aeruginosa, Burkholderia cepacia, or Aspergillus; allergic bronchopulmonary aspergillosis; massive hemoptysis; enteral feeding; and pancreatic insufficiency (136). Recurrence is 50% to 90% ipsilaterally and 46% contralaterally (136, 139). A pneumothorax carries an attributable mortality of 6% to 14% (140).
Mechanically, pneumothorax risk in CF may derive from effacement of normal lung structures, altered airflow dynamics, air trapping, use of inhaled medications, or noninvasive positive pressure ventilation (135). Risk does not correlate well with blebs or cysts (141). Among 21 CF carriers, one had pneumothorax and others had respiratory symptoms including nasal polyps causing obstruction (142); however, a larger study showed no difference in pneumothorax, sinusitis, or other respiratory conditions (143).
Management of pneumothorax in CF is debated. Some suggest early surgical intervention (144, 145); others urge caution given potential future lung transplantation (146, 147).
Others
There are reports of pneumothorax in other genetic syndromes. These include: Sotos syndrome (148), spinocerebellar ataxia type 1 (149), telomerase reverse transcriptase (TERT gene) mutations (emphysema and pneumothorax in smokers) (150), hereditary mucoepithelial dysplasia (151), and diffuse dendriform pulmonary ossification (152).
A Genetic Approach to Patients with Spontaneous Pneumothorax
Current clinical guidelines for the evaluation and management of pneumothorax do not address the familial, syndromic, or potentially syndromic cases (153, 154). We argue that establishing a genetic diagnosis is beneficial for patients and their relatives. For the index case, establishing a genetic etiology can guide pneumothorax management, including the need for pleurodesis after first pneumothorax. Moreover, given that the genetic forms of pneumothorax are associated with extrapulmonary complications, a genetic diagnosis enables targeted surveillance and prophylaxis. Examples include blood pressure control and echocardiography in Marfan syndrome and surveillance for renal tumors in BHDS. The potential benefits to family—whether pneumothorax has occurred in other family members or not—are no less significant. Identification of a pathogenic genetic variant in the index case enables testing, counseling, and surveillance/prophylaxis in family members carrying the same variant. For these reasons, we recommend that an underlying genetic diagnosis be routinely entertained in patients with spontaneous pneumothorax.
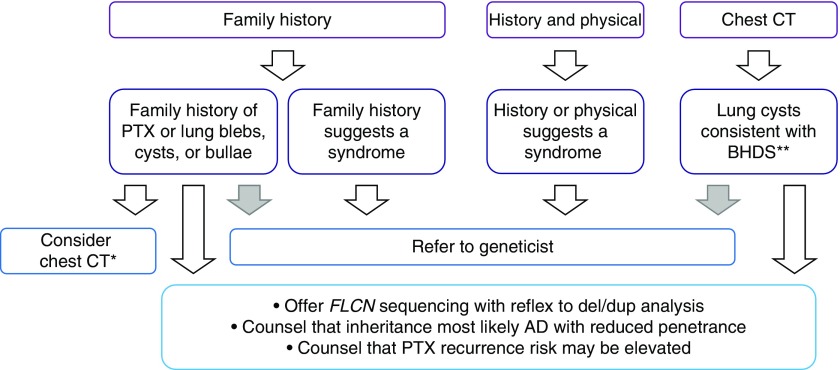
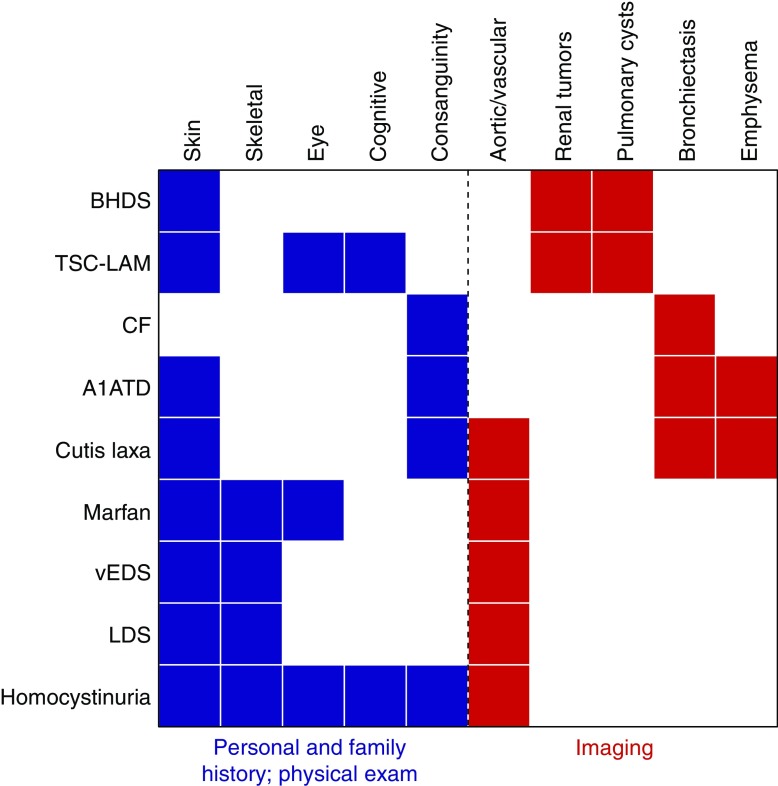
Figure 3 provides a basic algorithm to evaluate this question, emphasizing the importance of a focused family history, targeted history taking and physical examination, and careful review of imaging. Figure 4 provides a graphical representation of which findings are associated with which syndromes.
Figure 3.
Proposed algorithm for identifying spontaneous pneumothoraces with a genetic basis. Algorithm should be applied to all patients with spontaneous pneumothorax. Gray arrows are optional, based on the practitioner’s comfort. *FLCN testing in cases of absent family history is still reasonable, so long as a chest computed tomography (CT) scan, if previously obtained, is not inconsistent with Birt-Hogg-Dubé syndrome (BHDS). **Chest CT findings in BHDS include bilateral, multifocal, predominately lower-lobe cysts. AD = autosomal dominant; PTX = pneumothorax.
Figure 4.
History, physical examination, and imaging findings that raise the possibility of a syndromic cause of pneumothorax. The major features (x-axis) of syndromes predisposing to pneumothorax (y-axis) are shown. Personal history/family history/physical examination findings are in blue. Imaging findings are in red. The skin findings of Birt-Hogg-Dubé syndrome (BHDS) are age dependent; thus, skin examinations of the parents or even grandparents may at times be more informative than for the proband. A1ATD = alpha-1 antitrypsin deficiency; CF = cystic fibrosis; LDS = Loeys-Dietz syndrome; TSC-LAM = lymphangioleiomyomatosis in an individual with tuberous sclerosis; vEDS = vascular Ehlers-Danlos syndrome.
