Abstract
Background
This study aimed to undertake an analysis of ten years of real-world evidence (RWE) on overall survival (OS) following treatment of advanced gastrointestinal stromal tumor (GIST) with imatinib, sunitinib, and sorafenib using data from the Polish National Health Fund.
Material/Methods
Data from the Polish National Health Fund, the sole Polish public payer, identified 1,641 patients with advanced GIST who were treated with imatinib (n=1047), sunitinib (n=457), and sorafenib (n=137). The differences in overall survival (OS) were analyzed.
Results
For patients with advanced GIST, the median follow-up time for patients treated with imatinib was 71 months (95% CI, 64.8–79.2), the median OS was 56.9 months (95% CI, 50.4–61.2), with survival at 12 months (89.5%), 24 months (77.9%), 36 months (66.9%), and 60 months (48.4%). The median follow-up time for patients treated with sunitinib was 41.4 months (95% CI, 34.6–49.3), the median OS was 22.8 months (95% CI, 19.2–26.8), with survival at 12 months (68.2%), 24 months (47.1%), and 36 months (31%). The median follow-up time for patients treated with sorafenib was 17.4 months (95% CI, 14.6–22.9), the median OS was 16.9 months (95% CI, 13.7–24.3), with survival at 12 months (61.9%), at 24 months (36.2%), and at 36 months (16.8%).
Conclusions
Real-world data collected in a ten-year period confirmed the effectiveness of the use of imatinib, sunitinib, or sorafenib for the treatment of advanced GIST and was comparable with the findings from clinical trials.
MeSH Keywords: Abdominal Neoplasms, Population Control, Single-Payer System
Background
Drug treatment regimes in Poland are based on the outcome data from controlled clinical trials, and drug treatment programs that are publicly funded are a way to implement new therapies. However, data from real-world evidence (RWE) is increasingly recognized as a way to provide additional evidence for the effectiveness of drug treatment in clinical practice. A characteristic feature of drug programs is the strictly defined inclusion and exclusion criteria, drug regimens, patient monitoring, and follow-up to evaluate clinical outcome. In Poland, data from drug programs are made public from the Polish Ministry of Health [1].
Currently, the drug program that supports the treatment of gastrointestinal stromal tumor (GIST) is one of the most established programs in Poland (Table 1). As part of the GIST program, the first patient to be treated with imatinib was recorded 8th January 2007. In subsequent years, the GIST program included sunitinib, which had its first application on 6th September 2009, and sorafenib, which had its first application on 4th November 2014. Also, from1st March 2014, imatinib as adjuvant therapy and in children was added [1].
Table 1.
Eligibility criteria for the advanced gastrointestinal stromal tumor (GIST) drug program in Poland (ICD-10: C15–C18, C20, C48).
| 1. | ADJUVANT TREATMENT WITH IMATINIB: in patients at high risk of recurrence (≥50%) according to AJCC-NCCN-AFIP classification after radical tumor resection of KIT-CD117 GIST of the stomach, duodenum, small intestine and rectum and* palliative treatment with imatinib in patients with disseminated or inoperable gastrointestinal stromal tumor, which aims to stop the progression of the disease. |
| 1.1. | Inclusion criteria for treatment with imatinib in children and* adults |
| 1) | Diagnosis of gastrointestinal stromal tumor (GIST) confirmed histologically. |
| 2) | Expression of CD117 confirmed by immunohistochemistry. |
| 3) | Adjuvant treatment: high risk ≥50% of recurrence after radical resection of tumor with cKIT (CD117-positive GIST of the stomach, duodenum, small intestine and rectum, determined according to the AJCC-NCCN-AFIP classification); the time from primary GIST surgery to the implementation of adjuvant treatment should not exceed 4 months; the presence of KIT or PDGFR-α mutation excluding the PDGFR-αD842V mutation.* |
| 4) | Advanced disease treatment: the inability to perform resection or the presence of metastases documented by clinical examination or imaging. |
| 5) | Lesions measurable by computed tomography (CT). |
| 6) | World Health Organization (WHO) performance status 0–2. |
| 7) | Normal bone marrow examination results (platelet count ≥75,000/mm3, absolute neutrophil count ≥1,000/mm3, hemoglobin level ≥8.0 g/dL). |
| 8) | Normal liver and kidney parameters (≤2.5×ULN and ≤5×ULN for liver function tests in the case of liver metastases). |
