Abstract
Background and Aim:
The BreathID®Hp urea breath test provides several advantages over other 13C breath analyzers for the detection of Helicobacter pylori. We evaluated the sensitivity and specificity of a new BreathID®Hp Lab System (Exalenz Bioscience Ltd, Israel), a 13C-urea breath test system using breath sampling bags that facilitates multiple testing in a multicenter international clinical study.
Methods:
A total of 257 subjects with evaluable results for urea breath test, rapid urease test, and histology were enrolled into two study groups: 189 naïve subjects were included in the pre-therapy group, and 68 subjects comprised the post-eradication therapy group. Analytical studies were conducted to evaluate the stability, reproducibility, and repeatability of the 13C-urea breath test results using a delta over baseline cut-off value of 5.
Results:
Among the pre-therapy subjects evaluated with the composite results from the rapid urease test and histology/immunohistochemistry, 176 results matched those of the urea breath test, resulting in an overall agreement of 98.3% with a sensitivity of 100% and specificity of 97.9%. In the post-eradication therapy cohort, the overall agreement between the urea breath test and the biopsy diagnosis was 98.5%; the sensitivity of the urea breath test in this cohort was 92.3% and the specificity was 100%. There was uniformly high overall reproducibility (99.48%) of the test results over different batches of breath sample bags, when analyzed on different days and under different storage conditions, showing stability of the breath samples in the breath collection bags
Conclusion:
The BreathID®Hp Lab System is a highly accurate and dependable method for the diagnosis of H. pylori infection.
Keywords: diagnostic tests, Helicobacter pylori, lab mode, safety, sensitivity, specificity, urea breath test
Introduction
Biopsies taken via esophagogastroduodenoscopy (EGD) and the carbon-labeled urea breath test (UBT) are considered the ‘gold standard’ methods for the diagnosis of active Helicobacter pylori infection.1 The Maastricht V Consensus Report recommended 13C-UBT as the best approach for the diagnosis of H. pylori infection, due to its high sensitivity, specificity, and excellent performance, especially in patients in whom endoscopy is not indicated.2
The ¹³C-UBT is a noninvasive test for detecting the presence of H. pylori infection via changes in the ratio of 13CO2/12CO2 in exhaled breath.3 In the presence of H. pylori, ingested ¹³C-urea is metabolized to 13CO2. The resulting 13CO2/12CO2 ratio is compared with baseline values obtained before ingestion of the labeled urea. UBTs have high accuracy and reproducibility because they are functional tests that essentially sample the entire stomach. These tests are not prone to the same level of sampling error as biopsy-based tests, and false-positive results are uncommon.4 The sensitivity and specificity of the breath test range from 90% to 100% and, in most cases, it is above 95%.5–7
UBTs are the preferred method for epidemiological studies, screening dyspeptic patients, and assessing eradication or recurrence of the infection after treatment.3,8–10 A major disadvantage of the 13C-UBT is the inconvenience related to the 13CO2 analysis. In most medical centers, there is a need for collecting, storing, and transporting the samples to a central laboratory that is equipped with an isotope ratio mass spectrometer. This makes UBTs inconvenient to both the patient and physician.
A Food and Drug Administration (FDA)-cleared continuous UBT (using a nasal cannula) using the BreathID®Hp (Exalenz Bioscience Ltd, Modiin, Israel) provides several unique advantageous features. Instead of collecting and analyzing discrete breath samples, breath samples are continually evaluated, providing excellent accuracy (>99%) and enabling shortened breath testing procedures. Moreover, test results are available in real time for decision-making at the point of care.11,12 A user-friendly interface for operation and point-of-care testing is another advantage.
Although the BreathID®Hp Lab System has the advantage of real-time point-of-care analyses, using this system has its disadvantages: patients need to be present at the site where this system is located. In addition, only one patient’s breath can be measured at a time. This can limit the number of tests that can be done in a short time period. Using bags to collect breath samples allows accumulation of up to 10 sets of breath collection bags for up to 2 weeks. The BreathID®Hp Lab System performs automated analyses sequentially. This system may be located in a central laboratory or the system can be installed on-site. Its user-friendly interface, compact design, maintenance-free use, compatibility to lab information management system (LIMS), low cost compared to mass spectrometer, and automated operation make it an ideal method for H.pylori testing. Testing of each set of bags takes approximately 5 min. This study was aimed at validating the breath sampling bag test method in comparison to a composite reference standard of H. pylori status, comprising a combination of a histological examination for H. pylori and a rapid urease test (RUT).
