Abstract
Chronic obstructive pulmonary disease–associated chronic inflammation has been shown to lead to an autoimmune phenotype characterized in part by the presence of lung autoreactive antibodies. We hypothesized that ischemia–reperfusion injury (IRI) liberates epitopes that would facilitate preexisting autoantibody binding, thereby exacerbating lung injury after transplant. We induced emphysema in C57BL/6 mice through 6 months of cigarette smoke (CS) exposure. Mice with CS exposure had significantly elevated serum autoantibodies compared with non–smoke-exposed age-matched (NS) mice. To determine the impact of a full preexisting autoantibody repertoire on IRI, we transplanted BALB/c donor lungs into NS or CS recipients and analyzed grafts 48 hours after transplant. CS recipients had significantly increased lung injury and immune cell infiltration after transplant. Immunofluorescence staining revealed increased IgM, IgG, and C3d deposition in CS recipients. To exclude confounding alloreactivity and confirm the role of preexisting autoantibodies in IRI, syngeneic Rag1−/− (recombination-activating protein 1–knockout) transplants were performed in which recipients were reconstituted with pooled serum from CS or NS mice. Serum from CS-exposed mice significantly increased IRI compared with control mice, with trends in antibody and C3d deposition similar to those seen in allografts. These data demonstrate that pretransplant CS exposure is associated with increased IgM/IgG autoantibodies, which, upon transplant, bind to the donor lung, activate complement, and exacerbate post-transplant IRI.
Keywords: lung transplant, ischemia–reperfusion injury, autoantibodies, complement, chronic obstructive pulmonary disease
Despite recent advances in the field of transplantation, outcomes for lung transplant (LTx) recipients remains disproportionately poor. The reasons for this stagnation in outcomes are complex, but at least one culprit that can be identified as a major factor is primary graft dysfunction (PGD). PGD occurs in one-third to one-half of all LTx recipients and is associated with as much as a 33% increase in 90-day mortality (1, 2). Although the pathogenesis of PGD is multifactorial, clear associations have been made between PGD and the severity of ischemia–reperfusion injury (IRI) (3), with the long-term implications manifesting in the form of increased risk and incidence of early-onset bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome, which are two manifestations of chronic lung allograft dysfunction (4). Given these clinical data, it is incumbent on the LTx community to identify the effector mechanisms driving these outcomes, beginning specifically with the earliest donor–recipient interactions and how they shape IRI.
Increasing evidence indicates a role for recipient autoimmunity in the pathogenesis of graft rejection (5, 6). The earliest injury to the transplanted lung occurs as a consequence of IRI, which induces a proinflammatory microenvironment that is capable of shaping the adaptive immune response. Ischemic insult followed by reperfusion leads to the exposure of neoepitopes expressed on stressed/injured cells that may well be recognized by preformed extracellular matrix (ECM) reactive antibodies, which bind and activate the complement system, resulting in inflammation and injury (7–9). Preformed tissue-specific antibodies have recently been demonstrated as a contributor to IRI and PGD (10, 11). Studies examining the presence of a select few non–human leukocyte antigen (non-HLA) antibodies (Kα-1 tubulin, collagen type V, and collagen type I) have demonstrated that up to 30% of LTx recipients have elevated antibodies and that this may have significant implications for development of PGD and BOS (12). These autoantibodies are unaccounted for in the cross-match process; however, they are believed to be major contributors to peritransplant injury because they readily bind to neoepitopes exposed by IRI (10, 12, 13).
Patients with chronic obstructive pulmonary disease (COPD)/emphysema and idiopathic interstitial pneumonia (IIP) make up more than half of all LTx recipients; yet, these two groups of patients have some of the poorest long-term survival statistics, with median survival of 5.6 and 4.8 years, respectively (14). These trends hold when controlling for 3-month and 1-year perioperative survival. Furthermore, patients with COPD who develop chronic lung allograft dysfunction phenotypes such as BOS also have a significantly higher risk of mortality than similar patients with differing underlying diagnoses (15). The question of why these patient populations do poorly is complex. It is worth noting that one of the striking commonalities between patients with emphysema and patients with IIP is the documented presence of a large array of circulating ECM non-HLA autoreactive autoantibodies (16, 17). As a result of chronic inflammation, these patients develop an underlying autoimmune phenotype characterized by increased lung inflammation, injury, and the production of ECM humoral and cellular autoreactive immune responses (18–20). Indeed, emphysema has been shown to induce a far more diverse spectrum of ECM autoantibodies directed toward lung-specific collagen, elastin, and decorin than the handful of non-HLA autoantibodies studied previously in LTx (19–21). The impact of these heterogeneous autoantibody populations on post-LTx outcomes is largely unknown.
In the present study, we hypothesized that non-HLA lung autoreactive antibodies generated as a consequence of emphysema pathogenesis promote post-LTx injury that leads to an exacerbated IRI phenotype. We used the well-established murine orthotopic left-lung transplant model to demonstrate not only that mice with significant cigarette smoke (CS) exposure experience exacerbated IRI but also that CS-related antibodies contribute to allograft injury. Finally, our findings describe a novel model system that can be used to model the impact of a broad and comprehensive autoreactive immune response on LTx outcomes in future studies.
Methods
Animals
BALB/c (H-2kd), C57BL/6 (H-2kb), and Rag1−/− (recombination-activating protein 1–knockout; H-2kb) mice (The Jackson Laboratory) were housed at the Medical University of South Carolina. All procedures were performed in accordance with institutional animal care guidelines. Eight-week-old C57BL/6 mice were exposed to CS for 6 months (22). Upon completion, C57BL/6 (CS) and non–smoke-exposed age-matched control mice (NS) received a left-lung transplant from BALB/c donors. Allografts were harvested 48 hours after LTx.