In addition to pneumothorax, the family history should ascertain associated lung diseases (particularly emphysema, bronchiectasis, and CF), abnormal chest imaging (cysts/blebs/bullae), renal tumors (observed in BHDS and TSC), physical features consistent with pneumothorax-associated syndromes (Table 1), and consanguinity (which could heighten suspicion for an autosomal recessive condition). Although a positive family history can help flag patients, an absent family history should not dissuade pursuit of a genetic cause of pneumothorax; both FSP caused by FLCN mutations and dominant pneumothorax-associated syndromes can arise via de novo mutations (155) or display reduced penetrance, and autosomal recessive conditions frequently lack family history.
History taking and physical examination should include focused screening for features of pneumothorax-associated genetic syndromes (Table 1). A thorough examination of the skin is essential, as dermatologic manifestations of BHDS are often subtle. Skeletal anomalies, hyperextensibility, and distinct facial features could suggest a connective tissue disorder.
Current clinical guidelines do not recommend chest CT imaging in first-time, unilateral spontaneous pneumothorax (153, 154). However, a recent study demonstrating the cost effectiveness of CT imaging to detect diffuse cystic lung diseases in patients with first-time pneumothorax (156) is among the reasons some authors argue for the adoption of this practice (157). Although acknowledging that the risk–benefit profile is unknown (11), we suggest considering chest CT imaging in first-time pneumothorax if a family history is present or if the patient is female, given that the mutational burden to cause pneumothorax in women appears to be higher (S. Marciniak, unpublished results) and because of the possibility of LAM. The presence of multiple (158) and/or nonapical cysts (11) may suggest BHDS or FLCN-related FSP. Parenchymal lung disease might suggest A1AT deficiency or LAM, and airways disease CF. Aortic pathology might suggest Marfan syndrome or Loeys-Dietz syndrome.
We recommend genetic testing or evaluation in the following scenarios: An isolated family history of pneumothorax should prompt sequencing and deletion/duplication screening of FLCN. This will be positive in 17% to 50% of cases (5, 6). A family history of blebs/cysts/or bullae or a personal history of nonapical blebs/cysts/bullae should also prompt testing of FLCN. In the absence of positive family history and CT data, the merits of FLCN testing are not clear, although 10% of these patients will have pathogenic mutations as well. Should the personal or family medical history or the physical examination raise suspicion for a genetic syndrome, we recommend referral to a medical geneticist for a more detailed examination and consideration of genetic testing for pneumothorax-associated syndromes.
Conclusions
A genetic cause of pneumothorax can have high-impact clinical implications. The generalist, emergency physician, radiologist, pulmonologist, surgeon, or other specialist, armed with the above recommendation, is enabled to raise the possibility of a genetic/familial diagnosis in patients with pneumothorax. Additional studies—ideally prospective—that test the value of chest CT imaging, genetic testing, and genetics referral to uncover genetic causes of pneumothorax, and the appropriateness of surgery after first-time pneumothorax, will enable the official “society” guidelines to be updated. Furthermore, yet-undiscovered genetic contributions to pneumothorax likely exist, which researchers will uncover in time via assembly and study of patient cohorts and by other methods.
Supplementary Material
Footnotes
Supported by NHLBI grant U01HL131022-02 (E.P.H.); National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK102146-03 (E.P.H.); the U.S. Department of Defense Tuberous Sclerosis Medical Research Program (E.P.H.); the Engles Program in TSC and LAM Research (E.P.H.); NHLBI grants R01HL118455-04, R01HL123546-03, R01HL130974-02, and P01HL132825-02 (B.A.R.); and the Brigham and Women’s Precision Medicine Initiative (B.A.R.).
CME will be available for this article at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201807-1212CI on January 25, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bense L, Lewander R, Eklund G, Hedenstierna G, Wiman LG. Nonsmoking, non-alpha 1-antitrypsin deficiency-induced emphysema in nonsmokers with healed spontaneous pneumothorax, identified by computed tomography of the lungs. Chest. 1993;103:433–438. doi: 10.1378/chest.103.2.433. [DOI] [PubMed] [Google Scholar]
- 2.Withers JN, Fishback ME, Kiehl PV, Hannon JL. Spontaneous pneumothorax: suggested etiology and comparison of treatment methods. Am J Surg. 1964;108:772–776. doi: 10.1016/0002-9610(64)90030-3. [DOI] [PubMed] [Google Scholar]
- 3.Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest. 1987;92:1009–1012. doi: 10.1378/chest.92.6.1009. [DOI] [PubMed] [Google Scholar]
- 4.Abolnik IZ, Lossos IS, Zlotogora J, Brauer R. On the inheritance of primary spontaneous pneumothorax. Am J Med Genet. 1991;40:155–158. doi: 10.1002/ajmg.1320400207. [DOI] [PubMed] [Google Scholar]
- 5.Ren HZ, Zhu CC, Yang C, Chen SL, Xie J, Hou YY, et al. Mutation analysis of the FLCN gene in Chinese patients with sporadic and familial isolated primary spontaneous pneumothorax. Clin Genet. 2008;74:178–183. doi: 10.1111/j.1399-0004.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- 6.Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med. 2005;172:39–44. doi: 10.1164/rccm.200501-143OC. [DOI] [PubMed] [Google Scholar]
