Abstract
Mucus overproduction is a major contributor to morbidity and mortality in asthma. Mucus overproduction is induced by orchestrated actions of multiple factors that include inflammatory cytokines and γ-aminobutyric acid (GABA). GABA is produced only by pulmonary neuroendocrine cells (PNECs) in the mouse lung. Recent studies in a neonatal mouse model of allergic inflammation have shown that PNECs play an essential role in mucus overproduction by GABA hypersecretion. Whether PNECs mediate dysregulated GABA signaling for mucus overproduction in asthma is unknown. In this study, we characterized the cellular source of GABA in the lungs of nonhuman primates and humans and assessed GABA secretion and signaling in primate disease models. We found that like in mice, PNECs were the major source of GABA in primate lungs. In addition, an infant nonhuman primate model of asthma exhibited an increase in GABA secretion. Furthermore, subjects with asthma had elevated levels of expression of a subset of GABA type α (GABAα) and type β (GABAβ) receptors in airway epithelium compared with those of healthy control subjects. Last, employing a normal human bronchial epithelial cell model of preinduced mucus overproduction, we showed pharmaceutical blockade of GABAα and GABAβ receptor signaling reversed the effect of IL-13 on MUC5AC gene expression and goblet cell proliferation. Together, our data demonstrate an evolutionarily conserved intraepithelial GABA signaling that, in concert with IL-13, plays an essential role in mucus overproduction. Our findings may offer new strategies to ameliorate mucus overproduction in patients with asthma by targeting PNEC secretion and GABA signaling.
Keywords: pulmonary neuroendocrine cell, γ-aminobutyric acid, goblet cell hyperplasia, mucus overproduction, asthma
Mucus overproduction is a salient clinical feature of chronic airway diseases such as asthma and chronic obstructive pulmonary disease (COPD) (1). A major cell type in the airway epithelium that produces mucin is goblet cells. Patients with asthma and COPD have an increased number of goblet cells and mucus hypersecretion. Goblet cell hyperplasia is known to be triggered by inflammation in response to damaging exposures to environmental insults, such as allergen, ozone (O3), respiratory virus, and cigarette smoke. Excessive mucus in the airway blocks airflow, which causes disease exacerbation and contributes to disease mortality. To date, there is no drug that directly targets mucus overproduction.
Asthma often starts in early childhood. Both clinical and epidemiological findings reveal the association between early-life exposure to environmental insults and wheezing and asthma in young children (2, 3). Previous studies using rodent and nonhuman primate models indicated that the detrimental exposure in early life may cause long-term changes in lung structure and function, thereby contributing to the pathophysiology of asthma (4–6). Consistent with this hypothesis, the nervous system has been shown to undergo aberrant changes after early-life exposure (4, 5, 7–9) at least in part through the cross-talk between inflammation and the nerves (10). The nervous system in the lung is known to innervate airway smooth muscle cells and pulmonary neuroendocrine cells (PNECs). Genetic studies in the neonatal mouse models of allergen exposure have shown that changes in lung innervation alter the contractile phenotype of airway smooth muscle and induce the aberrant secretion from PNECs (4, 11, 12). Notably, PNECs express a variety of neuropeptides and bioactive amines that include γ-aminobutyric acid (GABA) and calcitonin-related gene peptide. GABA, which is produced only by PNECs and secreted through the regulation of nerves in the mouse lung, is essential for mucus overproduction (12–14). Whether PNEC innervation and secretion are altered after damaging exposures in early life in asthma is unknown.
In contrast to nonhuman primate and human lungs that normally have a significant number of goblet cells, the mouse lung has few goblet cells at baseline (1). After exposure to environmental insults in mice, secretory cells transdifferentiate into goblet cells through a process known as mucus metaplasia (15–17). However, despite the difference in the cell origin of goblet cells between mice and primates, mechanisms underlying mucus overproduction are conserved. The overproduction of mucus in patients with asthma and animal models is driven by inflammatory cytokines, including IL-13. In addition, several transcriptional factors that are essential for mucus overproduction in animal models of asthma, such as SPDEF (SAM pointed domain-containing Ets transcription factor) and FOXA3, have been shown to be upregulated in the airway epithelium of patients with asthma (17, 18). Furthermore, GABA has been shown to play an essential role in allergen- and nicotine-induced mucus overproduction (19, 20). GABA has two types of receptors: GABAα and GABAβ receptors. The GABAα receptor consists of five subunits and functions as a ligand-gated Cl− channel, whereas the GABAβ receptor consists of GABBR1 and GABBR2 and is a G protein–coupled receptor. Allergen exposure causes an increase in the expression of GABAα receptor subunits in airway epithelium of both mice and humans (19). Prenatal exposure to cigarette smoke in nonhuman primates has been shown to induce GABA production and GABAα receptor expression (21). Functional studies in mice have demonstrated that although GABA alone has no effect on the amount of mucus production, dysregulated GABA signaling is essential for IL-13–induced mucus overproduction in airway epithelium (12, 14). In addition, in a culture model of nonhuman primate airway epithelial cells, nicotine-induced mucus overproduction requires GABAα receptor signaling (20). So far, the role of the GABAβ receptor in mucus overproduction is unknown. More important, whether GABA signaling may serve as a drug target to reduce already-established mucus overproduction under the diseased condition has never been tested.