For reconstitution studies, Rag1−/− donor lungs were transplanted into Rag1−/− recipients. Thirty minutes before LTx, Rag1−/− recipients were inoculated with 200 μl of pooled serum from either CS or NS C57BL/6 mice. A subgroup of Rag1−/− recipients was reconstituted with pooled CS serum that had been depleted of specific antibody subtypes using a two-step process with IgG depleted by a protein G column (Santa Cruz Biotechnology), followed by IgM removal with anti-IgM microbeads (Thermo Fisher Scientific). IgM and IgG depletion was confirmed to be greater than 90% by ELISA (Bethyl Laboratories). For Rag1−/− transplants, recipient mice were killed 6 hours after LTx. Additional details on the methods used for micro–computed tomography (μ-CT), histology, autoantibodies, flow cytometry, and quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) are provided in the data supplement.
In Vitro Simulated Cold Storage and IRI
Mouse lung epithelial cells (MLE-15) and microvascular endothelial cells (Cedarlane) were cultured to confluence in microtiter ELISA plates and exposed to a simulated cold storage, hyperoxemia, and reperfusion process of LTx (23, 24). In brief, to recreate cold storage and reperfusion injury, media were replaced with Perfadex (XVIVO Perfusion), and cells were stored at 4°C for 18 hours in a sealed chamber containing 100% oxygen. Eighteen hours later, the Perfadex was removed and reperfused with 37°C culture media supplemented with 10% CS or NS pooled heat-inactivated serum for 6 hours, then incubated with either IgG or IgM (Bethyl Laboratories) primary antibodies and quantified by standard ELISA techniques. To determine antibody-mediated complement activity, experiments were modified such that after 6 hours of reperfusion with heat-inactivated NS or CS supplemented media, media were removed and then incubated with media containing freshly prepared 10% C6-deficient sera. C3 was quantified using anti-C3 antibody (Bethyl Laboratories) and detected as outlined above.
Statistics
Prism version 7.0 for Mac OS X software (GraphPad Software) was used for statistical analysis. Except when indicated, differences between groups were compared by use of one-way ANOVA with Dunnett’s multiple-comparisons test for post hoc analyses. For histological injury scores, the Kruskal-Wallis test was used, followed by post hoc analyses. P values less than 0.05 were considered significant. Values shown are mean ± SEM.
Results
CS Induces Autoreactive Antibodies
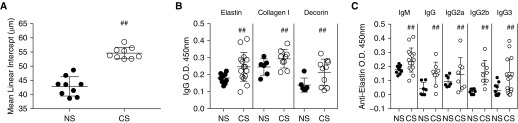
To confirm that our 6-month mouse model of CS exposure in C57BL/6 mice had pathophysiological features of emphysema, lungs were formalin inflated, and the presence of emphysema was confirmed by mean linear intercept measurements (Figure 1A). CS exposure resulted in an increased mean linear intercept as compared with age-matched NS control mice (55.6 μm vs. 42.4 μm; P < 0.01). Clinical COPD is associated with significantly increased production of a number of autoantibodies (25, 26). Consistent with previously published data (27), and to confirm the characteristics of our model system, we demonstrated in the present study that ECM-specific antibodies are upregulated in response to chronic CS exposure. Serum from the NS and CS cohorts was tested for specific ECM autoreactive antibodies. In CS mice, serum concentrations of antielastin, collagen type I, and decorin IgG antibodies were significantly elevated compared with NS mice (P < 0.05) (Figure 1B). Upon further analysis of the antielastin antibodies, IgM and IgG isotype analysis revealed that IgG subclasses IgG2 and IgG3 were elevated in CS mice. Of note, these immunoglobulin isotypes are associated with pronounced complement-activating ability (P < 0.05) (Figure 1C). Clinically, patients are required to quit smoking before selection for LTx; therefore, in the present study, we sought to determine whether smoking cessation altered the presence of ECM-specific antibodies in our model system. To test this, we modified our CS exposure protocol. Mice again received 6 months of CS exposure, after which they were returned to normal housing conditions for a period of 3 months. Measurement of antielastin antibodies demonstrated that cessation had no impact on antibody concentrations, with concentrations persisting through the smoking cessation period as compared with age-matched control mice (see Figure E1 in the data supplement).
Figure 1.
Prolonged cigarette smoke (CS) exposure promotes the induction of emphysema and the production of extracellular matrix autoantibodies in B6 mice. (A) Mean linear intercept of chronically CS-exposed mice demonstrates lung pathological changes associated with emphysema. ##P < 0.01. (B) Note the significant increase in antielastin, collagen I, and decorin IgG antibodies in chronically CS-exposed mice as compared with non–smoke-exposed age-matched control mice (NS), as determined by ELISA. ##P < 0.05. (C) Subtyping of elastin extracellular matrix antibodies and isotypes demonstrates significant increases in complement-activating antielastin IgM, IgG, IgG2, and IgG3 as measured by ELISA in CS as compared with NS. ##P < 0.05. O.D. = optical density.
