Abstract
Altered expression of syndecan-2 (SDC2), a heparan sulfate proteoglycan, has been associated with diverse types of human cancers. However, the mechanisms by which SDC2 may contribute to the pathobiology of lung adenocarcinoma have not been previously explored. SDC2 levels were measured in human lung adenocarcinoma samples and lung cancer tissue microarrays using immunohistochemistry and real-time PCR. To understand the role of SDC2 in vitro, SDC2 was silenced or overexpressed in A549 lung adenocarcinoma cells. The invasive capacity of cells was assessed using Matrigel invasion assays and measuring matrix metalloproteinase (MMP) 9 expression. Finally, we assessed tumor growth and metastasis of SDC2-deficient A549 cells in a xenograft tumor model. SDC2 expression was upregulated in malignant epithelial cells and macrophages obtained from human lung adenocarcinomas. Silencing of SDC2 decreased MMP9 expression and attenuated the invasive capacity of A549 lung adenocarcinoma cells. The inhibitory effect of SDC2 silencing on MMP9 expression and cell invasion was reversed by overexpression of MMP9 and syntenin-1. SDC2 silencing attenuated NF-κB p65 subunit nuclear translocation and its binding to the MMP9 promoter, which were restored by overexpression of syntenin-1. SDC2 silencing in vivo reduced tumor mass volume and metastasis. These findings suggest that SDC2 plays an important role in the invasive properties of lung adenocarcinoma cells and that its effects are mediated by syntenin-1. Thus, inhibiting SDC2 expression or activity could serve as a potential therapeutic target to treat lung adenocarcinoma.
Keywords: syndecan-2, syntenin-1, lung adenocarcinoma, metastasis
Lung cancer is the leading cause of cancer mortality in both men and women in the United States (1) and worldwide. An estimated 234,030 new lung cancer cases and 154,050 lung cancer deaths are projected to occur in the United States in 2018 (2). Despite advances in surgical techniques and novel therapeutic interventions, such as targeted therapy and immune checkpoint blockade, 5-year survival remains elevated at 17% (3), and the molecular mechanisms that drive lung tumorigenesis continue to be poorly understood.
Syndecans (SDCs), a family of type 1 transmembrane heparan sulfate proteoglycans, consisting of four members (SDC1–4), have been implicated in the regulation of several types of cancer (4–7). SDCs play a role in the control of cell adhesion, survival, proliferation, migration, and differentiation. These pleiotropic activities are mediated by interactions of the SDC ectodomain and its glycosaminoglycan chains with extracellular matrix and cell surface proteins, proteases, cytokines, and their receptors. Some of these interactions are transduced through a single transmembrane domain to a short, highly conserved, cytoplasmic tail that tethers SDCs to intracellular structural proteins and signaling pathways (8).
SDC2 is mainly expressed in cells of mesenchymal origin, but has been shown to exert effects on several cell types. We have previously demonstrated the role of SDC2 in epithelial cells and fibroblasts in the setting of idiopathic pulmonary fibrosis (9) and radiation-induced pulmonary fibrosis (10), respectively. Overexpression of this proteoglycan has also been associated with the tumorigenic properties and poor prognosis of various malignancies, including colon (11, 12) and prostate adenocarcinomas (13, 14), melanoma (15, 16), and fibrosarcoma (17). It has been shown to also potentiate angiogenesis, as downregulation of SDC2 expression at the surface of microvascular endothelial cells reduces angiogenic sprouting (18–20). Conversely, elevated levels of SDC2 have been associated with decreased migration of human osteosarcoma cells and increased sensitivity to chemotherapy-induced apoptosis (21).
The role of SDC2 in lung adenocarcinoma, the most common form of lung cancer, has not been well characterized. Here, we demonstrate that SDC2 is highly expressed in human lung adenocarcinomas and contributes to cancer cell invasion, matrix metalloproteinase (MMP)-9 expression, tumor growth, and metastasis. We further show that this is mediated by syntenin-1 and NF-κB activation.
Methods
Human Samples
All human samples were obtained under Institutional Review Board–approved protocols at the University of New Mexico, the University of Pittsburgh Cancer Institute, or Brigham and Women’s Hospital. Control human lung samples without malignancy were obtained from donor lungs deemed unsuitable for transplantation.
Antibodies
Anti-MMP9 and anti–NF-κB (p65) antibodies were purchased from Cell Signaling Technology, anti-SDC2 from Thermo Scientific, and anti–syntenin-1 from Santa Cruz Biotechnology. All other antibodies and associated reagents were purchased from Sigma-Aldrich.
Immunohistochemistry
Immunostaining of lung tissue from lung biopsies and tissue arrays was performed as previously described (22). Lung Cancer Tissue MicroArrays were obtained from Pantomics (cat. nos. LUC481, LUC961, and LUC962). Briefly, sections were incubated in citric acid buffer (pH 6.0) at 95°C for 30 minutes for the purpose of antigen retrieval. Sections were then incubated in 1% BSA for blocking nonspecific binding of the antibody. A polyclonal antibody for SDC2 was prepared in dilution of 1:500 and applied to the sections overnight at 4°C. Sections were stained using the HRP-DAB System (Cell and Tissue Staining Kit) according to the manufacturer’s instructions, and then counterstained with hematoxylin and eosin. The amount of staining was scored from 1 to 4 (lowest to highest, respectively). The score was determined by staining intensity in conjunction with the percentage of cells staining positively, counting 10 random fields at 200× magnification in tissue sections. Slides were independently scored by three investigators using an Olympus BX40 microscope (Olympus).
Cell Culture
A549 cells were obtained from American Type Tissue Culture Collection and cultured according to the manufacturer’s instructions in Dulbecco’s modified Eagle medium supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (10 μg/ml) (Invitrogen).
Statistical Analysis
Data are expressed as means (±SEM). Comparisons of mortality were made by analyzing Kaplan-Meier survival curves and log-rank tests to assess for differences in survival. For comparisons between two groups, we used Student’s unpaired t test and statistical significance defined as P less than 0.05. One-way ANOVA followed by Newman-Keuls or Tukey’s post-test analysis, was used for analysis of more than two groups. The numbers of samples per group (n), or the numbers of experiments, are specified in the figure legends.
For additional details regarding materials and methods, please see the data supplement.
