Summary
A fundamental challenge in developmental biology is to understand how forces produced by individual cells are patterned in space and time and then integrated to produce stereotyped changes in tissue- or embryo-level morphology. Ascidians offer a unique opportunity to address this challenge by studying how small groups of cells collectively execute complex, but highly stereotyped morphogenetic movements. Here we highlight recent progress and open questions in the study of ascidian morphogenesis, emphasizing the dynamic interplay of cell fate determination, cellular force generation and tissue-level mechanics.
Introduction
Embryos are shaped and remodeled by the collective actions of individual cells that change shape, divide, move and die. A fundamental challenge in developmental biology is to understand how the forces that drive these individual behaviors are produced locally, how they are patterned in space and time, and how they are integrated across multicellular tissues to produce stereotyped morphogenetic outcomes.
Ascidians are emerging as a powerful model system to study the dynamical interplay of patterning, force generation and morphogenesis. Ascidians develop rapidly from fertilized eggs into simple tadpole larvae with ~2000 cells, possessing the basic chordate body plan [1]. An invariant lineage [2] and highly stereotyped cleavage pattern [3] provide a unique opportunity to study the relationship between embryo geometry, cell division, and cell fate determination. The morphogenetic movements that position and shape individual larval tissues, are highly stereotyped, involve very few (~10-40) cells, and unfold rapidly in transparent (e.g. Phallusia mammillata), or semi-transparent (e.g. Ciona robusta) embryos, allowing direct observation in living embryos of cellular and subcellular dynamics in relation to tissue morphogenesis [4-8].
Here, we highlight recent progress and emerging insights in three areas: dynamic coupling of cell fate determination, cleavage plane position and cell geometry in pre-gastrula embryos, dynamics of notochord formation and the dynamics of neural tube closure after gastrulation. In all three cases, we emphasize how stereotyped architecture and morphology emerge as a collective outcome of individual cell behaviors that are patterned by cell fate and orchestrated by temporal dynamics of differentiation and cell cycle control.
Dynamic coupling of cell fate, geometry, and cleavage planes in pre-gastrula embryo.
In ascidian embryos, the major cell types are specified before the 112-cell stage through an invariant sequence of asymmetric cell divisions governed by internal segregation of cell fate determinants and/or polarizing inductive signals from neighboring cells [1,9]. This invariant pattern of cell fate specifications is accompanied by an equally invariant sequence of cleavage plane positions and orientations [3], providing a unique opportunity to study dynamic coupling of cell position and geometry to polarity and fate.
Until recently, studies of asymmetric cleavage in ascidians focused on three highly unequal cell divisions that occur at the posterior pole between the 8- and 64-cell stage. These unequal cleavages are directed by a subcellular structure called the centrosome-attracting body (CAB), which is inherited by the posterior daughter of each cleavage [(Figure 1A) 10]. The CAB is a subcortical domain containing putative germ plasm and rough cortical endoplasmic reticulum (cortical ER) that scaffolds a collection of so-called postplasmic/posterior end mark (PEM) mRNAs [11]. The CAB forms within a region of posterior vegetal cytoplasm (PVC) [12], that segregates to the posterior vegetal pole after fertilization, along with the PEM mRNAs. Among other things, PEM mRNAs encode the muscle determinant MACHO-1 [13], the germline-enriched factor VASA [14], and factors such as posterior end mark (PEM) [15], that mediate the CAB’s ability to capture the posterior spindle pole before each cleavage [16]. Thus the CAB coordinates localization of maternal factors with the positioning of cleavage planes to ensure their proper asymmetric inheritance [10,16-18].
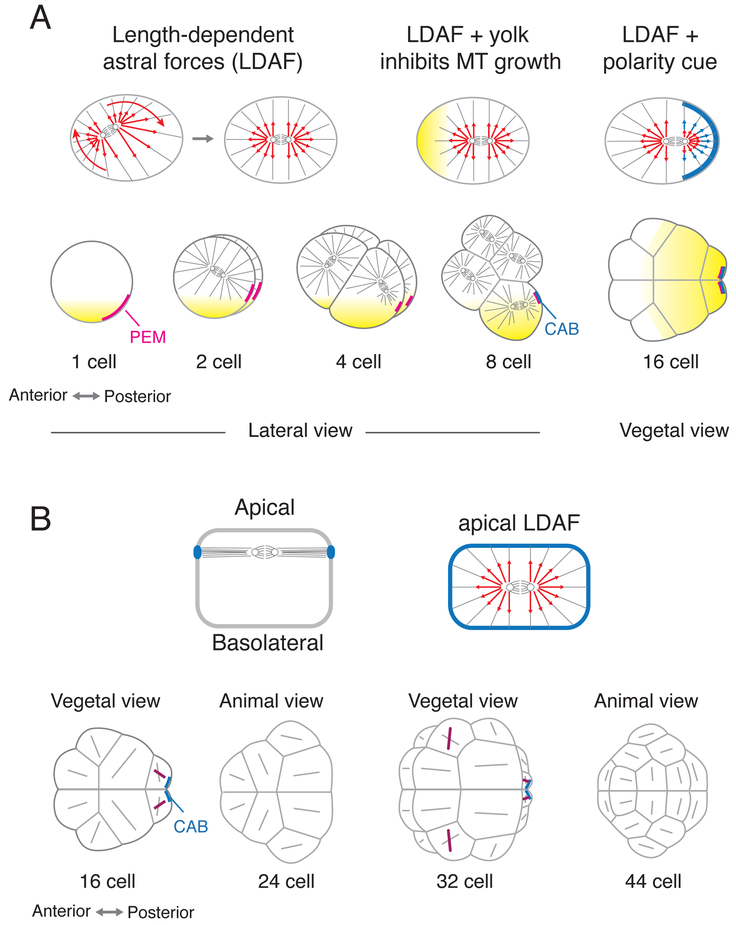
Figure 1. Mechanisms for spindle positioning in early ascidian embryos.
(A) Up to the 16-cell stage, spindle position/orientation is governed by a mechanical balance of length-dependent forces (red arrows) on astral microtubules (LDAF), biased by local inhibition of microtubule growth in yolk-rich (yellow) regions, and additional forces produced by cortical polarity domains (blue cortical patch and force arrows) acting on astral microtubules. (B) From the 16-64 cell stage, spindles are constrained to lie in the apical plane. For the majority of blastomeres, spindle orientations (gray lines) are predicted by a mechanical balance of length-dependent astral forces, acting in the apical plane, The few exceptions (purple lines) are asymmetrically dividing cells (see text for details).
