Abstract
Introduction: The polymorphic enzyme cytochrome P450 2D6 (CYP2D6) catalyzes a major step in the bioactivation of tamoxifen. Genotyping of clinically relevant CYP2D6 alleles and subsequent dose adjustment is a promising approach to individualize breast cancer therapy. The aim of this study was to investigate the relationship between the plasma levels of tamoxifen and its metabolites and different CYP2D6 genotypes under standard (20 mg/day) and dose-adjusted therapy (Registration ID in Iranian Registry of Clinical Trials: IRCT2015082323734N1).
Materials and Methods: Using TaqMan® assays common alleles of CYP2D6 (∗1, ∗2, ∗4, ∗5, ∗6, ∗10, ∗17, and ∗41) and gene duplication were identified in 134 breast cancer patients. Based on CYP2D6 genotypes patients with an activity score 1 (n = 15) and 0–0.5 (n = 2) were treated with tamoxifen adjusted dosage of 30 and 40 mg/day, respectively. The concentration of tamoxifen and its metabolites before and after 4 and 8 months of dose adjustment were measured using LC-MS/MS technology.
Results: At baseline, (Z)-endoxifen plasma concentrations (33 ± 15.5, 28.1 ± 14, 26.6 ± 23.4, 14.3 ± 8.6, and 10.7 ± 5.5 nmol/l for EM/EM, EM/IM, EM/PM, IM/IM and PM/PM, respectively) and the metabolic ratio (Z)-Endoxifen/N-desmethyltamoxifen (0.0558 ± 0.02, 0.0396 ± 0.0111, 0.0332 ± 0.0222, 0.0149 ± 0.0026, and 0.0169 ± 0.0177 for EM/EM, EM/IM, EM/PM, IM/IM, and PM/PM, respectively) correlated with CYP2D6 genotype (Kruskal–Wallis p = 0.013 and p < 0.0001, respectively). Dose escalation to 30 and 40 mg/day in patients with a CYP2D6 activity score of 1 (n = 15) and 0–0.5 (n = 2) resulted in a significant increase in (Z)-endoxifen plasma levels (22.17 ± 24.42, 34.43 ± 26.54, and 35.77 ± 28.89 nmol/l at baseline, after 4 and 8 months, respectively, Friedman p = 0.0388) along with the plasma concentrations of tamoxifen and its other metabolites. No severe side effects were recorded during dose escalation.
Conclusion: For the first time, we show the feasibility of dose escalation of tamoxifen in breast cancer patients with compromised CYP2D6 activity and Iranian ethnic background to increase the plasma concentrations of (Z)-endoxifen.
Keywords: CYP2D6, dose, endoxifen, genotype, metabolite, tamoxifen
Introduction
Breast cancer is one of the most common types of cancer and the leading cause of cancer related death in women worldwide (Torre et al., 2015). More than 70% of affected patients are ER-positive and therefore eligible for adjuvant endocrine therapy with tamoxifen (Early Breast Cancer Trialists’ Collaborative Group, 2005; Howlader et al., 2014). This drug has been the gold standard treatment of ER-positive breast cancer patients for more than three decades (Jordan, 2014). Tamoxifen acts as an estrogen antagonist and thus inhibits ER-dependent cell proliferation in breast cancer cells (Jordan, 1976). Tamoxifen decreases the risk of recurrence and mortality by 50 and 30%, respectively. Even so, the efficacy of tamoxifen shows a high degree of variation, with 30–50% of patients exhibiting drug resistance (Early Breast Cancer Trialists’ Collaborative Group, 2005).
Tamoxifen is extensively metabolized by several members of the cytochrome P450 enzymes (Desta et al., 2004; Brauch et al., 2009). Some of the resulting metabolites have higher anti-estrogenic effects in breast cancer tissue than tamoxifen itself. Among these, 4-hydroxytamoxifen (4-OH-Tam) and 4-hydroxy-N-desmethyl-tamoxifen (endoxifen) show the highest anti-estrogenic potency (Lim et al., 2006). As the steady state plasma concentration of endoxifen is approx. 10-times higher compared with levels of 4-OH-Tam, endoxifen is considered to be the major active tamoxifen metabolite (Stearns et al., 2003).
The bioactivation of tamoxifen to its main active metabolite endoxifen comprises two permutable steps, namely N-desmethylation and 4-hydroxylation (Figure 1; Brauch et al., 2009). The rate limiting step in the major route is the 4-hydroxylation via CYP2D6 (Desta et al., 2004).
FIGURE 1.
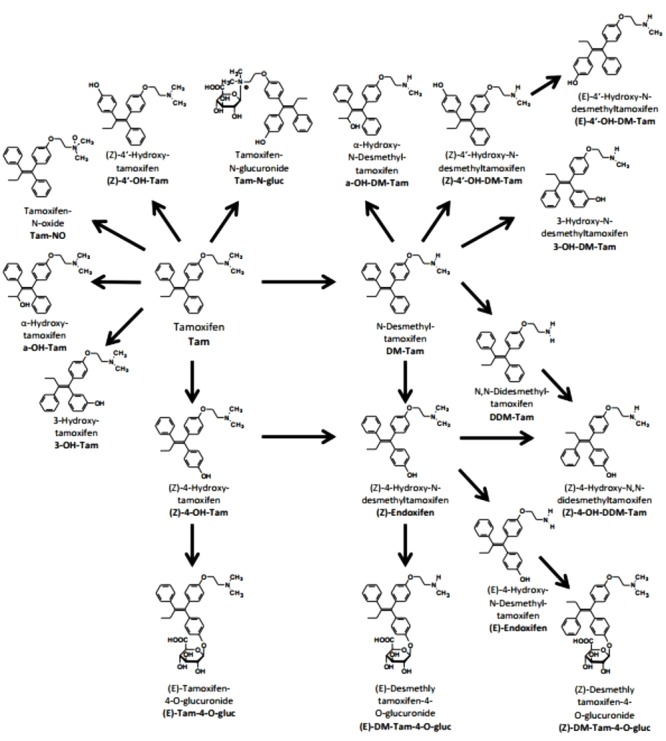
Metabolism of tamoxifen. Structures, names, and abbreviations of all metabolites which were quantified in the present study are shown (Brauch et al., 2009).
CYP2D6 is encoded by a highly polymorphic gene with more than 100 different alleles (Sim and Ingelman-Sundberg, 2010). It has been shown that a number of its genetic variants affect the activity of the enzyme and may decrease the levels of the active metabolites and thus result in decreased therapeutic response to tamoxifen (Goetz et al., 2005; Nowell et al., 2005; Schroth et al., 2009). According to the enzyme activity, four different phenotypic alleles including PM, IM, EM, and UM have been categorized (Bradford, 2002; Daly, 2003; Zanger et al., 2004; van Schaik, 2005; McGraw and Waller, 2012). There is a body of evidence that subjects carrying dysfunctional alleles of CYP2D6 have less benefit from tamoxifen therapy because of lower plasma concentrations of the active metabolite endoxifen (Jin et al., 2005; Lim et al., 2007; Madlensky et al., 2011; Karle et al., 2013; Saladores et al., 2015).
The frequency of CYP2D6 alleles varies in different populations. Various studies indicate that the prevalence of normal alleles (∗1 and ∗2) in the European population is around 70%. Twenty-six percent of the population in Europe is carrying one PM (∗3, ∗4, ∗5, and ∗6) allele and the most common PM allele is CYP2D6∗4. However, the frequency of PM alleles in the Asian population is less than 1%. In contrast, in the Asian population between the reduced function alleles (∗10, ∗17, and ∗41), CYP2D6∗10 is significantly more frequent by about 40–50% compared to Europeans (Bradford, 2002).
