Abstract
To examine the effect of the ectopic expression of three Arabidopsis genes, including WOX2, WOX8 and WOX9, on the regenerative competency of tissues and cells cultured in vitro, we developed a transgenic variety of Nicotiana tabacum, in which these genes were under the transcriptional control of a chemical-inducible expression system. We designed a two-step culture method to feasibly demonstrate the effect as follows. Leaf segments of approximately 10 mm2 were prepared from transgenic plants and their hybrids and cultured in a liquid medium based on modified Murashige and Skoog medium supplemented with an auxin, 2,4-dichrorophenoxyacetic acid and/or an expression inducer β-estradiol for 10 days in dark. The segments were subsequently cultured on a solidified medium in the absence of both the auxin and inducer in light for 3 weeks. We observed remarkable regeneration of plantlets only in segments derived from the hybrids possessing two transgenes, WOX2 combined with WOX8 or WOX9, but no regeneration in the segments derived from their parental lines. We also observed that free cells released from the hybrid explants in the liquid medium developed into embryo-like structures due to the transient application of the inducer. In a wide range of species including recalcitrants, the effect of the coexpression of these genes may be useful for developing an alternative to conventional protocols that requires cytokinin.
Keywords: Nicotiana tabacum, regeneration, WOX2, WOX8, WOX9
Introduction
The WUSCHEL related homeobox (WOX) gene family was defined in Arabidopsis thaliana and some family members; for example, WOX2, WOX8 and WOX9 showed a spatiotemporal expression pattern in the early phase of zygotic embryogenesis (Haecker et al. 2004; Palovaara et al. 2013). These genes play important roles in various developmental events, such as zygote polarization, embryonic tissue proliferation, embryonic pattern formation, stem cell niche maintenance and flower development and lateral organ development (Breuninger et al. 2008; Chung et al. 2016; Costanzo et al. 2014; Ueda and Laux 2012; Wu et al. 2007; Zhou et al. 2015). The WOX gene family is evolutionarily conserved throughout the plant kingdom (van der Graaff et al. 2009), and their orthologs also showed specific expression in zygotic and/or somatic embryogenesis in different species; for example, Vitis vinifera (Gambino et al. 2011), Populus tomentosa (Liu et al. 2014) and the gymnosperms Larix decidua (Rupps et al. 2016) and Picea abies (Hedman et al. 2013; Palovaara et al. 2010; Zhu et al. 2016), suggesting their functional conservation.
WUS was the first gene to be identified in the WOX family (Laux et al. 1996), and is expressed in the cells located at the center of the 16-cell-stage embryo and in the organizing center of the apical meristem to maintain the pluripotent stem cells (Mayer et al. 1998). This gene was rediscovered through functional screening for a regeneration-promoting (RP) gene using a LexA-VP16-estrogen receptor chimeric transcription activator system (XVE system) in A. thaliana (Zuo et al. 2002a). Such RP effects are useful for improving the transformation frequency of recalcitrant species in tissue cultures and developing a maker-free screening method (Zuo et al. 2002b). Several attempts to induce regeneration using the ectopic expression of WUS have previously been reported in some species other than A. thaliana, such as N. tabacum (Rashid et al. 2007), Coffea canephora (Arroyo-Herrera et al. 2008), Capsicum chinense (Solís-Ramos et al. 2009), white spruce (Klimaszewska et al. 2010) and cotton (Bouchabké-Coussa et al. 2013). However, it remains to be clarified whether the RP effect of WUS is applicable to more species.
The RP effect of WOX2, WOX8 and WOX9 on explants cultured in vitro remains unknown. Here, we described a two-step culture system using tobacco leaf segments to estimate the RP effect of transgenes and determine remarkable instances where the coexpression of WOX gene pairs confer this RP effect on the explants. Additionally, we showed that the coexpression was effective on free cell populations derived from leaf explants, which promoted the development of embryo-like structures.
Materials and methods
Vectors and bacteria
Total RNA was prepared from an inflorescence of A. thaliana (Columbia) using TRIzol (Life Technologies, Carlsbad, CA) and used for cDNA synthesis using M-MuLV reverse transcriptase (New England Biolabs, Ipswich, MA) according to the manufacturers' manuals. The cDNAs corresponding to WOX2, WOX8 and WOX9 were amplified by RT-PCR using PrimeStar MAX DNA polymerase (Takara, Kusatsu, Shiga) and primer sets containing sequences for generating restriction sites shown in lower case as follows: 5′-acacacctcagATG GAA AAC GAA GTA AAC GCA GGA A and 5′-tctctcactagtTTA CAA CCC ATT ACC ATT ACT ATC CA for WOX2, 5′-acacacgggcccATG TCC TCC TCA AAC AAA AAT TGG C and 5′-tctctcactagtCTA AAT AAG ATA ATA GAT TGC GCC GT for WOX8 and 5′-acacacctcagATG GCT TCT TCG AAT AGA CAC TGG and 5′-tctctcactagtCTA GAT CAG ATA GTA CGA GGC TC for WOX9. The amplified cDNAs for WOX2, WOX8 and WOX9 were digested with XhoI and SpeI, ApaI and SpeI, and XhoI and SpeI, respectively. The resulting fragments were ligated with pER8 (Zuo et al. 2000) digested with XhoI and SpeI or ApaI and SpeI. The three constructs were used for the transformation of XL1blue (Agilent Technology, Santa Clara, CA) on a medium with 100 mg L−1 spectinomycin and the reproduced plasmids were sequenced to confirm whether their T-DNA regions were designed. These plasmids harboring each WOX gene under the transcriptional control of the XVE system were referred to as pER8::WOX2, pER8::WOX8 and pER8::WOX9.