Recent work has begun to reveal a more comprehensive view of how cleavage planes are positioned in early ascidian embryos. Studies in other embryos, and in cultured cells, suggest that length-dependent pulling forces on astral microtubules (LDF) provide a “generic” mechanism for mitotic spindles to “read’ cell geometry, positioning themselves at the cell’s center and orienting themselves with the cell’s long axis (Figure 1A) [19]. Recent computational studies suggest that the spindle positions and orientations, observed up to the 16-cell stage in ascidian embryos, can be explained by LDF plus pulling forces exerted by the CAB, and local inhibition of microtubule growth in vegetal yolk-rich regions (Figure 1A) [*20]. Recent work suggests that local control of microtubule depolymerization by the kinesin Kif2, which colocalizes with PEM on CAB-enriched cortical ER, creates the net imbalance of forces on astral microtubules that pulls one centrosome towards the CAB [*21].
Between the 16- and 64-cell stages, mitotic spindles are further constrained to lie in the apical plane, likely through capture by junctional complexes, as in other epithelia [22,23]. But again, spindle position and orientation in the apical plane can be explained by LDF [*24], except for divisions controlled by the CAB [25], and a few other asymmetric cell divisions, where additional mechanisms align spindle position with cell polarity, either synergizing with, or overriding, LDF (Figure 1B) [26,27*]. Importantly, the same rules also correctly predict spindle position and orientation when the shapes of embryonic cells have been altered by ablating the CAB or inhibiting zygotic transcription, cell adhesion or apico-basal polarity [**20,*24].
These results highlight the important influence of cell geometry on spindle position and orientation, but of course spindle position and orientation also influence cell and embryo geometry. Computer simulations show that up to the 16-cell stage, cell geometries can be explained by a “generic” balance of interfacial forces (identical for all cell-cell interfaces) and resistance to cell compression [**20]. Beyond this stage, cells begin to exert additional fate-specific control over cell shape, with direct consequences for spindle position/orientation [*24,28]. One intriguing idea is that inductive signaling could be controlled by tuning the size of cell-cell contacts, thus the strength of signaling through those contacts, relative to sharp response thresholds [29-31]. By extending and combining methods for accurate 3D cell shape reconstruction [29,32,33], force inference [34-36] and mapping gene expression dynamics [37], it should soon be possible to gain deeper insights into how cell shape, fate, polarity and spindle position/orientation are coupled to ensure robust developmental trajectories.
Polarized motility and contractility drive multiple steps of notochord formation.
The ascidian notochord provides a unique opportunity to understand how an entire morphogenetic program unfolds within a single tissue. Notochord cells are specified before the 112-cell stage [38]; they divide twice during gastrulation to form a monolayer plate of 40 notochord cells (Figure 2A) [39]. Then a complex temporal program of notochord-specific gene expression [40,41] orchestrates a stereotyped sequence of morphogenetic events that transform this plate without any further cell division: first into a cylindrical rod made of coin-shaped cells, stacked end-to-end, then into an elongated tube with a fluid-filled lumen (Figure 2A) [42,43]. The majority of these events are highly conserved across species, although some species-specific differences have been noted, for example in the role of Wnt-5 in notochord specification [44-46], and in lumen formation during tubulogenesis [42,47].
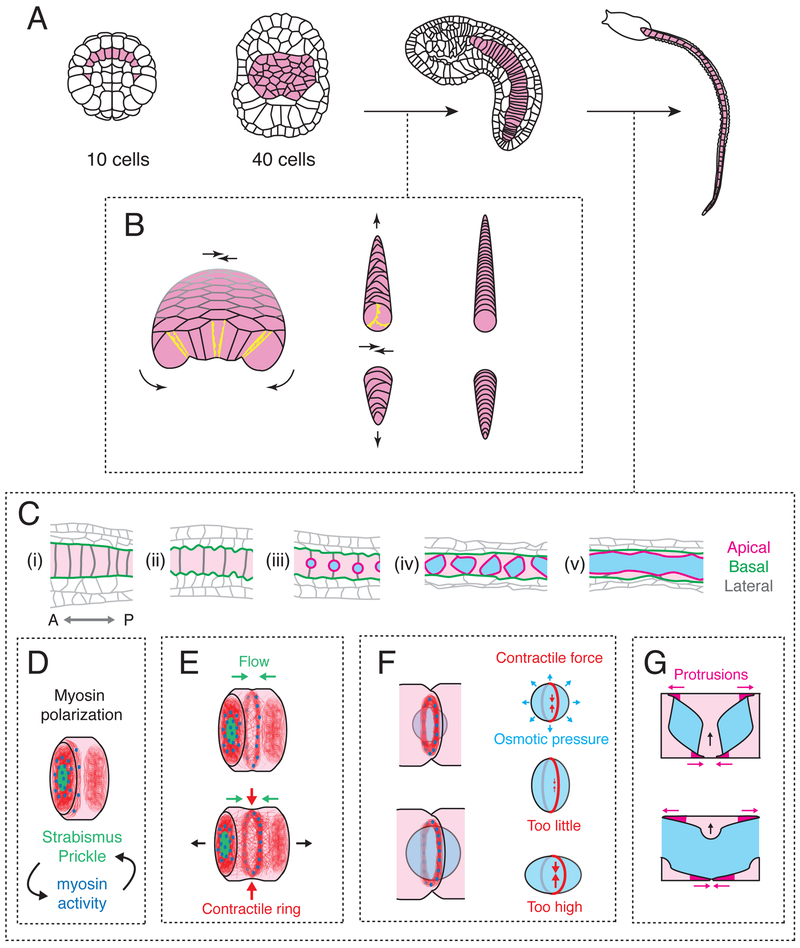
Figure 2. Cytomechanical basis for notochord elongation and tubulogenesis.