Regarding the Iranian population and the ethnic background few studies indicate the most relevant CYP2D6 alleles (Kouhi et al., 2009; Hashemi-Soteh et al., 2011; Bagheri et al., 2015). Accordingly, we decided to genotype the breast cancer patients of our study for the CYP2D6 alleles ∗1, ∗2, ∗4, ∗5, ∗6, ∗10, ∗17, and ∗41 as well as the gene duplication to comprehensively cover CYP2D6 alleles representative of the Iran population (Isfahan province). Based on their CYP2D6 alleles, the patients were assigned to different genotype groups discriminating for CYP2D6 phenotypes by CYP2D6 activity scores (AS) (Gaedigk et al., 2008). Plasma concentrations of tamoxifen and its metabolites following a standard tamoxifen dose of 20 mg/day were correlated with CYP2D6 phenotype. In patients with an activity score of one or less, the standard tamoxifen dose was adjusted accordingly to 40 mg. Follow-up after 4 and 8 months after dose adjustment was performed and plasma levels of tamoxifen and its metabolites as well as adverse events were assessed.
Materials and Methods
Patients
A total of 134 patients diagnosed with estrogen and/or progesterone receptor positive breast cancer, were enrolled in the Breast Cancer Research Center in Isfahan province, Iran. The main characteristics of the patients incorporated in the study, are presented in Table 1. Peripheral blood (5 ml) was collected from each patient in sterile tubes containing EDTA. A fixed time of blood collection was waived, since PBPK simulations showed only small fluctuations of endoxifen plasma levels within the dosing interval (Klopp-Schulze et al., 2018). The study was approved by the Ethical Committee of Isfahan University of Medical Sciences and written informed consent was obtained from all patients.
Table 1.
The main characteristics of the patients.
| Characteristics | All patients enrolled Number of patients (%) N = 134 (100) | Patients receiving 20 mg/day Number of patients (%) N = 117 (100) | Patients receiving 30 or 40 mg/day Number of patients (%) N = 17 (100) |
|---|---|---|---|
| Age (years) | |||
| Median | 45 | 45 | 46 |
| Range | 28–71 | 28–71 | 34–68 |
| BMI | |||
| Median | 22 | 22 | 23 |
| Range | 18–32 | 18–32 | 19.5–30 |
| Menopausal status | |||
| Premenopausal | 60 (44.8) | 57 (48.7) | 3 (17.7) |
| Postmenopausal | 70 (52.2) | 56 (47.9) | 14 (82.3) |
| Unknown | 4 (3.0) | 4 (3.4) | 0 (0.0) |
| Chemotherapy | |||
| Yes | 116 (86.6) | 100 (85.5) | 16 (94.1) |
| No | 15 (11.2) | 14 (12.0) | 1 (5.9) |
| Unknown | 3 (2.2) | 3 (2.5) | 0 (0.0) |
| Radiation | |||
| Yes | 122 (91.1) | 105 (89.7) | 17 (100) |
| No | 9 (6.7) | 9 (7.7) | 0 (0.0) |
| Unknown | 3 (2.2) | 3 (2.6) | 0 (0.0) |
| Duration of tamoxifen use | |||
| >4 months, ≤2 years | 81 (60.4) | 71 (60.7) | 10 (58.8) |
| >2 years | 53 (39.6) | 46 (39.3) | 7 (41.2) |
| Tumor size (cm) | |||
| ≤2 | 33 (24.6) | 31 (26.5) | 2 (11.8) |
| 2.1–5 | 67 (50.0) | 57 (48.7) | 10 (58.8) |
| >5 | 11 (8.2) | 9 (7.7) | 2 (11.8) |
| Unknown | 23 (17.2) | 20 (17.1) | 3 (17.6) |
| Grading | |||
| G1 | 16 (11.9) | 15 (12.8) | 1 (5.9) |
| G2 | 62 (46.3) | 54 (46.2) | 8 (47.1) |
| G3 | 32 (23.9) | 31 (26.5) | 1 (5.9) |
| Unknown | 24 (17.9) | 17 (14.5) | 7 (41.2) |
| Nodal status | |||
| Negative | 51 (38.0) | 42 (35.9) | 9 (52.9) |
| Positive | 62 (46.3) | 56 (47.9) | 6 (35.3) |
| Unknown | 21 (15.7) | 19 (16.2) | 2 (11.8) |
| Estrogen receptor status | |||
| Positive | 127 (94.8) | 111 (94.9) | 16 (94.1) |
| Negative | 2 (1.5) | 2 (1.7) | 0 (0.0) |
| Unknown | 5 (3.7) | 4 (3.4) | 1 (5.9) |
| Progesterone receptor status | |||
| Positive | 121 (90.3) | 105 (89.8) | 16 (94.1) |
| Negative | 6 (4.5) | 6 (5.1) | 0 (0.0) |
| Unknown | 7 (5.2) | 6 (5.1) | 1 (5.9) |
| HER-2 status | |||
| Positive | 16 (11.9) | 14 (12.0) | 2 (11.8) |
| Negative | 106 (79.1) | 94 (80.3) | 12 (70.6) |
| Unknown | 12 (9.0) | 9 (7.7) | 3 (17.6) |
BMI, Body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Genotyping
Genomic DNA was extracted from 200 μl of a peripheral blood sample of each patient using QIAamp DNA blood mini kit (Qiagen, Hilden, Germany). All patients included in this study were genotyped for CYP2D6∗2, ∗4, ∗6, ∗10, ∗17, and ∗41 alleles by TaqMan® allelic discrimination assays (Applied Biosystems, Foster City, CA, United States) according to the manufacturer’s instructions. Determination of CYP2D6 gene deletion (CYP2D6∗5) and duplication was performed using a pre-developed TaqMan® CYP2D6 Copy Number Assay (Hs00010001_cn, Thermo Fisher Scientific) and as a reference the TaqMan® Copy Number Reference Assay RNase P (Thermo Fisher Scientific) as previously described (Schroth; Front Pharmacol 2017). Real-time PCR analyses were carried out according to the manufacturer’s instructions on a 7900 Real-Time PCR System (Thermo Fisher Scientific) and data were analyzed with the SDS2.4 software. In the present study, alleles are assigned according to the nomenclature for human CYP2D6 alleles as previously published (Sim and Ingelman-Sundberg, 2010). Samples with one loss of function or reduced activity allele and a copy number of 3 were assigned EM phenotype. The allele frequencies were tested for deviation from the Hardy Weinberg equilibrium using the exact test1.
Clinical Trial Design and Endpoints
Pre- and postmenopausal breast cancer patients (n = 134) who were on standard tamoxifen therapy of 20 mg daily for at least 4 months with normal liver and kidney function were enrolled. Exclusion criteria as defined by the SmPC were pregnancy and breastfeeding and co-medication with a known CYP2D6 inhibitor. In addition, patients with a tamoxifen plasma concentration <100 nM at study baseline were excluded because this low plasma concentrations implied that these patients had missed at least one daily dose prior to sampling (Figure 2). The study design was as follows: Patients with EM/EM or EM/IM genotypes (activity score > 1) continued tamoxifen therapy at the standard dosage of 20 mg/day. Patients with EM/PM or IM/IM genotypes (activity score 1) received 30 mg/day (given as once 10 mg and once 20 mg) and patients with IM/PM or PM/PM genotypes (activity score 0.5 and 0, respectively) received 40 mg/day (given as 20 mg twice). Registration ID in IRCT was: IRCT2015082323734N12.
FIGURE 2.