Building on previous findings in mice that the nerve–PNEC–GABA axis was required for mucus overproduction after early-life allergen exposure (12), we sought to test whether this axis plays an evolutionarily conserved role in asthma by employing the infant nonhuman primate model of environmental insults and a normal human bronchial epithelial (NHBE) cell culture model of prestimulated mucus overproduction. By characterizing GABA and GABA receptor expression in vivo and functional studies of the GABAα and GABAβ receptors in vitro, we provide evidence in support of an essential role of GABA secreted from PNECs in mucus overproduction in primate models of asthma.
Methods
Human Cell/Tissue Study Approval
NHBE cells were obtained by the Tissue Procurement and Cell Culture Core at the University of North Carolina, Chapel Hill (institutional review board–approved protocol no. 03-1396). Human lungs from de-identified organ donors with no preexisting lung disease were procured from the International Institute for the Advancement of Medicine. This study used three adult donor lungs. Because the study did not involve the collection of data through an intervention or interaction with individual subjects or identifiable private information about living individuals, the study was deemed nonhuman research by the Partners Human Research Committee.
Nonhuman Primate Model of O3 and House Dust Mite Allergen Exposure
Infant rhesus monkeys were subjected to 11 episodes of exposure to O3 and house dust mite allergen (HDMA) starting around Postnatal Day 30 (22). Monkeys were killed at 6 months of age. All exposure experiments in nonhuman primates were performed at the California National Primate Research Center at the University of California, Davis (www.cnprc.ucdavis.edu/ourscience/respiratory-diseases/), under an animal protocol approved by the institutional animal care and use committee. Archived tissue blocks and serum samples were used for this study.
GABA Receptor Expression in Airway Brushing Samples of Healthy Donors and Donors with Asthma
Microarray datasets GOBMUC2A (patients with asthma; n = 7) and GOBMUC2H (healthy; n = 11) were downloaded from the Gene Expression Omnibus (GEO) website (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?andacc=GSe4302) (23). The Affymetrix CEL files are robust multiarray average normalized via the R/Bioconductor affy package. For the resulting data matrix of probes/genes, for each probe of GABA-related genes of interest, Wilcoxon’s rank-sum test was applied to assess the difference in median signal between the patients with asthma and healthy donor population. Any differences with P value less than 0.05 were deemed significant. To account for multiple hypothesis testing, we computed the corresponding positive false discovery rate analog of the P value called a q value for the 21 tested GABA receptor genes using the MATLAB (MathWorks) function mafdr (24).
Air–Liquid Interface Culture of NHBE Cells
NHBE cells (25) were expanded and cultured at an air–liquid interface (ALI) using an established protocol (26). To induce goblet cell hyperplasia, the ALI culture was treated with human IL-13 (10 ng/ml, catalogue no. 200-13; PeproTech) starting from Day 0 of ALI. The ALI culture was treated with the antagonists of the GABAα receptor (picrotoxin, 50 μM, catalogue no. P1675; MilliporeSigma) and the GABAβ receptor (CGP55845, 1 μM, catalogue no. SML0594; MilliporeSigma) starting at Day 14 of ALI. The culture medium was changed every other day. The ALI culture was analyzed at Day 21.
Statistical Analyses
Data represented mean ± SEM from at least three independent experiments. For comparisons between two conditions, statistical analysis was performed using unpaired Student’s t test. For the assessment of the effect of GABA receptor inhibitors in control and IL-13–treated conditions, statistical analysis was performed with one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. The difference between experimental groups was considered statistically significant if the P value was less than 0.05. Details of antibodies, immunohistochemistry, the ALI culture, and quantitative PCR are provided in the data supplement.
Results
Expression of GABA and GABA Receptors in the Lung of Nonhuman Primates and Humans
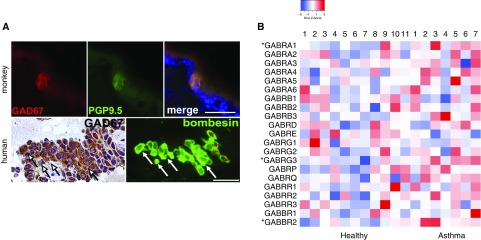
PNECs have been shown to be the only source of GABA in the mouse lung through the activity of biosynthetic enzyme glutamate decarboxylase 67 (GAD67) by a GAD67-GFP report mouse line and immunostaining using a monoclonal antibody (12, 13). No GAD65 expression in the mouse lung was found (12). However, a previous study suggested ubiquitous GAD65/67 expression in airway epithelium by immunostaining using a polyclonal GAD65/67 antibody (19). We suspected that the discrepancy may be caused by limited specificity of the polyclonal antibody. To resolve the issue of GABA production in the primate lung, we stained histological sections from rhesus monkeys and humans with the specific mouse monoclonal antibody against GAD67 (12). We detected GAD67 in clusters and singular cells in airway epithelium that coexpressed PNEC markers, such as PGP9.5 (protein gene product 9.5) in nonhuman primates and bombesin in humans (Figure 1A), and thus were PNECs. Notably, all PNECs expressed GAD67 in mice and in nonhuman primates (Figure 1A) (12, 13). We also did not find any change in PNEC expression of GAD67 after O3 and HDMA exposure, which is consistent with our findings in the mouse model of allergen exposure (12). However, in human donor lungs, approximately 80% of PNECs were labeled by the GAD67 antibody (Figure 1A). This finding indicates the heterogeneity of the PNEC population in humans. Although the antibody staining may exclude cells with low levels of GAD67 expression, we conclude that PNECs are the major source, if not the sole source, of GABA in the lung of nonhuman primates and humans.