- 7.Ding Y, Zou W, Zhu C, Min H, Ma D, Chen B, et al. Promoter methylation is not associated with FLCN irregulation in lung cyst lesions of primary spontaneous pneumothorax. Mol Med Rep. 2015;12:7770–7776. doi: 10.3892/mmr.2015.4341. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Ma D, Zou W, Ding Y, Zhu C, Min H, et al. A rapid NGS strategy for comprehensive molecular diagnosis of Birt-Hogg-Dubé syndrome in patients with primary spontaneous pneumothorax. Respir Res. 2016;17:64. doi: 10.1186/s12931-016-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobino K, Gunji Y, Kurihara M, Kunogi M, Koike K, Tomiyama N, et al. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol. 2011;77:403–409. doi: 10.1016/j.ejrad.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal PP, Gross BH, Holloway BJ, Seely J, Stark P, Kazerooni EA. Thoracic CT findings in Birt-Hogg-Dube syndrome. AJR Am J Roentgenol. 2011;196:349–352. doi: 10.2214/AJR.10.4757. [DOI] [PubMed] [Google Scholar]
- 11.Johannesma PC, Reinhard R, Kon Y, Sriram JD, Smit HJ, van Moorselaar RJ, et al. Amsterdam BHD Working Group. Prevalence of Birt-Hogg-Dubé syndrome in patients with apparently primary spontaneous pneumothorax. Eur Respir J. 2015;45:1191–1194. doi: 10.1183/09031936.00196914. [DOI] [PubMed] [Google Scholar]
- 12.Sousa I, Abrantes P, Francisco V, Teixeira G, Monteiro M, Neves J, et al. Multicentric genome-wide association study for primary spontaneous pneumothorax. PLoS One. 2016;11:e0156103. doi: 10.1371/journal.pone.0156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadikot RT, Greene T, Meadows K, Arnold AG. Recurrence of primary spontaneous pneumothorax. Thorax. 1997;52:805–809. doi: 10.1136/thx.52.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melton LJ, III, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379–1382. doi: 10.1164/arrd.1979.120.6.1379. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H, Konishiike J, Sugamura A, Takeno Y. Epidemiology of spontaneous pneumothorax in women. Chest. 1986;89:378–382. doi: 10.1378/chest.89.3.378. [DOI] [PubMed] [Google Scholar]
- 16.Gupta D, Hansell A, Nichols T, Duong T, Ayres JG, Strachan D. Epidemiology of pneumothorax in England. Thorax. 2000;55:666–671. doi: 10.1136/thorax.55.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenler-Petersen P, Grunnet N, Jespersen TW, Jaeger P. Familial spontaneous pneumothorax. Eur Respir J. 1990;3:342–345. [PubMed] [Google Scholar]
- 18.Guo Y, Xie C, Rodriguez RM, Light RW. Factors related to recurrence of spontaneous pneumothorax. Respirology. 2005;10:378–384. doi: 10.1111/j.1440-1843.2005.00715.x. [DOI] [PubMed] [Google Scholar]
- 19.Mikroulis D, Lukman AL, Didilis V, Bougioukas G. Familial spontaneous pneumothorax. Respirology. 2005;10:403. doi: 10.1111/j.1440-1843.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 20.Nickoladze GD. Surgical management of familial spontaneous pneumothorax. Respir Med. 1990;84:107–109. doi: 10.1016/s0954-6111(08)80011-x. [DOI] [PubMed] [Google Scholar]
- 21.Wilson WG, Aylsworth AS. Familial spontaneous pneumothorax. Pediatrics. 1979;64:172–175. [PubMed] [Google Scholar]
- 22.Cardy CM, Maskell NA, Handford PA, Arnold AG, Davies RJ, Morrison PJ, et al. Familial spontaneous pneumothorax and FBN1 mutations. Am J Respir Crit Care Med. 2004;169:1260–1262. doi: 10.1164/ajrccm.169.11.967. [DOI] [PubMed] [Google Scholar]
- 23.Yamada A, Takeda Y, Hayashi S, Shimizu K. Familial spontaneous pneumothorax in three generations and its HLA. Jpn J Thorac Cardiovasc Surg. 2003;51:456–458. doi: 10.1007/BF02719603. [DOI] [PubMed] [Google Scholar]
- 24.Sharpe IK, Ahmad M, Braun W. Familial spontaneous pneumothorax and HLA antigens. Chest. 1980;78:264–268. doi: 10.1378/chest.78.2.264. [DOI] [PubMed] [Google Scholar]
- 25.Cheng YJ, Chou SH, Kao EL. Familial spontaneous pneumothorax-report of seven cases in two families. Gaoxiong Yi Xue Ke Xue Za Zhi. 1992;8:390–394. [PubMed] [Google Scholar]
- 26.Painter JN, Tapanainen H, Somer M, Tukiainen P, Aittomäki K. A 4-bp deletion in the Birt-Hogg-Dubé gene (FLCN) causes dominantly inherited spontaneous pneumothorax. Am J Hum Genet. 2005;76:522–527. doi: 10.1086/428455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott RM, Henske EP, Raby B, Boone PM, Rusk RA, Marciniak SJ. Familial pneumothorax: towards precision medicine. Thorax. 2018;73:270–276. doi: 10.1136/thoraxjnl-2017-211169. [DOI] [PubMed] [Google Scholar]
- 28.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn G, Turner ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 29.Toro JR. Birt-Hogg-Dubé syndrome. Adam MP, Ardinger, HH, Pagon RA, Wallace, SE, Bean LJH, Stephens K, et al., editors. GeneReviews. Seattle, WA: University of Washington; 2006 [updated 2014 Aug 7; accessed 2018 Dec 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1522/ [Google Scholar]
- 30.Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR, Merino MJ, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet. 2005;76:1023–1033. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dal Sasso AA, Belém LC, Zanetti G, Souza CA, Escuissato DL, Irion KL, et al. Birt-Hogg-Dubé syndrome: state-of-the-art review with emphasis on pulmonary involvement. Respir Med. 2015;109:289–296. doi: 10.1016/j.rmed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Menko FH, van Steensel MA, Giraud S, Friis-Hansen L, Richard S, Ungari S, et al. European BHD Consortium. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199–1206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 33.Khoo SK, Giraud S, Kahnoski K, Chen J, Motorna O, Nickolov R, et al. Clinical and genetic studies of Birt-Hogg-Dubé syndrome. J Med Genet. 2002;39:906–912. doi: 10.1136/jmg.39.12.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zbar B, Alvord WG, Glenn G, Turner M, Pavlovich CP, Schmidt L, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393–400. [PubMed] [Google Scholar]
- 35.Skolnik K, Tsai WH, Dornan K, Perrier R, Burrowes PW, Davidson WJ. Birt-Hogg-Dubé syndrome: a large single family cohort. Respir Res. 2016;17:22. doi: 10.1186/s12931-016-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke C, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321–331. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med. 2007;175:1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houweling AC, Gijezen LM, Jonker MA, van Doorn MB, Oldenburg RA, van Spaendonck-Zwarts KY, et al. Renal cancer and pneumothorax risk in Birt-Hogg-Dubé syndrome; an analysis of 115 FLCN mutation carriers from 35 BHD families. Br J Cancer. 2011;105:1912–1919. doi: 10.1038/bjc.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johannesma PC, van den Borne BE, Gille JJ, Nagelkerke AF, van Waesberghe JT, Paul MA, et al. Spontaneous pneumothorax as indicator for Birt-Hogg-Dubé syndrome in paediatric patients. BMC Pediatr. 2014;14:171. doi: 10.1186/1471-2431-14-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamond JM, Kotloff RM. Recurrent spontaneous pneumothorax as the presenting sign of the Birt-Hogg-Dubé syndrome. Ann Intern Med. 2009;150:289–290. doi: 10.7326/0003-4819-150-4-200902170-00027. [DOI] [PubMed] [Google Scholar]