Rhizobium radiobacter AGL1 (ATC C BAA-101) that lacks RecA activity (Lazo et al. 1991) was purchased from Summit Pharmaceuticals International Corporation (Chuoku, Tokyo) and maintained according to the product sheet. The bacteria were transformed with the plasmids using an electroporator (Electro Cell Manipulator 600, BTX, San Diego, CA) under a pulse condition (1.25 kV, 129 ohm and 5.5 µF) in 50 µL of water containing 1 ng of plasmid and placed in a cuvette with 1 mm electrode distance. Transformants were screened on LB medium containing 100 mg L−1 spectinomycin and 2% agar. The bacterial lines that were confirmed to be carrying the above-mentioned constructs were cultured overnight in LB medium with 100 mg L−1 spectinomycin and used for plant transformation.
Plant transformation
For the basal medium for plant growth and leaf segment culture, we used the modified Murashige and Skoog medium (mMS; Murashige and Skoog 1962) that lacked growth regulators, KI, glycine, pyridoxine and nicotinic acid, but contained thiamine at a concentration of 1 mg L−1.
Leaves of young N. tabacum cv. Samsun were surface-sterilized, cut into segments (50 mm2) and placed in 10 mL of liquid mMS. Agroinfection was performed by adding 10 µL of overnight culture (LB medium) of transformed bacteria to the 10 mL mMS containing the leaf segments. After 16 h, the segments were rinsed with fresh medium and placed on filter papers to eliminate excess medium containing bacteria and then cultured on solidified mMS containing 10 µM of 6′-benzyladenine (BA) at 25°C in dark. To avoid the excess proliferation of bacteria, the medium was replaced with fresh medium every day for 3 days. If necessary, the segments were rinsed in liquid mMS to eliminate the excess bacteria. Subsequently, the segments were cultured on solidified mMS containing 10 µM of BA, 0.1 µM of 1-naphthaleneacetic acid (NAA), 200 mg L−1 of cefotaxime for suppressing bacterial proliferation and 50 mg L−1 of hygromycin B for screening tobacco transformants at 24–26°C in light. After 1 month, adventitious buds generated on the segments were excised and placed on solidified mMS containing 200 mg L−1 of cefotaxime, 0.1 µM of NAA and 50 mg L−1 of hygromycin B for rooting. The transformants were grown in a closed phytotron equipped only for transgenic plants (23–26°C, natural light) and their progeny and hybrids were obtained by selfing or crossing. For the demonstrative experiments, one individual per transgene was chosen and referred to as XVE::WOX2, XVE::WOX8 and XVE::WOX9. The hybrid lines between the transformants were referred to as follows: e.g., XVE::WOX2×XVE::WOX8.
Leaf segment culture for estimating the RP effect of transgenes
The details of methods and processes used for inducing regeneration are described in the Results section. The expression inducer 17-β-estradiol (Sigma-Aldrich, St. Louis, MO) used for activating the transcription factor XVE was solubilized in dimethyl sulfoxide (DMSO) at a concentration of 10 mM and sterilized by filtration using an Omni pore membrane (Merck Millipore, Burlington, MA).
Expression analysis
Using TRIzol, RNA was extracted from leaf segments cultured in mMS containing the expression inducer at concentrations of 0, 1, or 10 µM and used for cDNA synthesis using M-MuLV reverse transcriptase. The cDNA reaction mixtures were diluted 10 times with water, added to GoTaq Master Mix (Promega, Madison, WI, USA) and analyzed on agarose gel electrophoresis. Primer sequences for each transgene are described above. In expression analyses of the hybrid plants, PCR was performed by adding two sets of primer in one reaction mixture to reduce the total number of reactions.
Results
Through the agroinfection and subsequent culture, we obtained several transgenic plants for WOX2, WOX8 and WOX9 and their seeds by selfing or crossing. After expression analysis and preliminary experiments of tissue culture, we chose one transgenic plant per gene that showed a relatively high level of transgene expression in response to the inducer: XVE::WOX2, XVE::WOX8 and XVE::WOX9. Their seeds were aseptically sown on solidified mMS supplemented with 0.8% agar, 50 mg L−1 of hygromycin B and 0 or 1 µM of β-estradiol.