Figure 1.
Airway epithelial production of γ-aminobutyric acid (GABA) in primate lungs and GABA receptors in healthy humans and humans with asthma. (A) Representative glutamate decarboxylase 67 (GAD67) expression in pulmonary neuroendocrine cells (PNECs). Histological sections of the lung in rhesus monkeys (6 mo old) and infant human donors were double stained for GAD67 and PNEC markers PGP9.5 (protein gene product 9.5) and bombesin, respectively. *Bombesin+ PNECs that are GAD67−. Arrows mark nonspecific labeling by the GAD67 antibody in human lung mesenchyme. For quantification, 10 tissue sections from six monkeys were stained, and a total of 129 PNECs were all found to express GAD67. For the human lung, five tissue sections from three donor lungs were stained. Of 74 bombesin+ PNECs, 58 were GAD67+. Scale bars: 25 μm. (B) Relative GABA receptor gene expression in airway epithelium of healthy donors (n = 11) and donors with asthma (n = 7). Data were collected from microarray results of airway epithelial brushing samples deposited in the Gene Expression Omnibus GSE4302 public dataset (contact contributor, P. G. Woodruff). Each column represents one donor. *Genes with statistically significant increases in expression in asthmatic samples.
Exposure to allergen and nicotine has been shown to increase the expression of several GABAα receptor subunits in airway epithelium (19, 20). However, whether the expression of GABAα and GABAβ receptors is altered in patients with asthma is unknown. To evaluate the change in GABA receptor expression in asthma, we compared the relative gene expression levels of GABA receptor subunits in airway epithelial brushing samples collected from healthy donors and donors with asthma using published microarray datasets deposited at the GEO website (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?andacc=GSe4302) (23). Of the 21 GABA receptor genes, two GABAα receptor genes (GABRA1 and GABRG3) and one GABAβ receptor gene (GABBR2) exhibited an elevated level of expression in patients with asthma compared with healthy donors. No GABA receptor genes were downregulated in asthma. Notably, GABRA1 and GABRG3 were not among the upregulated GABAα receptor genes previously identified in allergen and nicotine exposure models (19, 20). However, both previous studies and our work uniformly identified an increase in GABAα receptor expression. Together, our findings support dysregulated GABAα signaling in the asthmatic airway epithelium. In addition, the observed increase in GABBR2 expression in patients with asthma suggests an additional role of the GABAβ receptor in mucus overproduction.
Aberrant GABA Secretion and PNEC Hyperinnervation in an Infant Nonhuman Primate Model of Childhood Asthma
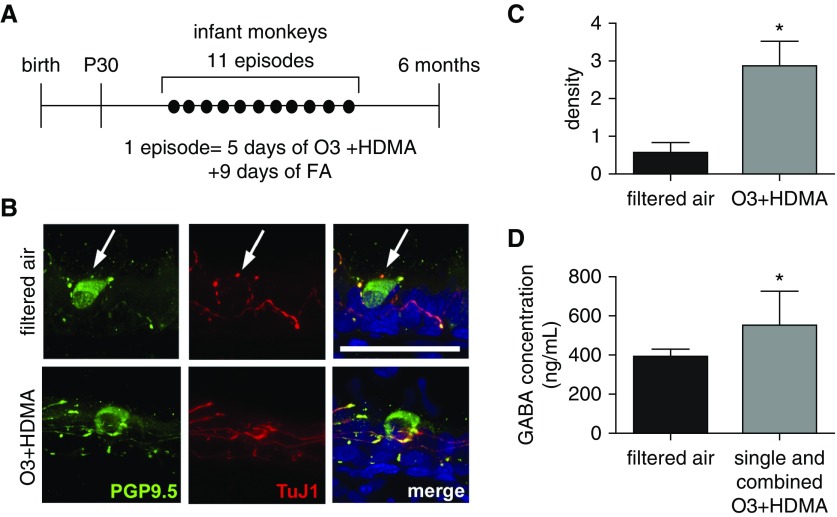
We showed previously in a neonatal mouse model that GABA secretion from PNECs was induced by a hyperactivated neurocircuitry after allergen exposure (12). Whether early-life exposure to environmental insults causes a similar increase in PNEC innervation and GABA hypersecretion, thereby contributing to mucus overproduction in childhood asthma, is completely unknown. This is due to technical difficulties of obtaining the lung tissue from young children. To circumvent this technical issue, we took advantage of an infant nonhuman primate model of O3 and HDMA exposure that has been established at the California National Primate Research Center at the University of California, Davis (Figure 2A) (7, 22). Rhesus monkeys that are exposed to O3 and HDMA during the first 6 months after birth have been shown to present clinical hallmarks of asthma and recapitulate disease progression (7, 22). To assess PNEC innervation in infant rhesus monkeys after the exposure, tissue sections (25 μm in thickness) were double stained for specific markers