Methods
Subjects
We determined the diagnostic accuracy of the BreathID®Hp Lab System in two prospectively enrolled sets of patients: initial diagnosis and post-eradication follow-up. This clinical study was conducted at 13 clinical sites in the USA and in Israel. Study participants were men and women 18 years of age or older who had a clinical indication for H. pylori testing for either initial diagnosis or post-eradication therapy verification (provided that their initial positive H. pylori diagnosis was confirmed through endoscopic biopsy). The study was approved by each clinical site’s Institutional Review Board or an Independent Ethics Committee (in the USA only) and was registered at clinicaltrials.gov. Each subject provided informed consent prior to participating in the study.
Subjects were included in the initial diagnosis cohort if they had not received H. pylori treatments in the preceding 18 months and had not been tested for H. pylori within the 6 months prior to enrollment. Subjects were included in the post-eradication therapy cohort if they had biopsy documentation of H. pylori infection prior to eradication therapy and had documentation of receiving H. pylori eradication therapy within 6 months and completion of therapy at least 6 weeks prior to the UBT.
Evaluation of H. pylori status
Each subject was evaluated for H. pylori status by three diagnostic methods: histology, RUT, and UBT. For histopathology and RUT, each subject underwent an EGD according to the standard clinical practice at each site. If the UBT was planned to be performed on the same day as the endoscopy, the subjects performed the UBT before the EGD. The American College of Gastroenterology guidelines1 recommend that a minimum of three biopsies be obtained (one from the angularis, one from the greater curvature of the corpus, and one from the greater curvature of the antrum) in order to maximize the diagnostic yield.13 In this study, all biopsies were taken in duplicate (for histology and RUT) resulting in a minimum of six biopsies: two from the angularis, two from the greater curvature of the corpus, and two from the greater curvature of the antrum. Biopsies from each of the three sites within the stomach were analyzed by histology and RUT.
Histopathology: at least three biopsy specimens were fixed with formalin, sectioned at a thickness of 4–5 μm and then stained with hematoxylin and eosin and by an immunohistochemistry (IHC) assay (Novacastra lyophilized polyclonal, product code NCL-HPp, purchased from Leica). The stain was performed on a Ventana ULTRA slide staining system. All slides were examined and analyzed by an experienced pathologist at a central laboratory.
RUT: at least three biopsy specimens, (similar to the ones taken by for histology) were analyzed on-site for urease activity after 1 h, with an FDA-cleared RUT (Pronto Dry®, Warsaw, Poland) according to the manufacturer’s instructions. The principle of the RUT test is as follows: if H. pylori is present in the gastric biopsy, it secretes the urease which results in breakdown of urea, which in turn causes the pH to increase, and the color of the pH indicator changes.
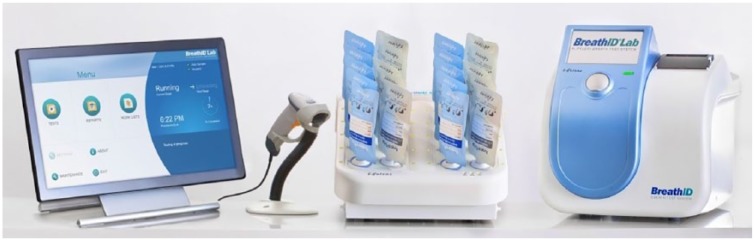
The UBT was performed within 1 week before or after the EGD. After fasting for at least 1 h, each participant filled two bags prior to the UBT test (baseline bags). Next, participants ingested a test solution containing the 13C-urea test solution (IDkitHp™ Two, Exalenz Bioscience Ltd) and filled two bags between 15 and 20 min after ingestion (test bags). The substrate of the drink contains 75 mg of 13C-urea and citric powder which are dissolved in a cup of tap water. Antibiotics and bismuth preparations were avoided by all participants for 4 weeks prior to the breath test for the pre-therapy cohort and for 6 weeks for the post-therapy cohort. Proton pump inhibitors or H2 blockers were avoided by all participants for 2 weeks prior to the breath test for both cohorts. Sample analysis using the BreathID®Hp Lab System (Figure 1) was performed either on-site or at a remote location.
Figure 1.
The BreathID® Lab System.