Results
SDC2 Expression Is Upregulated in Human Lung Adenocarcinoma
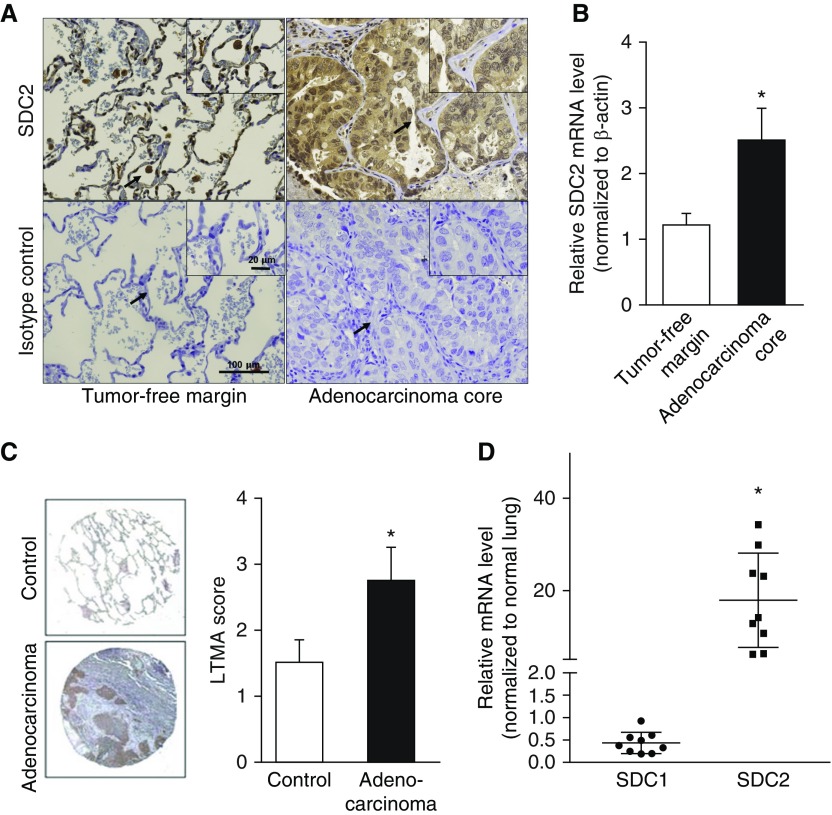
Immunostaining of lung tissue sections from patients with lung adenocarcinoma revealed that SDC2 was highly expressed in malignant epithelial cells in the tumor core, whereas, in the tumor-free margins, it was mainly expressed in macrophages (Figure 1A). SDC2 mRNA levels were also significantly elevated in the tumor core compared with the tumor-free margins (Figure 1B). We also assessed SDC2 expression using a Lung Cancer Tissue MicroArray and observed a significant increase in SDC2 staining in human lung adenocarcinoma tissue compared with control lung tissue (Figure 1C). SDC2 expression was also increased in other types of lung carcinomas (Figure E1 in the data supplement). To determine whether our findings were specific to SDC2, we measured syndecan-1 and SDC2 mRNA levels in lung adenocarcinoma tissue and control lungs without cancer (n = 9 per group). SDC2 mRNA was increased 5- to 35-fold in lung adenocarcinomas compared with controls (Figure 1D). In contrast, there was no significant difference in syndecan-1 mRNA expression.
Figure 1.
Syndecan (SDC)-2 is overexpressed in human lung adenocarcinoma. (A) SDC2 expression in lung adenocarcinoma tumor core and tumor-free margins was assessed by immunohistochemistry. SDC2 expression was detected in tumor-affected epithelium (upper right panel) and tissue-associated macrophages in tumor-free margins (upper left panel). Representative images are shown. The arrows indicate the area of magnification. (B) SDC2 mRNA levels were elevated in tumor core samples compared with tumor-free margin samples (n = 16; *P < 0.05). (C) Anti–human SDC2 antibody was applied to a Lung Cancer Tissue MicroArray (LTMA) in control lung tissue (n = 8) and lung adenocarcinoma (n = 24). Staining intensity was scored from 1 to 4 (lowest to highest, respectively), showing increased staining in adenocarcinoma tissue compared with controls (*P < 0.05). Representative images are shown. (D) SDC1 and SDC2 mRNA levels were measured in human lungs with adenocarcinoma and control lungs without cancer (n = 9). Data represent fold increase of SDC2 expression relative to normal lung tissue. SDC2 levels, but not SDC1 levels, were increased 5- to 35-fold compared with controls (*P < 0.05). Scale bars: 20 μm and 100 μm.
Taken together, these findings demonstrate that SDC2 expression is upregulated in lungs from patients with lung adenocarcinoma, and that malignant epithelial cells are the main source of this heparan sulfate proteoglycan.
SDC2 Increases MMP9 Expression and Activity, and Is Associated with Enhanced Invasive Activity of A549 Lung Adenocarcinoma Cells
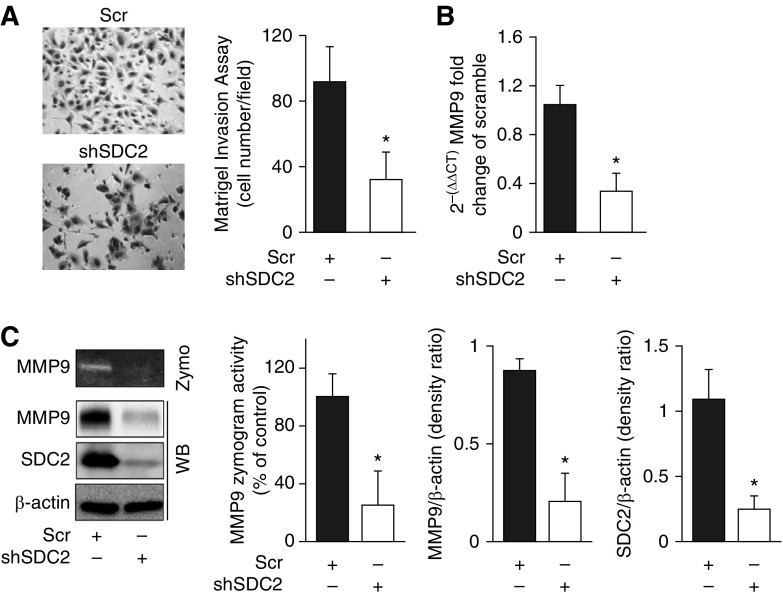
We next sought to establish the role of SDC2 in the malignant properties of lung adenocarcinoma. MMP9 has been strongly correlated with tumor malignant potential and poor patient survival, especially in lung cancer (23, 24). MMP9 is regulated by NF-κB signaling, which has been associated with poor prognosis in non–small-cell lung cancers (25). MMP9 inhibition has been reported to attenuate cancer cell invasion and metastasis (26, 27). We found an increased expression of SDC2 NF-κB (p65), and MMP9 in lung adenocarcinoma tissue (Figure E2). Although SDC2 has previously been shown to act as a docking protein for MMP7 in colon cancer (11), it is unknown whether it modulates MMP9 activity. Accordingly, we investigated the potential interaction between SDC2 and MMP9 in a lung adenocarcinoma cell line. Silencing of SDC2 in A549 cells reduced their invasive capacity, as determined by a Matrigel invasion assay (Figure 2A), significantly decreased MMP9 gene and protein expression, and attenuated its enzymatic activity (Figures 2B and 2C). SDC2 silencing had a similar effect on cell invasiveness and MMP9 expression in NCI-H23 cells, another lung adenocarcinoma cell line (Figure E3B).