of nerves and PNECs using a TuJ1 antibody and an antibody against PGP9.5, respectively. Nerves surrounding PNECs were visualized by confocal microscopy (Figure 2B). The nerve density was measured by normalizing TuJ1-immunoreactive area to the number of PNECs using Z-stacked confocal images. Compared with control animals that were exposed to filtered air, O3 and HDMA exposure significantly increased the nerve density surrounding PNECs by approximately fourfold (Figures 2B and 2C). We then measured GABA secretion from PNECs by ELISA using serum samples because PNECs are known to secrete basolaterally into the circulation (27). Owing to limited serum samples from the combined O3 + HDMA exposure group, we pooled all serum samples from rhesus monkeys that were subjected to single and combined exposures. The serum concentration of GABA from infant rhesus monkeys that were exposed to environmental insults was significantly higher than that in control animals (Figure 2D). Therefore, environmental exposures in infant nonhuman primates cause aberrant increases in PNEC innervation with associated GABA hypersecretion, which is similar to the neonatal mouse model of allergen exposure (12).
Figure 2.
PNEC hyperinnervation and GABA hypersecretion in a nonhuman primate model of early-life exposure. (A) Experimental scheme of rhesus monkey ozone (O3) and house dust mite allergen (HDMA) exposure during the first 6 months after birth. Control animals were exposed to filtered air (FA). Lungs and serum samples were harvested at 6 months of age. (B) Representative Z-stack confocal images of PNEC innervation in 6-month-old rhesus monkeys with and without O3 + HDMA exposure. PNEC innervation was assessed by double staining of proximal lung sections (25 μm in thickness) for PGP9.5 and neuron-specific β-tubulin III using a TuJ1 antibody. Arrows mark PNECs. Scale bar: 50 μm. (C) Quantification of PNEC innervation in infant rhesus monkeys with and without O3 and HDMA exposure. The density of nerves surrounding PNECs was calculated by normalizing TuJ1-immunoreactive area to the number of PNECs. At least five single PNECs and PNEC clusters from three lungs in each group were measured. (D) Serum concentrations of GABA in control animals (n = 7) and rhesus monkeys (n = 10) that were exposed to O3, HDMA, and combined O3 + HDMA. Data presented in C and D represent mean ± SEM. *P < 0.05.
GABAα and GABAβ Receptor Signaling in IL-13–induced Mucus Overproduction in ALI Culture of NHBE Cells
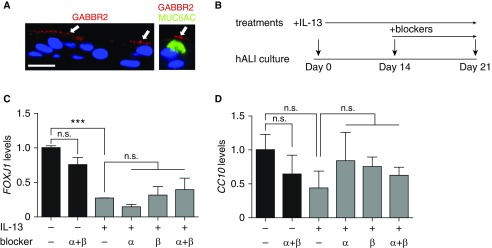
Previous studies in rodent and nonhuman primate models identified an essential role of GABA in the induction of mucus overproduction after allergen and nicotine exposure (19, 20). On the basis of our findings of the upregulation of GABA receptor expression in asthmatic airway brushing samples, we speculated that GABAα and GABAβ receptors may play an additional role in the maintenance and/or progression of mucus overproduction in asthma. To test this hypothesis, we employed an ALI culture of NHBE cells that was prestimulated with IL-13. The ALI culture contains multiple epithelial cells types during differentiation and thus provides an in vitro model for IL-13–induced goblet cell hyperplasia and mucus overproduction (25). Because previous studies focused on GABAα receptors (19, 20), we chose to validate GABBR2 expression in ALI culture at Day 14, when all differentiated cell types were readily identifiable (26, 28). Double staining for GABBR2 and MUC5AC showed a mostly apical distribution of GABBR2 in MUC5AC+ goblet cells and other epithelial cell types (Figure 3A). Quantification of the GABBR2+ and MUC5AC+ cells in three independent ALI cultures showed that approximately 40% of MUC5AC+ goblet cells expressed GABBR2 and that 17% of GABBR2+ cells were MUC5AC+ goblet cells.
Figure 3.