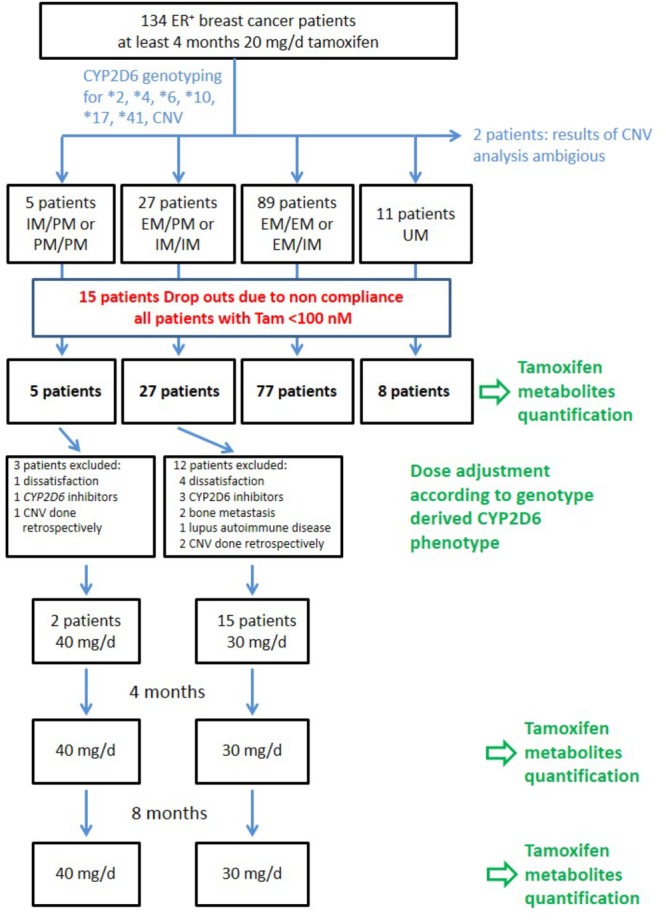
Flow chart of patients’ enrollment and sampling during the clinical trial.
During the follow-up period of 8 months plasma samples for the analysis of tamoxifen and its metabolites were drawn 4 and 8 months after inclusion of the patients. After 8 months the study was terminated. The decision whether treatment of patients with dose adjustment for tamoxifen should be continued was completely under the responsibility of the attending physician.
Measuring the Concentration of Tamoxifen and Its Metabolites in Plasma
The plasma concentrations of tamoxifen and its metabolites were analyzed before and after 4 and 8 months of dose adjustment using LC-MS/MS as previously described (Mürdter et al., 2011).
In brief, sample preparation was carried out by protein precipitation and dilution. For calibration and method validation, calibration samples and quality controls were prepared by adding mixtures of all reference compounds in acetonitrile to blank plasma. Final concentrations of calibration samples were chosen according to the concentrations of metabolites expected in plasma of patients under steady state condition (for calibration ranges see Supplementary Table S1). For α-OH-DM-Tam no pure reference material was available; plasma concentrations were calculated using the calibration data of α-OH-Tam.
Chromatographic separation of tamoxifen and its metabolites was performed using a ZORBAX Eclipse plus C18 (Agilent Technologies, Waldbronn, Germany) and 1200 rapid resolution LC-system (Agilent) with a binary pump using a gradient of 0.1% formic acid in acetonitrile in 0.1% formic acid in water. A 6460 triple quadrupole mass spectrometer (Agilent) equipped with a Jet Stream electrospray source (Agilent) was used for MRM. MRM transitions, retention times and internal standards are given in the Supplementary Table S1.
Toxicities Evaluation
Adverse events before and during the study were evaluated by questionnaire according to CTCAE v4.0. In addition, standard laboratory parameters such as ALT, AST, ALP, BUN, and creatinine were determined before and after 4 and 8 months.
Statistical Analysis
For statistical analysis, SPSS software version 16.0 (SPSS, Inc., Chicago, IL, United States) was utilized. The differences of the plasma concentrations of tamoxifen and its metabolites between different genotypes were evaluated using Kruskal–Wallis test followed by Dunn’s post hoc test. The concentrations of tamoxifen and its metabolites before and after tamoxifen dose escalation were compared using Friedman’s test followed by Dunn’s multiple comparison test as a post hoc which compared 0 with 4, 0 with 8, and 4 with 8 months. The Friedman’s test was also applied to evaluate the associations between the increase of tamoxifen dosage and the incidence of adverse events. The results were considered statistically significant if the p value was less than or equal to 0.05.
Results
Allele and Genotype Frequencies and Assigned CYP2D6 Phenotypes
CYP2D6 ∗1, ∗2, ∗4, ∗5, ∗6, ∗10, ∗17, and ∗41 alleles were successfully genotyped in 134 breast cancer patients. For copy number analysis 2 samples showed ambiguous results and therefore were excluded from further analysis (Figure 2). Allele and genotype frequencies of the study population are given in Supplementary Table S2. All genotypes were in Hardy–Weinberg equilibrium (p > 0.05).
Genotypes and the respective phenotypes based on the CYP2D6 activity score (AS) are shown in Table 2. The following phenotypes were assigned: UM (AS 3): ∗1/∗1, ∗1/∗2 or ∗2/∗2 and a copy number ≥ 3; EM/EM (AS: 2): ∗1/∗1, ∗1/∗2, ∗2/∗2, and ∗1/∗41 and ∗1/∗4 with copy number = 3; EM/IM (AS: 1.5): ∗1/∗10, ∗1/∗17, ∗1/∗41, and ∗2/∗41; EM/PM (AS: 1): ∗1/∗4; IM/IM (AS: 1): ∗10/∗10, ∗10/∗41, and ∗41/∗41; IM/PM (AS 0.5): ∗41/∗5; PM/PM (AS: 0): ∗4/∗4 and ∗4/∗6.
Table 2.
CYP2D6 phenotypes assigned according to the CYP2D6 activity scores and respective CYP2D6 genotypes.
| CYP2D6 phenotype | CYP2D6 | ||||
|---|---|---|---|---|---|
| (activity score) | n | (%) | genotype | n | (%) |
| UM (3) | 11 | (8.33) | ∗1/∗1xN | 2 | (1.52) |
| ∗1/∗2xN | 6 | (4.55) | |||
| ∗2/∗2xN | 3 | (2.27) | |||
| EM (2) | 68 | (51.51) | ∗1/∗1 | 16 | (12.12) |
| ∗1/∗2 | 23 | (17.42) | |||
| ∗2/∗2 | 26 | (19.7) | |||
| ∗1/∗41xN | 2 | (1.52) | |||
| ∗1/∗4xN | 1 | (0.76) | |||
| EM/IM (1.5) | 21 | (15.90) | ∗1/∗10 | 5 | (3.79) |
| ∗1/∗17 | 1 | (0.76) | |||
| ∗1/∗41 | 14 | (10.61) | |||
| ∗2/∗41 | 1 | (0.76) | |||
| EM/PM (1) | 23 | (17.42) | ∗1/∗4 | 19 | (14.39) |
| ∗1/∗5 | 3 | (2.27) | |||
| ∗2/∗5 | 1 | (0.76) | |||
| IM/IM (1) | 4 | (3.03) | ∗10/∗10 | 1 | (0.76) |
| ∗10/∗41 | 1 | (0.76) | |||
| ∗41/∗41 | 2 | (1.52) | |||
| IM/PM (0.5) | 1 | (0.76) | ∗41/∗5 | 1 | (0.76) |
| PM (0) | 4 | (3.03) | ∗4/∗4 | 2 | (1.52) |
| ∗4/∗5 | 1 | (0.76) | |||
| ∗4/∗6 | 1 | (0.76) |
Plasma Concentrations of Tamoxifen and Its Metabolites at Baseline
Steady-state plasma concentrations of tamoxifen and its metabolites before dose escalation were measured by LC-MS/MS (Table 3). The mean concentration of tamoxifen at baseline was 378 ± 176 nmol/l (n = 117). The most abundant metabolites of tamoxifen were N-desmethyl-tamoxifen (DM-Tam) whose concentration was approximately twice the concentration of tamoxifen followed by N,N-didesmethyltamoxifen (DDM-Tam), and (Z)-endoxifen. In contrast, the plasma concentrations of the other highly potent tamoxifen metabolite, (Z)-4-hydroxytamoxifen [(Z)-4-OH-Tam] was less than 1/5 of the concentration of (Z)-endoxifen. Of note, the less potent 3-hydroxylated metabolites 3-hydroxy-N-desmethyltamoxifen (3-OH-DM-Tam) and 3-hydroxytamoxifen (3-OH-Tam) showed only low concentrations compared to the respective 4-hydroxy-isomers. The concentrations of the (E)-isomers of both highly active metabolites, were highly variable and accounted for only 1/10 of that of (Z)-endoxifen and (Z)-4-OH-Tam, respectively. Interestingly, with respect to the glucuronides this difference in concentration was less pronounced (0.582 ± 0.377 nmol/l for (Z)-DM-Tam-4-O-Gluc vs. 1.9 ± 1.35 nmol/l for (E)-DM-Tam-4-O-Gluc) pointing to a more rapid glucuronidation of the (E)-isomer.