| 1.2. | Exclusion from the imatinib treatment program |
| 1) | Hypersensitivity to imatinib. |
| 2) | Relapse of GIST during adjuvant treatment, which may last up to a maximum of 36 months.* |
| 3) | Progression of the disease during treatment with the dose of imatinib up to 800 mg/day; especially primary resistance to imatinib; in children of the area body up to 1m2 progression of the disease when using the drug after increasing the dose of imatinib twice.* |
| 4) | Lack of efficacy after four months of using the drug (increase in the sum of lesions in the spiral CT by 20% or more, except when the density of lesions is less than 15% in relation to the initial density or the appearance of new lesion(s) of at least 10 mm). |
| 5) | Persistence of WHO toxicity of grade ≥ 3 (especially a 3-fold increase in bilirubin above ULN, a 5-fold increase in liver transaminases above the upper limit of normal (ULN), severe anemia, neutropenia or thrombocytopenia). |
| 6) | WHO performance status 3/4. |
| 7) | Significant co-morbidities or organ failure (to be assessed by the attending physician). |
| 8) | Heart disease class III or IV WHO or the New York Heart Association (NYHA). |
| 9) | Use of warfarin in full daily doses. |
| 10) | Pregnancy. |
| 11) | Breastfeeding. |
|
| |
| 2. | TREATMENT WITH SUNITINIB |
| 2.1. | Inclusion criteria for treatment with sunitinib for children and adults |
| 1) | Diagnosis of GIST confirmed histologically. |
| 2) | Expression of CD117 confirmed by immunohistochemistry. |
| 3) | The inability to perform resection or the presence of metastases documented by clinical examination or imaging. |
| 4) | Lesions measurable on CT. |
| 5) | Documented progression during imatinib treatment (resistance) or imatinib intolerance (toxicity of grade 3/4). |
| 6) | WHO performance status 0–3. |
| 7) | Results of a blood count analysis with a smear: platelet count ≥75 000/mm3, absolute neutrophil count ≥1 000/mm3, hemoglobin level ≥8.0 g/dL. |
| 8) | Normal liver and kidney parameters (≤2.5×ULN and ≤5×ULN for liver function tests in the case of liver metastases). |
| 2.2. | Exclusion from the sunitinib treatment program |
| 1 | Hypersensitivity to sunitinib. |
| 2) | Documented progression of the disease during the use of the drug. |
| 3) | Lack of efficacy (in the form of disease progression) after 3 months of using the drug, unacceptable, recurrent (despite dose modifications) toxicity of WHO grade ≥3 (especially 3-fold increase in bilirubin, 5-fold increase in liver transaminases, neutropenia or thrombocytopenia; symptoms of congestive heart failure, acute coronary insufficiency, uncontrolled hypertension and unstable arrhythmias requiring treatment). |
| 4) | WHO performance status 4. |
|
| |
| 3. | TREATMENT WITH SORAFENIB |
| 3.1. | Inclusion criteria for sorafenib treatment |
| 1) | ≥18 years of age. |
| 2) | Diagnosis of GIST confirmed histologically. |
| 3) | The inability to resect the primary lesions or the presence of metastases documented by clinical examination or imaging. |
| 4) | Lesions measurable on CT. |
| 5) | Documented failure of previous treatment with imatinib (progression during treatment with imatinib) and documented progression during treatment with sunitinib (resistance) or sunitinib intolerance. |
| 6) | Lack of metastases in the central nervous system; |
| 7) | Zubrod – WHO performance status 0/1. |
| 8) | Results of a blood count analysis with a smear: the number of plates blood ≥100 000/mm3, absolute neutrophil count ≥1,500/mm3, hemoglobin concentration ≥10.0 g/dL. |
| 9) | Normal liver and kidney parameters (≤2.5×ULN and ≤5×ULN for liver function tests in the case of liver metastases). |
| 10) | No contraindications to the use of sorafenib. |
| 3.2. | Exclusion from the sorafenib treatment program |
| 1) | Hypersensitivity to sorafenib. |
| 2) | Documented progression of the disease during the use of sorafenib; |
| 3) | Long-lasting (>28 days) adverse reactions of grade ≥3 (WHO) not susceptible to symptomatic treatment and dose reduction. |
| 4) | Persistent deterioration of Zubrod–WHO performance status 2–4. |
| 5) | Consent withdrawal. |
|
| |
| 4. | DURATION OF TREATMENT |
| Treatment lasts until the decision of the attending physician is taken to exclude the patient from the program in accordance with the exclusion criteria. | |
AJCC – American Joint Committee on Cancer; NCCN – National Comprehensive Cancer Network; AFIP – Armed Forces Institute of Pathology.