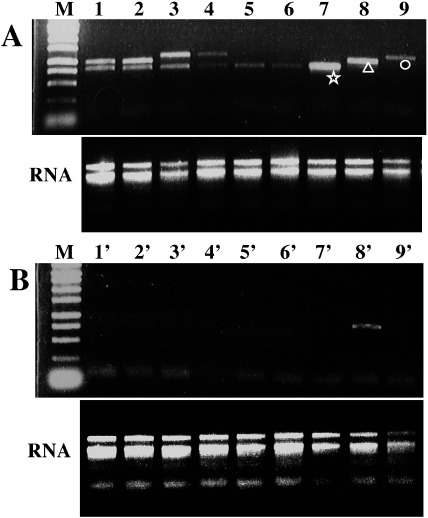
Morphological anomalies were not evident in the progenies of XVE::WOX8 (Figure 1B), XVE::WOX9 (Figure 1C) and hybrids of XVE::WOX8×XVE::WOX9 (data not shown) in the presence or absence of the inducer. However, different types of anomalies were found in the progenies of XVE::WOX2 and two hybrid lines (XVE::WOX2×XVE::WOX8 and XVE::WOX2×XVE::WOX9) in the presence of the inducer. The representative morphology in each transgenic population is shown in Figure 1: dwarfing in XVE::WOX2 progenies (Figure 1A), bulbous roots with shoot regeneration in XVE::WOX2×XVE::WOX8 (Figure 1D), roots with filamentous shoot bud-like structures (three plants centered in Figure 1E) and bulbous roots (two plants on the right side in Figure 1E) in XVE::WOX2×XVE::WOX9. Bulbous roots were also found in the back cross line of XVE::WOX2 (data not shown), suggesting that this phenotype resulted from the expression of WOX2 in the hemizygous genotype. In fact, the expression analysis shown in Figure 5 indicates that the two plants on the right side in Figure 1E expressed only WOX2 in the presence of the inducer.
Figure 1. Effect of expression of the transgenes on seedling morphology. (A) to (C) The seeds of each transgenic individual [XVE::WOX2 (A), XVE::WOX8 (B) and XVE::WOX9 (C)] were obtained through self-pollination and sown on mMS with 0.8% agar, 50 mg L−1 of hygromycin B and 0 or 1 µM of β-estradiol, and then kept in day-neutral light conditions at 25°C for 3 weeks. (D, E) The seeds of two hybrid lines were obtained by crossing XVE::WOX2 with XVE::WOX8 (D) or XVE::WOX9 (E) and sown as described above. (−) The two plants placed on the left in each panel were grown in the absence of the expression inducer as controls. (+) The other plants were grown in the presence of the inducer at the concentration of 1 µM. In the expression analysis (Figure 5), the two plants on the right in panel D and three plants in the center in panel E were proved to possess both transgenes WOX2 and WOX8 and WOX2 and WOX9, respectively. The dotted line in panel E means the boundary of the original images.
Figure 5. RT-PCR analysis of transgenes expressed in the first culture. Total RNA was extracted from the leaf segments cultured for 2 day in the presence (A) or absence (B) of the expression inducer. The pattern of RT-PCR products and total RNA are shown in the top and bottom in each panel, respectively. Lanes 1, 1′, 2, 2′, hybrids of XVE::WOX2×XVE::WOX8; lanes 3, 3′, 4, 4′, hybrids of XVE::WOX2×XVE::WOX9; lanes 5, 5′, 6, 6′, siblings of XVE::WOX2×XVE::WOX9 but not hybrid; lanes 7, 7′, progeny of XVE::WOX2; lanes 8, 8′, progeny of XVE::WOX8; lanes 9, 9′, progeny of XVE::WOX9; M, 250 bp ladder marker. Each reaction mix for 1–6 and 1′–6′ contained two sets of primers for the expected transgenes. The asterisk, triangle and circle indicate the amplified products corresponding to the coding sequences of WOX2, WOX8 and WOX9, respectively.
The aerial parts of the plants with anomalies in their roots (Figure 1) were excised and placed on solidified mMS for normal rooting. After several days, the plants that began rooting were replaced and aseptically grown on soil in vitro at 27–25°C under day-neutral conditions (12 h light/12 h dark) for 1 month. The resulting plants were used as materials for the leaf segment culture illustrated in Figure 2. For the progenies of XVE::WOX2, we used the plants grown in the absence of the inducer because plants with the dwarf phenotype (Figure 1A) did not recover their normal morphology in the subsequent culture in the absence of the inducer.