Table 3.
Steady state plasma concentrations of tamoxifen and its metabolites in breast cancer patients receiving 20 mg/day tamoxifen (n = 117∗).
| Compound | Mean ± SD (nM) |
|---|---|
| Tam | 378 ± 176 |
| Tam-NO | 21.7 ± 10.7 |
| DM-Tam | 675 ± 312 |
| DDM-Tam | 120.5 ± 52.9 |
| (Z)-Endoxifen | 29.6 ± 17.1 |
| (E)-Endoxifen | 4.92 ± 6.4 |
| (Z)-4’-OH-DM-Tam | 15.1 ± 7.7 |
| (E)-4’-OH-DM-Tam | 1.62 ± 1.28 |
| 3-OH-DM-Tam | 3.16 ± 2.14 |
| α-OH-DM-Tam// | 3.38 ± 1.84 |
| (Z)-4-OH-Tam# | 5.3 ± 3.14 |
| (Z)-4’-OH-Tam# | 5.83 ± 2.99 |
| 3-OH-Tam | 1.015 ± 0.647 |
| α-OH-Tam | 1.352 ± 0.721 |
| (Z)-OH-DDM-Tam# | 4.04 ± 2.29 |
| Tam-N-Gluc | 0.919 ± 0.755 |
| (Z)-DM-Tam-4-O-Gluc§ | 0.582 ± 0.377 |
| (E)-DM-Tam-4-O-Gluc$ | 1.9 ± 1.35 |
| (E)-Tam-4-O-Gluc$ | 0.551 ± 0.405 |
∗Non-adherent patients with plasma Tam concentrations <100 nM were excluded. //No reference compound available. Quantification was done using the calibration curve of α-OH-Tam. #The respective (E)-isomers could be quantified only in a minority of samples at low concentrations. §This glucuronide results from the conjugation of (E)-4-OH-DM-Tam. $These glucuronides result from conjugation of (Z)-endoxifen and (Z)-4-OH-Tam, respectively.
Relationship Between CYP2D6 Genotypes and Plasma Metabolite Concentrations
As CYP2D6 mediates a major step in the bioactivation of tamoxifen, we investigated the relationship between CYP2D6 genotypes and the concentrations of tamoxifen and its metabolites (Figure 3 and Supplementary Table S3). Using Kruskal–Wallis test, the mean concentrations of (Z)-endoxifen, (E)-endoxifen and their respective glucuronides, (Z)-4-OH-Tam, 3-OH-DM-Tam, and 3-OH-Tam were significantly different between genotype groups (p < 0.05). Despite considerable inter-individual variability within the genotype groups, all these metabolites showed increasing plasma concentrations with increasing CYP2D6 activity. However, 4′-hydroxy-N-desmethyltamoxifen (4′-OH-DM-Tam) showed an inverse correlation with lower plasma concentrations in patients with higher CYP2D6 activity (p = 0.0022). On the other hand, there was no significant difference in plasma concentrations of tamoxifen, tamoxifen-N-oxide, DM-Tam, DDM-Tam, α-hydroxytamoxifen (a-OH-Tam) and α-hydroxy-N-desmethyltamoxifen (a-OH-DM-Tam). As (Z)-endoxifen can undergo chemical or enzymatic isomerization into (E)-endoxifen we also analyzed the sum of both isomers revealing an even better correlation with CYP2D6 activity (p < 0.0001). As a surrogate parameter for the intrinsic enzyme activity the MR of (Z)-endoxifen to DM-Tam correlates very well with CYP2D6 activity (p < 0.0001).
FIGURE 3.
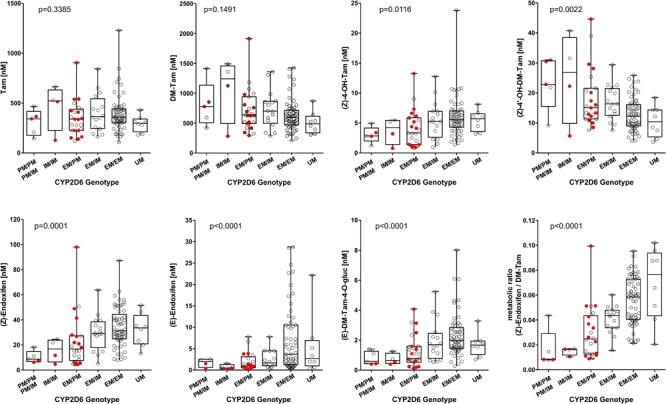
Plasma concentrations of selected tamoxifen metabolites stratified according to CYP2D6 genotype in 117 Iranian breast cancer patients receiving 20 mg/day tamoxifen for at least 4 months. Non-adherent patients with plasma tamoxifen concentrations <100 nM were excluded. The patients who were scheduled for the dose escalation trial are depicted in red. The differences between the genotype groups were assessed by Kruskal–Wallis Test.
Concentration of Tamoxifen and Its Metabolites Following Dose Adjustment
Dose adjustment in patients with an activity score of 0–0.5 (n = 2) and 1 (n = 15) to 40 and 30 mg/day, respectively, resulted in a significant increase in median plasma concentrations of tamoxifen and all metabolites except the (E)-isomers of endoxifen and 4′-OH-DM-Tam and (E)-Tam-4-O-Gluc (Supplementary Table S4). After 8 months the median plasma concentrations increased by about 26–122% compared to baseline. Plasma concentrations of tamoxifen and its main metabolites and the MR (Z)-endoxifen/DM-Tam during dose escalation are shown in Figure 4. Due to the unexplained high variability of isomerization the concentrations of the isomers of endoxifen were additionally evaluated in sum. Of note, the MR endoxifen/DM-Tam did not change upon dose escalation. In line with this data, the increased intake of tamoxifen did not change the MR of other reactions involved in the formation or clearance of the anti-estrogenic metabolites (Z)-4-OH-Tam and (Z)-endoxifen.
FIGURE 4.