(A) The notochord transforms from a set of ten precursor cells into a monolayer plate, then a cylindrical rod, then an elongated tube. (B) Transformation of the flat notochord plate into a cylindrical rod of coin-shaped cells is driven by basolateral crawling (yellow protrusions), which is polarized in the apico-basal plane to drive bending/invagination (curved black arrows) and planar polarized to drive convergent extension (straight black arrows). Modified from [5]). (C) Stages of notochord elongation and tubulogenesis. Schematics show a sagittal section along the notochord midline. Anterior is to the left. Cyan regions indicate lumen; apical, basal and lateral domains are shown in magenta, green and grey respectively. (D) Before elongation, polarized accumulation of Myosin II (blue) and PCP proteins (green) on anterior lateral cell contacts is governed by their reciprocal interactions. A basal actomyosin ring (red filaments and blue Myosin II) forms at the anterior edge of each cell. (E) Anterior enrichment of Myosin II and PCP proteins persists, and a self-centering cortical flow positions the basal actomyosin ring at the cell equator, where it contracts to drive cell elongation. (F) A peri-apical actomyosin contractile ring at cell contacts counteracts osmotic forces to control lumen expansion. (G) Bidirectional polarized basal crawling of notochord cells drives fusion of individual lumens into a central core.
Beginning shortly after gastrulation, the notochord plate undergoes simultaneous invagination, mediolateral convergence and axial extension to form a cylindrical rod of coin-shaped cells (Figure 2B) [4,5]. Analysis of local protrusive activity within the notochord plate suggested that these morphogenetic movements are driven by a single underlying cell behavior - active crawling of individual notochord cells on their adjacent neighbors – which is apico-basally polarized (or otherwise constrained) to drive invagination and planar-polarized to drive mediolateral intercalation and extension (Figure 2B) [5]. More recent work in other embryos, suggests that planar-polarized junction contraction and remodeling could also be important [48,49], although this has yet to be tested in ascidians.
Screens for notochord-enriched genes, and loss-of-function studies have identified a large number of factors that are required for polarized cell movements [41,50,51]. Planar polarized cell motility requires members of the canonical planar cell polarity PCP pathway, including Prickle, Dishevelled and Wnt-5, acting cell-autonomously within the notochord [52-54], and FGF signaling from the neighboring neural plate [55]. Tissue isolation, ablation and recombination experiments suggest that additional cues from neighboring tissues act redundantly to align planar polarity in the notochord with respect to the embryonic anterior-posterior AP axis [5]. Members of the classical PAR-3/PAR-6/aPKC polarity complex are apically localized in notochord cells during intercalation, and their loss or mis-localization leads to severe intercalation defects [56,57]. Basal deposition of laminin [53,56,58] and possibly also fibronectin [59], is essential to maintain a coherent interface between the basal notochord and surrounding tissue, and to constrain polarized motility into productive convergence and extension [53,56,58]. How apico-basal and planar polarity systems, and mechanical and/or biochemical inputs from neighboring tissues, bias and constrain motile and contractile force generation within the notochord to produce a single global axis of invagination and tissue extension, is now ripe for further exploration. In addition, more recent work has begun to reveal how stereotyped differences in notochord cell size and intercalation behavior, depending on lineage, initial position, PCP signaling, and regional differences in gene expression, feed into the core morphogenetic program to produce a notochord with a distinctive tapered shape [60-62].
In the second major step, known as tubulogenesis, the notochord transforms into an elongated tube with a central fluid-filled lumen. Detailed analysis in Ciona robusta [47] revealed the underlying sequence of events. Individual notochord cells first elongate along the AP axis, transforming from coin-shaped disks into elongated cylinders (Figure 2Ci-ii). As they elongate, notochord cells form apical domains centered on contacts with neighboring cells, and form apical lumens that expand through changes in osmotic pressure, driven by secretory activity (Fig 2Ciii-iv). Finally, these lumens fuse into a single central core, and polarized basal crawling of notochord cells drives the rearrangement of notochord cells into an outer layer of endothelial-like cells (Figure 2Cv).
Recent studies highlights how a dynamic interplay of cell polarity and actomyosin contractility controls multiple steps of this process (Figure 2C-G). First, as notochord cells begin to elongate, actomyosin becomes enriched on and near anterior contacts with neighboring cells, where it overlaps with PCP components Prickle and Strabismus [63,*64]. Actomyosin contractility is a well-known effector for PCP in other contexts [65], but in notochord cells, reciprocal interactions between actomyosin and PCP proteins are required to establish and maintain PCP asymmetries [63,64]. Actomyosin is also enriched in a basal equatorial ring of circumferentially aligned filaments, that contracts against an incompressible cytoplasm to drive axial cell elongation (Figure 2E) [66]. This ring is maintained by a continuous bidirectional flow of cortical actomyosin towards the equator, balanced by local disassembly, which concentrates and aligns filaments at the cell equator [67], as proposed originally for contractile ring assembly during cyokinesis [68], and documented recently in C. elegans embryos [69]. However, unlike the cytokinetic contractile ring, the basal contractile ring does not divide the cell into two, and it disappears before lumen fusion [66]. Interestingly, a precursor to the equatorial ring first forms near the anterior boundary of each cell, where actomyosin overlaps with PCP proteins, then relocates towards the cell equator (Figure 2D,E) [64,**70]. A combination of experiments and mathematical modeling suggests that an increase in contractile force leads to the spontaneous emergence of self-amplified cortical flow away from cell-cell contacts. The tendency of this flow to center itself between cell-cell contacts drives relocation of the contractile ring from its initial anterior position, biased by planar-polarized actin assembly, to the equator [**70]. Thus, a generic ability of actomyosin networks to produce long-range self-amplifying cortical flow, can be co-opted to pattern forces that drive cell and tissue elongation.
Finally, a second actomyosin-based “contractile ring”, forms at the edge of the apical domain during lumen expansion, and constrains isotropic osmotic expansion forces to favor longitudinal (A-P) over circumferential lumen expansion (Figure 2F) [*71]. Contractile forces within the ring are controlled by both actin assembly and myosin activation, and a correct balance of contractile and osmotic forces is essential: too little contraction attenuates longitudinal expansion and fusion with neighboring lumens to form the central tube; too much can drive hyper-contraction and internalization of the apical domain (Figure 2F). How forces generated by equatorial and peri-apical contractile rings are balanced against cellular and luminal pressures to coordinate cell elongation and luminal growth, remains an interesting question for future study [67,*71].
Dynamic control of cell motility, force generation, and tissue remodeling during neurulation.
Like many vertebrates, ascidians transform a neural plate into an elongated tube in three steps: invagination, followed by medial convergence and axial extension, then meeting and fusion of the neural folds along the dorsal midline [72]. However, ascidians use just 80 cells, each with a unique identity, to make a very simple neural tube, in which a single AP row of cells form the floor, sides and roof respectively [73,74].