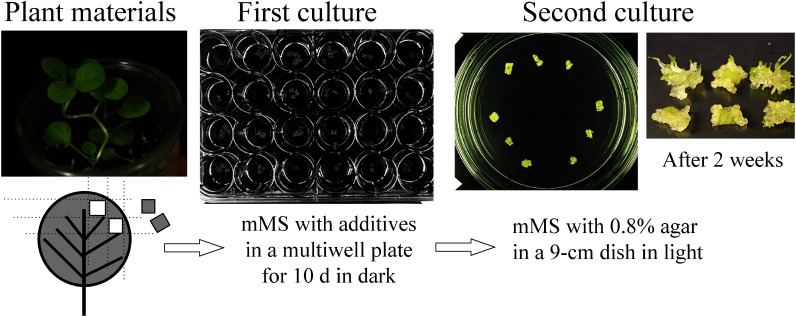
Figure 2. A two-step culture system to estimate the RP effect of transgenes. Small adult leaves of young plants grown aseptically were cut to approximately 10 mm2 and placed in liquid mMS to be treated with 2,4-D (0 to 1 µM) and β-estradiol (0 to 10 µM) (first culture). After 10 days, explants were placed onto solidified mMS without the auxin and the inducer. And they were kept in light (second culture) for 2–4 weeks. The cultures were replaced and arranged on a dish for recording the morphological traits as shown in Figure 3.
Using preliminary experiments, we designed a two-step culture method (Figure 2) to observe the effect of transgenes on the regenerative response. From young plants grown aseptically, adult round leaves (1–1.5 cm in diameter) were cut into segments of approximately 10 mm2 and placed in liquid mMS containing 1 µM of 2,4-dichlorophenoxyacetic acid (2,4-D) and inducer at a concentration of 0, 1, or 10 µM prepared in multiwell plates. Five leaf segments were placed on 0.4 mL of medium per well and incubated in dark at 25°C. This culture period was referred to as the first culture. After 2 days, two segments among the five were picked out from each well and used for the expression analysis as described later. Ten days after the beginning of the first culture, the remaining explants were rinsed in liquid mMS, blotted on filter papers, placed on solidified mMS and incubated in day-neutral light conditions (12 h light/12 h dark) at 25°C. This culture period was referred to as the second culture (Figure 2). After 2–4 weeks, the cultures from each individual were replaced and arranged on a dish for recording morphological traits as shown in Figure 3.
Figure 3. Effect of expression of the transgenes on morphogenesis in leaf segment culture. In each panel, the three cultures in the left, central and right columns were treated with the inducer at a concentration of 0, 1 and 10 µM, respectively, in the first culture. (A) to (C) Explants were prepared from a progeny of the transgenic individuals XVE::WOX2, XVE::WOX8 and XVE::WOX9, respectively. (D, E) Explants were prepared from hybrids by crossing between XVE::WOX2 and XVE::WOX8 and XVE::WOX2 and XVE::WOX9, respectively. (F) Explants were prepared from one of the siblings by crossing between XVE::WOX2 and XVE::WOX9, possessing the single WOX2 transgene.
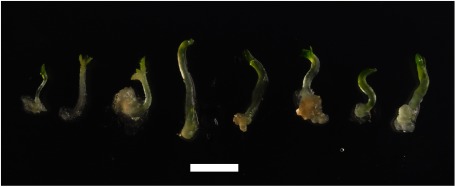
In the culture of leaf segments from XVE::WOX2×XVE::WOX8 and XVE::WOX2×XVE::WOX9, we found many shoots accompanied with calluses generated on the explants treated with the inducer at a concentration of 1 or 10 µM in the first culture (Figure 3D, E). In the early stage of the second culture, the regenerants were easily removed from the cultured tissues, suggesting that they were not tightly connected to the original tissue (Figure 4). They possessed no root, but showed vigorous growth and root development on solidified mMS (data not shown). No regeneration was observed in the progenies of XVE::WOX2, XVE::WOX8 and XVE::WOX9 (Figure 3A to C). In the explants from the progenies of XVE::WOX2, the growth of the callus was remarkable in response to the inducer but green spots were not observed (Figure 3A). An identical response was observed in the culture from one of the two plants on the right side in Figure 1E that possesses the single transgene WOX2 (Figure 3F). The explants treated with or without the inducer in the first culture of the XVE::WOX8 or XVE::WOX9 progeny (Figure 3B, C) showed no morphological features that were similar to the seedlings (Figure 1B, C). A similar result was observed in the cultures from the hybrid XVE::WOX8×XVE::WOX9 and also wild type (data not shown).
Figure 4. Morphology of the plantlets separated from the cultures developing regenerants in the second culture. The original explants were derived from the hybrid by crossing between XVE::WOX2 and XVE::WOX8. Note the hypocotyl-like structure in each plantlet. Bar, 5 mm.
For the expression analysis by RT-PCR, some leaf segments in the first culture were picked from the multiwell plates and used for RNA preparation. The expression levels of the transgenes in the presence and absence of the inducer are shown in Figure 5A and B, respectively. As shown at the bottom of each panel, the total RNA from each sample showed major bands of ribosomal RNA and was thus suitable for RT-PCR. In the absence of the inducer (Figure 5B), the expression levels of transgenes were very low (lanes 1′–7′ and 9′), except lane 8′. We speculated that this was due to occasional carryover of a small amount of the inducer from the medium for sowing. The explants from the progenies of XVE::WOX2, XVE::WOX8 and XVE::WOX9 showed single bands corresponding to the transcripts for WOX2, WOX8, and WOX9, respectively (Figure 5A, lanes 7–9). In case of hybrids (Figure 5A, lanes 1–4), two bands were detected in each lane, corresponding to the transcripts for WOX2 and WOX8 (lanes 1 and 2) or WOX2 and WOX9 (lanes 3 and 4). In case of two of the three plants shown in the center of Figure 1E, two bands were detected in each lane, corresponding to the transcripts for WOX2 and WOX9 (Figure 5A, lanes 3 and 4). In two plants possessing bulbous roots (shown on the right side of Figure 1E), only a single band corresponding to the transcripts for WOX2, but not WOX9, was detected (Figure 5A, lanes 5 and 6). This indicated that the differences in seedling morphology between the dwarf plants (Figure 1A) and those possessing bulbous roots (Figure 1E) were probably due to WOX2 transcription at different expression levels.