Figure 2.
SDC2 potentiates matrix metalloproteinase (MMP) 9 expression and enzymatic activity and enhances cell invasion in A549 lung adenocarcinoma cells. A549 cells were transfected with lentiviral particles carrying either scrambled (scr) or SDC2 shRNAs. (A) Transfected cells were transferred onto Matrigel-coated inserts and incubated for 24 hours to detect invasion. The invaded cells were stained with hematoxylin and eosin. Cells transfected with shSDC2 had significantly less invasion compared with Scr-transfected controls. shSDC2-transfected cells had significantly lower MMP9 (B) gene expression, as determined by RT-PCR and (C) protein levels and enzymatic activity, as determined via Western blot (WB) and zymography (Zymo). The data are presented as mean ± SEM, n = 3/group, with testing by Student’s unpaired t test (*P < 0.05, significant comparisons vs. Scr).
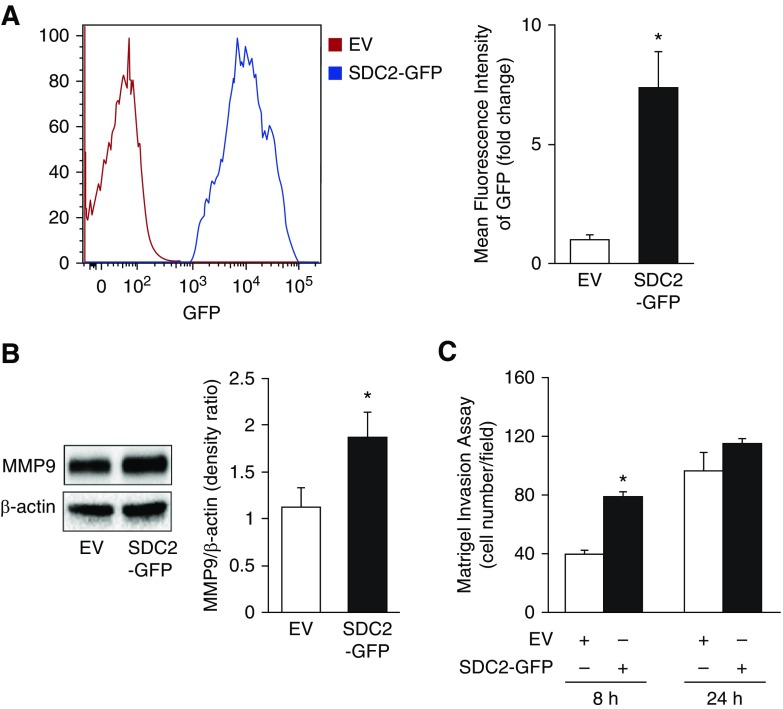
We hypothesized that SDC2 overexpression would conversely enhance MMP9 expression and cell invasion. A549 cells were lentivirally transfected with human SDC2 plasmids tagged with GFP (Figure 3A). Overexpression of SDC2 increased MMP9 expression and Matrigel invasion (Figures 3B and 3C). Cell invasion was significantly increased compared with controls at 8 hours, but not at 24 hours, possibly due to the high levels of endogenous SDC2 expressed in A549 cells, which may have diminished the early effects of SDC2 overexpression over time.
Figure 3.
Overexpression of SDC2 enhances MMP9 expression and A549 cell invasion. Cells were lentivirally transfected with empty vector (EV) or pLenti-SDC2-GFP (SDC2-GFP). (A) Transfection efficiency was confirmed by flow cytometry to detect GFP-positive cells. (B and C) Transfected cells were lysed and MMP9 expression and cell invasion were determined by Western blot or a Matrigel invasion assay, respectively. The data are presented as mean ± SEM, n = 3/group, with testing by Student’s unpaired t test (*P < 0.05, significant comparisons vs. EV).