- 41.Gupta N, Seyama K, McCormack FX. Pulmonary manifestations of Birt-Hogg-Dubé syndrome. Fam Cancer. 2013;12:387–396. doi: 10.1007/s10689-013-9660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta N, Kopras EJ, Henske EP, James LE, El-Chemaly S, Veeraraghavan S, et al. Spontaneous pneumothoraces in patients with Birt-Hogg-Dubé syndrome. Ann Am Thorac Soc. 2017;14:706–713. doi: 10.1513/AnnalsATS.201611-886OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johannesma PC, van de Beek I, van der Wel JW, Paul MA, Houweling AC, Jonker MA, et al. Risk of spontaneous pneumothorax due to air travel and diving in patients with Birt-Hogg-Dubé syndrome. Springerplus. 2016;5:1506. doi: 10.1186/s40064-016-3009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medvetz DA, Khabibullin D, Hariharan V, Ongusaha PP, Goncharova EA, Schlechter T, et al. Folliculin, the product of the Birt-Hogg-Dube tumor suppressor gene, interacts with the adherens junction protein p0071 to regulate cell-cell adhesion. PLoS One. 2012;7:e47842. doi: 10.1371/journal.pone.0047842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nahorski MS, Seabra L, Straatman-Iwanowska A, Wingenfeld A, Reiman A, Lu X, et al. Folliculin interacts with p0071 (plakophilin-4) and deficiency is associated with disordered RhoA signalling, epithelial polarization and cytokinesis. Hum Mol Genet. 2012;21:5268–5279. doi: 10.1093/hmg/dds378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kennedy JC, Khabibullin D, Henske EP. Mechanisms of pulmonary cyst pathogenesis in Birt-Hogg-Dube syndrome: the stretch hypothesis. Semin Cell Dev Biol. 2016;52:47–52. doi: 10.1016/j.semcdb.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Henske EP, McCormack FX. Lymphangioleiomyomatosis–a wolf in sheep’s clothing. J Clin Invest. 2012;122:3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryu JH, Moss J, Beck GJ, Lee JC, Brown KK, Chapman JT, et al. NHLBI LAM Registry Group. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen A, Iseman MD, Waldron JA, King TE. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous estrogens. Chest. 1987;91:782–785. doi: 10.1378/chest.91.5.782. [DOI] [PubMed] [Google Scholar]
- 50.Yu JJ, Robb VA, Morrison TA, Ariazi EA, Karbowniczek M, Astrinidis A, et al. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci USA. 2009;106:2635–2640. doi: 10.1073/pnas.0810790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yano S. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous oestrogen used for infertility treatment. Thorax. 2002;57:1085–1086. doi: 10.1136/thorax.57.12.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu J, Henske EP. mTOR activation, lymphangiogenesis, and estrogen-mediated cell survival: the “perfect storm” of pro-metastatic factors in LAM pathogenesis. Lymphat Res Biol. 2010;8:43–49. doi: 10.1089/lrb.2009.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc. 2000;75:591–594. doi: 10.4065/75.6.591. [DOI] [PubMed] [Google Scholar]
- 54.Henske EP, Jóźwiak S, Kingswood JC, Sampson JR, Thiele EA. Tuberous sclerosis complex. Nat Rev Dis Primers. 2016;2:16035. doi: 10.1038/nrdp.2016.35. [DOI] [PubMed] [Google Scholar]
- 55.Aubry MC, Myers JL, Ryu JH, Henske EP, Logginidou H, Jalal SM, et al. Pulmonary lymphangioleiomyomatosis in a man. Am J Respir Crit Care Med. 2000;162:749–752. doi: 10.1164/ajrccm.162.2.9911006. [DOI] [PubMed] [Google Scholar]
- 56.Au KS, Williams AT, Gambello MJ, Northrup H. Molecular genetic basis of tuberous sclerosis complex: from bench to bedside. J Child Neurol. 2004;19:699–709. doi: 10.1177/08830738040190091101. [DOI] [PubMed] [Google Scholar]
- 57.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Badri KR, Gao L, Hyjek E, Schuger N, Schuger L, Qin W, et al. Exonic mutations of TSC2/TSC1 are common but not seen in all sporadic pulmonary lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2013;187:663–665. doi: 10.1164/ajrccm.187.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujita A, Ando K, Kobayashi E, Mitani K, Okudera K, Nakashima M, et al. Detection of low-prevalence somatic TSC2 mutations in sporadic pulmonary lymphangioleiomyomatosis tissues by deep sequencing. Hum Genet. 2016;135:61–68. doi: 10.1007/s00439-015-1611-0. [DOI] [PubMed] [Google Scholar]
- 60.Sato T, Seyama K, Fujii H, Maruyama H, Setoguchi Y, Iwakami S, et al. Mutation analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangioleiomyomatosis. J Hum Genet. 2002;47:20–28. doi: 10.1007/s10038-002-8651-8. [DOI] [PubMed] [Google Scholar]
- 61.Karbowniczek M, Astrinidis A, Balsara BR, Testa JR, Lium JH, Colby TV, et al. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167:976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 62.McCormack FX, Travis WD, Colby TV, Henske EP, Moss J. Lymphangioleiomyomatosis: calling it what it is: a low-grade, destructive, metastasizing neoplasm. Am J Respir Crit Care Med. 2012;186:1210–1212. doi: 10.1164/rccm.201205-0848OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moss J, Avila NA, Barnes PM, Litzenberger RA, Bechtle J, Brooks PG, et al. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med. 2001;164:669–671. doi: 10.1164/ajrccm.164.4.2101154. [DOI] [PubMed] [Google Scholar]
- 64.Muzykewicz DA, Sharma A, Muse V, Numis AL, Rajagopal J, Thiele EA. TSC1 and TSC2 mutations in patients with lymphangioleiomyomatosis and tuberous sclerosis complex. J Med Genet. 2009;46:465–468. doi: 10.1136/jmg.2008.065342. [DOI] [PubMed] [Google Scholar]