The BreathID®Hp Lab System collects CO2 from exhaled breath and analyzes its different isotopes in real time based on specific optical-radiation emission and absorption by 13CO2 and 12CO2. The system calculates the change in the 13CO2/12CO2 ratio (R) from exhaled breath before and after ingestion of 13C-labeled urea and produces a delta over baseline (DOB) value. DOB is defined as [(13CO2(n)/12CO2[n]—13CO2(0)/12CO2(0))*1000]/[13CO2(PDB)/ 12CO2(PDB)], where PDB is the standard 13C/12C isotope ratio (=1.1273%), (0) is the baseline measurement and (n) is the measurement of interest. Normally, R remains constant in the expired breath of an individual patient. However, it can be changed via an external source of 13C. H. pylori bacteria decompose 13C-urea to ammonia and 13CO2. Administering 13C-enriched urea to a patient infected with H.pylori will cause an increase in the 13CO2/12CO2 ratio in the patient’s breath. The system uses the Exalenz MCS™ technology, with 13CO2 and 12CO2 discharge lamps as light sources. The light absorption will correlate directly to the presence of 13CO2 and 12CO2 in the gas samples. This approach results in highly sensitive and specific absorption curves which can detect minute (less than 1/1000) variations in 13CO2/12CO2 ratios.
A DOB ⩾ 5 indicates an H. pylori infection which was determined by preliminary studies (described in the Lab System’s publicly available package insert). The BreathID®Hp Lab System contains an autosampler unit and has an application to control the process that can measure up to 10 sets of bags consecutively and automatically within approximately 25 min. The bags are produced by Exalenz Bioscience Ltd. They are made of a printed flexible laminate LDPE 80 having a one-way valve and their volume (capacity) is 240 ml.
Based on the test results, the subject’s status was classified as H. pylori positive, H. pylori negative, or non-evaluable. These results were compared to the classification results obtained by histopathology, RUT, and both combined (composite) according to FDA classification guidelines.14 RUT was considered positive if any of the samples showed a positive result. If all samples were negative, the patient was classified as RUT negative. All biopsy samples were assessed together to provide a conclusive histology outcome. A subject was considered histologically positive when at least one of the samples showed positive IHC. Only if all three biopsy samples were IHC negative, the patient was classified as histologically negative. Finally, to determine if a patient was positive or negative when combining the RUT and histology results, FDA guidelines were used.14 If a patient was in the initial diagnosis cohort, only if there were concordant results between RUT and histology was the patient classified as positive or negative. Patients with discordant results were considered non-evaluable. For a patient in the post-eradication cohort, any positive outcome (RUT, histology or both) would render the subject’s classification as positive. Only if both RUT and histology were found to be negative would the post-eradication cohort patient be classified as negative.
The investigators remained blinded to the UBT results and the central pathology readings throughout the whole study to ensure that the patients would only be treated based on current clinical practice, without introducing bias from the UBT results or the central pathology laboratory assessments and to avoid any potential enrollment bias. Patient management decisions were made according to standard medical practice based on local testing results.
Stability of breath samples over time
To assess the stability of the breath samples in the breath sample bags, each pair of breath sample bags (before and after ingestion), obtained from the initial diagnosis cohort, was analyzed at a different time point up to 14 days after collection (on two different reading days). The stability was evaluated by the fact that the 13CO2/12CO2 ratio did not change between the different bag’s sampling. The first evaluable set of bags was used for the primary analysis. The second set of bags was collected and measured in order to assess the stability of the breath samples over time.
Reproducibility and repeatability results
Analytical studies were conducted to evaluate the reproducibility and precision (repeatability) of the results of the 13C-UBT for measurements by different technicians and using different BreathID®Hp Lab System, or when testing was done on different days and at different sites, and on samples that were stored up to 14 days at different temperature and humidity conditions. Three different gas isotope pairs were used with DOB values of 3.3, 6.4, and 15.5, as determined via a bench study. Reproducibility was assessed by two operators who were asked to operate each of three BreathID®Hp Lab System at three different sites for 5 days in order to measure the DOB values for samples from each of the three batches. Standard deviation (SD) was calculated. The package insert states that the SD is less than the natural variability of the DOB measurement, which is defined in the device specification as 1 DOB for results under 5 DOB and 20% for results over 5 DOB.
Statistical analysis
All statistical analyses were performed after the study was completed, and the database was locked. Statistical programming and analyses were performed using SAS® version 9.4. The results are presented in two-way contingency tables. The exact binomial distribution was used to calculate the lower and upper limits of the 95% confidence intervals (CI) for the performance statistics.
Results
A total of 189 subjects (78 women and 111 men, mean age 48.4 ± 14.9 years) were included in the initial diagnosis cohort. The post-eradication therapy cohort included 68 subjects (43 women and 25 men, mean age 49.7 ± 15.3 years). The characteristics of the study participants are summarized in Table 1. In both groups, the most common indications for EGD were heartburn and abdominal pain. The most common endoscopic finding was antral erythema.