Patients excluded from analysis.
Therefore, this study aimed to undertake an analysis of ten years of real-world evidence (RWE) on overall survival (OS) following treatment of advanced gastrointestinal stromal tumor (GIST) with imatinib, sunitinib, and sorafenib using data from the Polish National Health Fund, a database of publicly funded health care, which includes data from the GIST program.
Material and Methods
Data on health service provision for patients with gastrointestinal stromal tumor (GIST) were extracted from the database of the Polish National Health Fund, Narodowy Fundusz Zdrowia (NFZ), the sole Polish public healthcare payer. GIST was classified according to the International Classification of Diseases (ICD) criteria and included ICD-10 categories C15–C18, C20, and C48. Based on the unique patient ID, or Universal Electronic System of Population Register Number (PESEL), and the drug program identifier, the date of drug treatment, including treatment with imatinib, sunitinib, and sorafenib, in the GIST drug program were extracted. Detailed eligibility criteria in the GIST program are shown in Table 1.
Data were anonymized for the purpose of analysis. The median overall survival (OS) for patients undergoing imatinib, sunitinib, or sorafenib treatment was calculated using Kaplan-Meier statistics and survival tables. The median follow-up time was calculated based on the censored data.
To determine the efficacy of imatinib treatment in patients with advanced GIST, the survival time of patients receiving this treatment before the introduction of the imatinib as adjuvant therapy before 1st March 2014 was analyzed. For data analysis, the starting date of treatment in each case was the first administration of the drug, and the cutoff date was set on 30th October 2017. Censored observations referred to cases where the date of death of the patient was unavailable in the period analyzed (the patient was alive until the cutoff date). Dates of death were obtained from the PESEL database administered by the Polish Ministry of Health.
The SAS Enterprise Guide (EG) version 5.1 was used for statistical analysis. The differences in survival were assessed by a two-way log-rank test and Wilcoxon’s test. A P-value <0.05 was considered to be statistically significant.
Results
Patients in the gastrointestinal stromal tumor (GIST) program treated with imatinib
Patients diagnosed with gastrointestinal stromal tumor (GIST) who were treated with imatinib from the beginning of the GIST program in Poland to the introduction of adjuvant therapy with imatinib (1st March 2014) consisted of a cohort of 1,047 people, including 570 (54.4%) men and 477 (45.5%) women (Table 2). The median age of the male patients was 62 years (mean 62.4±11.5 years) (95% CI, 61.9–62.9). The median age of the female patients was 66 years (mean 64.8±12.7 years) (95% Cl, 64.2–65.6).
Table 2.
Data of imatinib, sunitinib and sorafenib treatment.
| Treatment data | Imatinib | Sunitinib | Sorafenib |
|---|---|---|---|
| No. of patients | 1047 | 456 | 137 |
| No. of complete observations (%) | 587 (56.1) | 299 (65.6) | 67 (48.9) |
| Median observation time (months) [95% CI] | 71 [64.8–79.2] | 41.4 [34.6–49.3] | 17.4 [14.6–22.9] |
| Median overall survival (months) [95% CI] | 56.9 [50.4–61.2] | 22.8 [19.2–26.8] | 16.9 [13.7–24.3] |
| Probability of surviving 12 months (%) | 89.5 | 68.2 | 61.9 |
| Probability of surviving 24 months (%) | 77.9 | 47.1 | 36.2 |
| Probability of surviving 36 months (%) | 66.9 | 31 | 16.8 |
| Probability of surviving 60 months (%) | 48.4 | Not reached | Not reached |
CI – confidence interval.