During gastrulation, local FGF/MEK/ERK, Delta/Notch and Nodal signals partition the neural primordium into progenitor domains that will give rise to the floor, sides and roof, respectively, of the axial nerve tube (Figure 3A) [75-77]}. Recent live imaging studies in Ciona robusta show that while all cells contribute to axial elongation of the neural primordium through AP oriented cell divisions, cells within each of the progenitor domains engage in unique behaviors to cohere while rearranging into a single AP row of cells [*78]. Floor plate progenitors undergo simple mediolateral intercalation, which appears to be driven by biased shortening of mediolateral junctions, as observed in the vertebrate neural plate [49]. Lateral progenitors engage in a modified intercalation behavior called “stacking” in which their descendants remain attached to one another and intercalate as groups into a single AP file (Figure 3A) [74,*78]. Finally, roof progenitors meet at the midline, establish new contacts (see below), and then undergo mediolateral intercalation (Figure 3A). Systematic perturbation of signaling during gastrulation suggests that early specification of floor plate and lateral fates during gastrulation by FGF and Nodal signals, respectively, is sufficient to ensure the autonomous expression of mediolateral intercalation and stacking behaviors in their descendants during neurulation. [*78]. How FGF and Nodal signaling induce these distinct cell behaviors, and how cell rearrangement is constrained to occur within, and not across, progenitor domains, remains unclear.
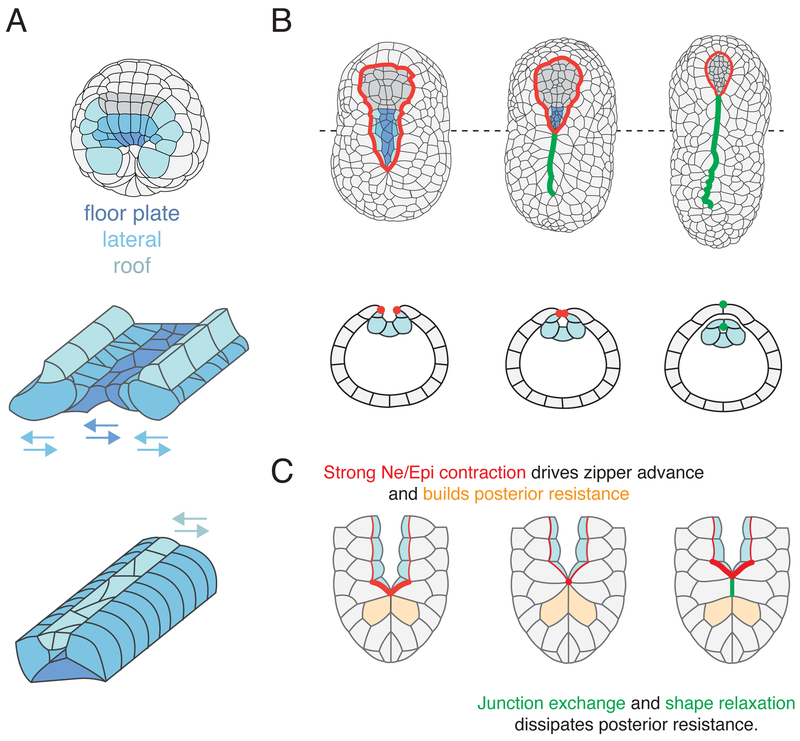
Figure 3. Dynamic control of cell behavior and tissue remodeling during neurulation.
(A) Presumptive floor plate, lateral and roof neural cells established by FGF and Nodal signals during gastrulation undergo distinct modes of rearrangement to form elongated rows during neurulation. (B) Neural tube closes by posterior-anterior “zippering”, in which pairs of neural (Ne) and epidermal (Epi) cells meet at the midline, followed by junctional exchange in which Ne/Epi junctions (red dots) rearrange to form Ne/Ne and Epi/Epi junctions (green dots). (C) Cytomechanical basis for zippering: Myosin is activated along all Ne/Epi junctions (thin red lines) ahead of the zipper. Strong myosin activation (thick red lines) just ahead of zipper drives zipper advance, stretching cells (orange) behind the zipper. Junction exchange and cell shape relaxation dissipates resistance behind the zipper.
In the final step of tail neural tube closure, the neural folds, consisting of adjacent rows of neural (roof) and epidermal precursors, meet at the midline, fuse, and undergo junctional rearrangements. Heterotypic Neural/Epidermal (Ne/Epi) junctions are converted to homotypic Neural/Neural (Ne/Ne) and Epidermal/Epidermal (Epi/Epi) junctions thus separating the closed neural tube from the overlying epidermis. This process proceeds in zipper-like fashion from posterior to anterior (Figure 3B) [74,**79]. Recent work in Ciona robusta, combining live imaging, laser ablations and computer simulations, reveals that spatiotemporal control of junctional tension by Myosin II plays a key role in driving zippering and neural tube closure (Figure 3B) [**79]. Active myosin is enriched on all Ne/Epi junctions ahead of the zipper, and reduced on newly formed Ne/Ne and Epi/Epi junctions behind the zipper. In addition, a wave of myosin activation just ahead of zipper drives rapid shortening of individual junctions to pull neural folds together and drive the zipper forward (Figure 3c). Computer simulations confirm that spatiotemporal regulation of junctional tension by myosin activity is sufficient to explain the dynamics of zipper progression, and also reveals the underlying design principles: First, higher levels of myosin activity ahead of the zipper and continuous dissipation of tissue resistance (through junctional rearrangements and cell shape relaxation) behind the zipper creates a tissue-level mechanical asymmetry that converts a sequence of local inherently symmetric junction contractions into unidirectional zipper progression. Second, localizing rapid “all-or-none” contractions just ahead of the zipper promotes highly efficient junctional exchange and local dissipation of tissue resistance behind the zipper, relative to a “purse string” mechanism in which uniformly strong contraction of the entire boundary leads to continuous shortening of all junctions and continuous buildup of tissue resistance (Figure 3C) [**68].