Table 1.
Subject baseline characteristics.
| Initial diagnosis cohort |
Post eradication therapy cohort |
|||
|---|---|---|---|---|
| Per protocol set | Per protocol set | |||
| Age (years) | ||||
| N | 189 | 68 | ||
| Mean (SD) | 48.4 (14.85) | 49.7 (15.33) | ||
| Median [range] | 48.3 [20.2; 82.8] | 50.0 [18.5; 82.3] | ||
| Gender | ||||
| Male | % (n/N) | 41.3% (78/189) | 36.8% (25/68) | |
| Female | % (n/N) | 58.7% (111/189) | 63.2% (43/68) | |
| Ethnic origin | ||||
| Caucasian | % (n/N) | 52.9% (100/189) | 23.5% (16/68) | |
| African-American | % (n/N) | 5.3% (10/189) | 5.9% (4/68) | |
| Asian-Pacific | % (n/N) | 2.1% (4/189) | 5.9% (4/68) | |
| Hispanic | % (n/N) | 38.6% (73/189) | 64.7% (44/68) | |
| Other | % (n/N) | 1.1% (2/189) | – | |
| BMI (kg/m2) | ||||
| Mean (SD) | 28.9 (6.12) | 30.6 (7.44) | ||
| Median [range] | 28.1 [16.3; 50.3] | 29.0 [19.7; 61.3] |
BMI: body mass index; SD: standard deviation.
Comparison of 13C-UBT results to endoscopy biopsy results
Initial diagnosis cohort
Among the initial diagnosis subjects evaluated with the composite results from the two endoscopy biopsy based methods (RUT and histological exam), 176 results matched those of the first evaluable UBT resulting in an overall agreement between the breath test and the reference biopsy result of 98.3% (95% CI: 95.2%, 99.7%) and the kappa (95% CI) was calculated to be 0.95; 37 results were positive, and 139 results were negative, showing a sensitivity of 100% (95% CI: 90.6%, 100.00%) and specificity of 97.9% (95% CI: 94.0%, 99.3%). Three subjects had false-positive results. However, 2 of the 3 false positives had a DOB result that was close to the predefined clinical cut-off value of 5 DOB. RUT and histology results alone were similar to those of the composite results (Table 2).
Table 2.
Comparative results of UBT, histology, RUT and composite test results.
| UBT | Composite |
RUT |
Histology (IHC) |
|||
|---|---|---|---|---|---|---|
| HP (+) | HP (–) | HP (+) | HP (–) | HP (+) | HP (–) | |
| Initial diagnosis | ||||||
| HP (+) | 37 | 3 | 37 | 7 | 41 | 3 |
| HP (–) | 0 | 139 | 5 | 140 | 1 | 144 |
| Post-eradication therapy | ||||||
| HP (+) | 12 | 0 | 11 | 1 | 12 | 0 |
| HP (–) | 1 | 55 | 0 | 56 | 1 | 55 |
HP: H. pylori; IHC: immunohistochemistry; RUT: rapid urease test; UBT: urea breath test.
Comparing the breath test to RUT only showed a sensitivity of 88.1% (95% CI: 75.0%, 94.8%) and a specificity of 95.2% (95% CI: 90.5%, 97.7%), and the kappa (95% CI) was 0.82 (Table 3). These results are slightly lower than the other sensitivity and specificity results presented in this study. This is mainly due to the addition of the subjects that were classified as non-evaluable per the composite reference standard when the RUT and histology results were discordant. In the majority of those cases, the breath test agreed with the histology results and not with the RUT result. RUT results can sometimes be ambiguous due to the need to determine a clear change in color by visual inspection. Comparing the breath test to the histology classification showed a sensitivity of 97.6% (95% CI: 87.7%, 99.6%) and a specificity of 98.0% (95% CI: 94.2%, 99.3%), and kappa (95% CI) was 0.94 (Table 3).
Table 3.
Diagnostic performance of the tests.
| Composite | RUT | Histology (IHC) | |
|---|---|---|---|
| Initial diagnosis | |||
| Sensitivity (%) | 100 (90.6–100.0) | 88.1 (75.0–94.8) | 97.6 (87.7–99.6) |
| Specificity (%) | 97.9 (94.0–99.3) | 95.2 (90.5–97.7) | 98.0 (94.2–99.3) |
| Kappa value | 0.95 | 0.82 | 0.94 |
| Post-eradication therapy | |||
| Sensitivity (%) | 92.3 (66.7–98.6) | 100 (74.1–100.0) | 92.3 (66.7–98.6) |
| Specificity (%) | 100 (93.5–100.0) | 98.3 (90.7–99.7) | 100 (93.5–100.0) |
| Kappa value | 0.95 | 0.95 | 0.95 |
IHC: immunohistochemistry; RUT: rapid urease test.