MMP9 Regulates Cell Invasiveness and Overexpression Reverses SDC2-mediated Cell Invasion
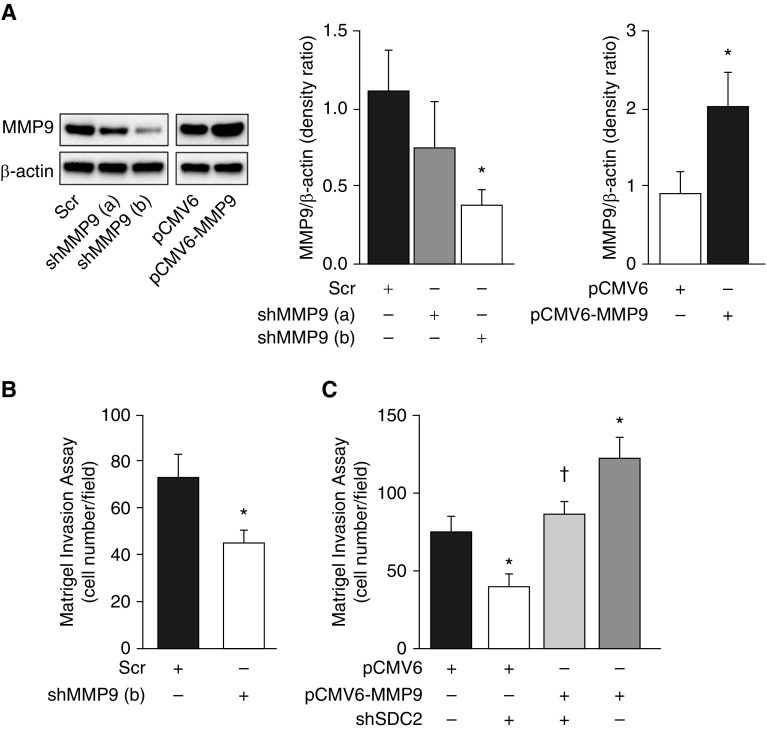
To confirm that MMP9 is an important contributor in lung adenocarcinoma invasiveness, we silenced MMP9 using shRNA lentiviral particles and found that this significantly reduced cell invasion in A549 cells (Figures 4A and 4B). Conversely, overexpression of MMP9 in A549 cells by pCMV6-MMP9 transfection enhanced cell invasion and completely reversed shSDC2-mediated inhibition of cell invasion (Figure 4C). These data suggest that MMP9 plays a critical role in A549 cell invasiveness and that SDC2 mediates cell invasion via regulation of MMP9.
Figure 4.
Silencing of MMP9 inhibits A549 cell invasion, whereas overexpression reverses shSDC2-mediated inhibition of cell invasion in A549 cells. (A) A549 cells were transfected with lentiviral particles carrying Scr or shMM9 (a and b) or transiently transfected with pCMV6 (EV) or pCMV6-MMP9. Cells were lysed and MMP9 expression was measured via Western blot. (B) The invasive capacity of Scr and shMMP9 (b)-transfected cells was measured using a Matrigel invasion assay. (C) Overexpression of MMP9 restored the invasive capacity of cells transfected with shSDC2. The data are presented as mean ± SEM, n = 3/group, with testing by Student’s unpaired t test or by one-way ANOVA (*P < 0.05, significant comparisons vs. EV; †P < 0.05, significant comparisons versus EV+shSDC2).
Syntenin-1 Facilitates SDC2-mediated MMP9 Expression and Cell Invasion
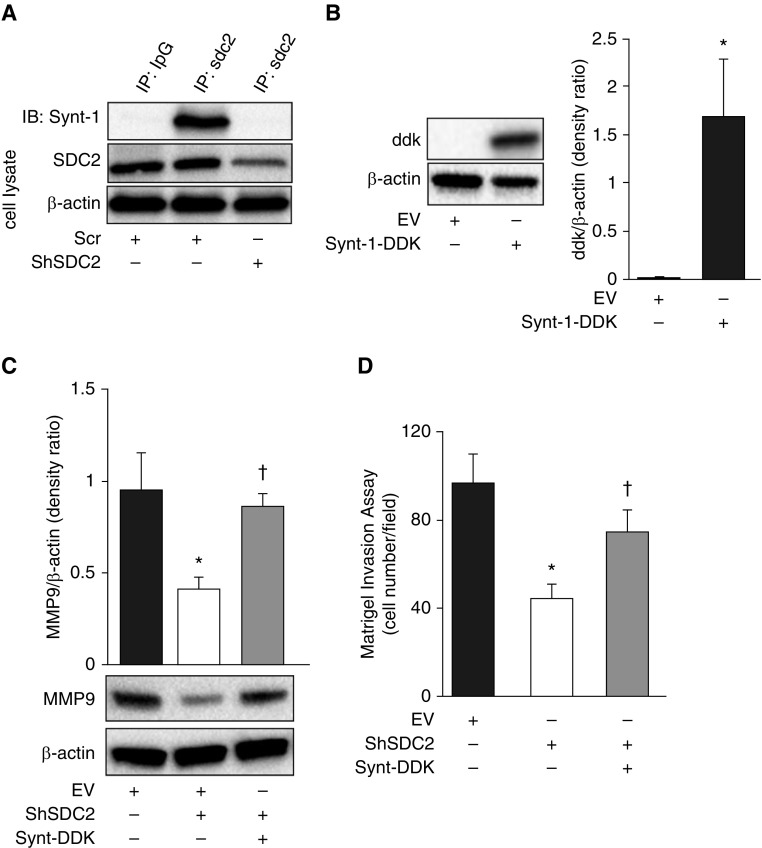
Syntenin-1 is an adaptor molecule involved in diverse cellular processes, including protein trafficking, cell adhesion and tumorigenesis. It has been previously shown that syntenin-1 can be activated by binding to the PDZ region of the cytosolic tail of SDC2 which, in turn, activates a variety of signaling molecules, such as mitogen-activated protein kinases and NF-κB (28–30). We confirmed that syntenin-1 binds to SDC2 in A549 cells, and that this binding is abolished by silencing of SDC2 (Figure 5A). We then assessed whether syntenin-1 mediates the invasive effects of SDC2 in A549 cells. Overexpression of syntenin-1 almost completely restored MMP9 expression and the invasive capacity of cells in which SDC2 was silenced, suggesting that syntenin-1 is necessary for SDC2-mediated invasion (Figures 5B–5D).
Figure 5.
Syntenin-1 overexpression attenuates shSDC2–mediated effects in A549 cells. (A) Scr and shSDC2-transfected cells were lysed and subjected to protein IP by anti-SDC2 antibody and immunoblotted against syntenin-1 (synt-1). (B) Cells were lentivirally transfected with pLenti-Syntenin-1-C-Myc-DDK (Synt-1-DDK) or pLenti-C-Myc-DDK (EV). Western blotting was used to measure syntenin-1 expression in cell lysates. (C) MMP9 expression was measured in cells transfected with shSDC2 with or without Synt-DDK. Cotransfection with Synt-1–DDK restored expression of MMP9 in cells transfected with shSDC2. (D) Cotransfection with Synt-1–DDK almost completely restored the invasive capacity of cells transfected with shSDC2. The data are presented as mean ± SEM, n = 3/group, with testing by Student’s unpaired t test or by one-way ANOVA (*P < 0.05, significant comparisons vs. EV; †P < 0.05, significant comparisons vs. EV+shSDC2).