Two key questions emerge from this work: First, how is myosin activity localized to the Ne/Epi boundary? Second, what patterns the posterior-to-anterior (P->A) wave of strong contraction that propagates along this boundary? Regarding the first question, it is likely that local activation of Myosin II along the entire Ne/Epi boundary is controlled by differential expression of signaling molecules or junctional transmembrane proteins [80-85]. Regarding the second question, recent work has shown that a P->A gradient in S-phase length patterns a wave of epidermal cell divisions that initiates just after zippering, and which is tightly coordinated with zipper progression [*86]. In principle, a P-A gradient of cell cycle timing could also pattern the wave of strong myosin activation that drives the zipper forward. Alternatively, the wave could be propagated by extracellular signals along the Ne/Epi boundary [87], or by a mechanochemical relay involving tension-dependent activation and/or stabilization of Myosin II [88-91], or by an activating signal, produced by the zipper, whose range extends with the zipper as it moves. Distinguishing these possibilities in ascidian embryos should provide valuable clues about spatiotemporal control of contractility and tissue morphogenesis in many other contexts.
Concluding remarks
Studies in ascidians are beginning to reveal some of the myriad ways in which cell fate and polarity are coupled to local force generation and transmission to achieve highly stereotyped morphogenetic outcomes. We refer the interested reader to additional exemplary work on spatiotemporal control of myosin activity during gastrulation [7], induced polarity, asymmetric division and transcriptional control of cell migration in the cardiac lineage [31,92-95], dynamic coordination of cell division and tissue morphogenesis during neurulation [*86,96], and dynamic control of cleavage plane orientation in the epidermis during tail elongation [97]. These, and the works covered here, highlight the wealth of insight to be gained through detailed analysis of cases in which a few cells collectively execute complex stereotyped morphogenetic transformations.
Acknowledgments
We thank the anonymous reviewers for helpful comments. We gratefully acknowledge support from NICHD, 1R01HD088831-01
References and recommended reading
* of special interest
** of outstanding interest
- 1.Lemaire P: Unfolding a chordate developmental program, one cell at a time: invariant cell lineages, short-range inductions and evolutionary plasticity in ascidians. Dev. Biol. 2009, 332:48–60. [DOI] [PubMed] [Google Scholar]
- 2.Nishida H: Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. III. Up to the tissue restricted stage. Dev. Biol. 1987, 121:526–541. [DOI] [PubMed] [Google Scholar]
- 3.McDougall A, Chenevert J, Pruliere G, Costache V, Hebras C, Salez G, Dumollard R: Centrosomes and spindles in ascidian embryos and eggs. Methods Cell Biol. 2015, 129:317–339. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto DM, Crowther RJ: Formation of the notochord in living ascidian embryos. J Embryol Exp Morphol 1985, 86:1–17. [PubMed] [Google Scholar]
- 5.Munro EM, Odell GM: Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development 2002, 129:13–24. [DOI] [PubMed] [Google Scholar]
- 6.Passamaneck YJ, Hadjantonakis A-K, Di Gregorio A: Dynamic and polarized muscle cell behaviors accompany tail morphogenesis in the ascidian Ciona intestinalis. PLoS ONE 2007, 2:e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherrard K, Robin F, Lemaire P, Munro E: Sequential activation of apical and basolateral contractility drives ascidian endoderm invagination. Curr Biol 2010, 20:1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veeman M, Reeves W: Quantitative and in toto imaging in ascidians: working toward an image-centric systems biology of chordate morphogenesis. genesis 2015, 53:143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Negishi T, Nishida H: Asymmetric and Unequal Cell Divisions in Ascidian Embryos. Results Probl Cell Differ 2017, 61:261–284. [DOI] [PubMed] [Google Scholar]
- 10.Hibino T, Nishikata T, Nishida H: Centrosome-attracting body: a novel structure closely related to unequal cleavages in the ascidian embryo. Dev Growth Differ 1998, 40:85–95. [DOI] [PubMed] [Google Scholar]
- 11.Makabe KW, Nishida H: Cytoplasmic localization and reorganization in ascidian eggs: role of postplasmic/PEM RNAs in axis formation and fate determination. Wiley Interdiscip Rev Dev Biol 2012, 1:501–518. [DOI] [PubMed] [Google Scholar]
- 12.Conklin EG: The organization and cell-lineage of the ascidian egg / by Edwin G. Conklin. [Academy of Natural Sciences; ]; 1905. [Google Scholar]
- 13.Nishida H, Sawada K: macho-1 encodes a localized mRNA in ascidian eggs that specifies muscle fate during embryogenesis. Nature 2001, 409:724–729. [DOI] [PubMed] [Google Scholar]
- 14.Shirae-Kurabayashi M, Nishikata T, Takamura K, Tanaka KJ, Nakamoto C, Nakamura A: Dynamic redistribution of vasa homolog and exclusion of somatic cell determinants during germ cell specification in Ciona intestinalis. Development 2006, 133:2683–2693. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida S, Marikawa Y, Satoh N: Posterior end mark, a novel maternal gene encoding a localized factor in the ascidian embryo. Development 1996, 122:2005–2012. [DOI] [PubMed] [Google Scholar]
- 16.Negishi T, Takada T, Kawai N, Nishida H: Localized PEM mRNA and protein are involved in cleavage-plane orientation and unequal cell divisions in ascidians. Current Biology 2007, 17:1014–1025. [DOI] [PubMed] [Google Scholar]
- 17.Nishikata T, Hibino T, Nishida H: The Centrosome-Attracting Body, Microtubule System, and Posterior Egg Cytoplasm Are Involved in Positioning of Cleavage Planes in the Ascidian Embryo [Internet]. Dev. Biol. 1999, 209:72–85. [DOI] [PubMed] [Google Scholar]
- 18.Paix A, Yamada L, Dru P, Lecordier H, Pruliere G, Chenevert J, Satoh N, Sardet C: Cortical anchorages and cell type segregations of maternal postplasmic/PEM RNAs in ascidians. Dev. Biol. 2009, 336:96–111. [DOI] [PubMed] [Google Scholar]
- 19.Minc N, Piel M: Predicting division plane position and orientation. Trends Cell Biol 2012, 22:193–200. [DOI] [PubMed] [Google Scholar]
- 20.Pierre A, Sallé J, Wühr M, Minc N: Generic Theoretical Models to Predict Division Patterns of Cleaving Embryos. Dev. Cell 2016, 39:667–682.** This paper shows how a few “self-organizing” rules that couple cell geometry and cleavage plane position can explain morphological dynamics (cell geometry and division patterns) in early embryos of four different species, including ascidians; Length dependent astral microtubule forces, biased by yolk gradients and cortical polarity, can explain spindle position and orientation, while a balance of intracellular pressure and interfacial tension can explain cell shape and packing. This work highlights how cell geometry and cleavage plane positioning are dynamically coupled to shape morphology.
