Abstract
Plants grow under threats of environmental changes that could injure cellular viability and damage whole-plant physiology. To defend themselves against such threats, plants induce protective responses, including the production of defense molecules. The red/purple pigment anthocyanin is synthesized upon leaf and fruit development as well as environmental stimuli such as excess light exposure. Therefore, the anthocyanin biosynthesis is considered as a model signaling pathway of the integration of developmental and environmental responses. This integration is tightly regulated by transcription factors, but the integrative mode of these signaling pathways has received little attention. In this study, using an Arabidopsis mutant with mutation in two ETHYLENE RESPONSE FACTOR (ERF) genes, AtERF4 and AtERF8, we investigated the regulatory signaling pathway that leads to the production of anthocyanin in response to light. We detected the accumulation of anthocyanin in detached leaves after incubation on water under light illumination and intact leaves after being transferred into the strong light condition, suggesting that the photoinhibition mediated the production of anthocyanin. Our results demonstrated that the erf mutant decreased the rate and extent of the production of anthocyanin in association with changes of the transcript levels of anthocyanin-biosynthetic genes. As these ERF genes are known regulators of leaf senescence—the final stage of leaf development—we provide an insight into the ERF-mediated integration of two regulatory pathways of the light response and developmental age.
Keywords: anthocyanin, ERF, gene expression, light stress, transcription factors
Plants respond to environmental changes and induce various protective responses to cope with harmful environmental conditions. Transcription factors (TFs) act as a hub of many regulatory pathways in response to such environmental changes and are capable of coordinating with the developmental program of plants (Kim et al. 2016; Nakashima et al. 2014). TFs usually contribute to the changes of the expression profile of their downstream genes.
Extensive studies have revealed that plant-specific ETHYLENE RESPONSE FACTOR (ERF) transcription factors both positively and negatively regulate gene expression for protection against environmental changes (Mizoi et al. 2012; Nakano et al. 2006). The class II ERFs comprise repressors of transcription and possess the motif for this repression (Fujimoto et al. 2000; Hiratsu et al. 2003; Ohta et al. 2000, 2001). Since the class II ERFs are responsive to many environmental inputs (Fujimoto et al. 2000; Kitajima et al. 2000; McGrath et al. 2005; Nasir et al. 2005; Nishiuchi et al. 2004) as well as developmental cues (Kitajima et al. 2000; Koyama et al. 2001), we predicted that the class II ERF would integrate stress and developmental signaling pathways.
We previously reported that the class II repressors, primarily NtERF3, AtERF4, and AtERF8, regulated the expression of leaf senescence-associated genes and stimulated the onset of leaf senescence—the final stage of leaf development (Koyama et al. 2013; Koyama 2014). To further explore our previous findings, we first observed the distinctive responses to light in Arabidopsis thaliana (Arabidopsis plants) Columbia-0 and the aterf4 aterf8 mutant (Koyama et al. 2013). The seeds of these genotypes were individually grown in a plastic pot filled with soil for 4 wk at 22°C under a light/dark cycle of 16 h at 50 to 75 µmol m−2 s−1/8 h in a plant growth chamber (CFH-415, TOMY).
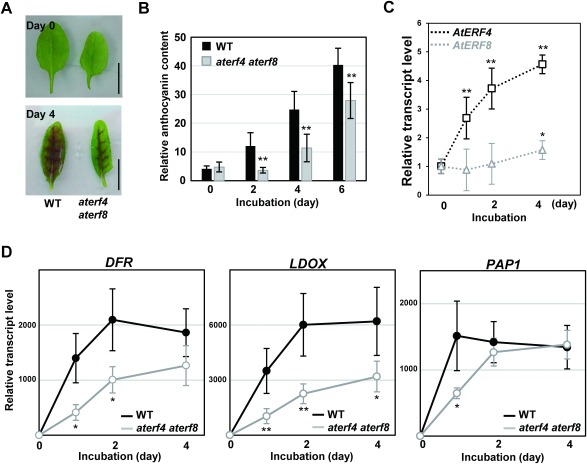
In the light condition at 50 µmol m−2 s−1, intact leaves of wild type (WT) remained green, whereas the detached leaves at the sixth position after incubation on water for four days changed color from green to purple (Figure 1A). This contrasted a senescence response, as detached leaves change their color to yellow after incubation in dark. Since aterf4 aterf8 delayed the progression of leaf senescence (Koyama et al. 2013), we investigated whether detached leaves of the mutant might also delay responses to light. We assessed the phenotype of the detached leaves of aterf4 aterf8 after incubation on water under the control light condition. Our results demonstrated that detached aterf4 aterf8 leaves delayed the induction of pigmentation (Figure 1A).