SDC2 Regulates NF-κB Activation through Syntenin-1
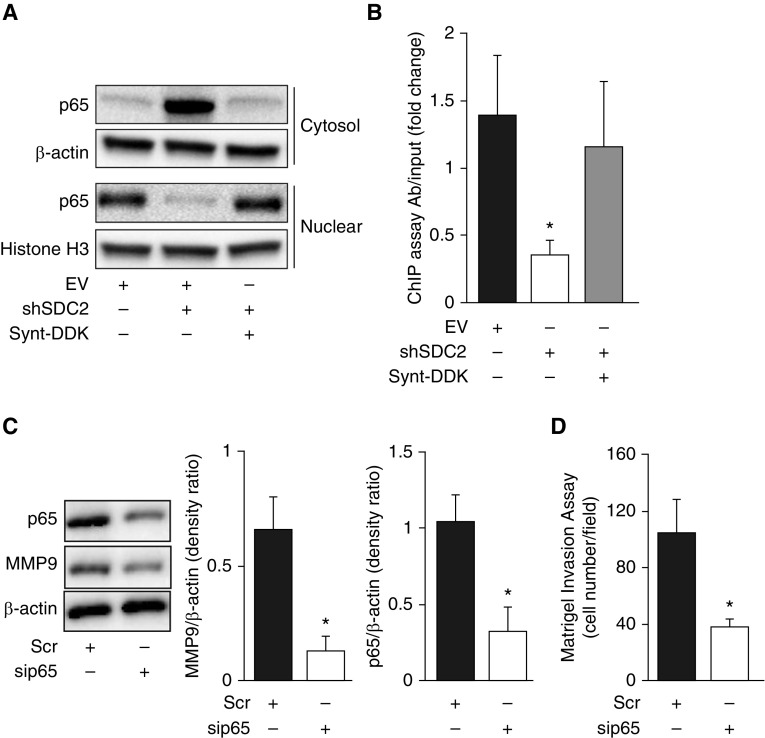
It has previously been suggested that syntenin-1 may be involved in the activation of NF-κB (31, 32), a master regulator of cancer cell metastasis and gene expression. In light of our findings showing that NF-κB expression is elevated in human lung adenocarcinomas (Figure E2) and that SDC2 regulates A549 cell invasion via syntenin-1, we asked whether SDC2 also modulates NF-κB activation. As shown in Figure 6A, nuclear translocation of the p65 subunit of NF-κB was significantly lower in shSDC2-transfected A549 cells than in scramble-transfected cells. Syntenin-1 overexpression restored p65 nuclear translocation. Furthermore, silencing of SDC2 significantly attenuated p65 binding to the MMP9 promoter, but not in cells overexpressing syntenin-1 (Figure 6B). Knockdown of p65 significantly reduced MMP9 expression and cell invasion (Figures 6C and 6D). These data strongly suggest that SDC2 regulates MMP9 expression via syntenin-1 and NF-κB activation to facilitate cell invasion.
Figure 6.
Silencing of SDC2 inhibits nuclear accumulation of NF-κB subunit p65 and its binding to the MMP9 promoter in A549 cells. Cells were lentivirally transfected with shSDC2 with or without Synt-1–DDK. (A) Cell lysates underwent nuclear/cytosol fractionation to measure p65 localization. (B) A chromatin immunoprecipitation (ChIP) assay was used to measure p65 binding to the MMP9 promoter. (C and D) Cells were transiently transfected with scr or sip65 RNAs for 24 hours before being collected to measure MMP9 expression or for the Matrigel invasion assay. The data are presented as mean ± SEM, n = 3/group, with testing by Student’s unpaired t test (*P < 0.05, significant comparisons vs. EV or Scr).
SDC2 Silencing Attenuates Growth and Metastasis of A549 Cells in Severe Combined Immunodeficient Mice
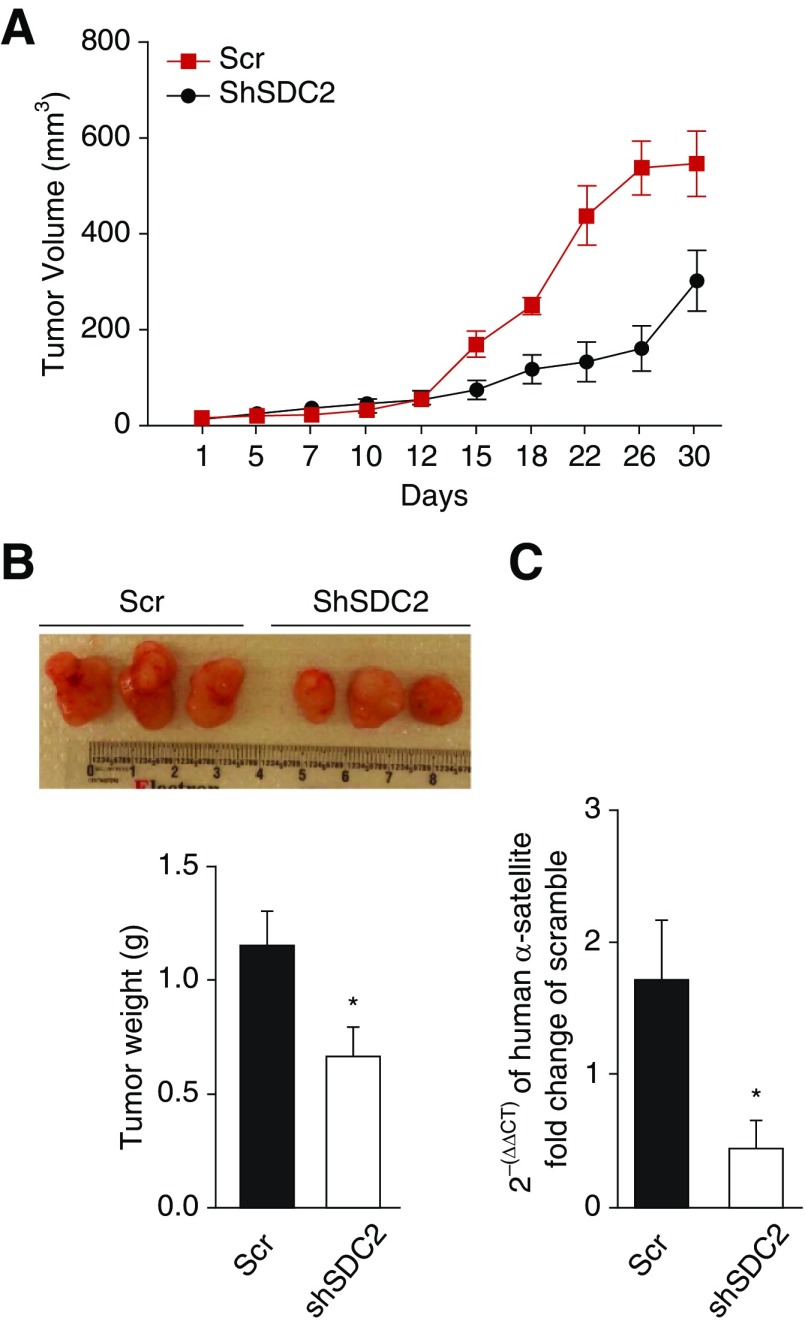
To evaluate the effect of SDC2 on tumor progression in vivo, scramble or shSDC2-transfected A549 adenocarcinoma cells were subcutaneously injected into severe combined immunodeficient mice. Consistent with our in vitro findings, silencing of SDC2 with targeted shRNA significantly attenuated tumor growth (Figures 7A and 7B) and micrometastasis to the lungs (Figure 7C) in severe combined immunodeficient mice, further highlighting the role of SDC2 in potentiating adenocarcinoma cell invasion.