- 21.Costache V, Hebras C, Pruliere G, Besnardeau L, Failla M, Copley RR, Burgess D, Chenevert J, McDougall A: Kif2 localizes to a subdomain of cortical endoplasmic reticulum that drives asymmetric spindle position. Nat Commun 2017, 8:917.* This paper identifies a molecular motor, Kif2, that co-localizes with the centrosome attracting body (CAB) and appears to mediate centrosome attraction by acting as a depolymerase to shift the balance of forces on astral microtubules near the CAB towards a net pulling force.
- 22.Nakajima Y-I, Meyer EJ, Kroesen A, McKinney SA, Gibson MC: Epithelial junctions maintain tissue architecture by directing planar spindle orientation. Nature 2013, 500:359–362. [DOI] [PubMed] [Google Scholar]
- 23.Ragkousi K, Gibson MC: Cell division and the maintenance of epithelial order. The Journal of Cell Biology 2014, 207:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumollard R, Minc N, Salez G, Aicha SB, Bekkouche F, Hebras C, Besnardeau L, McDougall A: The invariant cleavage pattern displayed by ascidian embryos depends on spindle positioning along the cell's longest axis in the apical plane and relies on asynchronous cell divisions. Elife 2017, 6:495.* Building on the work of Minc and colleagues [14, **15], this paper shows that length-dependent astral forces, and confinement of spindles to the apical surface, provides a generic explanation for spindle position and orientation for most cells between the 16- and 64-cell stage in ascidian embryos. The exceptions to these rules highlight cells in which spindles are oriented with respect to cell polarity (see [*22])
- 25.Prodon F, Chenevert J, Hebras C, Dumollard R, Faure E, Gonzalez-Garcia J, Nishida H, Sardet C, McDougall A: Dual mechanism controls asymmetric spindle position in ascidian germ cell precursors. Development 2010, 137:2011–2021. [DOI] [PubMed] [Google Scholar]
- 26.Shi W, Levine M: Ephrin signaling establishes asymmetric cell fates in an endomesoderm lineage of the Ciona embryo. Development 2008, 135:931–940. [DOI] [PubMed] [Google Scholar]
- 27.Negishi T, Yasuo H: Distinct modes of mitotic spindle orientation align cells in the dorsal midline of ascidian embryos. Dev. Biol. 2015, 408:66–78.* The authors document multiple mechanisms, operating in different blastomeres, that orient spindles with respect to cell polarity during asymmetric cell division in early ascidian embryos.
- 28.Dumollard R, Hebras C, Besnardeau L, McDougall A: Beta-catenin patterns the cell cycle during maternal-to-zygotic transition in urochordate embryos. Dev. Biol. 2013, 384:331–342. [DOI] [PubMed] [Google Scholar]
- 29.Tassy O, Daian F, Hudson C, Bertrand V, Lemaire P: A quantitative approach to the study of cell shapes and interactions during early chordate embryogenesis. Current Biology 2006, 16:345–358. [DOI] [PubMed] [Google Scholar]
- 30.Ohta N, Waki K, Mochizuki A, Satou Y: A Boolean Function for Neural Induction Reveals a Critical Role of Direct Intercellular Interactions in Patterning the Ectoderm of the Ascidian Embryo. PLoS Comput. Biol. 2015, 11:e1004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cota CD, Davidson B: Mitotic Membrane Turnover Coordinates Differential Induction of the Heart Progenitor Lineage. Dev. Cell 2015, 34:505–519. [DOI] [PubMed] [Google Scholar]
- 32.Robin FB, Dauga D, Tassy O, Sobral D, Daian F, Lemaire P: Creating 3D digital replicas of ascidian embryos from stacks of confocal images. Cold Spring Harb Protoc 2011, 2011:1251–1261. [DOI] [PubMed] [Google Scholar]
- 33.Stegmaier J, Amat F, Lemon WC, McDole K, Wan Y, Teodoro G, Mikut R, Keller PJ: Real-Time Three-Dimensional Cell Segmentation in Large-Scale Microscopy Data of Developing Embryos. Dev. Cell 2016, 36:225–240. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuis JH, Ehsandar A, Maître J-L, Hiiragi T, Cox S, Brodland GW: Inferring cellular forces from image stacks. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2017, 372:20160261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiou KK, Hufnagel L, Shraiman BI: Mechanical stress inference for two dimensional cell arrays. PLoS Comput. Biol. 2012, 8:e1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishihara S, Sugimura K, Cox SJ, Bonnet I, Bellaïche Y, Graner F: Comparative study of non-invasive force and stress inference methods in tissue. Eur Phys J E Soft Matter 2013, 36:9859. [DOI] [PubMed] [Google Scholar]
- 37.Tassy O, Dauga D, Daian F, Sobral D, Robin F, Khoueiry P, Salgado D, Fox V, Caillol D, Schiappa R, et al. : The ANISEED database: digital representation, formalization, and elucidation of a chordate developmental program. Genome Res. 2010, 20:1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakatani Y, Nishida H: Induction of notochord during ascidian embryogenesis. Dev. Biol. 1994, 166:289–299. [DOI] [PubMed] [Google Scholar]
- 39.Munro EM, Odell G: Morphogenetic pattern formation during ascidian notochord formation is regulative and highly robust. Development 2002, 129:1–12. [DOI] [PubMed] [Google Scholar]
- 40.Katikala L, Aihara H, Passamaneck YJ, Gazdoiu S, José-Edwards DS, Kugler JE, Oda-Ishii I, Imai JH, Nibu Y, Di Gregorio A: Functional Brachyury binding sites establish a temporal read-out of gene expression in the Ciona notochord. PLoS Biol. 2013, 11:e1001697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reeves WM, Wu Y, Harder MJ, Veeman MT: Functional and evolutionary insights from the Ciona notochord transcriptome. Development 2017, 144:3375–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang D, Smith WC: Ascidian notochord morphogenesis. Dev Dyn 2007, 236:1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denker E, Jiang D: Ciona intestinalis notochord as a new model to investigate the cellular and molecular mechanisms of tubulogenesis. Semin. Cell Dev. Biol. 2012, 23:308–319. [DOI] [PubMed] [Google Scholar]
- 44.Takatori N, Kumano G, Saiga H, Nishida H: Segregation of germ layer fates by nuclear migration-dependent localization of Not mRNA. Dev. Cell 2010, 19:589–598. [DOI] [PubMed] [Google Scholar]