Post-eradication therapy cohort
There were 68 evaluable histology, RUT and composite reference assessments with corresponding breath test results. In 67 subjects, the first evaluable breath test results matched those of the composite reference standard biopsy results: 55 biopsy results were negative and 13 results were positive. In one subject, the first evaluable breath test results did not match the composite reference standard biopsy result as classified for post-eradication. This subject classified as a false negative had a breath test result that was close to the predefined clinical cut-off value of 5 DOB, but they were classified as positive per the composite reference standard based on a positive histology assessment despite the fact that all three RUT samples produced a negative result.
The overall agreement between the UBT diagnosis and the biopsy diagnosis in the post-eradication therapy cohort was 98.5% (95% CI: 92.1%, 100.0%) and the kappa (95% CI) was 0.95. The sensitivity of the breath test in this cohort was 92.3% (95% CI: 66.7%, 98.6%) and its specificity was 100.0% (95% CI: 93.5%, 100.0%; Table 3).
Comparing the breath test to RUT only showed a sensitivity of 100.0% (95% CI: 74.1%, 100.0%) and a specificity of 98.3% (95% CI: 90.7%, 99.7%), and kappa (95% CI) 0.95. Comparing the breath test to histology demonstrated a sensitivity of 92.3% (95% CI: 66.7%, 98.6%) and a specificity of 100.0% (95% CI: 93.5%, 100.0%), and kappa (95% CI) 0.95; Table 3).
Safety
Overall, there were a total of four adverse events in the initial diagnosis cohort and one adverse event in the post-eradication therapy cohort: one subject had a cyst found on the epiglottis, one had a gastric ulcer, two patients had nausea for approximately 2 min after drinking the breath test mixture and one felt lightheaded due to fasting for the EGD procedure. None of them were serious or severe, and none were related to the study device. Hence, the test procedure itself was found to be very safe and well-tolerated by all subjects.
Stability of breath samples over time
The stability of the breath samples over a period of 14 days was evaluated on samples from 191 subjects from the pre-therapy cohort who had two breath test bags per subject analyzed on two separate occasions. This analysis also included subjects who were not evaluable based on the biopsy results. Of 45 samples that were positive on the first measurement, 44 of the samples in the same bags remained positive on the second measurement [percent-positive agreement: 97.8% (95% CI: 88.43, 99.61)]. Out of 146 samples negative on the first measurement, all 146 remained negative on the second measurement [percent negative agreement: 100.0% (95% CI: 97.44, 100.0)].
Reproducibility and repeatability results
The results demonstrated that the SD and overall reproducibility of the results of the 13C-UBT were stable over different batches for both the operator, the devices, and between days. The reproducibility SD was 0.65 or less for all batches, and the between days, devices and operators SD was 0.66 or less in all cases; this is less than the natural variability of the DOB measurement (Table 4). Repeatability was assessed by measuring the DOB values for samples from each of the three batches twice a day for 12 days. The results demonstrated that the SD and overall repeatability were stable over different batches and different days. The repeatability SD was 0.64 or less and the overall between-days SD was 0.72 or less; this is less than the natural variability of the DOB measurement (Table 5).
Table 4.
Results of the reproducibility analytical study.
| Expected DOB | Parameter | SD value | 95% CI | CV (%) |
|---|---|---|---|---|
| DOB: 3.3‰ | ||||
| Reproducibility | 0.53 | [0.46–0.63] | 14.8 | |
| Between days precision | 0.54 | [0.46–0.60] | 14.9 | |
| Between devices precision | 0.54 | [0.45–0.59] | 14.9 | |
| Between operators precision | 0.53 | [0.44–0.58] | 14.8 | |
| DOB: 6.4‰ | ||||
| Reproducibility | 0.60 | [0.52–0.71] | 9.7 | |
| Between days precision | 0.62 | [0.54–0.68] | 10.0 | |
| Between devices precision | 0.60 | [0.51–0.65] | 9.7 | |
| Between operators precision | 0.60 | [0.51–0.70] | 9.7 | |
| DOB: 15.5‰ | ||||
| Reproducibility | 0.65 | [0.57–0.77] | 4.3 | |
| Between days precision | 0.65 | [0.56–0.72] | 4.3 | |
| Between devices precision | 0.66 | [0.56–0.73] | 4.4 | |
| Between operators precision | 0.65 | [0.55–0.76] | 4.3 |
DOB: delta over baseline; CI: confidence interval; CV: coefficient of variance; SD: standard deviation.