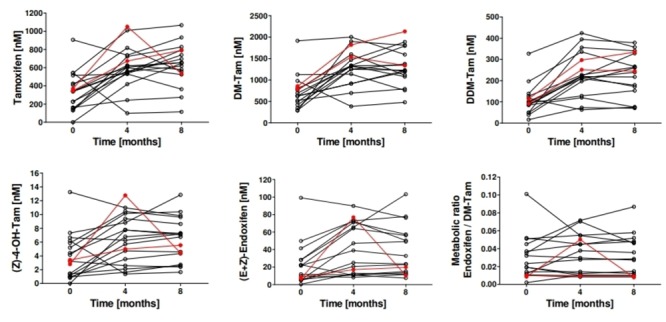
Plasma concentrations of tamoxifen and its main metabolites and the MR (Z)-endoxifen/DM-Tam in patients (n = 17) before and after dose escalation. Patients genotyped for CYP2D6 as intermediate metabolizer (n = 15) (EM/PM or IM/IM) received 30 mg/day of tamoxifen and patients genotyped as poor metabolizers (n = 2) (PM/PM, IM/PM) received 40 mg/day. Plasma samples were drawn 4 and 8 months after dose adjustment and tamoxifen and its metabolites were quantified by UHPLC-MS/MS. The two patients who received 40 mg tamoxifen are shown in red.
Adherence to Therapy
Among the 17 patients who completed the dose adjustment protocol compliance of study medication has been considered entirely satisfactory: 47.1% (n = 8) did not miss any tamoxifen dose, 29.4% (n = 5) and 17.6% (n = 3) missed 1–5 and 6–10 doses, respectively. One patient reported more than 10 missing tamoxifen doses.
Toxicities and Adverse Events
No woman was withdrawn from the study due to metastasis, thromboembolic or other serious adverse events. Vomiting, nausea, sexual pain, bloating, and irritability were significantly more frequent in the patients under tamoxifen dose escalation (Table 4; Friedman test, P < 0.05). No changes in ALT, AST, ALP, BUN, and creatinine levels have been observed upon dose escalation.
Table 4.
Side effects are shown in patients without dose escalation (n = 117) and for dose adjusted patients (n = 17) before, after 4 and 8 months of dose adjustment.
| Test Side effects | Tamoxifen dosage | Time point | Number of patients reporting |
Friedman p-value | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| Hot flashes | 20 mg/day (n = 117) | 13 | 34 | 40 | 30 | ||
| Dose escalation | Baseline | 2 | 4 | 6 | 5 | 0.439 | |
| group (n = 15) | 4 months | 3 | 5 | 5 | 4 | ||
| 8 months | 5 | 2 | 4 | 6 | |||
| Cold sweat | 20 mg/day (n = 117) | 58 | 20 | 22 | 17 | ||
| Dose escalation | Baseline | 9 | 3 | 3 | 2 | 0.358 | |
| group (n = 15) | 4 months | 8 | 6 | 1 | 2 | ||
| 8 months | 6 | 4 | 4 | 3 | |||
| Night sweat | 20 mg/day (n = 117) | 44 | 27 | 25 | 21 | ||
| Dose escalation | Baseline | 7 | 4 | 3 | 3 | 0.509 | |
| group (n = 15) | 4 months | 6 | 8 | 2 | 1 | ||
| 8 months | 6 | 4 | 4 | 3 | |||
| Vaginal irritation | 20 mg/day (n = 117) | 72 | 22 | 21 | 2 | ||
| Dose escalation | Baseline | 10 | 3 | 4 | 0 | 0.152 | |
| group (n = 15) | 4 months | 7 | 3 | 4 | 3 | ||
| 8 months | 6 | 3 | 2 | 6 | |||
| Vaginal bleeding | 20 mg/day (n = 117) | 102 | 3 | 8 | 4 | ||
| Dose escalation | Baseline | 15 | 0 | 1 | 1 | 0.662 | |
| group (n = 15) | 4 months | 13 | 1 | 3 | 0 | ||
| 8 months | 13 | 1 | 1 | 2 | |||
| Vaginal dryness | 20 mg/day (n = 117) | 55 | 44 | 17 | 1 | ||
| Dose escalation | Baseline | 7 | 7 | 3 | 0 | 0.549 | |
| group (n = 15) | 4 months | 7 | 4 | 4 | 2 | ||
| 8 months | 7 | 3 | 3 | 4 | |||
| Sexual unwillingness | 20 mg/day (n = 117) | 44 | 26 | 26 | 21 | ||
| Dose escalation | Baseline | 6 | 3 | 4 | 4 | 0.692 | |
| group (n = 15) | 4 months | 7 | 3 | 4 | 3 | ||
| 8 months | 6 | 3 | 5 | 3 | |||
| Sexual pain | 20 mg/day (n = 117) | 58 | 44 | 9 | 6 | ||
| Dose escalation | Baseline | 8 | 8 | 0 | 1 | 0.002 | |
| group (n = 15) | 4 months | 6 | 5 | 2 | 4 | ||
| 8 months | 6 | 3 | 4 | 4 | |||
| Weight gain | 20 mg/day (n = 117) | 53 | 34 | 12 | 18 | ||
| Dose escalation | Baseline | 8 | 4 | 2 | 3 | 0.309 | |
| group (n = 15) | 4 months | 6 | 5 | 2 | 4 | ||
| 8 months | 7 | 4 | 3 | 3 | |||
| Dizziness | 20 mg/day (n = 117) | 54 | 53 | 4 | 6 | ||
| Dose escalation | Baseline | 8 | 8 | 0 | 1 | 0.727 | |
| group (n = 15) | 4 months | 7 | 7 | 0 | 3 | ||
| 8 months | 8 | 6 | 0 | 3 | |||
| Vomiting | 20 mg/day (n = 117) | 101 | 12 | 4 | 0 | ||
| Dose escalation | Baseline | 15 | 2 | 0 | 0 | 0.009 | |
| group (n = 15) | 4 months | 12 | 4 | 1 | 0 | ||
| 8 months | 10 | 6 | 1 | 0 | |||
| Diarrhea | 20 mg/day (n = 117) | 104 | 13 | 0 | 0 | ||
| Dose escalation | Baseline | 15 | 2 | 0 | 0 | 0.819 | |
| group (n = 15) | 4 months | 14 | 3 | 0 | 0 | ||
| 8 months | 14 | 2 | 0 | 1 | |||
| Bloating | 20 mg/day (n = 117) | 46 | 34 | 22 | 15 | 0.023 | |
| Dose escalation | Baseline | 7 | 4 | 3 | 3 | ||
| group (n = 15) | 4 months | 4 | 3 | 6 | 4 | ||
| 8 months | 5 | 4 | 5 | 3 | |||
| Mood swing | 20 mg/day (n = 117) | 14 | 66 | 26 | 11 | ||
| Dose escalation | Baseline | 2 | 9 | 4 | 2 | 0.423 | |
| group (n = 15) | 4 months | 3 | 4 | 7 | 3 | ||
| 8 months | 4 | 4 | 6 | 3 | |||
| Irritability | 20 mg/day (n = 117) | 24 | 50 | 31 | 12 | ||
| Dose escalation | Baseline | 3 | 7 | 5 | 2 | 0.006 | |
| group (n = 15) | 4 months | 3 | 2 | 6 | 6 | ||
| 8 months | 4 | 1 | 7 | 5 | |||
Discussion
There is increasing evidence that personalized therapy in oncology contributes significantly to better treatment outcome with a tolerable safety (Meyer et al., 2013; Relling and Evans, 2015). Recently, genotyping of CYP2D6 in patients undergoing tamoxifen therapy for premenopausal and postmenopausal breast cancer patients has been proposed to improve treatment outcome (Province et al., 2014;Goetz et al., 2018). Previous clinical trials in breast cancer showed first evidence that CYP2D6 dose-adjusted treatment of tamoxifen resulted in significantly increased plasma concentrations of endoxifen, the major active metabolite of tamoxifen (Barginear et al., 2011; Irvin et al., 2011; Kiyotani et al., 2012; de Duenas et al., 2014; Dezentjé et al., 2015; Fox et al., 2016; Hertz et al., 2016).