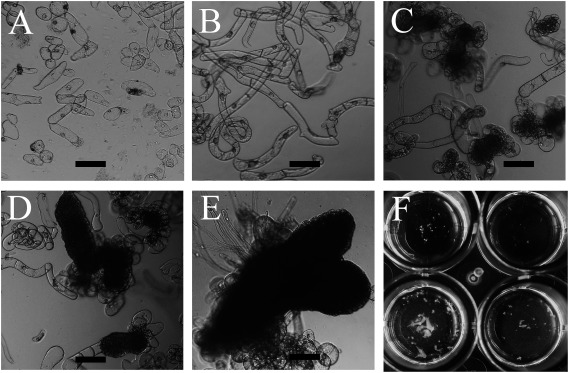
In the culture of leaf segments, we occasionally found a small number of single cells in the basal medium (mMS with 1 µM of 2,4-D) of the first culture after transferring the explants to the second culture (Figure 6A). These cells seemed to be derived from the mesophyll tissue or root primordia developed around vascular bundles in the explants. We continued culturing for 1 month, during which the single cells proliferated and generated free cell populations, showing an aspect similar to tobacco BY-2 cells (Nagata et al. 1992), although with a much lower division rate (Figure 6B). Such free cell population was observed in the culture of each transgenic plant. To observe their response to the expression inducer, we added an equal volume of fresh basal medium (mMS with 1 µM of 2,4-D) to the culture and divided it into two wells that already contained one thousandth volume of a β-estradiol (1 mM in DMSO) or DMSO (control) drop; the final concentrations of β-estradiol and DMSO were 1 µM and 0.1%, respectively. After incubating these cultures at 25°C in dark for 2 weeks, we found cell clusters with high optical density only in the presence of the inducer (Figure 6C). Some cluster surfaces became smooth and compacted due to the reduction of cell size accompanying active cell division (Figure 6D). The generation of cell cluster was observed in the culture of the XVE::WOX2×XVE::WOX8 hybrid and XVE::WOX2×XVE::WOX9 hybrid, but not XVE::WOX2, XVE::WOX8 and XVE::WOX9 progeny.
Figure 6. Aspects of development of embryo-like structures from free cells derived from leaf explants of XVE::WOX2×XVE::WOX8 hybrid. (A) Free cells released from leaf segments in the first culture (mMS with 1 µM of 2,4-D). (B) Cell growth and division were observed in the subsequent culture. (C) Cell clusters were generated after adding the expression inducer. (D) Cell clusters with a smooth surface appeared in the subsequent culture. (E) Embryo-like structures developed in the process of diluting the medium with fresh mMS. (F) Aspects of the culture wells with developing plantlets. The diameter of the well is 1 cm. Bars, 200 µm.
To develop the embryo-like structures into plantlets, it seemed feasible to decrease the concentrations of the inducer and auxin in the medium, considering the conditions for regeneration in the leaf segment culture. Therefore, we added an equal volume (approximately 200 µL) of fresh mMS to the culture well and removed an equal volume of the supernatant to keep the culture volume constant. This procedure was repeated every 3 days to decrease the concentrations of the auxin and inducer. After 2 weeks, several plantlets were observed in some wells (Figure 6E, F). The embryo-like structure and regeneration was observed in the culture of the XVE::WOX2×XVE::WOX8 hybrid but not XVE::WOX2×XVE::WOX9 hybrid so far. However, it seems possible to induce regeneration also in the latter hybrid by improving the culture conditions and the timing of operation through some more trials.
Discussion
In plant biotechnology, it is important to find genes whose ectopic expression induces regenerative responses in cells or tissues because such genes may be useful for producing transgenic plants more efficiently, especially in crops that are recalcitrant to the traditional regeneration protocol requiring cytokinin. RP genes have been previously found in many studies of A. thaliana, in which overexpression of these genes caused shoot formation or embryogenesis (Motte et al. 2014; Radoeva and Weijers 2014). As described in the Introduction, WUS may be a prime candidate for an RP gene applicable to a wide range of species. Several attempts have been reported for genes other than WUS using BABY BOOM in tobacco (Srinivasan et al. 2007), pepper (Heidmann et al. 2011) and cacao (Florez et al. 2015); LEAFY COTYLEDON 1 (LEC1) and LEC2 in tobacco (Guo et al. 2013); LEC1 homolog in conifer (Klimaszewska et al. 2010; Uddenberg et al. 2016); AGAMOUS-LIKE15 in soybean (Harding et al. 2003). However, whether these results are widely applicable to other cultivars or relative species remains unclear and the number of RP genes is still limited. Taken together, there is immediate need to identify additional RP genes.