Table 5.
Results of the precision analytical study.
| Expected DOB | Parameter | SD value | 95% CI | CV (%) |
|---|---|---|---|---|
| DOB: 3.3‰ | ||||
| Repeatability | 0.56 | [0.44–0.78] | 16.9 | |
| Between days precision | 0.63 | [0.52–0.80] | 17.4 | |
| DOB: 6.4‰ | ||||
| Repeatability | 0.59 | [0.46–0.82] | 9.2 | |
| Between days precision | 0.68 | [0.56–0.87] | 10.6 | |
| DOB: 15.5‰ | ||||
| Repeatability | 0.64 | [0.50–0.89] | 4.3 | |
| Between days precision | 0.72 | [0.60–0.92] | 4.8 |
DOB: delta over baseline; CI: confidence interval; CV: coefficient of variance; SD: standard deviation.
Breath sample bags were stored at two different storage conditions representing the two extreme temperatures of the recommended storage range (15°C and 35°C) and at the high limit for the recommended relative humidity (70%). The DOB values for samples from each storage condition were measured on the BreathID®Hp Lab System seven times during 14 consecutive days for each storage condition, specifically on days 2, 4, 8, 9, 10, 11, and 14. The results demonstrated that the SD and overall repeatability were stable over different batches, days, and storage conditions. The overall repeatability SD and the between days precision SD were 0.60 or less; this is less than the natural variability of the DOB measurement (Table 6).
Table 6.
Results of the bag storage analytical study per storage condition.
| Expected DOB | Storage condition | Parameter | SD value | 95% CI | CV (%) |
|---|---|---|---|---|---|
| DOB: 3.3‰ | |||||
| 15°C | Overall repeatability | 0.57 | [0.45–0.78] | 15.0 | |
| Between days precision | 0.57 | [0.45–0.68] | 15.0 | ||
| 35°C + 70% humidity | Overall repeatability | 0.60 | [0.48–0.82] | 16.9 | |
| Between days precision | 0.60 | [0.47–0.72] | 16.9 |
DOB: delta over baseline; CI: confidence interval; CV: Coefficient of Variance; SD: standard deviation.
Discussion
Active H. pylori testing is the preferred modality according to guidelines by the American College of Gastroenterology, the American Gastroenterological Association, and the European and Japanese societies in the test-and-treat approach to dyspepsia.1,2,15 Additional support for this concept came when Cigna became the first large national payer in the United States to decide that it will no longer reimburse serology testing as of 15 August 2014. This provided a great opportunity to further convert serology testing into active H. pylori testing via either the UBT or stool antigen test for initial diagnosis or to confirm eradication.
The BreathID®Hp was launched in the United States in the second half of 2010, offering a cannula-based test kit that features continuous breath sampling and an expected 10 min total test time. It also offers a bag-based test kit for those practices that prefer this method. The cannula kit is simple for patients and staff and provides real-time results in 10–15 min. This improved convenience has enabled physicians to bring H. pylori breath testing in-house. An additional advantage of the device is that it is a relatively small and portable instrument that may be located at large-volume patients clinics such as a hospital outpatient gastroenterology clinic, preventing the requirement for the transportation of the bags for breath testing. However, as we discussed earlier, the BreathID®Hp may also have some negative aspects such as limitation of its use to only a single patient at a time and the inability to evaluate mailed or transported samples. The BreathID®Hp and the cannula-based method of testing are differentiated from the BreathID®Hp Lab System, the subject of this article, which is a bag-based breath collection method and offer notable advantages. These include significantly larger sample size (approximately 50–75 times more breath samples for the same time period), shortening the testing time by approximately 50% and maximizing accuracy.