- 45.Hudson C, Kawai N, Negishi T, Yasuo H: β-Catenin-driven binary fate specification segregates germ layers in ascidian embryos. Curr Biol 2013, 23:491–495. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Makabe KW, Nishida H: The functional analysis of Type I postplasmic/PEM mRNAs in embryos of the ascidian Halocynthia roretzi. [Internet]. Dev Genes Evol 2006, 216:69–80. [DOI] [PubMed] [Google Scholar]
- 47.Dong B, Horie T, Denker E, Kusakabe T, Tsuda M, Smith WC, Jiang D: Tube formation by complex cellular processes in Ciona intestinalis notochord. Dev. Biol. 2009, 330:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shindo A, Wallingford JB: PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science 2014, 343:649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishimura T, Honda H, Takeichi M: Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 2012, 149:1084–1097. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi H, Hotta K, Erives A, Di Gregorio A, Zeller RW, Levine M, Satoh N: Brachyury downstream notochord differentiation in the ascidian embryo. Genes Dev. 1999, 13:1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hotta K, Takahashi H, Asakura T, Saitoh B, Takatori N, Satou Y, Satoh N: Characterization of Brachyury-downstream notochord genes in the Ciona intestinalis embryo. Dev. Biol. 2000, 224:69–80. [DOI] [PubMed] [Google Scholar]
- 52.Keys DN, Levine M, Harland RM, Wallingford JB: Control of intercalation is cell-autonomous in the notochord of Ciona intestinalis. Dev. Biol. 2002, 246:329–340. [DOI] [PubMed] [Google Scholar]
- 53.Jiang D, Munro EM, Smith WC: Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Current Biology 2005, 15:79–85. [DOI] [PubMed] [Google Scholar]
- 54.Niwano T, Takatori N, Kumano G, Nishida H: Wnt5 is required for notochord cell intercalation in the ascidian Halocynthia roretzi. Biol. Cell 2009, 101:645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi W, Peyrot SM, Munro E, Levine M: FGF3 in the floor plate directs notochord convergent extension in the Ciona tadpole. Development 2009, 136:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oda-Ishii I, Ishii Y, Mikawa T: Eph regulates dorsoventral asymmetry of the notochord plate and convergent extension-mediated notochord formation. PLoS ONE 2010, 5:e13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denker E, Bocina I, Jiang D: Tubulogenesis in a simple cell cord requires the formation of bi-apical cells through two discrete Par domains. Development 2013, 140:2985–2996. [DOI] [PubMed] [Google Scholar]
- 58.Veeman MT, Nakatani Y, Hendrickson C, Ericson V, Lin C, Smith WC: Chongmague reveals an essential role for laminin-mediated boundary formation in chordate convergence and extension movements. Development 2008, 135:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Segade F, Cota C, Famiglietti A, Cha A, Davidson B: Fibronectin contributes to notochord intercalation in the invertebrate chordate, Ciona intestinalis. Evodevo 2016, 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carlson M, Reeves W, Veeman M: Stochasticity and stereotypy in the Ciona notochord. Dev. Biol. 2015, 397:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veeman MT, Smith WC: Whole-organ cell shape analysis reveals the developmental basis of ascidian notochord taper. Dev. Biol. 2013, 373:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reeves W, Thayer R, Veeman M: Anterior-posterior regionalized gene expression in the Ciona notochord. Dev Dyn 2014, 243:612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kourakis MJ, Reeves W, Newman-Smith E, Maury B, Abdul-Wajid S, Smith WC: A one-dimensional model of PCP signaling: polarized cell behavior in the notochord of the ascidian Ciona. Dev. Biol. 2014, 395:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newman-Smith E, Kourakis MJ, Reeves W, Veeman M, Smith WC: Reciprocal and dynamic polarization of planar cell polarity core components and myosin. Elife 2015, 4:e05361.* This work describes the novel finding that reciprocal interactions between PCP signaling and myosin activity polarize PCP proteins, myosin activity and nuclear position during notochord morphogenesis in ascidian embryos.
- 65.Wallingford JB: Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol 2012, 28:627–653. [DOI] [PubMed] [Google Scholar]
- 66.Dong B, Deng W, Jiang D: Distinct cytoskeleton populations and extensive crosstalk control Ciona notochord tubulogenesis. Development 2011, 138:1631–1641. [DOI] [PubMed] [Google Scholar]
- 67.Sehring IM, Dong B, Denker E, Bhattachan P, Deng W, Mathiesen BT, Jiang D: An equatorial contractile mechanism drives cell elongation but not cell division. PLoS Biol. 2014, 12:e1001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White JG, Borisy GG: On the mechanisms of cytokinesis in animal cells. J. Theor. Biol. 1983, 101:289–316. [DOI] [PubMed] [Google Scholar]
- 69.Reymann A-C, Staniscia F, Erzberger A, Salbreux G, Grill SW: Cortical flow aligns actin filaments to form a furrow. Elife 2016, 5:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sehring IM, Recho P, Denker E, Kourakis M, Mathiesen B, Hannezo E, Dong B, Jiang D: Assembly and positioning of actomyosin rings by contractility and planar cell polarity. Elife 2015, 4:e09206.** This work combines experiments and computer simulations to show how a self centering cortical flow positions an equatorial actomyosin ring that drives elongation of notochord cells to drive tail elongation in ascidian embryos.
- 71.Denker E, Sehring IM, Dong B, Audisso J, Mathiesen B, Jiang D: Regulation by a TGFβ-ROCK-actomyosin axis secures a non-linear lumen expansion that is essential for tubulogenesis. Development 2015, 142:1639–1650.* During notochord tubulogenesis in ascidians, apical lumens form at cell-cell contacts, expand and fuse to form a single lumen. This paper describes how notochord cells harness a peri-apical actomyosin contractile ring to constrain apical lumen expansion and ensure the orderly fusion of growing lumens into a single central core.