In this study, we showed for the first time that the coexpression of a pair of WOXs in combinations WOX2 and WOX8 or WOX2 and WOX9 conferred an RP effect on the leaf segments. This was sufficiently supported by a series of experiments demonstrated in the Results section and its reproducibility was confirmed through the similar experiments using several progeny and hybrid lines derived from the plants independently transformed with each gene. As mentioned in the Introduction section, these genes and their homologs are expressed in the early stages of zygotic or somatic embryogenesis in A. thaliana and other species. Therefore, it seems reasonable that their ectopic expression causes the RP effect, resulting in organogenesis or embryogenesis in the seedling or explants in vitro. To apply this mechanism to actual transformation, we designed a binary vector harboring both WOX2 and WOX8 under the control of the XVE system and proved its functionality in A. thaliana and few Nicotiana species (data not shown).
As shown in Figure 2, we developed an assay system to estimate the RP effect induced by the expression of the transgenes by culturing leaf segments prepared from young transgenic plants. This consisted of two phases: the first period for inducing transgene expression in the presence of β-estradiol and second for inducing regeneration. In case of A. thaliana transgenic for WUS (Zuo et al. 2002a), it was noted that continuous expression of the transgene was not suitable for regeneration. Also, in our experiments, when the explants were kept in the first culture medium throughout, the color of the calluses generated on the explants darkened and the frequency of regeneration was reduced (data not shown).
In the presence of both the regulators (auxin and inducer), the cells around the edge of the explants seemed to proliferate and develop to primordia of the origin of regeneration. The primordia then developed into plantlets in the absence of both the regulators. An example of a developmental event that requires such a temporal change in growth regulators is well known in carrot somatic embryogenesis (Fujimura 2014; Komamine et al. 2005); i.e., exogenous auxin is required during phase 0 in which the competent single cells (state 0) develop into embryogenic cell clusters (state 1), but not in the subsequent process (phases 1–3) in which cell clusters develop into globular, heart and torpedo stages. The progression from single cell (state 0) to multicellular clumps with embryogenic competency (state 1) could be demonstrated in a culture system established by Nomura and Komamine (1985). In the present study, we observed a similar process in generating embryo-like structures in freely suspended cell populations derived from tobacco leaf segments (Figure 6B to D). This process seems to be corresponding to phase 0 (state 0 and 1) in the carrot system and is the first example of tobacco somatic embryogenesis in a free cell population.
Since the classic studies by Skoog and Miller (1957) and Steward et al. (1958), tobacco and carrot have been used as model species in the field of cell and tissue culture. Interestingly, it is well known that the two species show different regeneration behaviors in vitro; the explants from the former easily develop adventitious shoots in response to cytokinin, but rarely show somatic embryogenesis, whereas those from the latter easily develop embryogenic calluses in response to auxin, but rarely develop adventitious shoots. There are some reports regarding somatic embryogenesis in tobacco tissue culture (Gill and Saxena 1993; Guo et al. 2013; Pathi et al. 2013; Srinivasan et al. 2007); however, the researchers may have observed organogenesis but not embryogenesis, judging from their use of shoot inducers (cytokinin) in the culture and morphological aspect and greenish color shown in the figures in their reports. Previously, in tobacco tissue and cell culture, cytokinin was essential for regeneration with the exception of pollen embryogenesis (Kyo et al. 2014). Therefore, it is interesting that the coexpression of two WOX genes induced regeneration from the explants and freely suspended cells in the absence of cytokinin, suggesting crucial roles of some orthologous WOX genes for embryogenic response in carrot cells and tobacco pollen in vitro.
Acknowledgments
We thank the previous laboratory members H. Sakimori, M. Tada, Y. Kawanishi and T. Okada for their cooperation in this study. We also thank Dr. K. Higashi at Teikyo University of Science for the discussion about somatic embryogenesis in A. thaliana. This study was partly supported by a JSPS KAKENHI Grant (No. 24568007).