The aim of this article is to evaluate the BreathID®Hp Lab System, when breath is collected into bags that are then either tested on-site or delivered to a central laboratory, instead of using the original continuous collection system. The results show a high diagnostic accuracy for both pre- and post-eradication setting. Accuracy reaches 100% sensitivity and 97.9% specificity for initial diagnosis of H.pylori than composite reference standard. UBT had a better sensitivity than biopsy urease test (approximately 90%).2 These could be attributed to sampling error associated with endoscopic biopsy, due to patchy distribution of H. pylori, a very low number of H. pylori in the tissue sample or sampling of gastric atrophy or intestinal metaplasia that associated with decreasing H. pylori colonization.16 Another reason for false-negative results is the recent use of proton pump inhibitors, bismuth, or antibiotics. Nonetheless, all of the tests for active infection including RUT, histology, UBT, and culture may become false negative during the use of these drugs. In addition, the possible effect of the storage of the bags was evaluated, and it was found that storage for up to 14 days and under different conditions does not significantly affect breath test results.
The BreathID®Hp Lab System has several advantages compared to previous BreathID®Hp device: First, the BreathID®Hp Lab System can perform sequential diagnosis on 10 pairs of breath collection bags within approximately 25 min via a fully automated process, thereby minimizing potential human error, as opposed to the previous BreathID®Hp Lab System that measures only one subject at a time. Second, it allows performing the breath test in locations that do not have the device itself (due to cost or other reason), and the test cannot be performed on-site. Third, this test takes 15 min with high accuracy than composite reference standard (97%). Finally, this is a reliable system, user-friendly with touch-screen operation, maintenance free, compact, and the system has availability to be connected to a laboratory Lab Information Management Software (LIMS) system.
The optimal 13C-UBT conditions for diagnosing H. pylori infection are still being perfected. The optimal diagnostic cut-off point discriminating between positive and negative 13C-UBT results is still a controversial issue. Therefore, the results for 13C-UBT often affect the diagnostic accuracy when the results are very close to the cutoff as at the onset of the infection or when the level lies in a so-called gray zone.3 The BreathID®Hp Lab System shares with its predicate device the same underlying technology, test substrate, and diagnostic capabilities. Both the subject and predicate systems use molecular correlation spectroscopy (MCS) technology and measure the ratio of 13CO2/12CO2 in exhaled breath prior to and after administration of the test substrate (13C-Urea). MCS technology measures the light absorbance of the sample by infrared spectrometry; this correlates to the CO2 concentrations of the different carbon isotpoes in the breath sample. The output results from both systems are the DOB, and a positive/negative determination is based on the same assay cutoff (⩾5 DOB). Indeed, this study has shown that the cutoff of 5 DOB for the BreathID®Hp Lab System is precise and accurate when compared to the gold standard (EGD biopsy results). However, when assessing gastric biopsies, it should be noted that RUT is considered to be less accurate than IHC assessments.16 Indeed, comparison of the UBT results in our study, to RUT and histology, respectively, revealed higher agreement with IHC, a finding consistent with a recent publication.17
There are several limitations to this study. First, the breath test was not compared to culture which is one of the recommended reference standards, as the efforts needed to insure proper conditions for culture growing were very difficult logistically to arrange in a clinical study and prone to human error. The FDA accepts a reference composite score using RUT and histology alone. Furthermore, the RUT test, whose results are determined by change in color of the substrate, can be interpreted differently by different users in borderline cases (pink is positive and yellow is negative), contributing to human error.
In conclusion, the BreathID®Hp Lab System (Exalenz Bioscience Ltd) has been demonstrated to be as safe and effective as its predicate device, that is, the FDA-cleared Exalenz Bioscience Ltd BreathID®Hp Lab System. It is substantially equivalent to BreathID®Hp without raising new safety or efficacy issues. Based on this study, the BreathID®Hp Lab System also received marketing clearance from FDA for H. pylori detection in November 2016.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Exalenz Bioscience Ltd.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: H.S. has received grants and stock options from Exalenz Bioscience Ltd. None of other authors report conflicts.
Trial Registry: NCT02528721, for protocol titled:#DM2-HP-0715 (https://clinicaltrials.gov/ct2/results?cond=&term=NCT02528721&cntry=&state=&city=&dist=)
ORCID iD: Vered Richter  https://orcid.org/0000-0001-6003-9220
https://orcid.org/0000-0001-6003-9220
Contributor Information
Vered Richter, Institute of Gastroenterology, Liver Diseases and Nutrition, Assaf Harofeh Medical Center, Tel-Aviv University, Zerifin, Israel.
Jeff O. Gonzalez, Palmetto Research, LLC, Hialeah, FL, USA
Sabine Hazan, Ventura Clinical Trials, Ventura, CA, USA.
Gary Gottlieb, Del Sol Research Management, LLC, Tucson, AZ, USA.
Keith Friedenberg, Great Lakes Medical Research, Mentor, OH, USA.
David Gatof, Innovative Clinical Research, Lafayette, CO, USA.