- 72.Munro E, Robin F, Lemaire P: Cellular morphogenesis in ascidians: how to shape a simple tadpole. Curr. Opin. Genet. Dev. 2006, 16:399–405. [DOI] [PubMed] [Google Scholar]
- 73.Nicol D, Meinertzhagen IA: Development of the central nervous system of the larva of the ascidian, Ciona intestinalis L. I. The early lineages of the neural plate. Dev. Biol. 1988, 130:721–736. [DOI] [PubMed] [Google Scholar]
- 74.Nicol D, Meinertzhagen IA: Development of the central nervous system of the larva of the ascidian, Ciona intestinalis L. II. Neural plate morphogenesis and cell lineages during neurulation. Dev. Biol. 1988, 130:737–766. [DOI] [PubMed] [Google Scholar]
- 75.Hudson C, Yasuo H: Patterning across the ascidian neural plate by lateral Nodal signalling sources. Development 2005, 132:1199–1210. [DOI] [PubMed] [Google Scholar]
- 76.Hudson C, Lotito S, Yasuo H: Sequential and combinatorial inputs from Nodal, Delta2/Notch and FGF/MEK/ERK signalling pathways establish a grid-like organisation of distinct cell identities in the ascidian neural plate. Development 2007, 134:3527–3537. [DOI] [PubMed] [Google Scholar]
- 77.Roure A, Lemaire P, Darras S: An otx/nodal regulatory signature for posterior neural development in ascidians. PLoS Genet. 2014, 10:e1004548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Navarrete IA, Levine M: Nodal and FGF coordinate ascidian neural tube morphogenesis. Development 2016, 143:4665–4675.* This paper establishes a link between specification of distinct (floor, lateral and roof) cell fates in the neural plate by FGF and Nodal signals and the fate-specific morphogenetic behaviors that form the floor, sides and roof of the elongated neural tube.
- 79.Hashimoto H, Robin FB, Sherrard KM, Munro EM: Sequential contraction and exchange of apical junctions drives zippering and neural tube closure in a simple chordate. Dev. Cell 2015, 32:241–255.** This paper combines experiments and modeling to determine the cytomechanical basis for zippering and neural tube closure in ascidians. They show that zippering is driven a wave of myosin activation and junction contraction that sweeps from posterior to anterior along the neural/epidermal boundary Tissue-level asymmetry in myosin activity and continuous dissipation of tissue resistance through junctional rearrangements behind the zipper converts a sequence of local junction contractions into unidirectional zipper progression.
- 80.Major RJ: Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. 2005, 132:3823–3833. [DOI] [PubMed] [Google Scholar]
- 81.Röper K: Anisotropy of Crumbs and aPKC Drives Myosin Cable Assembly during Tube Formation. 2013, 23:939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laplante C, Nilson LA: Asymmetric distribution of Echinoid defines the epidermal leading edge during Drosophila dorsal closure. The Journal of Cell Biology 2011, 192:335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paré AC, Vichas A, Fincher CT, Mirman Z, Farrell DL, Mainieri A, Zallen JA: A positional Toll receptor code directs convergent extension in Drosophila. Nature 2014, 515:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fagotto F: Regulation of cell adhesion and cell sorting at embryonic boundaries. Curr. Top. Dev. Biol. 2015, 112:19–64. [DOI] [PubMed] [Google Scholar]
- 85.Umetsu D, Kuranaga E: Planar polarized contractile actomyosin networks in dynamic tissue morphogenesis. Curr. Opin. Genet. Dev. 2017, 45:90–96. [DOI] [PubMed] [Google Scholar]
- 86.Ogura Y, Sasakura Y: Developmental Control of Cell-Cycle Compensation Provides a Switch for Patterned Mitosis at the Onset of Chordate Neurulation. Dev. Cell 2016, 37:148–161.* This paper (and [75]) describe mechanisms that coordinate a posterior-anterior wave of cell division with shape changes that drive neural tube closure in ascidian embryos.
- 87.Aoki K, Kondo Y, Naoki H, Hiratsuka T, Itoh RE, Matsuda M: Propagating Wave of ERK Activation Orients Collective Cell Migration. Dev. Cell 2017, 43:305–317.e5. [DOI] [PubMed] [Google Scholar]
- 88.Fernandez-Gonzalez R, Simões S de M, Röper J-C, Eaton S, Zallen JA: Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 2009, 17:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Effler JC, Kee Y-S, Berk JM, Tran MN, Iglesias PA, Robinson DN: Mitosis-specific mechanosensing and contractile-protein redistribution control cell shape. Current Biology 2006, 16:1962–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitrossilis D, Röper J-C, Le Roy D, Driquez B, Michel A, Ménager C, Shaw G, Le Denmat S, Ranno L, Dumas-Bouchiat F, et al. : Mechanotransductive cascade of Myo-II-dependent mesoderm and endoderm invaginations in embryo gastrulation. Nat Commun 2017, 8:13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kee Y-S, Ren Y, Dorfman D, Iijima M, Firtel R, Iglesias PA, Robinson DN: A mechanosensory system governs myosin II accumulation in dividing cells. Mol Biol Cell 2012, 23:1510–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christiaen L, Davidson B, Kawashima T, Powell W, Nolla H, Vranizan K, Levine M: The transcription/migration interface in heart precursors of Ciona intestinalis. Science 2008, 320:1349–1352. [DOI] [PubMed] [Google Scholar]
- 93.Cooley J, Whitaker S, Sweeney S, Fraser S, Davidson B: Cytoskeletal polarity mediates localized induction of the heart progenitor lineage. Nat. Cell Biol. 2011, 13:952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Norton J, Cooley J, Islam AFMT, Cota CD, Davidson B: Matrix adhesion polarizes heart progenitor induction in the invertebrate chordate Ciona intestinalis. Development 2013, 140:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gline S, Kaplan N, Bernadskaya Y, Abdu Y, Christiaen L: Surrounding tissues canalize motile cardiopharyngeal progenitors towards collective polarity and directed migration. Development 2015, 142:544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogura Y, Sakaue-Sawano A, Nakagawa M, Satoh N, Miyawaki A, Sasakura Y: Coordination of mitosis and morphogenesis: role of a prolonged G2 phase during chordate neurulation. Development 2011, 138:577–587. [DOI] [PubMed] [Google Scholar]
- 97.Negishi T, Miyazaki N, Murata K, Yasuo H, Ueno N: Physical association between a novel plasma-membrane structure and centrosome orients cell division. Elife 2016, 5:525. [DOI] [PMC free article] [PubMed] [Google Scholar]