Figure 7.
Silencing of SDC2 attenuates A549 cell tumor growth and micrometastasis in a xenograft tumor model. A total of 12 mice were randomly assigned to two groups and subcutaneously injected with 3 × 106 Scr A549 cells or shSDC2 A549 cells (n = 6 per group). (A) Tumor volume was measured every 2–3 days for 30 days. The mean tumor volume ± SEM is shown. *P < 0.05 vs. shSDC2. (B) Tumors were excised and weighed at Day 30 after cell injection. Representative specimens are shown. (C) Micrometastasis of A549 cells to the lung was assessed by measuring human DNA in mouse lungs using RT-PCR, as described in Methods. The data are presented as mean ± SEM, n = 6/group, with testing by Student’s unpaired t test (*P < 0.05, significant comparisons vs. shSDC2).
Discussion
Tumor metastasis is the primary cause of morbidity and mortality in patients with cancer (33). It is a complex process in which diverse interactions between cancer cells and their microenvironment enable malignant cells to invade the tissue surrounding the primary neoplasm, enter the circulation via the bloodstream or lymphatics, and populate distant locations (33). Proteolysis of tissue barriers is an essential component of the invasive process and has been linked to the production of degradative enzymes by cancer cells, the most intensively studied being MMPs (34). MMPs are a group of proteins with a variety of cellular and extracellular functions, including extracellular matrix degradation (24). To date, 23 different MMPs have been identified in humans. MMP9, also known as gelatinase B, has been shown to play a prominent role in tumor malignant potential and lung-specific metastasis (23, 35). Higher levels of MMP9 are correlated with poor patient prognosis in non–small-cell lung cancer (36), and MMP9 inhibition has been reported to attenuate cancer cell invasion and metastasis (26, 27).
Syndecans interact with multiple proteins in the extracellular environment, including cytokines and their receptors, through their highly divergent ectodomains (37). High levels of SDC2 have been detected in diverse cancers, and are associated with cancer cell migration, invasion, and proliferation. On the other hand, shed SDC2 has been shown to inhibit angiogenesis, a critical feature of metastasis, by activating membrane-bound protein tyrosine phosphotase eta (38). The role of SDC2 in lung adenocarcinoma has not been extensively studied (39).
In this study, we report, for the first time, that SDC2 is significantly elevated in malignant lung epithelial cells of patients with lung adenocarcinoma as well as other lung cancer types. Using loss- and gain-of-function experiments, we demonstrate that SDC2 plays an important role in lung adenocarcinoma cell invasion and MMP9 expression and activity. Furthermore, silencing of SDC2 in A549 cells significantly reduced tumor growth and cancer cell metastasis in a xenograft tumor model. Interestingly, SDC2 was also detected in the tumor stroma of lung adenocarcinoma (Figure 1A). Elevated expression of syndecan-1 has been observed in tumor stromal cells and is associated with poor prognosis (40), and expression of syndecan-1 in fibroblasts enhances tumor growth and angiogenesis in vivo and in vitro (41). Future studies should be dedicated to evaluate the role of SDC2 in cancer-associated stromal cells, as well as to correlate SDC2 expression to lung adenocarcinoma histological subtypes, lymphovascular invasion, metastases, and clinical outcomes.
In contrast to our findings, Munesue and colleagues (42) demonstrated that overexpression of SDC2 is associated with decreased metastatic potential in cells cloned from Lewis lung carcinoma 3LL by suppressing the activity of MMP2. However, the 3LL clones used in these experiments only expressed MMP2 in the extracellular matrix, suggesting that the biological effects of SDC2 are highly dependent on their interactions with specific heparin-binding extracellular ligands, and that the balance between MMP2 and MMP9 expression may be critical in determining the role of SDC2 in the tumor microenvironment.
The cytoplasmic domain of SDC2 has been shown to interact with different adapter proteins. These include PDZ proteins, such as syntenin. Syntenin, a PDZ domain–containing adapter protein, originally identified as a syndecan-binding protein, is known to be involved in the organization of protein complexes in the plasma membrane. Syntenin plays a well-described role in cell migration and invasion in different cancer cell types (30–32, 43, 44). In this study, we demonstrate that syntenin-1 interacts with SDC2 and that overexpression of syntenin-1 restores the invasive phenotype in SDC2-silenced lung adenocarcinoma cells. It has recently been shown that syntenin-1 tightly regulates the epithelial–mesenchymal transition in transforming growth factor-β–induced lung adenocarcinoma epithelial cells (45, 46). Further investigation is needed to understand the role of SDC2 in epithelial–mesenchymal transition.