- 65.Cudzilo CJ, Szczesniak RD, Brody AS, Rattan MS, Krueger DA, Bissler JJ, et al. Lymphangioleiomyomatosis screening in women with tuberous sclerosis. Chest. 2013;144:578–585. doi: 10.1378/chest.12-2813. [DOI] [PubMed] [Google Scholar]
- 66.Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, et al. Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 67.Gupta N, Finlay GA, Kotloff RM, Strange C, Wilson KC, Young LR, et al. ATS Assembly on Clinical Problems. Lymphangioleiomyomatosis diagnosis and management: high-resolution chest computed tomography, transbronchial lung biopsy, and pleural disease management. An Official American Thoracic Society/Japanese Respiratory Society clinical practice guideline. Am J Respir Crit Care Med. 2017;196:1337–1348. doi: 10.1164/rccm.201709-1965ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCormack FX, Gupta N, Finlay GR, Young LR, Taveira-DaSilva AM, Glasgow CG, et al. ATS/JRS Committee on Lymphangioleiomyomatosis. Official American Thoracic Society/Japanese Respiratory Society clinical practice guidelines: lymphangioleiomyomatosis diagnosis and management. Am J Respir Crit Care Med. 2016;194:748–761. doi: 10.1164/rccm.201607-1384ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seyama K, Kumasaka T, Souma S, Sato T, Kurihara M, Mitani K, et al. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol. 2006;4:143–152. doi: 10.1089/lrb.2006.4.143. [DOI] [PubMed] [Google Scholar]
- 70.Young LR, Inoue Y, McCormack FX. Diagnostic potential of serum VEGF-D for lymphangioleiomyomatosis. N Engl J Med. 2008;358:199–200. doi: 10.1056/NEJMc0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steagall WK, Glasgow CG, Hathaway OM, Avila NA, Taveira-Dasilva AM, Rabel A, et al. Genetic and morphologic determinants of pneumothorax in lymphangioleiomyomatosis. Am J Physiol Lung Cell Mol Physiol. 2007;293:L800–L808. doi: 10.1152/ajplung.00176.2007. [DOI] [PubMed] [Google Scholar]
- 72.Almoosa KF, McCormack FX, Sahn SA. Pleural disease in lymphangioleiomyomatosis. Clin Chest Med. 2006;27:355–368. doi: 10.1016/j.ccm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Hagaman JT, Schauer DP, McCormack FX, Kinder BW. Screening for lymphangioleiomyomatosis by high-resolution computed tomography in young, nonsmoking women presenting with spontaneous pneumothorax is cost-effective. Am J Respir Crit Care Med. 2010;181:1376–1382. doi: 10.1164/rccm.200910-1553OC. [DOI] [PubMed] [Google Scholar]
- 74.Young L, Lee HS, Inoue Y, Moss J, Singer LG, Strange C, et al. MILES Trial Group. Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med. 2013;1:445–452. doi: 10.1016/S2213-2600(13)70090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taveira-DaSilva AM, Burstein D, Hathaway OM, Fontana JR, Gochuico BR, Avila NA, et al. Pneumothorax after air travel in lymphangioleiomyomatosis, idiopathic pulmonary fibrosis, and sarcoidosis. Chest. 2009;136:665–670. doi: 10.1378/chest.08-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen MM, Freyer AM, Johnson SR. Pregnancy experiences among women with lymphangioleiomyomatosis. Respir Med. 2009;103:766–772. doi: 10.1016/j.rmed.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 77.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCarty KS, Jr, Mossler JA, McLelland R, Sieker HO. Pulmonary lymphangiomyomatosis responsive to progesterone. N Engl J Med. 1980;303:1461–1465. doi: 10.1056/NEJM198012183032506. [DOI] [PubMed] [Google Scholar]
- 79.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 80.Dietz H. Marfan syndrome. Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al., editors. GeneReviews. Seattle, WA: University of Washington; 2001 [updated 2017 Oct 12; accessed 2018 Dec 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1335/ [Google Scholar]
- 81.Dwyer EM, Jr, Troncale F. Spontaneous pneumothorax and pulmonary disease in the Marfan syndrome: report of two cases and review of the literature. Ann Intern Med. 1965;62:1285–1292. doi: 10.7326/0003-4819-62-6-1285. [DOI] [PubMed] [Google Scholar]
- 82.Giske L, Stanghelle JK, Rand-Hendrikssen S, Strøm V, Wilhelmsen JE, Røe C. Pulmonary function, working capacity and strength in young adults with Marfan syndrome. J Rehabil Med. 2003;35:221–228. doi: 10.1080/16501970306095. [DOI] [PubMed] [Google Scholar]
- 83.Karpman C, Aughenbaugh GL, Ryu JH. Pneumothorax and bullae in Marfan syndrome. Respiration. 2011;82:219–224. doi: 10.1159/000322958. [DOI] [PubMed] [Google Scholar]
- 84.Lesur O, Delorme N, Fromaget JM, Bernadac P, Polu JM. Computed tomography in the etiologic assessment of idiopathic spontaneous pneumothorax. Chest. 1990;98:341–347. doi: 10.1378/chest.98.2.341. [DOI] [PubMed] [Google Scholar]
- 85.Amjadi K, Alvarez GG, Vanderhelst E, Velkeniers B, Lam M, Noppen M. The prevalence of blebs or bullae among young healthy adults: a thoracoscopic investigation. Chest. 2007;132:1140–1145. doi: 10.1378/chest.07-0029. [DOI] [PubMed] [Google Scholar]
- 86.Wood JR, Bellamy D, Child AH, Citron KM. Pulmonary disease in patients with Marfan syndrome. Thorax. 1984;39:780–784. doi: 10.1136/thx.39.10.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hall JR, Pyeritz RE, Dudgeon DL, Haller JA., Jr Pneumothorax in the Marfan syndrome: prevalence and therapy. Ann Thorac Surg. 1984;37:500–504. doi: 10.1016/s0003-4975(10)61142-3. [DOI] [PubMed] [Google Scholar]
- 88.Suzuki T, Akiba T, Miyake R, Marushima H, Morikawa T. Familial spontaneous pneumothorax in two adult siblings with Marfan syndrome. Ann Thorac Cardiovasc Surg. 2010;16:362–364. [PubMed] [Google Scholar]
- 89.Yellin A, Shiner RJ, Lieberman Y. Familial multiple bilateral pneumothorax associated with Marfan syndrome. Chest. 1991;100:577–578. doi: 10.1378/chest.100.2.577. [DOI] [PubMed] [Google Scholar]
- 90.Viveiro C, Rocha P, Carvalho C, Zarcos MM. Spontaneous pneumothorax as manifestation of Marfan syndrome. BMJ Case Rep. 2013;2013:bcr2013201697. doi: 10.1136/bcr-2013-201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sensenig DM, LaMarche P. Marfan’s syndrome and spontaneous pneumothorax. Am J Surg. 1980;139:602–604. doi: 10.1016/0002-9610(80)90345-1. [DOI] [PubMed] [Google Scholar]