GABAα and GABAβ signaling has no effect on baseline and IL-13–induced differentiation of air–liquid interface (hALI) cultures of normal human bronchial epithelial (NHBE) cells. (A) GABAβ receptor (GABBR2) expression in ALI cultures at Day 14 of differentiation by double staining for GABBR2 and MUC5AC. Nuclei were stained by DAPI. Arrows mark GABBR2 expression in MUC5AC+ goblet cells and other epithelial cell types. Scale bar: 50 μm. (B) Scheme of the treatment with GABA receptor blockers in control and IL-13–stimulated ALI cultures. IL-13 (10 ng/ml) stimulation started at Day 0. Treatment with single and combined GABAα receptor blocker (50 μM picrotoxin) and GABAβ receptor blocker (1 μM CGP55845) started at Day 14. (C and D) Cultures were analyzed at Day 21 for the gene expression of a ciliated cell marker (FOXJ1) (C) and a club cell marker (CC10) (D). Data represent mean ± SEM of three independent experiments of NHBE cells from each donor and a total of three donors for each condition. ***P < 0.001. n.s. = not significant.
After validating the expression of GABA receptors in NHBE cells, we evaluated the presence of GABA in the ALI culture system. We detected GABA in the culture medium itself at concentrations within the 1–3 mM range, possibly due to the bovine pituitary extract as a supplement. Because only very few PNECs formed in the ALI culture (29), PNECs are unlikely to have contributed to the GABA concentration in culture. Therefore, the ALI culture contains endogenous GABA in abundance. This finding may explain why IL-13 is sufficient to induce goblet cell hyperplasia in the ALI culture without the addition of exogenous GABA.
For our assay, NHBE cells were prestimulated with IL-13 for the first 2 weeks of the ALI culture, which has been shown to robustly induce mucus overproduction (30, 31). The ALI culture was then treated for 1 week with specific pharmaceutical blockers of GABAα and GABAβ receptors (picrotoxin and CGP55845, respectively) at the continuous presence of IL-13 (Figure 3B). The ALI culture was analyzed at Day 21. Compared with untreated control cultures, IL-13 significantly impaired ciliated cell differentiation as shown by a decrease in mRNA expression of FOXJ1 (a ciliated cell marker) (Figure 3C). These findings are consistent with a previous report that IL-13–mediated STAT6 (signal transducer and activator of transcription 6) signaling represses FOXJ1 expression in the ALI culture (32). In contrast, IL-13 had no effect on the differentiation of secretory cells assayed by CC10 mRNA concentrations (Figure 3D). Furthermore, GABAα and GABAβ receptor blockers had no effect on the differentiation of ciliated and secretory cells in ALI culture of NHBE cells at baseline and at the presence of IL-13 (Figures 3C and 3D).
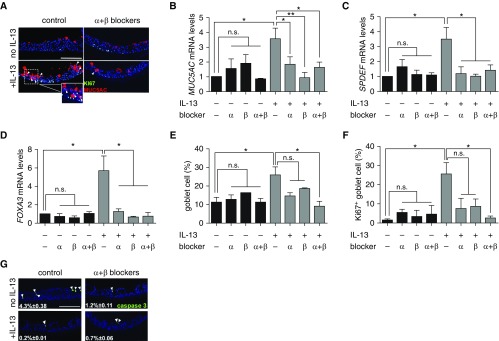
We then assessed the effect of GABA receptor blockers on goblet cell differentiation and proliferation. Approximately 10% of NHBE cells in Day 21 ALI culture were goblet cells at baseline, as shown by their expression of MUC5AC (Figures 4A and 4E). Treatment of ALI cultures with GABAα and GABAβ receptor blockers had no effect on the baseline level of MUC5AC gene expression and the number of MUC5AC+ goblet cells (Figures 4A, 4B, and 4E). The mRNA expression of other transcriptional factors known to be essential for mucus overproduction, such as SPDEF and FOXA3, was also unaffected by the inhibitors at baseline (Figures 4D and 4E). IL-13 stimulation elevated the level of MUC5AC gene expression by 3.5-fold and doubled the number of goblet cells (Figures 4A, 4B, and 4E). Double staining for MUC5AC and a cell proliferation marker, Ki-67, showed that IL-13 stimulation caused a significant increase in goblet cell proliferation (Figure 4F). We found that 7-day treatment with the GABAα and GABAβ receptor blockers, either alone or in combination, prevented the increase in IL-13–induced mRNA expression of MUC5AC, SPDEF, and FOXA3 (Figures 4B–4D). In addition, treatment with a combination of GABAα and GABAβ receptor blockers significantly reduced goblet cell proliferation in IL-13–prestimulated ALI cultures to baseline amounts (Figures 4A and 4F). Compared with the combination approach, individual GABA receptor blockers had a smaller negative effect on goblet cell proliferation that failed to reach statistical significance (Figure 4F). Last, GABA receptor blockers had no general side effects on cell apoptosis assayed by staining for activated cleaved caspase 3 (Figure 4G). Notably, IL-13 treatment reduced the percentage of apoptotic cells in culture (Figure 4G), which is consistent with a role of IL-13 in promoting the expression of an antiapoptotic gene, BCL2, in airway epithelial cells (33). Together, our findings indicate that GABA signaling may be required for the maintenance of IL-13–induced goblet differentiation and mucus overproduction and thus may serve as a drug target under the established disease condition.