CS Exposure in Recipient Mice Exacerbates IRI
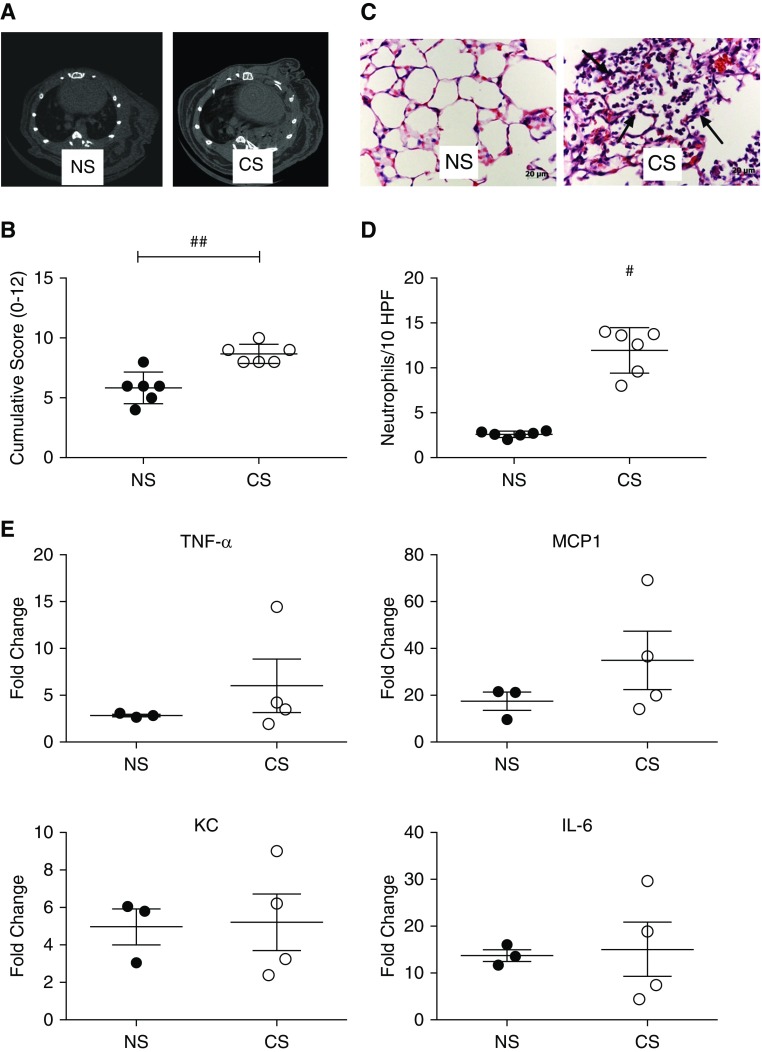
Fully allogeneic left-lung transplants were performed to investigate the impact of recipient CS exposure on peritransplant IRI. Age-matched transplants were performed into NS or CS mice. μ-CT performed 48 hours after LTx (Figure 2A) demonstrated the presence of minimal functional lung/airspace and dramatically increased opacification in CS recipients as compared with NS mice, suggestive of edema and inflammation. μ-CT− findings were corroborated by pathologic scoring performed on lungs harvested 48 hours after LTx. CS mice demonstrated significantly worse pathologic injury (Figures 2B and 2C). In keeping with our histopathological findings, immunohistochemistry for localization and quantification of neutrophils demonstrated that CS mice had significantly greater innate immune infiltrates (Figure 2D). Given the increased evidence of injury and inflammation, we performed qRT-PCR for key innate cytokines associated with IRI. Although increases were seen in CS as compared with NS for TNF-α and MCP-1 (monocyte chemoattractant protein 1), no significant differences were noted for any of the analyzed proinflammatory cytokines, which also included KC (keratinocyte chemoattractant) and IL-6 (Figure 2E).
Figure 2.
Pretransplant recipient chronic CS exposure exacerbates lung transplant ischemia–reperfusion injury. Prolonged recipient CS exposure predisposes to (A) increased radiographically detectable lung injury and (B) histologically scored injury. ##P < 0.01. Representative histological images of lung injury show increased neutrophilic infiltrates, alveolar red blood cell accumulation, and fibrin deposition (C, arrows). Representative of n = 6. (D) Immunohistochemical quantification of neutrophils shows a significant increase in neutrophil numbers in CS versus NS as determined by image analysis of computerized randomly generated high-power fields (HPF). #P < 0.01. (E) Analysis of proinflammatory gene expression after transplant showed no significant differences in TNF-α, monocyte chemoattractant protein 1 (MCP-1), keratinocyte chemoattractant (KC), and IL-6.
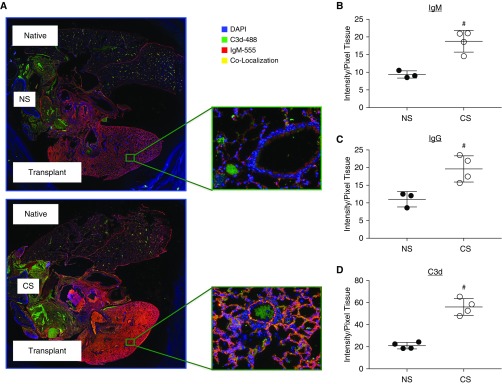
To delineate the role of preformed antibodies in peri-LTx IRI in CS versus NS recipients, immunofluorescence staining was performed. Given the predominance of complement fixing that immunoglobulin subtypes demonstrated in the serum of CS mice before transplant, we performed colocalization studies with either IgM or IgG detection together with C3d, a complement C3 split product, and opsonin as an indicator of complement activity (Figure 3A). Using automated quantification, immunofluorescence staining demonstrated significantly increased IgM, total IgG, and C3d in CS recipients compared with NS (Figures 3B–3D). Immunoglobulin and complement immunostaining was seen on both epithelial and endothelial cells, with the distributions of IgM and IgG not dissimilar from each other. Antibody staining colocalized with C3d on both epithelial and endothelial cells (Figure 3A).
Figure 3.
Antibody and complement deposition are significantly elevated in the transplanted lung of chronic CS-exposed recipients. (A) Immunofluorescent localization of IgM and C3d shows increased binding in CS mice versus NS. Quantification of pixel fluorescence intensity normalized to tissue area demonstrates significant increases in (B) IgM, (C) IgG, and (D) C3d in CS versus NS. #P < 0.05.