Previous reports suggest that the prometastatic role of syntenin is mediated by NF-κB activation (44, 45). NF-κB is well established as a master regulator of multiple genes involved in cancer cell metastasis, including MMPs (47). Our findings clearly demonstrate that SDC2 regulates the NF-κB p65 subunit cytosol to nuclear translocation and its binding to the MMP9 promoter via syntenin-1. This further supports the role of SDC2 in enhancing the invasive capacity of lung cancer cells.
Taken together, these findings demonstrate the important role of SDC2 in mediating lung adenocarcinoma cell invasion, and highlight its potential as a target for therapeutic interventions to treat lung adenocarcinomas.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Yuanyuan Shi and Xiaomeng Tang for their technical support.
Footnotes
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) grant P01 HL114501 (I.O.R.), NHLBI grant T32 5T32HL007633-32 (K.T.), and National Cancer Institute Cancer Center support grant P30CA11800 (S.A.B.).
Author Contributions: K.T., J.C.O., S.G.C., and I.O.R. designed the study; K.T., J.C.O., I.E.F., S.P.D.F., L.S., Y.C., C.S.T., and S.A.B. performed the experiments, contributed materials, and analyzed data; J.M.S., M.A.P., and S.E.-C. contributed intellectual input; K.T., J.C.O., S.G.C., and I.O.R. wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0118OC on December 18, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 4.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labropoulou VT, Skandalis SS, Ravazoula P, Perimenis P, Karamanos NK, Kalofonos HP, et al. Expression of syndecan-4 and correlation with metastatic potential in testicular germ cell tumours. BioMed Res Int. 2013;2013:214864. doi: 10.1155/2013/214864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suhovskih AV, Mostovich LA, Kunin IS, Boboev MM, Nepomnyashchikh GI, Aidagulova SV, et al. Proteoglycan expression in normal human prostate tissue and prostate cancer. ISRN Oncol. 2013;2013:680136. doi: 10.1155/2013/680136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen TL, Grizzle WE, Zhang K, Hameed O, Siegal GP, Wei S. Syndecan-1 overexpression is associated with nonluminal subtypes and poor prognosis in advanced breast cancer. Am J Clin Pathol. 2013;140:468–474. doi: 10.1309/AJCPZ1D8CALHDXCJ. [DOI] [PubMed] [Google Scholar]
- 8.Choi Y, Chung H, Jung H, Couchman JR, Oh ES. Syndecans as cell surface receptors: unique structure equates with functional diversity. Matrix Biol. 2011;30:93–99. doi: 10.1016/j.matbio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Gochuico BR, Yu G, Tang X, Osorio JC, Fernandez IE, et al. Syndecan-2 exerts antifibrotic effects by promoting caveolin-1–mediated transforming growth factor-β receptor I internalization and inhibiting transforming growth factor-β1 signaling. Am J Respir Crit Care Med. 2013;188:831–841. doi: 10.1164/rccm.201303-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsoyi K, Chu SG, Patino-Jaramillo NG, Wilder J, Villalba J, Doyle-Eisele M, et al. Syndecan-2 attenuates radiation-induced pulmonary fibrosis and inhibits fibroblast activation by regulating PI3K/Akt/ROCK pathway via CD148. Am J Respir Cell Mol Biol. 2018;58:208–215. doi: 10.1165/rcmb.2017-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park H, Kim Y, Lim Y, Han I, Oh ES. Syndecan-2 mediates adhesion and proliferation of colon carcinoma cells. J Biol Chem. 2002;277:29730–29736. doi: 10.1074/jbc.M202435200. [DOI] [PubMed] [Google Scholar]
- 12.Choi S, Kim JY, Park JH, Lee ST, Han IO, Oh ES. The matrix metalloproteinase-7 regulates the extracellular shedding of syndecan-2 from colon cancer cells. Biochem Biophys Res Commun. 2012;417:1260–1264. doi: 10.1016/j.bbrc.2011.12.120. [DOI] [PubMed] [Google Scholar]
- 13.Contreras HR, Ledezma RA, Vergara J, Cifuentes F, Barra C, Cabello P, et al. The expression of syndecan-1 and -2 is associated with Gleason score and epithelial–mesenchymal transition markers, E-cadherin and β-catenin, in prostate cancer. Urol Oncol. 2010;28:534–540. doi: 10.1016/j.urolonc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Popović A, Demirović A, Spajić B, Stimac G, Kruslin B, Tomas D. Expression and prognostic role of syndecan-2 in prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:78–82. doi: 10.1038/pcan.2009.43. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Park H, Chung H, Choi S, Kim Y, Yoo H, et al. Syndecan-2 regulates the migratory potential of melanoma cells. J Biol Chem. 2009;284:27167–27175. doi: 10.1074/jbc.M109.034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baljinnyam E, Iwatsubo K, Kurotani R, Wang X, Ulucan C, Iwatsubo M, et al. Epac increases melanoma cell migration by a heparan sulfate–related mechanism. Am J Physiol Cell Physiol. 2009;297:C802–C813. doi: 10.1152/ajpcell.00129.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park H, Han I, Kwon HJ, Oh ES. Focal adhesion kinase regulates syndecan-2–mediated tumorigenic activity of HT1080 fibrosarcoma cells. Cancer Res. 2005;65:9899–9905. doi: 10.1158/0008-5472.CAN-05-1386. [DOI] [PubMed] [Google Scholar]
- 18.Fears CY, Gladson CL, Woods A. Syndecan-2 is expressed in the microvasculature of gliomas and regulates angiogenic processes in microvascular endothelial cells. J Biol Chem. 2006;281:14533–14536. doi: 10.1074/jbc.C600075200. [DOI] [PubMed] [Google Scholar]
- 19.Chen E, Hermanson S, Ekker SC. Syndecan-2 is essential for angiogenic sprouting during zebrafish development. Blood. 2004;103:1710–1719. doi: 10.1182/blood-2003-06-1783. [DOI] [PubMed] [Google Scholar]
- 20.Noguer O, Villena J, Lorita J, Vilaró S, Reina M. Syndecan-2 downregulation impairs angiogenesis in human microvascular endothelial cells. Exp Cell Res. 2009;315:795–808. doi: 10.1016/j.yexcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Orosco A, Fromigué O, Bazille C, Entz-Werle N, Levillain P, Marie PJ, et al. Syndecan-2 affects the basal and chemotherapy-induced apoptosis in osteosarcoma. Cancer Res. 2007;67:3708–3715. doi: 10.1158/0008-5472.CAN-06-4164. [DOI] [PubMed] [Google Scholar]