Abbreviations
- 2,4-D
2,4-dichlorophenoxyacetic acid
- mMS
modified Murashige and Skoog medium
- RP
regeneration-promoting
- WOX
WUSCHEL related homeobox
- WUS
WUSCHEL
- XVE
LexA-VP16-estrogen receptor chimeric transcription activator
References
- Arroyo-Herrera A, Gonzalez AK, Moo RC, Quiroz-Figueroa FR, Loyola-Vargas VM, Rodriguez-Zapata LC, Hondt CB, Suarez-Solis VM, Castano E (2008) Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell Tissue Organ Cult 94: 171–180 [Google Scholar]
- Bouchabké-Coussa O, Obellianne M, Linderme D, Montes E, Maia-Grondard A, Vilaine F, Pannetier C (2013) Wuschel overexpression promotes somatic embryogenesis and induces organogenesis in cotton (Gossypium hirsutum L.) tissues cultured in vitro. Plant Cell Rep 32: 675–686 [DOI] [PubMed] [Google Scholar]
- Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T (2008) Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis Embryo. Dev Cell 14: 867–876 [DOI] [PubMed] [Google Scholar]
- Chung K, Sakamoto S, Mitsuda N, Suzuki K, Ohme-Takagi M, Fujiwara S (2016) WUSCHEL-RELATED HOMEOBOX 2 is a transcriptional repressor involved in lateral organ formation and separation in Arabidopsis. Plant Biotechnol 33: 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo E, Trehin C, Vandenbussche M (2014) The role of WOX genes in flower development. Ann Bot (Lond) 114: 1545–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez SL, Erwin RL, Maximova SN, Guiltinan MJ, Curtis WR (2015) Enhanced somatic embryogenesis in Theobroma cacao using the homologous BABY BOOM transcription factor. BMC Plant Biol 15: 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T (2014) Carrot somatic embryogenesis: A dream comes true? Plant Biotechnol Rep 8: 23–28 [Google Scholar]
- Gambino G, Minuto M, Boccacci P, Perrone I, Vallania R, Gribaudo I (2011) Characterization of expression dynamics of WOX homeodomain transcription factors during somatic embryogenesis in Vitis vinifera. J Exp Bot 62: 1089–1101 [DOI] [PubMed] [Google Scholar]
- Gill R, Saxena PK (1993) Somatic embryogenesis in Nicotiana tabacum L.: Induction by thidiazuron of direct embryo differentiation from cultured leaf discs. Plant Cell Rep 12: 154–159 [DOI] [PubMed] [Google Scholar]
- Guo F, Liu C, Xia H, Bi Y, Zhao C, Zhao S, Hou L, Li F, Wang X (2013) Induced expression of AtLEC1 and AtLEC2 differentially promotes somatic embryogenesis in transgenic tobacco plants. PLoS ONE 8: e71714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff E, Laux T, Rensing SA (2009) The WUS homeobox-containing (WOX) protein family. Genome Biol 10: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T (2004) Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131: 657–668 [DOI] [PubMed] [Google Scholar]
- Harding EW, Tang W, Nichols KW, Fernandez DE, Perry SE (2003) Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-Like 15. Plant Physiol 133: 653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman H, Zhu T, von Arnold S, Sohlberg JJ (2013) Analysis of the WUSCHEL-RELATED HOMEOBOX gene family in the conifer Picea abies reveals extensive conservation as well as dynamic patterns. BMC Plant Biol 13: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann I, de Lange B, Lambalk J, Angenent GC, Boutilier K (2011) Efficient sweet pepper transformation mediated by the BABY BOOM transcription factor. Plant Cell Rep 30: 1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimaszewska K, Pelletier G, Overton C, Stewart D, Rutledge RG (2010) Hormonally regulated overexpression of Arabidopsis WUS and conifer LEC1 (CHAP3A) in transgenic white spruce: Implications for somatic embryo development and somatic seedling growth. Plant Cell Rep 29: 723–734 [DOI] [PubMed] [Google Scholar]
- Komamine A, Murata N, Nomura K (2005) 2004 SIVB Congress symposium proceeding: mechanisms of somatic embryogenesis in carrot suspension cultures: Morphology, physiology, biochemistry, and molecular biology. In Vitro Cell Dev Biol Plant 41: 6–10 [Google Scholar]
- Kyo M, Nagano A, Yamaji N, Hashimoto Y (2014) Timing of the G1/S transition in tobacco pollen vegetative cells as a primary step towards androgenesis in vitro. Plant Cell Rep 33: 1595–1606 [DOI] [PubMed] [Google Scholar]
- Laux T, Mayer KFX, Berger J, Jurgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96 [DOI] [PubMed] [Google Scholar]
- Lazo GR, Stein PA, Ludwig RA (1991) A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Biotechnology (N Y) 9: 963–967 [DOI] [PubMed] [Google Scholar]
- Liu B, Wang L, Zhang J, Li J, Zheng H, Chen J, Lu M (2014) WUSCHEL related homeobox genes in Populus tomentosa: Diversified expression patterns and a functional similarity in adventitious root formation. BMC Genomics 15: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KFX, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815 [DOI] [PubMed] [Google Scholar]
- Motte H, Vereecke D, Geelen D, Werbrouck S (2014) The molecular path to in vitro shoot regeneration. Biotechnol Adv 32: 107–121 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S (1992) Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol 132: 1–30 [Google Scholar]