Simulated Cold Static Storage and Reperfusion Exposes Epitopes Recognized by CS Serum Autoantibodies
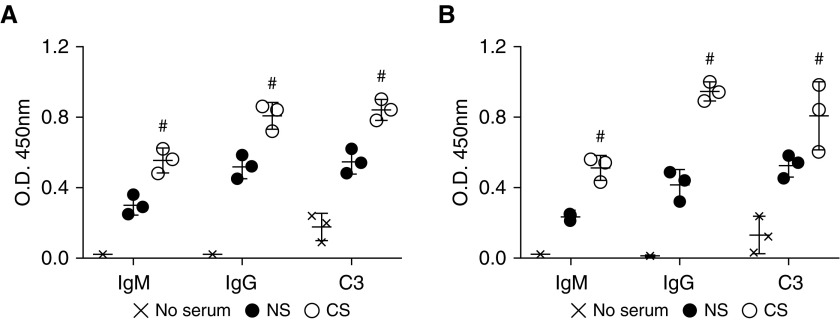
Our in vivo data suggest that the presence of preexisting autoantibodies leads to antibody binding and complement activation. To test antibody binding and complement deposition more directly, we exposed mouse lung epithelial cells and endothelial cells to simulated cold storage and reperfusion injury using a recently described cell culture model (23). After 18 hours of cold storage, cells subsequently exposed to media containing CS serum had significantly elevated IgM and IgG binding as compared with NS (Figures 4A and 4B). To determine whether these antibodies fix complement, we repeated these experiments, but this time, after 6 hours of exposure to either NS or CS serum-containing media, the cells were subsequently exposed to media with fresh C6-deficient serum as a source of active complement and incubated for a further 6 hours. C6-deficient serum was used to inhibit complement-mediated cell lysis. As anticipated, C3 deposition was increased in the CS group compared with the NS group (Figures 4A and 4B).
Figure 4.
Simulated cold storage and reperfusion injury results in increased endothelial and epithelial deposition of IgM, IgG, and C3 by cells reperfused with pooled serum of CS-exposed mice. Note that CS serum promotes increased IgM, IgG, and C3 deposition after cold storage and reperfusion-induced injury of (A) epithelial and (B) endothelial cells. #P < 0.01 CS versus NS.
To rule out the potential confounding impact of alloimmunity, CS and NS pooled serum was assayed to determine the presence of alloantibody using standard donor splenocyte serum incubations and flow cytometry as previously described (28). No significant differences in alloantibody mean fluorescence intensities between donor splenocytes incubated with CS or with NS serum were determined (Figure E2).
Autoantibodies Present in CS Serum Reconstitutes IRI in Antibody-Deficient Recipients
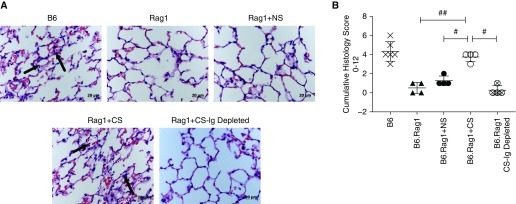
To more thoroughly implicate CS-associated preformed autoantibodies in the exacerbation of lung IRI after LTx, we performed reconstitution experiments using syngeneic left-lung transplants with immunodeficient B6.Rag1 (Rag1)-knockout donors and recipients. Serum isolated from C57BL/6 NS or CS mice was reconstituted into Rag1 recipients 30 minutes before receiving a Rag1 donor lung. To enable comparison and to minimize batch variability, samples from each CS and NS batch were pooled and quality controlled to confirm development of ECM autoreactivity in CS-exposed mice, as demonstrated in Figure 1. Analysis of total IgM and IgG concentrations by ELISA confirmed that no significant difference in total IgM and IgG was seen between pooled CS and NS sera (data not shown). After 6 hours of reperfusion, graft histology showed significant neutrophil infiltration, edema, and red blood cell accumulation in the alveoli of Rag1 recipients reconstituted with pooled serum from CS-exposed C57BL/6 mice. These features were seen to a much lesser degree in NS reconstituted recipients and were all but absent in non–serum-reconstituted Rag1 control mice (Figures 5A and 5B). To demonstrate that the effect noted in CS serum-reconstituted mice was antibody dependent, we depleted IgM and IgG from serum (depletion confirmed by ELISA; data not shown). In the absence of IgM and IgG, CS serum failed to reconstitute significant injury (Figures 5A and 5B).
Figure 5.
Autoreactive antibodies generated by chronic smoke exposure reconstitute ischemia–reperfusion injury in antibody-deficient recipients. Histologic images of left lungs 6 hours after treatment are shown. (A) Representative hematoxylin and eosin–stained lung sections. B6 recipients demonstrate immune cell infiltrates, alveolar red blood cells, and neutrophil infiltrates (arrows). Serum reconstitution with either serum type–induced injury, but alveolar accumulation of neutrophils and red blood cells was significantly worse in CS-exposed mice (arrows) versus NS and recombination-activating protein 1 (Rag1) control mice. Reconstitution with antibody-depleted CS serum (Ig) resulted in an injury profile consistent with Rag1 control mice. (B) Histopathological quantification of injury demonstrates that adoptive transfer of CS serum induces significantly more injury. Scale bars: 20 μm. #P = 0.02 for CS versus NS; #P = 0.02 for CS versus Rag1 + CS-Ig-depleted serum control; ##P = 0.01 for CS versus Rag1 control mice.