Figure 4.
GABA is required for IL-13–induced goblet cell hyperplasia in ALI cultures of NHBE cells. (A) Representative images of double staining for Ki-67 and MUC5AC in Day 21 ALI cultures of NHBE cells with and without the treatment of IL-13 and combined GABAα and GABAβ receptor blockers. Arrowheads mark MUC5AC+Ki-67+ cells. The inset shows an enlarged image of double-positive cells in IL-13–treated cultures. (B–D) MUC5AC, SPDEF (SAM pointed domain-containing Ets transcription factor), and FOXA3 gene expression in Day 21 ALI cultures of NHBE cells under different culture conditions. (E) Quantification of the percentages of MUC5AC+ goblet cells in Day 21 ALI cultures under different culture conditions. (F) Goblet cell proliferation in Day 21 ALI cultures under different culture conditions. Proliferation was quantified by normalizing the number of MUC5AC+Ki-67+ cells to the total MUC5AC+ goblet cells. More than 500 cells from each donor were counted for each condition. (G) Representative images of staining for cleaved caspase 3 in Day 21 ALI cultures with and without the treatment of IL-13 and combined GABAα and GABAβ receptor blockers. Data in B–D represent mean ± SEM of three independent experiments in triplicates for each donor, three donors in total. *P < 0.05 and **P < 0.01. Arrowheads mark caspase 3+ cells. Scale bars: 50 μm.
Discussion
Previous studies in mice demonstrated that genetic disruption of GABA signaling prevented mucus overproduction after early-life allergen exposure. The present study indicates that the GABAα and GABAβ receptor pathways may serve as an integral, evolutionarily conserved component of mucus production in primates. This extends previous observations in mice in which genetic disruption of GABA signaling prevented mucus overproduction after early-life antigen exposure (12). In patients with asthma, there is elevated expression of three genes representing both subtypes of GABA receptors, which suggests aberrant GABA signaling in airway epithelium. We also show that GABA is mostly produced by PNECs through the enzymatic activities of GAD67 in both nonhuman primates and humans. In addition, early-life exposure to environmental insults in nonhuman primates causes aberrant PNEC innervation and GABA hypersecretion. The observed increase in PNEC innervation is aligned with previous findings that early-life environmental exposures cause airway epithelium hyperinnervation in nonhuman primates (7). Collectively, these findings suggest that deregulation of the nerve–PNEC–GABA axis plays an essential role in both the initiation and the progression of IL-13–induced mucus overproduction. If true, GABA secretion and GABA receptor signaling may serve as valid drug targets to directly block/reverse mucus overproduction in patients with asthma who have higher concentrations of IL-13.
We provide evidence that in the presence of IL-13, the GABAα and GABAβ receptors play a similar role in MUC5AC gene expression and synergistically contribute to goblet cell proliferation. We speculate that the molecular signaling mechanism of the GABA pathway in mucus overproduction may involve ionotropic and metabotropic receptor functions. The GABAα receptor is a chloride channel. GABA binding to the GABAα receptor triggers an inward current in cultured airway epithelial cells by electrophysiological assays. This finding indicates that GABA is an excitatory signal (18, 19), which is different from a predominantly inhibitory neurotransmitter function of GABA in the central nervous system. GABA also has been shown to block apical-to-basolateral transport of Cl− in alveolar type II cells (34). Notably, cystic fibrosis transmembrane conductance regulator is also a Cl− channel. Mutations in cystic fibrosis transmembrane conductance regulator cause cystic fibrosis, a lung disease with clinical features of abnormal mucus secretion and clearance. Whether the GABAα receptor contributes to mucus production and secretion in allergic inflammation by regulating Cl− outflux in airway epithelial cells warrants future investigation. In addition to ionotropic GABAα receptors, the GABAβ receptor is a metabotropic, G protein–coupled receptor that regulates cAMP production, a secondary messenger that may act together with IL-13 to regulate MUC5AC gene expression (35). Interestingly, GABBR2 is broadly expressed by primary human airway epithelium cells, and not all goblet cells express GABBR2. These findings raise the possibility that the GABAβ pathway may signal directly in goblet cells and indirectly by acting on other epithelial cells and triggering paracrine mechanisms to affect goblet cells. These possibilities need to be tested in future studies. It is important to emphasize that GABA signaling alone has no effect on mucus production in the airway. As shown in our previous work in vivo (12) and this work in vitro, the role of GABA signaling in mucus overproduction requires IL-13. It is possible that IL-13 may compromise the barrier function of airway epithelium so that basally located GABA can diffuse to the apical side of epithelial cells to interact with GABA receptors.
In addition to asthma, mucus overproduction is a major factor in the exacerbation of COPD. COPD is strongly associated with cigarette smoking. Using a nonhuman primate epithelial cell culture model, previous studies showed that the GABAα receptors played an essential role in nicotine-induced mucus overproduction without the involvement of IL-13 (20). In that study, nicotine upregulated GABA production and GABAα receptor expression in the airway epithelium to induce mucus overproduction.