- 92.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee JJ, Galatioto J, Rao S, Ramirez F, Costa KD. Losartan attenuates degradation of aorta and lung tissue micromechanics in a mouse model of severe Marfan syndrome. Ann Biomed Eng. 2016;44:2994–3006. doi: 10.1007/s10439-016-1616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:8–26. doi: 10.1002/ajmg.c.31552. [DOI] [PubMed] [Google Scholar]
- 95.Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, et al. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci USA. 1975;72:1314–1316. doi: 10.1073/pnas.72.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pepin MG, Murray ML, Byers PH. Vascular Ehlers-Danlos syndrome. Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al., editors. GeneReviews. Seattle, WA: University of Washington; 1999 [updated 2015 Nov 19; accessed 2018 Dec 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1494/. [Google Scholar]
- 97.Oderich GS, Panneton JM, Bower TC, Lindor NM, Cherry KJ, Noel AA, et al. The spectrum, management and clinical outcome of Ehlers-Danlos syndrome type IV: a 30-year experience. J Vasc Surg. 2005;42:98–106. doi: 10.1016/j.jvs.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 98.Dowton SB, Pincott S, Demmer L. Respiratory complications of Ehlers-Danlos syndrome type IV. Clin Genet. 1996;50:510–514. doi: 10.1111/j.1399-0004.1996.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 99.Hatake K, Morimura Y, Kudo R, Kawashima W, Kasuda S, Kuniyasu H. Respiratory complications of Ehlers-Danlos syndrome type IV. Leg Med (Tokyo) 2013;15:23–27. doi: 10.1016/j.legalmed.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 100.McLees BD, Schleiter G, Pinnell SR. Isolation of type III collagen from human adult parenchymal lung tissue. Biochemistry. 1977;16:185–190. doi: 10.1021/bi00621a004. [DOI] [PubMed] [Google Scholar]
- 101.Clark JG, Kuhn C, III, Uitto J. Lung collagen in type IV Ehlers-Danlos syndrome: ultrastructural and biochemical studies. Am Rev Respir Dis. 1980;122:971–978. doi: 10.1164/arrd.1980.122.6.971. [DOI] [PubMed] [Google Scholar]
- 102.Watanabe A, Shimada T. Vascular type of Ehlers-Danlos syndrome. J Nippon Med Sch. 2008;75:254–261. doi: 10.1272/jnms.75.254. [DOI] [PubMed] [Google Scholar]
- 103.Murray RA, Poulton TB, Saltarelli MG, Dweik RA, Litwin DK, Kirby TJ, et al. Rare pulmonary manifestation of Ehlers-Danlos syndrome. J Thorac Imaging. 1995;10:138–141. doi: 10.1097/00005382-199521000-00012. [DOI] [PubMed] [Google Scholar]
- 104.Corrin B, Simpson CG, Fisher C. Fibrous pseudotumours and cyst formation in the lungs in Ehlers-Danlos syndrome. Histopathology. 1990;17:478–479. doi: 10.1111/j.1365-2559.1990.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 105.Kawabata Y, Watanabe A, Yamaguchi S, Aoshima M, Shiraki A, Hatamochi A, et al. Pleuropulmonary pathology of vascular Ehlers-Danlos syndrome: spontaneous laceration, haematoma and fibrous nodules. Histopathology. 2010;56:944–950. doi: 10.1111/j.1365-2559.2010.03574.x. [DOI] [PubMed] [Google Scholar]
- 106.Ishiguro T, Takayanagi N, Kawabata Y, Matsushima H, Yoshii Y, Harasawa K, et al. Ehlers-Danlos syndrome with recurrent spontaneous pneumothoraces and cavitary lesion on chest X-ray as the initial complications. Intern Med. 2009;48:717–722. doi: 10.2169/internalmedicine.48.1818. [DOI] [PubMed] [Google Scholar]
- 107.Abrahamsen BJ, Kulseth MA, Paus B. A 19-year-old man with relapsing bilateral pneumothorax, hemoptysis, and intrapulmonary cavitary lesions diagnosed with vascular Ehlers-Danlos syndrome and a novel missense mutation in COL3A1. Chest. 2015;147:e166–e170. doi: 10.1378/chest.13-3002. [DOI] [PubMed] [Google Scholar]
- 108.Dar RA, Wani SH, Mushtaque M, Kasana RA. Spontaneous hemo-pneumothorax in a patient with Ehlers-Danlos syndrome. Gen Thorac Cardiovasc Surg. 2012;60:587–589. doi: 10.1007/s11748-012-0047-x. [DOI] [PubMed] [Google Scholar]
- 109.Watanabe A, Kosho T, Wada T, Sakai N, Fujimoto M, Fukushima Y, et al. Genetic aspects of the vascular type of Ehlers-Danlos syndrome (vEDS, EDSIV) in Japan. Circ J. 2007;71:261–265. doi: 10.1253/circj.71.261. [DOI] [PubMed] [Google Scholar]
- 110.MacCarrick G, Black JH, III, Bowdin S, El-Hamamsy I, Frischmeyer-Guerrerio PA, Guerrerio AL, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–587. doi: 10.1038/gim.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chambers JE, Dalton LE, Subramanian DN, Gooptu B, Balan A, Park SM, et al. Spontaneous pneumothorax can be associated with TGFBR2 mutation. Eur Respir J. 2015;46:1832–1835. doi: 10.1183/13993003.00952-2015. [DOI] [PubMed] [Google Scholar]
- 112.Loeys BL, Dietz HC. Loeys-Dietz syndrome. Adam MP, Ardinger, HH, Pagon RA, Wallace, SE, Bean LJH, Stephens K, et al., editors. GeneReviews. Seattle, WA: University of Washington; 2008 [updated 2018 Mar 1; accessed 2018 Dec 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1133/ [Google Scholar]
- 113.Sacharow SJ, Picker JD, Levy HL. Homocystinuria caused by cystathionine beta-synthase deficiency. Adam MP, Ardinger, HH, Pagon RA, Wallace, SE, Bean LJH, Stephens K, et al., editors. GeneReviews. Seattle, WA: University of Washington; 2004 [updated 2017 May 18; accessed 2018 Dec 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1524/ [PubMed]
- 114.Bass HN, LaGrave D, Mardach R, Cederbaum SD, Fuster CD, Chetty M. Spontaneous pneumothorax in association with pyridoxine-responsive homocystinuria. J Inherit Metab Dis. 1997;20:831–832. doi: 10.1023/a:1005384121649. [DOI] [PubMed] [Google Scholar]
- 115.Cochran FB, Sweetman L, Schmidt K, Barsh G, Kraus J, Packman S. Pyridoxine-unresponsive homocystinuria with an unusual clinical course. Am J Med Genet. 1990;35:519–522. doi: 10.1002/ajmg.1320350415. [DOI] [PubMed] [Google Scholar]
- 116.Berk DR, Bentley DD, Bayliss SJ, Lind A, Urban Z. Cutis laxa: a review. J Am Acad Dermatol. 2012;66:842, e1–17. doi: 10.1016/j.jaad.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 117.McCarthy CF, Warin RP, Read AE. Loose skin (cutis laxa) associated with systemic abnormalities. Arch Intern Med. 1965;115:62–67. doi: 10.1001/archinte.1965.03860130064011. [DOI] [PubMed] [Google Scholar]