Figure 1. Light responses in detached WT and aterf4 terf8 leaves during incubation under the moderate light illumination. (A) Photographs of the detached WT and aterf4 terf8 leaves after incubation of indicated days. Bars=1 cm. (B) Measurement of the relative anthocyanin content in the detached WT and aterf4 aterf8 leaves. The values are presented as arbitrary units and error bars indicate standard deviation of seven biological replicates. Asterisks represent significant differences compared with the WT values by a Student’s t-test (**p<0.01). (C) The transcript levels of AtERF4 and AtERF8 in the detached WT leaves after incubation of the indicated number of days. Error bars indicate standard deviation of four biological replicates. Asterisks represent significant differences compared with the values at Day 0 by a Student’s t-test (*p<0.05, **p<0.01). (D) The transcript levels of DFR, LDOX and PAP1 in the detached leaves of WT and aterf4 aterf8 after incubation of the indicated number of days. Error bars indicate standard deviation of four biological replicates. Asterisks represent significant differences compared with the WT values by a Student’s t-test (*p<0.05, **p<0.01).
To measure the relative anthocyanin content, the sixth leaves of four-week-old Arabidopsis plants were extracted by methanol containing HCl (1% v/v) at 4°C. The relative anthocyanin content was determined using spectrophotometric analysis and was normalized by the fresh weight of leaves (Laby et al. 2000). As expected, the detached WT leaves increased the content of anthocyanin in response to light, but the detached aterf4 aterf8 leaves exhibited a delayed production of anthocyanin (Figure 1B). As detached leaves floated on water usually suffer from a photoinhibition response (Kato et al. 2002; Nishiyama et al. 2006), it is possible that the photoinhibition mediated the production of anthocyanin.
Our gene expression analysis demonstrated the rapid and moderate increase in the levels of AtERF4 and AtERF8 transcripts, respectively (Figure 1C). To prepare RT-PCR samples, total RNA was prepared from Arabidopsis leaves detached at the sixth position using an RNAeasy Plant Mini Kit (Qiagen) and was reverse-transcribed with poly-dT primers (Invitrogen) using SuperScript II (Life Technologies). Aliquots of the complementary DNAs were amplified using the iQ CYBR Green PCR Supermix (Bio-Rad) with a real-time PCR system (CFX96, Bio-Rad) and the appropriate primer sets for AtERF4 (GGT GCA TGT TGC GAC GAA GAT and CTC CAT CCC ACC TTC GAA ATC A) and AtERF8 (CGT AAG ATC CCG CTT GTG CAT and CCA CAC GTC GTC ATC TTT GGA). The transcript levels were determined using standard curves derived from a reference sample and were normalized to the UBIQUITIN1 level, amplified using the set of primers (TGA GCC TTC CTT GAT GAT GCT and GCA CTT GCG GCA AAT CAT CT). The values of the WT at the initiating time of the incubation were set as 1.
We also demonstrated that the detached WT leaves under the control light condition markedly increased the levels of transcripts of anthocyanin biosynthetic enzyme genes, DIHYDROFLAVONOL 4-REDUCTASE (DFR) and LEUCOANTHOCYANIDIN DIOXYGENASE (LDOX), and their transcriptional activator gene PRODUCTION OF ANTHOCYANIN PIGMENT1 (PAP1), as revealed by our RT-PCR analysis using the primer sets for DFR (CGT GGC AAC ACC CAT GGA TTT and CTT CGT ACG GTC TTT GCC TTA ACA), LDOX (TGG CCT AAG ACA CCA AGT GAT TAC and ACC GAC AGA GAG AGC CTT GAA) and PAP1 (GGT GCT TGG ACT ACT GAA GAA GAT and ACC GGT TTA GCC CAG CTC TTA), respectively (Figure 1D). In contrast, the rate and extent of their light-inducible expression significantly decreased in the aterf4 aterf8 leaves (Figure 1D). These results demonstrated that the aterf4 aterf8 mutations reduced the light-responsive anthocyanin biosynthesis.