To the best of our knowledge currently no data are available demonstrating the feasibility of individualized CYP2D6 guided tamoxifen therapy in an Iranian population of breast cancer patients. Therefore, we initiated a prospective clinical pilot study to adjust tamoxifen dosage in ER+ breast cancer patients with Iranian ethnic background. Considering major differences of CYP2D6 allele frequencies world-wide (Zanger and Schwab, 2013), genotyping of CYP2D6 was performed covering CYP2D6 alleles relevant for the Middle East population (Sistonen et al., 2007; Bagheri et al., 2015). Based on our study protocol and in agreement with previous studies elucidating the impact of CYP2D6-guided tamoxifen dose adjustment in patients with compromised CYP2D6 activity (PM and IM), plasma concentrations of tamoxifen and selected metabolites were quantified before, after 4 and 8 months of dose escalation. In addition to DM-Tam, (Z)-endoxifen, and 4-OH-Tam which are suggested to be clinically relevant, we comprehensively measured other tamoxifen metabolites by a previously established and validated sensitive and highly specific LC-MS/MS method (Mürdter et al., 2011). This method includes various hydroxylated metabolites and glucuronides (Figure 1; Brauch et al., 2009).
At baseline, the inter-individual variability of tamoxifen and selected metabolites in the Iranian population was similar to data in other ethnic populations of breast cancer patients (e.g., Europe, America and Asians) (Saladores et al., 2015). Regarding (Z)-endoxifen, our data confirms the close relationship between CYP2D6 genotypes and plasma concentrations proposed by other studies (Lim et al., 2011; Antunes et al., 2015; Schroth et al., 2017; Woo et al., 2017). Due to the small number of patients who were enrolled in our pilot study to evaluate dose escalation of tamoxifen in Iranian breast cancer, data analyses have been performed by combining IM and PM patients treated with 30 and 40 mg tamoxifen per day, respectively. Generally our data demonstrate a significant increase in plasma concentrations of tamoxifen and its metabolites following dose escalation for 4 and 8 months. Of note, (Z)-endoxifen plasma concentrations in patients with compromised CYP2D6 activity and dose escalation were similar to those in patients with CYP2D6 EM phenotype at standard dosage.
These data are in line with previous studies demonstrating that dose escalation of tamoxifen in CYP2D6 compromised breast cancer patients result in higher plasma levels of the active metabolite endoxifen. In the study by Irvin et al. (2011) in a US American population the concentration of endoxifen was increased in both, the IM and PM patients, after doubling the tamoxifen dosage to 40 mg/day for 4 months. The endoxifen plasma levels in the IM group receiving 40 mg/day tamoxifen were similar to the EM group treated with 20 mg/day (21.8 vs. 29.2 ng/ml; p = 0.84). In contrast, patients in the PM group failed to reach similar endoxifen plasma levels (12.9 vs. 29.2 ng/ml; p = 0.016) despite dose escalation. Barginear et al. (2011) increased the tamoxifen dosage from 20 to 30 mg/day based on plasma endoxifen concentration (<40 nmol/l) in US patients with various ethnic background and IM CYP2D6 phenotype. Dose adjustment resulted in elevated levels of (Z)-endoxifen, (Z)-4′-OH-DM-Tam, (Z)-4-OH-Tam, and (Z)-4′-OH-Tam. Noteworthy, in line with our data lower plasma concentrations of (Z)-4′-OH-DM-Tam in patients with an EM CYP2D6 activity score compared to IM/PM patients were reported, indicating a metabolic shift from (Z)-4′-OH-DM-Tam to endoxifen. In an Asian population Kiyotani et al. (2012) investigated dose escalation of tamoxifen to 30 and 40 mg/day in patients who were heterozygous and homozygous for CYP2D6 IM alleles, respectively. After 8 weeks, concentrations of tamoxifen, N-DM-Tam, 4-OH-Tam and endoxifen were increased in IM patients resulting in endoxifen and 4-OH-Tam levels similar to those detected in EM patients. After enrollment of the patients in the CYPTAM study, tamoxifen dose escalation for IM and PM patients was calculated by the formula: 20 mg × (average endoxifen serum concentration in EMs divided by the patient’s baseline endoxifen serum concentration) and led to tamoxifen doses between 30–100 mg for the IMs and 60–120 mg for PMs (Dezentjé et al., 2015). Dose adjustment resulted in elevated levels of endoxifen in both PMs and IMs. There was no significant difference with the mean endoxifen level in EMs (=33.7 nM), and IMs after dose escalation (=30.3 nM; p = 0.20), although the mean endoxifen level in PMs after dose escalation was significantly lower (=27.3 nM; p = 0.03).
Building on the success of the pilot study by Irvin et al. (2011) and Hertz et al. (2016) expanded enrollment to 500 patients and again they doubled the tamoxifen dosage from 20 to 40 mg/day in both, the IM and PM patients. After 4 months, the endoxifen plasma levels in the genotype-dosed IM patients, had risen by 48% to 10.74 ng/mL, which was no longer different from, and was in fact nominally greater than, the endoxifen concentrations in EM/UM patients (9.30 ng/mL, p = 0.08). Endoxifen concentrations in genotype-dosed PM patients also rose by approximately 61% to 5.52 ng/mL but remained significantly lower than in EM/ UM patients (9.30 ng/ml; p = 0.009). In the Australian TADE study patients with basal endoxifen concentrations <30 nmol/l underwent a sequential dose escalation scheme with a maximum tamoxifen dose of 60 mg/day resulting in endoxifen concentrations >30 nmol/l in 76% (93 of 122) of participants (Fox et al., 2016). Of note patients with a PM phenotype, i.e., carrying two non-functional CYP2D6 alleles were unable to achieve the target endoxifen concentration of 30 nmol/l despite dose escalation.
Independent from CYP2D6 genotype increased dosing of tamoxifen to 30 and 40 mg/day resulted in significantly higher plasma levels of tamoxifen compared to tamoxifen standard treatment (20 mg/day).
Since altered pharmacokinetics may result in a higher rate of ADR, we systematically monitored tamoxifen specific side effects as well as laboratory parameters indicating liver and kidney toxicity. No severe side effects (e.g., thromboembolism) occurred during dose escalation. Moreover there was no significant increase in the occurrence of hot flashes and only slightly increased frequencies of vomiting and agitation. These data corroborate previous studies demonstrating that doses escalation of tamoxifen in CYP2D6 compromised breast cancer patients is well tolerated (Barginear et al., 2011; Irvin et al., 2011; Kiyotani et al., 2012; Dezentjé et al., 2015; Fox et al., 2016; Hertz et al., 2016).
Besides CYP2D6, CYP2C9, and CYP2C19 as well as some phase 2 enzymes have also been reported to participate in tamoxifen pharmacokinetics, however, with far less impact than CYP2D6 (Mürdter et al., 2011; Saladores et al., 2015; Marcath et al., 2017; Sutiman et al., 2016). Therefore, this study was focused on CYP2D6.