- 118.Genevieve D, Baumann C, Huber C, Faivre L, Sanlaville D, Bodemer C, et al. A novel form of syndromic cutis laxa with facial dysmorphism, cleft palate, and mental retardation. J Med Genet. 2004;41:e77. doi: 10.1136/jmg.2003.013573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nascimento GM, Nunes CS, Menegotto PF, Raskin S, Almeida Nd. Cutis laxa: case report. An Bras Dermatol. 2010;85:684–686. doi: 10.1590/s0365-05962010000500013. [DOI] [PubMed] [Google Scholar]
- 120.Stoller JK, Lacbawan FL, Aboussouan LS. Alpha-1 antitrypsin deficiency. Adam MP, Ardinger, HH, Pagon RA, Wallace, SE, Bean LJH, Stephens K, et al., editors. GeneReviews. Seattle, WA: University of Washington; 2006 [updated 2017 Jan 19; accessed 2018 Dec 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1519/
- 121.Gishen P, Saunders AJ, Tobin MJ, Hutchison DC. Alpha 1-antitrypsin deficiency: the radiological features of pulmonary emphysema in subjects of Pi type Z and Pi type SZ: a survey by the British Thoracic Association. Clin Radiol. 1982;33:371–377. doi: 10.1016/s0009-9260(82)80297-3. [DOI] [PubMed] [Google Scholar]
- 122.Needham M, Stockley RA. Alpha 1-antitrypsin deficiency: 3. Clinical manifestations and natural history. Thorax. 2004;59:441–445. doi: 10.1136/thx.2003.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mostafavi S, Lieberman J. Intermediate alpha 1-antitrypsin deficiency with apical lung bullae and spontaneous pneumothorax: presence of a Z variant in an American black. Chest. 1991;99:1545–1546. doi: 10.1378/chest.99.6.1545. [DOI] [PubMed] [Google Scholar]
- 124.Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency--a model for conformational diseases. N Engl J Med. 2002;346:45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- 125.Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin: the Alpha-1-Antitrypsin Deficiency Registry Study Group. Am J Respir Crit Care Med. 1998;158:49–59. doi: 10.1164/ajrccm.158.1.9712017. [DOI] [PubMed] [Google Scholar]
- 126.Larsson C. Natural history and life expectancy in severe alpha1-antitrypsin deficiency, Pi Z. Acta Med Scand. 1978;204:345–351. doi: 10.1111/j.0954-6820.1978.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 127.Tanash HA, Nilsson PM, Nilsson JA, Piitulainen E. Survival in severe alpha-1-antitrypsin deficiency (PiZZ) Respir Res. 2010;11:44. doi: 10.1186/1465-9921-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin YC, Chiu WK, Chang H, Cheng YL, Chen JC. Spontaneous pneumothorax in flight as first manifestation of alpha-1 antitrypsin deficiency. Aviat Space Environ Med. 2008;79:704–706. doi: 10.3357/asem.2224.2008. [DOI] [PubMed] [Google Scholar]
- 129.Lepiorz M, Großer C, Hofmann HS, Pfeifer M. A rare cause of a spontaneous pneumothorax [in German] Pneumologie. 2017;71:590–593. doi: 10.1055/s-0043-112886. [DOI] [PubMed] [Google Scholar]
- 130.Sakula A. Antitrypsin deficiency in lung disease. Lancet. 1970;1:302–303. doi: 10.1016/s0140-6736(70)90668-9. [DOI] [PubMed] [Google Scholar]
- 131.Eriksson S. Studies in alpha 1-antitrypsin deficiency. Acta Med Scand Suppl. 1965;432:1–85. [PubMed] [Google Scholar]
- 132.Pawlowicz A, Droszcz W. Pulmonary function and alpha-1-antitrypsin levels in patients after so-called idiopathic spontaneous pneumothorax. Bull Eur Physiopathol Respir. 1987;23:1–4. [PubMed] [Google Scholar]
- 133.Serapinas D, Obrikyte V, Vaicius D, Balciuviene R, Valavicius A, Sakalauskas R. Alpha-1 antitrypsin deficiency and spontaneous pneumothorax: possible causal relationship. Pneumologia. 2014;63:32–35. [PubMed] [Google Scholar]
- 134.Janus ED, Phillips NT, Carrell RW. Smoking, lung function, and alpha 1-antitrypsin deficiency. Lancet. 1985;1:152–154. doi: 10.1016/s0140-6736(85)91916-6. [DOI] [PubMed] [Google Scholar]
- 135.Kioumis IP, Zarogoulidis K, Huang H, Li Q, Dryllis G, Pitsiou G, et al. Pneumothorax in cystic fibrosis. J Thorac Dis. 2014;6:S480–S487. doi: 10.3978/j.issn.2072-1439.2014.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest. 2005;128:720–728. doi: 10.1378/chest.128.2.720. [DOI] [PubMed] [Google Scholar]
- 137.Hafen GM, Ukoumunne OC, Robinson PJ. Pneumothorax in cystic fibrosis: a retrospective case series. Arch Dis Child. 2006;91:924–925. doi: 10.1136/adc.2006.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Spector ML, Stern RC. Pneumothorax in cystic fibrosis: a 26-year experience. Ann Thorac Surg. 1989;47:204–207. doi: 10.1016/0003-4975(89)90269-5. [DOI] [PubMed] [Google Scholar]
- 139.Rich RH, Warwick WJ, Leonard AS. Open thoracotomy and pleural abrasion in the treatment of spontaneous pneumothorax in cystic fibrosis. J Pediatr Surg. 1978;13:237–242. doi: 10.1016/s0022-3468(78)80393-5. [DOI] [PubMed] [Google Scholar]
- 140.Flume PA. Pneumothorax in cystic fibrosis. Chest. 2003;123:217–221. doi: 10.1378/chest.123.1.217. [DOI] [PubMed] [Google Scholar]
- 141.McLaughlin FJ, Matthews WJ, Jr, Strieder DJ, Khaw KT, Schuster S, Shwachman H. Pneumothorax in cystic fibrosis: management and outcome. J Pediatr. 1982;100:863–869. doi: 10.1016/s0022-3476(82)80502-7. [DOI] [PubMed] [Google Scholar]
- 142.De Rose AF, Giglio M, Gallo F, Romano L, Carmignani G. Congenital bilateral absence of the vasa deferentia and related respiratory disease. Arch Ital Urol Androl. 2003;75:214–216. [PubMed] [Google Scholar]
- 143.Castellani C, Quinzii C, Altieri S, Mastella G, Assael BM. A pilot survey of cystic fibrosis clinical manifestations in CFTR mutation heterozygotes. Genet Test. 2001;5:249–254. doi: 10.1089/10906570152742317. [DOI] [PubMed] [Google Scholar]
- 144.Henry M, Arnold T, Harvey J Pleural Diseases Group, Standards of Care Committee, British Thoracic Society. BTS guidelines for the management of spontaneous pneumothorax. Thorax. 2003;58:ii39–ii52. doi: 10.1136/thorax.58.suppl_2.ii39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Curtis HJ, Bourke SJ, Dark JH, Corris PA. Lung transplantation outcome in cystic fibrosis patients with previous pneumothorax. J Heart Lung Transplant. 2005;24:865–869. doi: 10.1016/j.healun.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 146.Rosenblatt RL. Lung transplantation in cystic fibrosis. Respir Care. 2009;54:777–786. [Discussion, pp. 786–787.]. doi: 10.4187/002013209790983197. [DOI] [PubMed] [Google Scholar]