- 22.Valencia JC, Pacheco-Rodriguez G, Carmona AK, Xavier J, Bruneval P, Riemenschneider WK, et al. Tissue-specific renin–angiotensin system in pulmonary lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2006;35:40–47. doi: 10.1165/rcmb.2005-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Kempen LC, Coussens LM. MMP9 potentiates pulmonary metastasis formation. Cancer Cell. 2002;2:251–252. doi: 10.1016/s1535-6108(02)00157-5. [DOI] [PubMed] [Google Scholar]
- 24.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 25.Gu L, Wang Z, Zuo J, Li H, Zha L. Prognostic significance of NF-κB expression in non–small cell lung cancer: a meta-analysis. PLoS One. 2018;13:e0198223. doi: 10.1371/journal.pone.0198223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YM, Tsoyi K, Jang HJ, Park EJ, Park SW, Kim HJ, et al. CKD712, a synthetic isoquinoline alkaloid, enhances the anti-cancer effects of paclitaxel in MDA-MB-231 cells through regulation of PTEN. Life Sci. 2014;112:49–58. doi: 10.1016/j.lfs.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Li G, He Y, Yao J, Huang C, Song X, Deng Y, et al. Angelicin inhibits human lung carcinoma A549 cell growth and migration through regulating JNK and ERK pathways. Oncol Rep. 2016;36:3504–3512. doi: 10.3892/or.2016.5166. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann P, Tomatis D, Rosas M, Grootjans J, Leenaerts I, Degeest G, et al. Characterization of syntenin, a syndecan-binding PDZ protein, as a component of cell adhesion sites and microfilaments. Mol Biol Cell. 2001;12:339–350. doi: 10.1091/mbc.12.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Dürr J, et al. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci USA. 1997;94:13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kegelman TP, Das SK, Hu B, Bacolod MD, Fuller CE, Menezes ME, et al. MDA-9/syntenin is a key regulator of glioma pathogenesis. Neuro Oncol. 2014;16:50–61. doi: 10.1093/neuonc/not157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boukerche H, Aissaoui H, Prévost C, Hirbec H, Das SK, Su ZZ, et al. Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-κB. Oncogene. 2010;29:3054–3066. doi: 10.1038/onc.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boukerche H, Su ZZ, Emdad L, Sarkar D, Fisher PB. MDA-9/syntenin regulates the metastatic phenotype in human melanoma cells by activating nuclear factor-κB. Cancer Res. 2007;67:1812–1822. doi: 10.1158/0008-5472.CAN-06-3875. [DOI] [PubMed] [Google Scholar]
- 33.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 34.Herszényi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci. 2012;13:13240–13263. doi: 10.3390/ijms131013240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 36.Gong L, Wu D, Zou J, Chen J, Chen L, Chen Y, et al. Prognostic impact of serum and tissue MMP-9 in non–small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7:18458–18468. doi: 10.18632/oncotarget.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonova EI, Galzitskaya OV. Cell communication using intrinsically disordered proteins: what can syndecans say? J Biomol Struct Dyn. 2015;33:1037–1050. doi: 10.1080/07391102.2014.926256. [DOI] [PubMed] [Google Scholar]
- 38.De Rossi G, Evans AR, Kay E, Woodfin A, McKay TR, Nourshargh S, et al. Shed syndecan-2 inhibits angiogenesis. J Cell Sci. 2014;127:4788–4799. doi: 10.1242/jcs.153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munesue S, Kusano Y, Oguri K, Itano N, Yoshitomi Y, Nakanishi H, et al. The role of syndecan-2 in regulation of actin-cytoskeletal organization of Lewis lung carcinoma–derived metastatic clones. Biochem J. 2002;363:201–209. doi: 10.1042/0264-6021:3630201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szarvas T, Reis H, Kramer G, Shariat SF, Vom Dorp F, Tschirdewahn S, et al. Enhanced stromal syndecan-1 expression is an independent risk factor for poor survival in bladder cancer. Hum Pathol. 2014;45:674–682. doi: 10.1016/j.humpath.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 41.Maeda T, Desouky J, Friedl A. Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene. 2006;25:1408–1412. doi: 10.1038/sj.onc.1209168. [DOI] [PubMed] [Google Scholar]
- 42.Munesue S, Yoshitomi Y, Kusano Y, Koyama Y, Nishiyama A, Nakanishi H, et al. A novel function of syndecan-2, suppression of matrix metalloproteinase-2 activation, which causes suppression of metastasis. J Biol Chem. 2007;282:28164–28174. doi: 10.1074/jbc.M609812200. [DOI] [PubMed] [Google Scholar]
- 43.Das SK, Guo C, Pradhan AK, Bhoopathi P, Talukdar S, Shen XN, et al. Knockout of MDA-9/syntenin (SDCBP) expression in the microenvironment dampens tumor-supporting inflammation and inhibits melanoma metastasis. Oncotarget. 2016;7:46848–46861. doi: 10.18632/oncotarget.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talukdar S, Das SK, Pradhan AK, Emdad L, Shen XN, Windle JJ, et al. Novel function of MDA-9/syntenin (SDCBP) as a regulator of survival and stemness in glioma stem cells. Oncotarget. 2016;7:54102–54119. doi: 10.18632/oncotarget.10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwangbo C, Tae N, Lee S, Kim O, Park OK, Kim J, et al. Syntenin regulates TGF-β1–induced Smad activation and the epithelial-to-mesenchymal transition by inhibiting caveolin-mediated TGF-β type I receptor internalization. Oncogene. 2016;35:389–401. doi: 10.1038/onc.2015.100. [DOI] [PubMed] [Google Scholar]
- 46.Wang LK, Pan SH, Chang YL, Hung PF, Kao SH, Wang WL, et al. MDA-9/syntenin-slug transcriptional complex promote epithelial–mesenchymal transition and invasion/metastasis in lung adenocarcinoma. Oncotarget. 2016;7:386–401. doi: 10.18632/oncotarget.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2:823–830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.