- Nomura K, Komamine A (1985) Identification and isolation of single cells that produce somatic embryos at a high frequency in a carrot suspension culture. Plant Physiol 79: 988–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathi KM, Tula S, Tuteja N (2013) High frequency regeneration via direct somatic embryogenesis and efficient Agrobacterium-mediated genetic transformation of tobacco. Plant Signal Behav 8: e24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palovaara J, Hallberg H, Stasolla C, Hakman I (2010) Comparative expression pattern analysis of WUSCHEL-related homeobox 2 (WOX2) and WOX8/9 in developing seeds and somatic embryos of the gymnosperm Picea abies. New Phytol 188: 122–135 [DOI] [PubMed] [Google Scholar]
- Palovaara J, Saiga S, Weijers D (2013) Transcriptomics approaches in the early Arabidopsis embryo. Trends Plant Sci 18: 514–521 [DOI] [PubMed] [Google Scholar]
- Radoeva T, Weijers D (2014) A roadmap to embryo identity in plants. Trends Plant Sci 19: 709–716 [DOI] [PubMed] [Google Scholar]
- Rashid SZ, Yamaji N, Kyo M (2007) Shoot formation from root tip region: A developmental alteration by WUS in transgenic tobacco. Plant Cell Rep 26: 1449–1455 [DOI] [PubMed] [Google Scholar]
- Rupps A, Raschke J, Rummler M, Linke B, Zoglauer K (2016) Identification of putative homologs of Larix decidua to BABY BOOM (BBM), LEAFY COTYLEDON1 (LEC1), WUSCHEL-related HOMEOBOX2 (WOX2) and SOMATIC EMBRYOGENESIS RECEPTOR-like KINASE (SERK) during somatic embryogenesis. Planta 243: 473–488 [DOI] [PubMed] [Google Scholar]
- Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissue cultured in vitro. Symp Soc Exp Biol 11: 118–130 [PubMed] [Google Scholar]
- Steward FC, Mapes MO, Mears K (1958) Growth and organized development of cultured cells. II: Organization in cultures grown from freely suspended cells. Am J Bot 45: 705–708 [Google Scholar]
- Solís-Ramos LY, Gonzalez-Estrada T, Nahuath-Dzib S, Zapata-Rodriguez LC, Castano E (2009) Overexpression of WUSCHEL in C. chinense causes ectopic morphogenesis. Plant Cell Tissue Organ Cult 96: 279–287 [Google Scholar]
- Srinivasan C, Liu Z, Heidmann I, Supena EDJ, Fukuoka H, Joosen R, Lambalk J, Angenent G, Scorza R, Custers JBM, et al. (2006) Heterologous expression of the BABY BOOM AP2/ERF transcription factor enhances the regeneration capacity of tobacco (Nicotiana tabacum L.). Planta 225: 341–351 [DOI] [PubMed] [Google Scholar]
- Ueda M, Laux T (2012) The origin of the plant body axis. Curr Opin Plant Biol 15: 578–584 [DOI] [PubMed] [Google Scholar]
- Uddenberg D, Abrahamsson M, von Arnold S (2016) Overexpression of PaHAP3A stimulates differentiation of ectopic embryos from maturing somatic embryos of Norway spruce. Tree Genet Genomes 12: 18 [Google Scholar]
- Wu X, Chory J, Weigel D (2007) Combinations of WOX activities regulate tissue proliferation during Arabidopsis embryonic development. Dev Biol 309: 306–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liu X, Engstrom EM, Nimchuk ZL, Pruneda-Paz JL, Tarr PT, Yan A, Kay SA, Meyerowitz EM (2015) Control of plant stem cell function by conserved interacting transcriptional regulators. Nature 517: 377–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Moschou PN, Alvarez JM, Sohlberg JJ, von Arnold S (2016) WUSCHEL-RELATED HOMEOBOX 2 is important for protoderm and suspensor development in the gymnosperm Norway spruce. BMC Plant Biol 16: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Chua N (2000) An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24: 265–273 [DOI] [PubMed] [Google Scholar]
- Zuo J, Niu Q, Frugis G, Chua N (2002a) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30: 349–359 [DOI] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Ikeda Y, Chua NH (2002b) Marker-free transformation: Increasing transformation frequency by the use of regeneration-promoting genes. Curr Opin Biotechnol 13: 173–180 [DOI] [PubMed] [Google Scholar]



![Figure 1. Effect of expression of the transgenes on seedling morphology. (A) to (C) The seeds of each transgenic individual [XVE::WOX2 (A), XVE::WOX8 (B) and XVE::WOX9 (C)] were obtained through self-pollination and sown on mMS with 0.8% agar, 50 mg L−1 of hygromycin B and 0 or 1 µM of β-estradiol, and then kept in day-neutral light conditions at 25°C for 3 weeks. (D, E) The seeds of two hybrid lines were obtained by crossing XVE::WOX2 with XVE::WOX8 (D) or XVE::WOX9 (E) and sown as described above. (−) The two plants placed on the left in each panel were grown in the absence of the expression inducer as controls. (+) The other plants were grown in the presence of the inducer at the concentration of 1 µM. In the expression analysis (Figure 5), the two plants on the right in panel D and three plants in the center in panel E were proved to possess both transgenes WOX2 and WOX8 and WOX2 and WOX9, respectively. The dotted line in panel E means the boundary of the original images.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/d86f/6543738/108f6c555db2/plantbiotechnology-35-1-18.0126a-figure01.jpg)