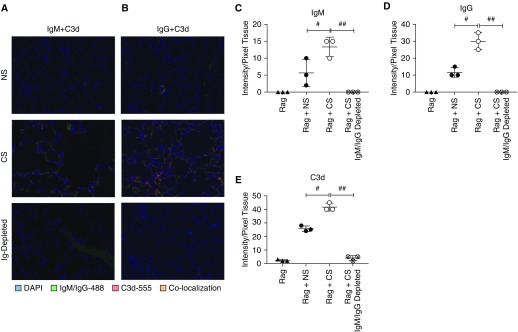
Finally, to confirm that the effects of serum reconstitution were associated with antibody binding in the lung, we assessed IgM, IgG, and C3d deposition in reconstituted mice. As anticipated, nonreconstituted and IgM/IgG-depleted serum control Rag1 transplants had no IgM or IgG staining, and complement C3d deposition was minimal (Figures 6A–6E). In Rag1 recipients of CS serum, significantly greater IgM, IgG, and C3d than with NS serum-reconstituted recipients was noted (Figures 6A–6E).
Figure 6.
Reconstituted antibodies colocalize and activate complement. Colocalization of (A) IgM and C3d, and (B) IgG and C3d shows increased antibody binding and complement activation in Rag1 recipients reconstituted with pooled serum of CS mice. Antibody and complement colocalization can be seen on epithelial and endothelial cells in a pattern not dissimilar to that seen in allograft transplants. n = 3 in all groups. IgM, IgG, and C3d immunofluorescent staining was quantified in all groups. Note the significant in increase in (C) IgM, (D) IgG, and (E) C3d in CS serum-reconstituted mice as compared with NS and CS Ig-depleted serum-reconstituted mice. #P < 0.01 for NS versus CS; ##P < 0.001 for CS versus CS IgM- and IgG-depleted serum.
Discussion
In the present study, we demonstrated that chronically CS-exposed recipients had an increased injury profile and that CS-induced preformed autoreactive antibodies can promote complement activation and lung injury. A role for autoreactive antibodies in LTx has previously been demonstrated in studies focused on a handful of particularly compelling non-HLA antibodies (collagen type V and K-α1 tubulin) that were shown to develop de novo after LTx. The development of these non-HLA antibodies after transplant was associated with an increased risk of PGD and obliterative bronchiolitis (5, 10, 12, 29). Furthermore, adoptive transfer of either collagen type V or K-α1 antibodies at supraphysiological concentrations caused lung inflammation and fibrosis when administered at the time of transplant in a rodent syngeneic LTx model (5, 11). Although these elegant studies provided important clues to the injurious pathways induced by singular adoptively transferred autoreactive antibodies, they did not take into consideration the full spectrum of specificities that may develop in the recipient before transplant, nor did they consider/model the impact of immunoglobulin autoantibody subtypes, such as IgM, IgG1, IgG2, and IgG3, that may have differing Fcγ- or complement-mediated functions.
In the present study, we used a rodent model of chronic CS exposure as a tool to develop a full spectrum of pretransplant autoreactivity in a clinically relevant paradigm. Our rationale for using a chronic CS exposure model of emphysema was as follows: 1) The model is well validated (30–33); 2) animals develop a broad array of ECM autoreactive antibodies with specificities that are also seen in humans (21, 27, 34); and 3) emphysema is a key indication for LTx, and thus our studies have greater clinical relevance. Although other chronic lung diseases, such as idiopathic pulmonary fibrosis (IPF), have been shown to develop autoreactive antibodies (16, 35), and although recipients with IPF similarly have poorer outcomes, animal models of IPF tend to be acute and do not display a robust autoreactive immune phenotype that mirrors clinical findings. We acknowledge that chronic CS exposure will likely alter a host of metabolic and immunological factors, both pro- and antiinflammatory, but in the present study we focused, through our reconstitution studies, on the mechanistic interplay between CS-induced antibodies and demonstrated that complement activation appears to be central to this exacerbated phenotype. The findings described in our studies expand on previous clinical and preclinical findings regarding the roles of recipient preformed autoantibodies such as those found in high prevalence in patients with COPD/emphysema, and they highlight a potential benefit for targeting complement activation early after transplant in COPD/IIP recipients to improve overall outcomes in this at-risk population.
In the present study, we sought to examine how the full repertoire of emphysema-related autoantibodies in a clinically relevant in vivo system would impact the severity of IRI after LTx, as well as if this diverse array of antibodies could drive IRI by themselves. We demonstrated significantly worse cellular lung injury, edema, pulmonary hemorrhage, and immune cell infiltration in the transplanted lungs of CS recipients versus NS control mice. Despite the minimal cold and warm ischemia times, an obvious difference in injury was seen in CS recipients, and importantly, this difference correlated to significantly increased antibody deposition. It is also important to note that the antibody binding that we demonstrated by immunofluorescence was almost entirely absent in the native nontransplanted right lung, indicating that the documented antibody activity was in response to epitope exposure resulting from the transplant process itself (i.e., cold storage and IRI). Although we demonstrated increases in proinflammatory gene expression in transplant lungs in CS as compared with NS, we could not demonstrate any significance, which led us to consider complement activation as a prime inducer of injury.
Complement-dependent activation/cytotoxicity represents a main pathway by which antibodies propagate immune responses and inflammation (8). In this study, we demonstrated that a large proportion of the pretransplant antibodies present in emphysema represents isotypes associated with complement fixation. We and others have previously demonstrated that transplant IRI can, in and of itself, activate the complement cascade, with a resultant increase in allograft neutrophil and macrophage infiltration leading to tissue inflammation, injury, and acute rejection (8, 36). Given the elevated level of complement-driven inflammation in these studies using otherwise healthy (young, without CS exposure) mice as recipients, it stands to reason that additional antibody-mediated complement activation in CS-exposed mice would only serve to further exacerbate injury and graft dysfunction after LTx, which we demonstrated with the increased antibody and complement colocalization seen in CS recipients after LTx.