Mechanisms that regulate GABA receptor expression in the airway epithelium by nicotine and inflammation warrant future investigation. In an acute model of allergen exposure in mice, we found no change in airway epithelial expression of the GABA receptors (12). However, three GABA receptor genes exhibit an increase in expression in patients with asthma. We speculate that chronic allergic inflammation may contribute to the upregulation of GABA receptor gene expression in patients with asthma.
In summary, our studies have demonstrated that PNEC-derived GABA is required for the induction of mucus overproduction in both rodent and primate models of asthma (12, 18). GABA signaling also contributes to nicotine-induced mucus overproduction, which has implications in COPD (12, 19, 20). In addition, calcitonin-related gene peptide secreted by PNECs promotes inflammation by activating innate lymphoid cells (14). Furthermore, PNEC hyperplasia has been associated with pulmonary disorders in young children and unusual forms of interstitial lung disease (36–38). Because PNECs are a critical source of neuropeptides and bioactive amines in airway epithelium, it is possible that aberrant PNEC secretion associated with PNEC hyperplasia may contribute to the pathogenesis of these rare diseases. Our studies show that PNEC secretion is regulated by a neurocircuitry that innervates PNECs (12). In this context, the identification of neural pathways that regulate PNEC secretion may provide new drug targets for the treatment of a variety of lung diseases.
Acknowledgments
Acknowledgment
The authors thank the entire Ai laboratory for critical discussion of the project.
Footnotes
Supported by National Institute of Health Grants F31HL126443 (J.B.) and 1R01HL132991 (X.A.), P51OD011107 (CNPRC), and R01 HL127332 (K.G.T.).
Author Contributions: J.B. and L.A.: performed experiments and analyzed data; J.A.M., J.-A.P., and S.H.R.: assisted normal human bronchial epithelial cell culture; S.H.R.: provided normal human bronchial epithelial cells; A.T.K. and K.G.T.: designed and performed bioinformatic analysis of γ-aminobutyric acid receptor expression in airway epithelium of healthy donors and donors with asthma; L.A.M.: provided lung sections and serum samples from nonhuman primates; X.A.: designed experiments and provided funding; and J.B. and X.A.: wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0179OC on December 20, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med. 2002;53:477–498. doi: 10.1146/annurev.med.53.082901.103921. [DOI] [PubMed] [Google Scholar]
- 3.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aven L, Paez-Cortez J, Achey R, Krishnan R, Ram-Mohan S, Cruikshank WW, et al. An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity. FASEB J. 2014;28:897–907. doi: 10.1096/fj.13-238212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herring MJ, Putney LF, St George JA, Avdalovic MV, Schelegle ES, Miller LA, et al. Early life exposure to allergen and ozone results in altered development in adolescent rhesus macaque lungs. Toxicol Appl Pharmacol. 2015;283:35–41. doi: 10.1016/j.taap.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu ZX, Hunter DD, Kish VL, Benders KM, Batchelor TP, Dey RD. Prenatal and early, but not late, postnatal exposure of mice to sidestream tobacco smoke increases airway hyperresponsiveness later in life. Environ Health Perspect. 2009;117:1434–1440. doi: 10.1289/ehp.0800511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson SD, Schelegle ES, Walby WF, Gershwin LJ, Fanuccihi MV, Evans MJ, et al. Postnatal remodeling of the neural components of the epithelial-mesenchymal trophic unit in the proximal airways of infant rhesus monkeys exposed to ozone and allergen. Toxicol Appl Pharmacol. 2004;194:211–220. doi: 10.1016/j.taap.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Yu M, Zheng X, Peake J, Joad JP, Pinkerton KE. Perinatal environmental tobacco smoke exposure alters the immune response and airway innervation in infant primates. J Allergy Clin Immunol. 2008;122:640–647.e1. doi: 10.1016/j.jaci.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 9.Wu ZX, Hunter DD, Batchelor TP, Dey RD. Side-stream tobacco smoke-induced airway hyperresponsiveness in early postnatal period is involved nerve growth factor. Respir Physiol Neurobiol. 2016;223:1–8. doi: 10.1016/j.resp.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Barrios J, Ai X. Neurotrophins in asthma. Curr Allergy Asthma Rep. 2018;18:10. doi: 10.1007/s11882-018-0765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel KR, Bai Y, Trieu KG, Barrios J, Ai X. Targeting acetylcholine receptor M3 prevents the progression of airway hyperreactivity in a mouse model of childhood asthma. FASEB J. 2017;31:4335–4346. doi: 10.1096/fj.201700186R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrios J, Patel KR, Aven L, Achey R, Minns MS, Lee Y, et al. Early life allergen-induced mucus overproduction requires augmented neural stimulation of pulmonary neuroendocrine cell secretion. FASEB J. 2017;31:4117–4128. doi: 10.1096/fj.201700115R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnorbusch K, Lembrechts R, Pintelon I, Timmermans JP, Brouns I, Adriaensen D. GABAergic signaling in the pulmonary neuroepithelial body microenvironment: functional imaging in GAD67-GFP mice. Histochem Cell Biol. 2013;140:549–566. doi: 10.1007/s00418-013-1093-x. [DOI] [PubMed] [Google Scholar]