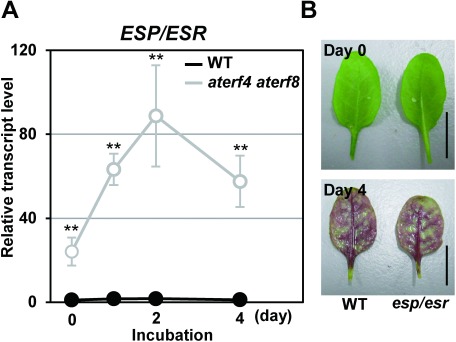
We previously reported that AtERF4 and AtERF8 directly target EPITHIOSPECIFIER PROTEIN/EPITHIOSPECIFYING SENESCENCE REGULATOR (ESP/ESR) gene for the progression of leaf senescence (Koyama et al. 2013). Our RT-PCR analysis, using the primer pair for ESP/ESR (GTG AGG TAT GGC CTG ATC TC and TCC AAC GCA TAT CCC TCA TTG GA), showed that detached aterf4 aterf8 leaves exhibited a remarkable increase in ESP/ESR transcripts throughout the incubation under light illumination (Figure 2A). This demonstrated that the aterf4 aterf8 mutation released the repression of ESP/ESR gene. In contrast, the esp/esr mutant (SALK_055029C; Miao and Zentgraf 2007) accumulated purple pigments to a similar extent as WT (Figure 2B). These results suggested that the delayed accumulation of anthocyanin in aterf4 aterf8 mutants was independent from the function of the ESP/ESR gene.
Figure 2. ESP/ESR gene acts downstream of AtERF4 and AtERF8 but is independent on the light-mediated accumulation of anthocyanin. (A) The transcript level of the ESP/ESR gene in the detached WT leaves and aterf4 aterf8 after incubation of the indicated number of days. Error bars indicate standard deviation of four biological replicates. Asterisks represent significant differences compared with the WT values by a Student’s t-test (**p<0.01). (B) Photographs of the detached WT and esp/esr leaves after incubation of indicated days. Bars=1 cm.
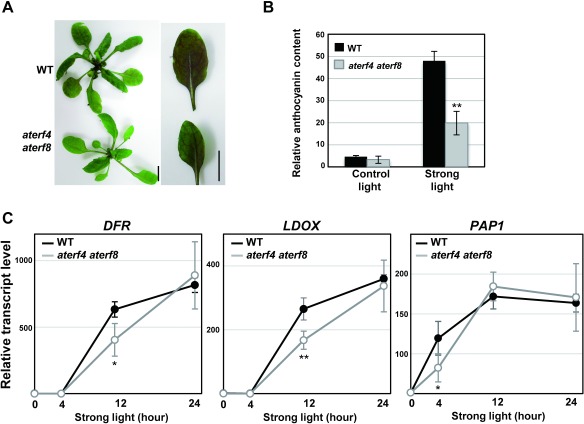
Since a strong light condition stimulates the photoinhibition of plants (Nishiyama et al. 2006), we analyzed the phenotypes of intact WT and aterf4 aterf8 plants under the strong light condition at 300 µmol m−2 s−1 after the 4-wk growth period under the light condition at 50 to 75 µmol m−2 s−1. The intact WT leaves quickly changed color from green to purple after being transferred into the strong light condition and, thus, increased their anthocyanin content. In contrast, the intact aterf4 aterf8 leaves exhibited moderate color change and accumulation of anthocyanin (Figure 3A, 3B). Furthermore, our RT-PCR analysis revealed that the rates of the strong light induction of anthocyanin-biosynthetic enzyme genes LDOX, DFR, and PAP1 had decreased in the intact aterf4 aterf8 leaves (Figure 3C). The PAP1 transcript was increased prior to the induction of LDOX and DFR transcripts, suggesting the PAP1 TF-mediated activation of LDOX and DFR genes.
Figure 3. Responses of intact WT and aterf4 terf8 plants after being transferred to the strong light condition. (A) Photographs of the rosettes (left) and the intact leaves (right) of WT and aterf4 terf8 at two days after being transferred to the strong light condition. Bars=1 cm. (B) Measurement of the relative anthocyanin content in the intact WT and aterf4 aterf8 leaves. The sixth leaves were harvested at 0 or 2 day after being transferred to the strong light condition. The values are presented as arbitrary units, and error bars indicate standard deviation of 12 biological replicates. Asterisks represent significant differences compared with the WT values by a Student’s t-test (**p<0.01). (C) The transcript levels of DFR, LDOX, and PAP1 in the intact leaves of WT and aterf4 aterf8 plants at indicated time points after being transferred to the strong light condition. A detailed description is presented in the legend of Figure 1D.
An important finding of this study is that AtERF4 and AtERF8 regulate the anthocyanin biosynthesis in light responses. As AtERF4 and AtERF8 act as transcriptional repressors, it is possible that that these ERFs repress the expression of a negative regulator of the anthocyanin biosynthesis. This negative regulator acts independently on the ESP/ESR gene and, thus, other genes downstream from AtERF4 and AtERF8 could mediate a signal from the light stress to the induction of anthocyanin biosynthesis. Our results also demonstrate that the levels of AtERF4 and AtERF8 transcripts are differentially increased under light stress. In addition, the activities of the class II ERF repressors are controlled in the mRNA and protein levels as observed in other signaling pathways (Koyama et al. 2003; Koyama et al. 2013; Lyons et al. 2013). Future research will illustrate the temporal and flexible modulation of the AtERF4 and AtERF8 activities, which operate under continuous stressful conditions during leaf development.