A limitation of our study is the number of patients included. Previous studies have shown a low frequency of CYP2D6 PM subjects in the Iranian population (Kouhi et al., 2009; Hashemi-Soteh et al., 2011; Bagheri et al., 2015). In line with these findings, the number of PM patients was not high enough to analyze those patients separately. Moreover no outcome data are available.
Taken together, for the first time we showed that the concept of dose escalation of tamoxifen in CYP2D6 compromised breast cancer patients is feasible in an Iranian population resulting in significantly higher plasma concentrations of the active metabolite (Z)-endoxifen. Our study supports ongoing and future activities to comprehensively elucidate the impact of CYP2D6- guided dose escalation on clinical outcome in prospective clinical trials. The strategy of dose escalation in patients with compromised genetics for drug metabolizing enzymes regarding other drugs as tamoxifen appears to be a promising approach which warrants future trials.
Ethics Statement
This study was carried out in accordance with the recommendations of the Ethical Committee of Isfahan University of Medical Sciences with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Ethical Committee of Isfahan University of Medical Sciences.
Author Contributions
ZK, ZB, PN, HK, RS, and ManS designed the project and involved in doing the experiments. MatS and TM performed the measurements of tamoxifen metabolite concentrations. ES performed the CYP2D6 CNV analysis. ZK, ZB, MatS, TM, ManS, and PN analyzed the data and wrote the manuscript. FM was the clinician of the project, who helped us in designing the project, sample collection, and data analysis. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all the participants for their blood donation to the Breast Cancer Research Center in Isfahan, Iran. We also gratefully acknowledge Markus König for excellent technical assistance. As well, we would like to express our utmost gratitude and appreciation to the Dr. Mohajeri laboratory and Farzaneh Mahmoodi for their help in experiments.
Abbreviations
- ADR
adverse drug reaction
- ALP
alkaline phosphatase
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- BUN
blood urea nitrogen
- CTCAE v4.0
Common Toxicity Criteria for Adverse Events, version 4
- CYP2D6
cytochrome P450 2D6
- DDM
N, N-DiDesmethyl
- DM
N-desmethyl
- EM
extensive metabolizer
- ER
estrogen receptor
- Gluc
Glucuronides
- IM
intermediate metabolizer
- IRCT
Iranian Registry of Clinical Trials
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- MR
metabolic ratio
- MRM
multiple reaction monitoring
- OH
hydroxyl
- PM
poor metabolizer
- SPSS
Statistical Package for Social Sciences
- Tam
tamoxifen
- UM
ultra-rapid metabolizer
Funding. This work was financially supported by Isfahan University of Medical Sciences, Isfahan, Iran and also in part by the German Research Foundation, Bonn, Germany (Grants MU 1727/2-1 and SCHR 1323/2-1), the Robert Bosch Stiftung, Stuttgart, Germany, and the Horizon 2020-PHC-2015 grant U-PGx 668353.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00530/full#supplementary-material
References
- Antunes M. V., Da Fontoura Timm T. A., De Oliveira V., Staudt D. E., Raymundo S., Gossling G., et al. (2015). Influence of CYP2D6 and CYP3A4 phenotypes, drug interactions, and vitamin D status on tamoxifen biotransformation. Ther. Drug Monitor. 37 733–744. 10.1097/FTD.0000000000000212 [DOI] [PubMed] [Google Scholar]
- Bagheri A., Kamalidehghan B., Haghshenas M., Azadfar P., Akbari L., Sangtarash M. H., et al. (2015). Prevalence of the CYP2D6∗ 10 (C100T),∗ 4 (G1846A), and∗ 14 (G1758A) alleles among Iranians of different ethnicities. Drug Des. Devel. Ther. 13 2627–2634. 10.2147/DDDT.S79709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barginear M., Jaremko M., Peter I., Yu C., Kasai Y., Kemeny M., et al. (2011). Increasing tamoxifen dose in breast cancer patients based on CYP2D6 genotypes and endoxifen levels: effect on active metabolite isomers and the antiestrogenic activity score. Clin. Pharmacol. Ther. 90 605–611. 10.1038/clpt.2011.153 [DOI] [PubMed] [Google Scholar]
- Bradford L. D. (2002). CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 3 229–243. [DOI] [PubMed] [Google Scholar]
- Brauch H., Mürdter T. E., Eichelbaum M., Schwab M. (2009). Pharmacogenomics of tamoxifen therapy. Clin. Chem. 55 1770–1782. 10.1373/clinchem.2008.121756 [DOI] [PubMed] [Google Scholar]
- Daly A. K. (2003). Pharmacogenetics of the major polymorphic metabolizing enzymes. Fundam. Clin. Pharmacol. 17 27–41. [DOI] [PubMed] [Google Scholar]
- de Duenas E. M., Aranda E. O., Lopez-Barajas I. B., Magdalena T. F., Moya F. B., Garcia L. M. C., et al. (2014). Adjusting the dose of tamoxifen in patients with early breast cancer and CYP2D6 poor metabolizer phenotype. Breast 23 400–406. 10.1016/j.breast.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Desta Z., Ward B. A., Soukhova N. V., Flockhart D. A. (2004). Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J. Pharmacol. Exp. Ther. 310 1062–1075. [DOI] [PubMed] [Google Scholar]
- Dezentjé V., Opdam F., Gelderblom H., Van Der Straaten T., Vree R., Maartense E., et al. (2015). CYP2D6 genotype-and endoxifen-guided tamoxifen dose escalation increases endoxifen serum concentrations without increasing side effects. Breast Cancer Res. Treat. 153 583–590. 10.1007/s10549-015-3562-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group (2005). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365 1687–1717. [DOI] [PubMed] [Google Scholar]
- Fox P., Balleine R. L., Lee C., Gao B., Balakrishnar B., Menzies A. M., et al. (2016). Dose escalation of tamoxifen in patients with low endoxifen level: evidence for therapeutic drug monitoring—the TADE Study. Clin. Cancer Res. 22 3164–3171. 10.1158/1078-0432.CCR-15-1470 [DOI] [PubMed] [Google Scholar]
- Gaedigk A., Simon S., Pearce R., Bradford L., Kennedy M., Leeder J. (2008). The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther. 83 234–242. [DOI] [PubMed] [Google Scholar]
- Goetz M. P., Rae J. M., Suman V. J., Safgren S. L., Ames M. M., Visscher D. W., et al. (2005). Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J. Clin. Oncol. 23 9312–9318. [DOI] [PubMed] [Google Scholar]
- Goetz M. P., Sangkuhl K., Guchelaar H. J., Schwab M., Province M., Whirl-Carrillo M., et al. (2018). Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin. Pharmacol. Ther. 103 770–777. 10.1002/cpt.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi-Soteh S. M., Sarzare F., Merat F., Salehifar E., Shiran M. R. (2011). Frequencies of three CYP2D6 nonfunctional alleles (CYP2D6∗3, ∗4, and ∗6) within an Iranian population (Mazandaran). Genet. Test Mol. Biomark. 15 821–825. 10.1089/gtmb.2011.0033 [DOI] [PubMed] [Google Scholar]
- Hertz D. L., Deal A., Ibrahim J. G., Walko C. M., Weck K. E., Anderson S., et al. (2016). Tamoxifen dose escalation in patients with diminished CYP2D6 activity normalizes endoxifen concentrations without increasing toxicity. Oncologist 21 795–803. 10.1634/theoncologist.2015-0480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N., Chen V. W., Ries L. A., Loch M. M., Lee R., Desantis C., et al. (2014). Overview of breast cancer collaborative stage data items–their definitions, quality, usage, and clinical implications: a review of SEER data for 2004-2010. Cancer 120(Suppl. 23) 3771–3780. 10.1002/cncr.29059 [DOI] [PubMed] [Google Scholar]
