Cystic fibrosis (CF) is a genetic disease characterized by the inheritance of two defective genes encoding CF transmembrane conductance regulator (CFTR), an anion channel that is responsible for conducting chloride (Cl−) and bicarbonate (HCO3−) ions across the epithelia (1). Although CFTR variants are causal for CF, the functional consequences of these variants can be impacted by other disease-modifying genes that may encode additional ion transporters in airway epithelial cells (2). As a result of complex CF phenotypes due to diverse genotypes, combinatorial pharmacology for optimal management of CF is becoming the norm for each patient (3). The advent of CFTR potentiators and correctors has ushered in a new area of pharmacological intervention in patients with CF that can result in increased lung function and quality of life (4). Because of their efficacy, CFTR-targeting pharmacological treatments have been revolutionary for patients, but they should not overshadow complementary pharmacological approaches that could address distinct components of CF pathology, such as inhibitors of the epithelium sodium channel (5), phosphodiesterase 4 (6), and ATP-binding cassette transporter C4 (7, 8). In this issue of the Journal, Kim and colleagues (pp. 705–716) report that pendrin (SCL26A4), a member of the solute carrier family of proteins, represents an additional candidate target that may be important for normalization of ion and second messenger function in epithelial cells from individuals with CF (9).
A key pathological feature of CF airways is an abnormal airway surface lining (ASL) fluid composition and volume resulting from dysregulated ion transport into and out of airway epithelial cells (2). Although Cl− and Na+ have been implicated in regulating ASL fluid volume, HCO3− is a key ion that regulates pH and needs to be precisely controlled for optimal host defenses against inhaled pathogens (2). HCO3− has long been recognized as being regulated by CFTR and transmembrane member 16A (TMEM16A) (2), and now Kim and colleagues mechanistically define pendrin as an additional player involved in HCO3− ion transport and ASL fluid volume and pH regulation.
Kim and colleagues used primary human nasal and bronchial epithelia to demonstrate the ability of pendrin to work with CFTR to regulate ion transport and fluid secretion (9). Using a combination of fresh human lung samples, isolated primary cells, immunostaining, gene expression analysis, intracellular pH measurements, and short-circuit current recordings, they showed that pendrin and CFTR colocalized in the apical membrane of ciliated epithelial cells, and that pendrin was upregulated by IL-4 exposure, resulting in changes in intracellular and ASL fluid pH and volume.
The demonstration that pendrin can function as a HCO3− transporter increases our understanding of how ASL fluid pH and volume are regulated, which in turn can impact the viscosity and activity of antimicrobial host defense peptides secreted by airway epithelial cells (10, 11). Although there is controversy regarding the presence of an acidified ASL fluid in CF (12, 13), the findings of Kim and colleagues are independent of this debate because the majority of their studies focused on airway epithelial cells from healthy subjects. To elucidate pendrin’s role in the context of lung health and disease, investigations of the relationship between epithelial cell–secreted ASL fluid pH, volume, and antimicrobial host defense peptide activities will also need to consider CFTR and TMEM16A (2).
A major strength of this study is the use of primary human airway epithelial cell samples and lung material for mechanistic and observational studies of pendrin biology. Indeed, the results were not fully conserved in the Calu-3 epithelial cell line, which is frequently used for CFTR studies, demonstrating the importance of considering the cell type when interrogating pendrin biology. Interestingly, the characterizations of pendrin from primary cell materials were reported to be consistent between donors, suggesting that patient-to-patient variability in pendrin function may be limited.
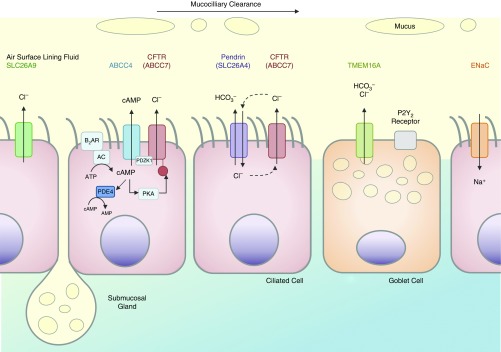
Despite the use of primary cells for the characterization of pendrin, only observational experiments of expression profiles were performed using samples from donors with CF. Characterization of pendrin’s function in donor samples from individuals carrying the f508 del or G551D CFTR variant would facilitate the translation of these findings to combinatorial pharmacology for CF management (3, 7). Importantly, it is not just pendrin that will need to be revisited in the context of CFTR variants, but also the (likely) complex interaction of the key ion transporters that regulate ASL fluid properties, such as the epithelium sodium channel, TMEM16A, and ATP-binding cassette transporter C4 (2, 7) (Figure 1).
Figure 1.