- 14.Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018;360:eaan8546. doi: 10.1126/science.aan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reader JR, Tepper JS, Schelegle ES, Aldrich MC, Putney LF, Pfeiffer JW, et al. Pathogenesis of mucous cell metaplasia in a murine asthma model. Am J Pathol. 2003;162:2069–2078. doi: 10.1016/S0002-9440(10)64338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SW, Verhaeghe C, Nguyenvu LT, Barbeau R, Eisley CJ, Nakagami Y, et al. Distinct roles of FOXA2 and FOXA3 in allergic airway disease and asthma. Am J Respir Crit Care Med. 2009;180:603–610. doi: 10.1164/rccm.200811-1768OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, et al. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13:862–867. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 20.Fu XW, Wood K, Spindel ER. Prenatal nicotine exposure increases GABA signaling and mucin expression in airway epithelium. Am J Respir Cell Mol Biol. 2011;44:222–229. doi: 10.1165/rcmb.2010-0109OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh SP, Gundavarapu S, Peña-Philippides JC, Rir-Sima-ah J, Mishra NC, Wilder JA, et al. Prenatal secondhand cigarette smoke promotes Th2 polarization and impairs goblet cell differentiation and airway mucus formation. J Immunol. 2011;187:4542–4552. doi: 10.4049/jimmunol.1101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, et al. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol Appl Pharmacol. 2003;191:74–85. doi: 10.1016/s0041-008x(03)00218-7. [DOI] [PubMed] [Google Scholar]
- 23.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storey JD. A direct approach to false discovery rates. J R Stat Soc Ser A Stat Soc. 2002;64:479–498. [Google Scholar]
- 25.Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol. 2013;945:109–121. doi: 10.1007/978-1-62703-125-7_8. [DOI] [PubMed] [Google Scholar]
- 26.Park JA, Tschumperlin DJ. Chronic intermittent mechanical stress increases MUC5AC protein expression. Am J Respir Cell Mol Biol. 2009;41:459–466. doi: 10.1165/rcmb.2008-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linnoila RI. Functional facets of the pulmonary neuroendocrine system. Lab Invest. 2006;86:425–444. doi: 10.1038/labinvest.3700412. [DOI] [PubMed] [Google Scholar]
- 28.Ross AJ, Dailey LA, Brighton LE, Devlin RB. Transcriptional profiling of mucociliary differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol. 2007;37:169–185. doi: 10.1165/rcmb.2006-0466OC. [DOI] [PubMed] [Google Scholar]
- 29.Gu X, Karp PH, Brody SL, Pierce RA, Welsh MJ, Holtzman MJ, et al. Chemosensory functions for pulmonary neuroendocrine cells. Am J Respir Cell Mol Biol. 2014;50:637–646. doi: 10.1165/rcmb.2013-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchel JA, Antoniak S, Lee JH, Kim SH, McGill M, Kasahara DI, et al. IL-13 augments compressive stress-induced tissue factor expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2016;54:524–531. doi: 10.1165/rcmb.2015-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007;36:244–253. doi: 10.1165/rcmb.2006-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomperts BN, Kim LJ, Flaherty SA, Hackett BP. IL-13 regulates cilia loss and foxj1 expression in human airway epithelium. Am J Respir Cell Mol Biol. 2007;37:339–346. doi: 10.1165/rcmb.2006-0400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chand HS, Harris JF, Tesfaigzi Y. IL-13 in LPS-induced inflammation causes Bcl-2 expression to sustain hyperplastic mucous cells. Sci Rep. 2018;8:436. doi: 10.1038/s41598-017-18884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin N, Kolliputi N, Gou D, Weng T, Liu L. A novel function of ionotropic γ-aminobutyric acid receptors involving alveolar fluid homeostasis. J Biol Chem. 2006;281:36012–36020. doi: 10.1074/jbc.M606895200. [DOI] [PubMed] [Google Scholar]
- 35.Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Annu Rev Physiol. 2008;70:405–429. doi: 10.1146/annurev.physiol.70.113006.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popler J, Gower WA, Mogayzel PJ, Jr, Nogee LM, Langston C, Wilson AC, et al. Familial neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2010;45:749–755. doi: 10.1002/ppul.21219. [DOI] [PubMed] [Google Scholar]
- 37.Young LR, Brody AS, Inge TH, Acton JD, Bokulic RE, Langston C, et al. Neuroendocrine cell distribution and frequency distinguish neuroendocrine cell hyperplasia of infancy from other pulmonary disorders. Chest. 2011;139:1060–1071. doi: 10.1378/chest.10-1304. [DOI] [PubMed] [Google Scholar]
- 38.Reyes LJ, Majó J, Perich D, Morell F. Neuroendocrine cell hyperplasia as an unusual form of interstitial lung disease. Respir Med. 2007;101:1840–1843. doi: 10.1016/j.rmed.2005.10.024. [DOI] [PubMed] [Google Scholar]