- Irvin W. J., Jr., Walko C. M., Weck K. E., Ibrahim J. G., Chiu W. K., Dees E. C., et al. (2011). Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: a multicenter study. J. Clin. Oncol. 29 3232–3239. 10.1200/JCO.2010.31.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Desta Z., Stearns V., Ward B., Ho H., Lee K. H., et al. (2005). CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J. Natl. Cancer Inst. 97 30–39. [DOI] [PubMed] [Google Scholar]
- Jordan C. (2014). Tamoxifen as the first targeted long term adjuvant therapy for breast cancer. Endocr. relat. Cancer 21 R235–R246. 10.1530/ERC-14-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan V. C. (1976). Antiestrogenic and antitumor properties of tamoxifen in laboratory animals. Cancer Treat. Rep. 60 1409–1419. [PubMed] [Google Scholar]
- Karle J., Bolbrinker J., Vogl S., Kreutz R., Denkert C., Eucker J., et al. (2013). Influence of CYP2D6-genotype on tamoxifen efficacy in advanced breast cancer. Breast Cancer Res. Treat. 139 553–560. 10.1007/s10549-013-2565-3 [DOI] [PubMed] [Google Scholar]
- Kiyotani K., Mushiroda T., Imamura C. K., Tanigawara Y., Hosono N., Kubo M., et al. (2012). Dose-adjustment study of tamoxifen based on CYP2D6 genotypes in Japanese breast cancer patients. Breast Cancer Res. Treat. 131 137–145. 10.1007/s10549-011-1777-7 [DOI] [PubMed] [Google Scholar]
- Klopp-Schulze L., Joerger M., Wicha S. G., Ter Heine R., Csajka C., Parra-Guillen Z. P., et al. (2018). Exploiting pharmacokinetic models of tamoxifen and endoxifen to identify factors causing subtherapeutic concentrations in breast cancer patients. Clin. Pharmacokinet. 57 229–242. 10.1007/s40262-017-0555-z [DOI] [PubMed] [Google Scholar]
- Kouhi H., Hamzeiy H., Barar J., Asadi M., Omidi Y. (2009). Frequency of five important CYP2D6 alleles within an Iranian population (Eastern Azerbaijan). Genet. Test Mol. Biomark. 13 665–670. 10.1089/gtmb.2009.0009 [DOI] [PubMed] [Google Scholar]
- Lim H. S., Ju Lee H., Seok Lee K., Sook Lee E., Jang I. J., Ro J. (2007). Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J. Clin. Oncol. 25 3837–3845. [DOI] [PubMed] [Google Scholar]
- Lim J. S., Chen X. A., Singh O., Yap Y. S., Ng R. C., Wong N. S., et al. (2011). Impact of CYP2D6, CYP3A5, CYP2C9 and CYP2C19 polymorphisms on tamoxifen pharmacokinetics in Asian breast cancer patients. Br. J. Clin. Pharmacol. 71 737–750. 10.1111/j.1365-2125.2011.03905.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. C., Li L., Desta Z., Zhao Q., Rae J. M., Flockhart D. A., et al. (2006). Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J. Pharmacol. Exp. Ther. 318 503–512. [DOI] [PubMed] [Google Scholar]
- Madlensky L., Natarajan L., Tchu S., Pu M., Mortimer J., Flatt S. W., et al. (2011). Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin. Pharmacol. Ther. 89 718–725. 10.1038/clpt.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcath L. A., Deal A. M., Van Wieren E., Danko W., Walko C. M., Ibrahim J. G., et al. (2017). Comprehensive assessment of cytochromes P450 and transporter genetics with endoxifen concentration during tamoxifen treatment. Pharmacogenet. Genom. 27 402–409. 10.1097/FPC.0000000000000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw J., Waller D. (2012). Cytochrome P450 variations in different ethnic populations. Expert Opin. Drug Metab. Toxicol. 8 371–382. 10.1517/17425255.2012.657626 [DOI] [PubMed] [Google Scholar]
- Meyer U. A., Zanger U. M., Schwab M. (2013). Omics and drug response. Annu. Rev. Pharmacol. Toxicol. 53 475–502. 10.1146/annurev-pharmtox-010510-100502 [DOI] [PubMed] [Google Scholar]
- Mürdter T., Schroth W., Bacchus-Gerybadze L., Winter S., Heinkele G., Simon W., et al. (2011). Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin. Pharmacol. Ther. 89 708–717. 10.1038/clpt.2011.27 [DOI] [PubMed] [Google Scholar]
- Nowell S. A., Ahn J., Rae J. M., Scheys J. O., Trovato A., Sweeney C., et al. (2005). Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res. Treat. 91 249–258. [DOI] [PubMed] [Google Scholar]
- Province M. A., Goetz M. P., Brauch H., Flockhart D. A., Hebert J. M., Whaley R., et al. (2014). CYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populations. Clin. Pharmacol. Ther. 95 216–227. 10.1038/clpt.2013.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relling M. V., Evans W. E. (2015). Pharmacogenomics in the clinic. Nature 526:343. 10.1038/nature15817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladores P., Mürdter T., Eccles D., Chowbay B., Zgheib N., Winter S., et al. (2015). Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenom. J. 15 84. 10.1038/tpj.2014.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth W., Goetz M. P., Hamann U., Fasching P. A., Schmidt M., Winter S., et al. (2009). Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA 302 1429–1436. 10.1001/jama.2009.1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth W., Winter S., Mürdter T., Schaeffeler E., Eccles D., Eccles B., et al. (2017). Improved prediction of endoxifen metabolism by CYP2D6 genotype in breast cancer patients treated with tamoxifen. Front. Pharmacol. 8:582. 10.3389/fphar.2017.00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S. C., Ingelman-Sundberg M. (2010). The human cytochrome P450 (CYP) allele nomenclature website: a peer-reviewed database of CYP variants and their associated effects. Hum. Genom. 4:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistonen J., Sajantila A., Lao O., Corander J., Barbujani G., Fuselli S. (2007). CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet. Genom. 17 93–101. [DOI] [PubMed] [Google Scholar]
- Stearns V., Johnson M. D., Rae J. M., Morocho A., Novielli A., Bhargava P., et al. (2003). Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J. Nat. Cancer Inst. 95 1758–1764. [DOI] [PubMed] [Google Scholar]
- Sutiman N., Lim J. S. L., Muerdter T. E., Singh O., Cheung Y. B., Ng R. C. H., et al. (2016). Pharmacogenetics of UGT1A4, UGT2B7 and UGT2B15 and their influence on tamoxifen disposition in asian breast cancer patients. Clin. Pharmacokin. 55 1239–1250. [DOI] [PubMed] [Google Scholar]
- Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J., Jemal A. (2015). Global cancer statistics, 2012. CA Cancer J. Clin. 65 87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- van Schaik R. H. (2005). Cancer treatment and pharmacogenetics of cytochrome P450 enzymes. Invest. New Drugs 23 513–522. [DOI] [PubMed] [Google Scholar]
- Woo H. I., Lee S. K., Kim J., Kim S. W., Yu J., Bae S. Y., et al. (2017). Variations in plasma concentrations of tamoxifen metabolites and the effects of genetic polymorphisms on tamoxifen metabolism in Korean patients with breast cancer. Oncotarget 8 100296–100311. 10.18632/oncotarget.22220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger U. M., Raimundo S., Eichelbaum M. (2004). Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch. Pharmacol. 369 23–37. [DOI] [PubMed] [Google Scholar]
- Zanger U. M., Schwab M. (2013). Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 138 103–141. 10.1016/j.pharmthera.2012.12.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