In these acute studies, we proposed that epitopes exposed by organ harvest, cold storage, and IRI would be bound by a wide range of preformed ECM autoantibodies that are present in emphysema, resulting in subsequent immune activation and injury. To more mechanistically test this, we used a recently described simulated cold storage IRI in vitro model and demonstrated that incubation with CS serum not only increased antibody deposition on both epithelial and endothelial cells but also resulted in increased C3 deposition after reperfusion (23). We confirmed these in vitro findings with our acute in vivo reconstitution experiments that also demonstrated increased IgM and IgG deposition and complement activation in CS serum-reconstituted LTx recipients. Taken together, our data show increased antibody binding and C3 deposition, which might account for the increased injury seen in the lungs of CS recipients in vivo. Our qRT-PCR data showed no differences in KC, a key neutrophil chemotactic factor in the lung, which was in contrast to our immunohistochemical data that clearly demonstrated increased neutrophil infiltrates. Although these and the other mRNA data point to no significant differences in proinflammatory gene expression, it is important to note that complement activation fragments can directly impact immune cell infiltration via C3a- and C5a-mediated chemotaxis (37–40). Therefore, we speculate that increased injury and immune cell infiltration could be directly impacted by the degree of complement activation. In keeping with this, increased complement activation has been shown to associate with increased incidence of PGD in clinical studies (41), and it is believed to promote injury and collagen deposition in models of obliterative bronchiolitis (42).
Moving forward, the insights derived from these studies and from the extension of these studies could help to inform the management of patients who constitute a majority of LTx recipients: patients with COPD and potentially patients with IIP. The underlying mechanisms that appeared to drive the worsened post-transplant injury seen in CS-exposed mice in the present study appear to be the same mechanisms that have recently been implicated in the clinical literature to worsen long-term outcomes: a marked increase in circulating lung-specific autoreactive antibodies (6), recipient pretransplant CS exposure (4), and pretransplant diagnosis of COPD (4, 14). Indeed, Bharat and colleagues had previously documented that 30% of their LTx cohort had positive results for at least one of the three IgG non-HLA antibodies they tested. It is also worth noting that approximately 70% of their patient population, and over half of the antibody-positive patients, were patients with COPD and patients with IIP (12). On the basis of our data, it is reasonable to suggest that increasing the antibody screen or investigating immunoglobulin subtypes and their association with outcomes would yield even higher numbers. As more is learned about the role of pretransplant non-HLA autoantibodies, the implementation of screening for certain isotypes, or even entire panels, will enable more appropriate patient-specific care.
Given these data, the use of short-term complement inhibition may provide relief from autoantibody-driven injury (8, 43). With the current availability of U.S. Food and Drug Administration–approved complement therapeutics and ongoing research to develop a number of different complement-inhibitory compounds (44, 45), this may represent a significant step forward in the care of this patient population. Factoring in the documented ability of glucocorticoids to increase complement production only serves to support complement-targeted therapy as an adjunct or alternative to existing immunosuppressive regimens (46). Studies more thoroughly exploring these topics, using more clinically relevant model systems with brain death and cold ischemia, may provide more definitive evidence regarding the utility of these interventions.
Although our data support a role for antibody-induced injury, we acknowledge that further studies are needed to see how preexisting ECM T-cell autoreactivity, as described in humans and mice (47, 48), will impact post-transplant outcomes. Given the complex role of T cells in organ rejection, a role that is even further obscured in the context of lung transplantation as Kreisel and colleagues have demonstrated through a series of publications (49–51), we believed that the task of addressing the mechanisms by which CS alters T-cell subsets and effector/memory/regulatory functions was beyond the scope of our present study. They remain extremely important to carry out, however, given the well-documented survival statistics demonstrating disproportionately poor long-term outcomes for patients with COPD and patients with IIP (14).
In conclusion, LTx recipients with certain underlying pathologies develop a wide array of lung-specific autoantibodies before transplant that are clinically relevant to the peritransplant period. In the present study, we focused on the murine emphysema model to demonstrate that the full range and/or exacerbated pretransplant titers of autoantibodies negatively contribute to the severity of IRI. The injury mediated through the post-LTx binding of these antibodies appears to be driven by the complement cascade, which may represent a logical therapeutic target.
Footnotes
Supported by grants from the National Institutes of Health (NIH) (National Heart, Lung, and Blood Institute grants 1R01090144 and R01 HL140470-0181 [C.A.]); a Lee Patterson Allen Foundation Award (S.N.N. and C.A.); NIH Institutional Postdoctoral Training Grant NIH-HL-007260 (K.J.P. and J.K.); American Heart Association/Enduring Hearts Clinical Scientist Training Grant 17CPOST3367120 (K.J.P.); and the South Carolina Clinical & Translational Research Institute, Medical University of South Carolina Clinical and Translational Science Award (NIH/National Center for Advancing Translational Sciences grant UL1TR000062).