Ravi Ganeshappa, Digestive Disease Center of South Texas, P.L.L.C, San Antonio, TX, USA.
Jorge-Shmuel Delgado, Barzilai Medical Center, Ben-Gurion University of the Negev, Ashkelon, Israel.
Dov Abramowitz, Institute of Gastroenterology, Liver Diseases and Nutrition, Assaf Harofeh Medical Center, Tel-Aviv University, Zerifin, Israel.
Robert Hardi, Chevy Chase Clinical Research, Chevy Chase, MD, USA.
Allan Coates, West Michigan Clinical Research Center, Wyoming, MI, USA.
Mahmudul Haq, Hope Clinical Research, Kissimmee, FL, USA.
Nilesh Mehta, Digestive Disease Care PC, New Hyde Park, NY, USA.
Blake A. Jones, Innovative Clinical Research, Rapid City, SD, USA
Steven F. Moss, Brown University, Providence, RI, USA
Haim Shirin, Institute of Gastroenterology, Liver Diseases and Nutrition, Assaf Harofeh Medical Center, Tel-Aviv University, Zerifin, Israel.
References
- 1. Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102: 1808–1825. [DOI] [PubMed] [Google Scholar]
- 2. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 3. Gisbert JP, Pajares JM. 13C-urea breath test in the diagnosis of Helicobacter pylori infection—a critical review. Aliment Pharmacol Ther 2004; 20: 1001–1017. [DOI] [PubMed] [Google Scholar]
- 4. Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Am J Gastroenterol 1998; 93: 2330–2338. [DOI] [PubMed] [Google Scholar]
- 5. Chey WD. Accurate diagnosis of Helicobacter pylori. 14C-urea breath test. Gastroenterol Clin North Am 2000; 29: 895–902. [DOI] [PubMed] [Google Scholar]
- 6. Graham DY, Klein PD. Accurate diagnosis of Helicobacter pylori. 13C-urea breath test. Gastroenterol Clin North Am 2000; 29: 885–893. [DOI] [PubMed] [Google Scholar]
- 7. Perri F. Diagnosis of Helicobacter pylori infection: which is the best test? The urea breath test. Dig Liver Dis 2000; 32: S196–S198. [DOI] [PubMed] [Google Scholar]
- 8. Nocon M, Kuhlmann A, Leodolter A, et al. Efficacy and cost-effectiveness of the 13C-urea breath test as the primary diagnostic investigation for the detection of Helicobacter pylori infection compared to invasive and non-invasive diagnostic tests. GMS Health Technol Assess 2009; 5: Doc14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gisbert JP, Calvet X. Helicobacter Pylori ‘test-and-treat’ strategy for management of dyspepsia: a comprehensive review. Clin Transl Gastroenterol 2013; 4: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leodolter A, Dominguez-Munoz JE, von Arnim U, et al. Validity of a modified 13C-urea breath test for pre- and posttreatment diagnosis of Helicobacter pylori infection in the routine clinical setting. Am J Gastroenterol 1999; 94: 2100–2104. [DOI] [PubMed] [Google Scholar]
- 11. Schmilovitz-Weiss H, Sehayek-Shabat V, et al. Applicability of a short/rapid 13C-urea breath test for Helicobacter pylori: retrospective multicenter chart review study. BMC Gastroenterol 2012; 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Broide E, Shirin H. Evaluation of Exalenz Bioscience’s BreathID for Helicobacter pylori detection. Expert Rev Mol Diagn 2015; 15: 299–312. [DOI] [PubMed] [Google Scholar]
- 13. el-Zimaity HM. Accurate diagnosis of Helicobacter pylori with biopsy. Gastroenterol Clin North Am 2000; 29: 863–869. [DOI] [PubMed] [Google Scholar]
- 14. Staff FDA. Characteristics of in vitro diagnostic devices for the detection of Helicobacter pylori, 2010, www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm425025.htm
- 15. Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 2010; 15: 1–20. [DOI] [PubMed] [Google Scholar]
- 16. Miftahussurur M, Yamaoka Y. Diagnostic methods of Helicobacter pylori infection for epidemiological studies: critical importance of indirect test validation. Biomed Res Int 2016; 2016: 4819423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kocsmar E, Szirtes I, Kramer Z, et al. Sensitivity of Helicobacter pylori detection by Giemsa staining is poor in comparison with immunohistochemistry and fluorescent in situ hybridization and strongly depends on inflammatory activity. Helicobacter 2017; 22: 12387. [DOI] [PubMed] [Google Scholar]