Airway epithelial cell ion transport mechanisms responsible for regulating airway surface lining fluid volume, pH, chloride concentration, and innate immune function of the respiratory mucosa, highlighting ciliated cells (pink) and goblet cells (orange). ABCC4 = ATP-binding cassette transporter C4; AC = adenylyl cyclase; β2AR = β2 adrenoceptor; CFTR = cystic fibrosis transmembrane conductance regulator; ENaC = epithelium sodium channel; P2Y2 = purinergic receptor subtype; PDE4 = phosphodiesterase 4; PDZK1 = PDZ domain containing 1; PKA = protein kinase A; SLC26A9 = solute carrier family 26 member 9; TMEM16A = transmembrane member 16A.
Although Kim and colleagues performed only a few mechanistic studies using CFTR variants, it should be emphasized that by design, their findings relate more broadly to chronic respiratory diseases beyond CF. Indeed, the use of the T-helper cell type 2 cytokine IL-4 to upregulate pendrin expression and activity in primary human airway epithelial cells from individuals with wild-type CFTR suggests that the biology of pendrin could be relevant in asthma. Pendrin expression and function have been implicated in chronic rhinosinusitis (14), further supporting the notion that this transporter plays a role in the regulation of epithelial cell secretory functions. Furthermore, TMEM16A is also upregulated by IL-4 and may work in tandem with pendrin on goblet cells and ciliated cells, respectively, to regulate mucin production in the lungs of individuals with asthma (15).
Translating the basic science results reported by Kim and colleagues to individualized patient care in CF (or other chronic respiratory diseases) will be a long road. By keeping an “ion” the prize and characterizing the fundamental biology of epithelial cell ion transport using clinically relevant primary human cell samples, Kim and colleagues have contributed to a strong foundation of knowledge required for downstream applications.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mall MA, Galietta LJ. Targeting ion channels in cystic fibrosis. J Cyst Fibros. 2015;14:561–570. doi: 10.1016/j.jcf.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell. 2016;27:424–433. doi: 10.1091/mbc.E14-04-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle MP, De Boeck K. A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. Lancet Respir Med. 2013;1:158–163. doi: 10.1016/S2213-2600(12)70057-7. [DOI] [PubMed] [Google Scholar]
- 5.Reihill JA, Walker B, Hamilton RA, Ferguson TE, Elborn JS, Stutts MJ, et al. Inhibition of protease-epithelial sodium channel signaling improves mucociliary function in cystic fibrosis airways. Am J Respir Crit Care Med. 2016;194:701–710. doi: 10.1164/rccm.201511-2216OC. [DOI] [PubMed] [Google Scholar]
- 6.Turner MJ, Matthes E, Billet A, Ferguson AJ, Thomas DY, Randell SH, et al. The dual phosphodiesterase 3 and 4 inhibitor RPL554 stimulates CFTR and ciliary beating in primary cultures of bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2016;310:L59–L70. doi: 10.1152/ajplung.00324.2015. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadi S, Bozoky Z, Di Paola M, Xia S, Li C, Wong AP, et al. Phenotypic profiling of CFTR modulators in patient-derived respiratory epithelia. NPJ Genom Med. 2017;2:12. doi: 10.1038/s41525-017-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conner GE, Ivonnet P, Gelin M, Whitney P, Salathe M. H2O2 stimulates cystic fibrosis transmembrane conductance regulator through an autocrine prostaglandin pathway, using multidrug-resistant protein-4. Am J Respir Cell Mol Biol. 2013;49:672–679. doi: 10.1165/rcmb.2013-0156OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D, Huang J, Billet A, Abu-Arish A, Goepp J, Matthes E, et al. Pendrin mediates bicarbonate secretion and enhances cystic fibrosis transmembrane conductance regulator function in airway surface epithelia. Am J Respir Cell Mol Biol. 2019;60:705–716. doi: 10.1165/rcmb.2018-0158OC. [DOI] [PubMed] [Google Scholar]
- 10.Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abou Alaiwa MH, Beer AM, Pezzulo AA, Launspach JL, Horan RA, Stoltz DA, et al. Neonates with cystic fibrosis have a reduced nasal liquid pH; a small pilot study. J Cyst Fibros. 2014;13:373–377. doi: 10.1016/j.jcf.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz A, Puvvadi R, Borisov SM, Shaw NC, Klimant I, Berry LJ, et al. Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat Commun. 2017;8:1409. doi: 10.1038/s41467-017-00532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seshadri S, Lu X, Purkey MR, Homma T, Choi AW, Carter R, et al. Increased expression of the epithelial anion transporter pendrin/SLC26A4 in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015;136:1548–1558, e7. doi: 10.1016/j.jaci.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorrieri G, Scudieri P, Caci E, Schiavon M, Tomati V, Sirci F, et al. Goblet cell hyperplasia requires high bicarbonate transport to support mucin release. Sci Rep. 2016;6:36016. doi: 10.1038/srep36016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.