Author Contributions: Conception and design: K.J.P. and C.A.; performed experiments: K.J.P., Q.C., S.S., D.P.A., C.L., J.K., R.F., V.M.-C., S.E., C.V., and M.G.; and data analysis and manuscript drafting: K.J.P., Q.C., S.N.N., and C.A.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0224OC on December 20, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Prekker ME, Nath DS, Walker AR, Johnson AC, Hertz MI, Herrington CS, et al. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2006;25:371–378. doi: 10.1016/j.healun.2005.11.436. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki Y, Cantu E, Christie JD. Primary graft dysfunction. Semin Respir Crit Care Med. 2013;34:305–319. doi: 10.1055/s-0033-1348474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuntz CL, Hadjiliadis D, Ahya VN, Kotloff RM, Pochettino A, Lewis J, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant. 2009;23:819–830. doi: 10.1111/j.1399-0012.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 4.Koutsokera A, Royer PJ, Antonietti JP, Fritz A, Benden C, Aubert JD, et al. SysCLAD Consortium. Development of a multivariate prediction model for early-onset bronchiolitis obliterans syndrome and restrictive allograft syndrome in lung transplantation. Front Med (Lausanne) 2017;4:109. doi: 10.3389/fmed.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiriveedhi V, Gautam B, Sarma NJ, Askar M, Budev M, Aloush A, et al. Pre-transplant antibodies to Kα1 tubulin and collagen-V in lung transplantation: clinical correlations. J Heart Lung Transplant. 2013;32:807–814. doi: 10.1016/j.healun.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T. Antibodies to K-α1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12:2164–2171. doi: 10.1111/j.1600-6143.2012.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, et al. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkinson C, Qiao F, Yang X, Zhu P, Reaves N, Kulik L, et al. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury. Circulation. 2015;131:1171–1180. doi: 10.1161/CIRCULATIONAHA.114.010482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, et al. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol (1985) 1999;86:938–942. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 10.Bharat A, Chiu S, Zheng Z, Sun H, Yeldandi A, DeCamp MM, et al. Lung-restricted antibodies mediate primary graft dysfunction and prevent allotolerance after murine lung transplantation. Am J Respir Cell Mol Biol. 2016;55:532–541. doi: 10.1165/rcmb.2016-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian V, Ramachandran S, Banan B, Bharat A, Wang X, Benshoff N, et al. Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am J Transplant. 2014;14:2359–2366. doi: 10.1111/ajt.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, Lee J, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report—2010. J Heart Lung Transplant. 2010;29:1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Fakhro M, Broberg E, Algotsson L, Hansson L, Koul B, Gustafsson R, et al. Double lung, unlike single lung transplantation might provide a protective effect on mortality and bronchiolitis obliterans syndrome. J Cardiothorac Surg. 2017;12:100. doi: 10.1186/s13019-017-0666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyne GF, Elliott H, Mutsaers SE, Prêle CM. Idiopathic pulmonary fibrosis and a role for autoimmunity. Immunol Cell Biol. 2017;95:577–583. doi: 10.1038/icb.2017.22. [DOI] [PubMed] [Google Scholar]
- 17.Wen L, Krauss-Etschmann S, Petersen F, Yu X. Autoantibodies in chronic obstructive pulmonary disease. Front Immunol. 2018;9:66. doi: 10.3389/fimmu.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandsma CA, Kerstjens HA, Geerlings M, Kerkhof M, Hylkema MN, Postma DS, et al. The search for autoantibodies against elastin, collagen and decorin in COPD. Eur Respir J. 2011;37:1289–1292. doi: 10.1183/09031936.00116710. [DOI] [PubMed] [Google Scholar]
- 19.Cottin V, Fabien N, Khouatra C, Moreira A, Cordier JF. Anti-elastin autoantibodies are not present in combined pulmonary fibrosis and emphysema. Eur Respir J. 2009;33:219–221. doi: 10.1183/09031936.00140208. [DOI] [PubMed] [Google Scholar]
- 20.Greene CM, Low TB, O’Neill SJ, McElvaney NG. Anti-proline-glycine-proline or antielastin autoantibodies are not evident in chronic inflammatory lung disease. Am J Respir Crit Care Med. 2010;181:31–35. doi: 10.1164/rccm.200904-0545OC. [DOI] [PubMed] [Google Scholar]
- 21.Low TB, Greene CM, O’Neill SJ, McElvaney NG. Quantification and evaluation of the role of antielastin autoantibodies in the emphysematous lung. Pulm Med. 2011;2011:826160. doi: 10.1155/2011/826160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodell A, Jones BW, Williamson T, Schnabolk G, Tomlinson S, Atkinson C, et al. A targeted inhibitor of the alternative complement pathway accelerates recovery from smoke-induced ocular injury. Invest Ophthalmol Vis Sci. 2016;57:1728–1737. doi: 10.1167/iovs.15-18471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao W, Zhao J, Kim H, Xu S, Chen M, Bai X, et al. α1-Antitrypsin inhibits ischemia reperfusion-induced lung injury by reducing inflammatory response and cell death. J Heart Lung Transplant. 2014;33:309–315. doi: 10.1016/j.healun.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Cardella JA, Keshavjee S, Mourgeon E, Cassivi SD, Fischer S, Isowa N, et al. A novel cell culture model for studying ischemia-reperfusion injury in lung transplantation. J Appl Physiol (1985) 2000;89:1553–1560. doi: 10.1152/jappl.2000.89.4.1553. [DOI] [PubMed] [Google Scholar]
- 25.Leidinger P, Keller A, Heisel S, Ludwig N, Rheinheimer S, Klein V, et al. Novel autoantigens immunogenic in COPD patients. Respir Res. 2009;10:20. doi: 10.1186/1465-9921-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feghali-Bostwick CA, Gadgil AS, Otterbein LE, Pilewski JM, Stoner MW, Csizmadia E, et al. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:156–163. doi: 10.1164/rccm.200701-014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandsma CA, Timens W, Geerlings M, Jekel H, Postma DS, Hylkema MN, et al. Induction of autoantibodies against lung matrix proteins and smoke-induced inflammation in mice. BMC Pulm Med. 2010;10:64. doi: 10.1186/1471-2466-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Jiang J, Liu W, Kubelik D, Chen G, Gies D, et al. Prevention of acute vascular rejection by a functionally blocking anti-C5 monoclonal antibody combined with cyclosporine. Transplantation. 2005;79:1121–1127. doi: 10.1097/01.tp.0000161218.58276.9a. [DOI] [PubMed] [Google Scholar]