- 147.Flume PA, Mogayzel PJ, Jr, Robinson KA, Rosenblatt RL, Quittell L, Marshall BC Clinical Practice Guidelines for Pulmonary Therapies Committee; Cystic Fibrosis Foundation Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: pulmonary complications: hemoptysis and pneumothorax. Am J Respir Crit Care Med. 2010;182:298–306. doi: 10.1164/rccm.201002-0157OC. [DOI] [PubMed] [Google Scholar]
- 148.Balasubramanian M, Shearing E, Smith K, Chavasse R, Taylor R, Tatton-Brown K, et al. Pneumothorax from subpleural blebs-a new association of Sotos syndrome? Am J Med Genet A. 2014;164A:1222–1226. doi: 10.1002/ajmg.a.36406. [DOI] [PubMed] [Google Scholar]
- 149.Fukazawa T, Sasaki H, Kikuchi S, Hamada K, Hamada T, Tashiro K. Spinocerebellar ataxia type 1 and familial spontaneous pneumothorax. Neurology. 1997;49:1460–1462. doi: 10.1212/wnl.49.5.1460. [DOI] [PubMed] [Google Scholar]
- 150.Stanley SE, Chen JJ, Podlevsky JD, Alder JK, Hansel NN, Mathias RA, et al. Telomerase mutations in smokers with severe emphysema. J Clin Invest. 2015;125:563–570. doi: 10.1172/JCI78554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Witkop CJ, Jr, White JG, King RA, Dahl MV, Young WG, Sauk JJ., Jr Hereditary mucoepithelial dysplasia: a disease apparently of desmosome and gap junction formation. Am J Hum Genet. 1979;31:414–427. [PMC free article] [PubMed] [Google Scholar]
- 152.Tsai AP, English JC, Murphy D, Sin DD. Recurrent pneumothorax related to diffuse dendriform pulmonary ossification in genetically predisposed individual. Respirol Case Rep. 2016;5:e00211. doi: 10.1002/rcr2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.MacDuff A, Arnold A, Harvey J BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65:ii18–ii31. doi: 10.1136/thx.2010.136986. [DOI] [PubMed] [Google Scholar]
- 154.Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, et al. AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590–602. doi: 10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- 155.Menko FH, Johannesma PC, van Moorselaar RJ, Reinhard R, van Waesberghe JH, Thunnissen E, et al. A de novo FLCN mutation in a patient with spontaneous pneumothorax and renal cancer; a clinical and molecular evaluation. Fam Cancer. 2013;12:373–379. doi: 10.1007/s10689-012-9593-8. [DOI] [PubMed] [Google Scholar]
- 156.Gupta N, Langenderfer D, McCormack FX, Schauer DP, Eckman MH. Chest computed tomographic image screening for cystic lung diseases in patients with spontaneous pneumothorax is cost effective. Ann Am Thorac Soc. 2017;14:17–25. doi: 10.1513/AnnalsATS.201606-459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gupta N. Primary spontaneous pneumothorax: looking beyond the usual. Acad Emerg Med. 2018;25:470–472. doi: 10.1111/acem.13363. [DOI] [PubMed] [Google Scholar]
- 158.Kunogi M, Kurihara M, Ikegami TS, Kobayashi T, Shindo N, Kumasaka T, et al. Clinical and genetic spectrum of Birt-Hogg-Dube syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J Med Genet. 2010;47:281–287. doi: 10.1136/jmg.2009.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.DeMartino ES, Wieland CN, Allen MS, Utz JP. What pneumothoraces and fibrofolliculomas convey: Birt-Hogg-Dubé. Am J Respir Crit Care Med. 2016;194:634–635. doi: 10.1164/rccm.201603-0508IM. [DOI] [PubMed] [Google Scholar]
- 160.De Maio F, Fichera A, De Luna V, Mancini F, Caterini R. Orthopaedic aspects of Marfan syndrome: the experience of a referral center for diagnosis of rare diseases. Adv Orthop. 2016;2016:8275391. doi: 10.1155/2016/8275391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sridhar J, Chang JS. Marfan’s syndrome with ectopia lentis. N Engl J Med. 2017;377:1076. doi: 10.1056/NEJMicm1406002. [DOI] [PubMed] [Google Scholar]
- 162.Sobey G. Ehlers-Danlos syndrome: how to diagnose and when to perform genetic tests. Arch Dis Child. 2015;100:57–61. doi: 10.1136/archdischild-2013-304822. [DOI] [PubMed] [Google Scholar]
- 163.Vilacosta I, Cañadas Godoy V. Images in clinical medicine: bifid uvula and aortic aneurysm. N Engl J Med. 2008;359:e2. doi: 10.1056/NEJMicm070582. [DOI] [PubMed] [Google Scholar]
- 164.Stoller JK, Aboussouan LS. A review of α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185:246–259. doi: 10.1164/rccm.201108-1428CI. [DOI] [PubMed] [Google Scholar]
- 165.Morava E, Guillard M, Lefeber DJ, Wevers RA. Autosomal recessive cutis laxa syndrome revisited. Eur J Hum Genet. 2009;17:1099–1110. doi: 10.1038/ejhg.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sweetser DA, Lin AE, Troulis MJ, Chen TC, Westra SJ. Case 34-2016: a 17-year-old boy with myopia and craniofacial and skeletal abnormalities. N Engl J Med. 2016;375:1879–1890. doi: 10.1056/NEJMcpc1610096. [DOI] [PubMed] [Google Scholar]
- 167.Kaliaperumal S, Kumar KP, Bhuvaneshwari Varied phenotypic presentations of homocystinuria in two siblings. Indian J Ophthalmol. 2014;62:93–94. doi: 10.4103/0301-4738.126190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Northrup H, Krueger DA International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243–254. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ha SM, Yoon HK, Lee SK. Rare lung manifestation of multifocal micronodular pneumocyte hyperplasia in a teenage girl with tuberous sclerosis complex. J Korean Soc Radiol. 2016;75:133–137. [Google Scholar]
- 170.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 171.Morris AA, Kožich V, Santra S, Andria G, Ben-Omran TI, Chakrapani AB, et al. Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis. 2017;40:49–74. doi: 10.1007/s10545-016-9979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sandhaus RA, Turino G, Brantly ML, Campos M, Cross CE, Goodman K, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis (Miami) 2016;3:668–682. doi: 10.15326/jcopdf.3.3.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: cystic fibrosis foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.