Acknowledgments
We thank the Arabidopsis Biological Resource Center for providing esp/esr seeds.
Abbreviations
- Arabidopsis
Arabidopsis thaliana
- ERF
ETHYLENE RESPONSE FACTOR
- ESP/ESR
EPITHIOSPECIFIER PROTEIN/EPITHIOSPECIFYING SENESCENCE REGULATOR
- LDOX
LEUCOANTHOCYANIDIN DIOXYGENASE
- PAP1
PRODUCTION OF ANTHOCYANIN PIGMENT1
- TF
transcription factor
- WT
wild type
References
- Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M (2000) Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12: 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsu K, Matsui K, Koyama T, Ohme-Takagi M (2003) Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J 34: 733–739 [DOI] [PubMed] [Google Scholar]
- Kato MC, Hikosaka K, Hirose T (2002) Leaf discs floated on water are different from intact leaves in photosynthesis and photoinhibition. Photosynth Res 72: 65–70 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Nam HG, Lim PO (2016) Regulatory network of NAC transcription factors in leaf senescence. Curr Opin Plant Biol 33: 48–56 [DOI] [PubMed] [Google Scholar]
- Kitajima S, Koyama T, Ohme-Takagi M, Shinshi H, Sato F (2000) Characterization of gene expression of NsERFs, transcription factors of basic PR genes from Nicotiana sylvestris. Plant Cell Physiol 41: 817–824 [DOI] [PubMed] [Google Scholar]
- Koyama T (2014) The roles of ethylene and transcription factors in the regulation of onset of leaf senescence. Front Plant Sci 5: 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Kitajima S, Sato F (2001) Expression of PR-5d and ERF genes in cultured tobacco cells and their NaCl stress-response. Biosci Biotechnol Biochem 65: 1270–1273 [DOI] [PubMed] [Google Scholar]
- Koyama T, Nii H, Mitsuda N, Ohta M, Kitajima S, Ohme-Takagi M, Sato F (2013) A regulatory cascade involving class II ETHYLENE RESPONSE FACTOR transcriptional repressors operates in the progression of leaf senescence. Plant Physiol 162: 991–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Okada T, Kitajima S, Ohme-Takagi M, Shinshi H, Sato F (2003) Isolation of tobacco ubiquitin-conjugating enzyme cDNA in a yeast two-hybrid system with tobacco ERF3 as bait and its characterization of specific interaction. J Exp Bot 54: 1175–1181 [DOI] [PubMed] [Google Scholar]
- Laby RJ, Kincaid MS, Kim D, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23: 587–596 [DOI] [PubMed] [Google Scholar]
- Lyons P, Iwase A, Gänsewig T, Sherstnev A, Duc C, Barton GJ, Hanada K, Higuchi-Takeuchi M, Matsui M, Sugimoto K, et al. (2013) The RNA-binding protein FPA regulates flg22-triggered defense responses and transcription factor activity by alternative polyadenylation. Sci Rep 3: 2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath KC, Dombrecht B, Manners JM, Schenk PM, Edgar CI, Udvardi MK, Kazan K (2005) Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression. Plant Physiol 139: 949–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Zentgraf U (2007) The antagonist function of Arabidopsis WRKY53 and ESR / ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell 19: 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) AP2 / ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta 1819: 86–96 [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140: 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (2014) The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci 5: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir KH, Takahashi Y, Ito A, Saitoh H, Matsumura H, Kanzaki H, Shimizu T, Ito M, Fujisawa S, Sharma PC, et al. (2005) High-throughput in planta expression screening identifies a class II ethylene-responsive element binding factor-like protein that regulates plant cell death and non-host resistance. Plant J 43: 491–505 [DOI] [PubMed] [Google Scholar]
- Nishiuchi T, Shinshi H, Suzuki K (2004) Rapid and transient activation of transcription of the ERF3 gene by wounding tobacco leaves. J Biol Chem 279: 55355–55361 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757: 742–749 [DOI] [PubMed] [Google Scholar]
- Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M (2001) Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Ohme-Takagi M, Shinshi H (2000) Three ethylene-responsive transcription factors in tobacco with distinct transactivation functions. Plant J 22: 29–38 [DOI] [PubMed] [Google Scholar]