- 29.Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624–631. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong Z, Leme AS, Ray P, Shapiro SD, Lee JS. CX3CR1+ lung mononuclear phagocytes spatially confined to the interstitium produce TNF-α and IL-6 and promote cigarette smoke-induced emphysema. J Immunol. 2011;186:3206–3214. doi: 10.4049/jimmunol.1003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voss M, Wolf L, Kamyschnikow A, Wonnenberg B, Honecker A, Herr C, et al. Il-17A contributes to maintenance of pulmonary homeostasis in a murine model of cigarette smoke-induced emphysema. Am J Physiol Lung Cell Mol Physiol. 2015;309:L188–L195. doi: 10.1152/ajplung.00388.2014. [DOI] [PubMed] [Google Scholar]
- 32.Motz GT, Eppert BL, Sun G, Wesselkamper SC, Linke MJ, Deka R, et al. Persistence of lung CD8 T cell oligoclonal expansions upon smoking cessation in a mouse model of cigarette smoke-induced emphysema. J Immunol. 2008;181:8036–8043. doi: 10.4049/jimmunol.181.11.8036. [DOI] [PubMed] [Google Scholar]
- 33.Mahadeva R, Shapiro SD. Chronic obstructive pulmonary disease. 3: Experimental animal models of pulmonary emphysema. Thorax. 2002;57:908–914. doi: 10.1136/thorax.57.10.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkham PA, Caramori G, Casolari P, Papi AA, Edwards M, Shamji B, et al. Oxidative stress-induced antibodies to carbonyl-modified protein correlate with severity of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:796–802. doi: 10.1164/rccm.201010-1605OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li FJ, Surolia R, Li H, Wang Z, Kulkarni T, Liu G, et al. Autoimmunity to vimentin is associated with outcomes of patients with idiopathic pulmonary fibrosis. J Immunol. 2017;199:1596–1605. doi: 10.4049/jimmunol.1700473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkinson C, Floerchinger B, Qiao F, Casey S, Williamson T, Moseley E, et al. Donor brain death exacerbates complement-dependent ischemia/reperfusion injury in transplanted hearts. Circulation. 2013;127:1290–1299. doi: 10.1161/CIRCULATIONAHA.112.000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan X, Shan M, You R, Frazier MV, Hong MJ, Wetsel RA, et al. Activation of C3a receptor is required in cigarette smoke-mediated emphysema. Mucosal Immunol. 2015;8:874–885. doi: 10.1038/mi.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Q, Patel K, Lei B, Rucker L, Allen DP, Zhu P, et al. Donor pretreatment with nebulized complement C3a receptor antagonist mitigates brain-death induced immunological injury post-lung transplant. Am J Transplant. 2018;18:2417–2428. doi: 10.1111/ajt.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monsinjon T, Gasque P, Chan P, Ischenko A, Brady JJ, Fontaine MC. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17:1003–1014. doi: 10.1096/fj.02-0737com. [DOI] [PubMed] [Google Scholar]
- 40.Denk S, Taylor RP, Wiegner R, Cook EM, Lindorfer MA, Pfeiffer K, et al. Complement C5a-induced changes in neutrophil morphology during inflammation. Scand J Immunol. 2017;86:143–155. doi: 10.1111/sji.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah RJ, Emtiazjoo AM, Diamond JM, Smith PA, Roe DW, Wille KM, et al. Plasma complement levels are associated with primary graft dysfunction and mortality after lung transplantation. Am J Respir Crit Care Med. 2014;189:1564–1567. doi: 10.1164/rccm.201312-2121LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki H, Lasbury ME, Fan L, Vittal R, Mickler EA, Benson HL, et al. Role of complement activation in obliterative bronchiolitis post-lung transplantation. J Immunol. 2013;191:4431–4439. doi: 10.4049/jimmunol.1202242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu P, Bailey SR, Lei B, Paulos CM, Atkinson C, Tomlinson S. Targeted complement inhibition protects vascularized composite allografts from acute graft injury and prolongs graft survival when combined with subtherapeutic cyclosporine A therapy. Transplantation. 2017;101:e75–e85. doi: 10.1097/TP.0000000000001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cernoch M, Viklicky O. Complement in kidney transplantation. Front Med (Lausanne) 2017;4:66. doi: 10.3389/fmed.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damman J, Schuurs TA, Ploeg RJ, Seelen MA. Complement and renal transplantation: from donor to recipient. Transplantation. 2008;85:923–927. doi: 10.1097/TP.0b013e3181683cf5. [DOI] [PubMed] [Google Scholar]
- 46.Coulpier M, Andreev S, Lemercier C, Dauchel H, Lees O, Fontaine M, et al. Activation of the endothelium by IL-1α and glucocorticoids results in major increase of complement C3 and factor B production and generation of C3a. Clin Exp Immunol. 1995;101:142–149. doi: 10.1111/j.1365-2249.1995.tb02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meghraoui-Kheddar A, Pierre A, Sellami M, Audonnet S, Lemaire F, Le Naour R. Elastin receptor (S-gal) occupancy by elastin peptides modulates T-cell response during murine emphysema. Am J Physiol Lung Cell Mol Physiol. 2017;313:L534–L547. doi: 10.1152/ajplung.00465.2016. [DOI] [PubMed] [Google Scholar]
- 48.Bhavani S, Yuan X, You R, Shan M, Corry D, Kheradmand F. Loss of peripheral tolerance in emphysema. phenotypes, exacerbations, and disease progression. Ann Am Thorac Soc. 2015;12(Suppl 2):S164–S168. doi: 10.1513/AnnalsATS.201503-115AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008;180:4754–4762. doi: 10.4049/jimmunol.180.7.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182:3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krupnick AS, Lin X, Li W, Higashikubo R, Zinselmeyer BH, Hartzler H, et al. Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest. 2014;124:1130–1143. doi: 10.1172/JCI71359. [DOI] [PMC free article] [PubMed] [Google Scholar]