In the group of 1,047 patients treated with imatinib, the number of deaths was 587 (56.1%), and the number of censored observations was 460 (43.9%). The median follow-up time was 71 months (95% CI, 64.8–79.2). The median overall survival (OS) was 56.9 months (95% CI, 50.4–61.2). The probability of 12-month survival was 89.5%, 24-month survival was 77.9%, 36-month survival was 66.9%, and 60-month survival was 48.4% (Figure 1).
Figure 1.
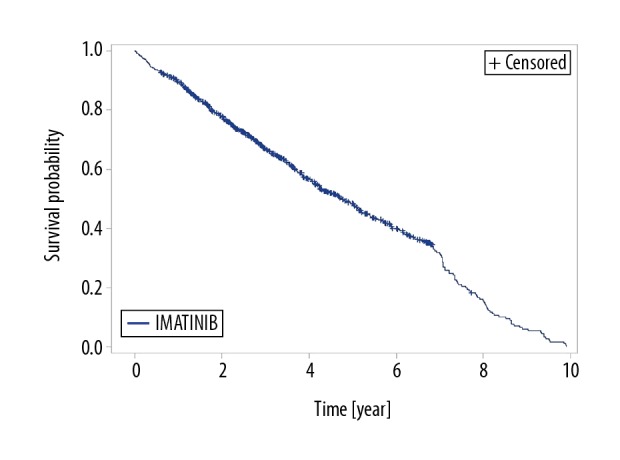
Kaplan-Meier estimation of overall survival (OS) for patients treated with imatinib.
Patients in the GIST program treated with sunitinib
Patients diagnosed with GIST who were treated with sunitinib from the beginning of the GIST program in Poland (1st Oct 2009) until the cutoff date consisted of a cohort of 456 people, including 260 (57.1%) men and 196 (42.9%) women (Table 2). The median age of the male patients was 63 years (mean 62.1±11.5 years) (95% CI, 61.1–63.2), and the median age of the female patients was 63 years (mean 61.41±14.2 years) (95% Cl, 60.0–62.9).
In the group of 456 patients treated with sunitinib, the number of deaths was 299 (65.6%), and the number of censored observations was 157 (34.4%). The median follow-up time was 41.4 months (95% CI, 34.6–49.3). The median OS was 22.8 months (95% CI, 19.2–26.8). The probability of 12-month survival was 68.2%, 24-month survival was 47.1%, and 36-month survival was 31% (Figure 2).
Figure 2.
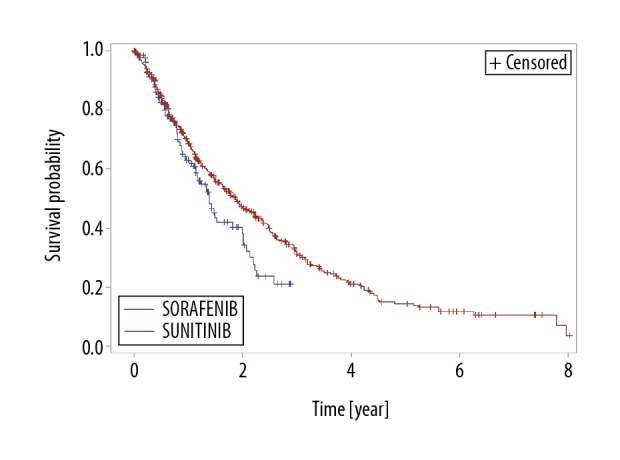
Kaplan-Meier estimation of overall survival (OS) for patients treated with sorafenib or sunitinib.
Patients in the GIST program treated with sorafenib
Patients diagnosed with GIST who were treated with sorafenib from the beginning of the GIST program in Poland (1st Nov 2014) until the cutoff date consisted of a cohort of 137 people, including 84 (61.3%) men and 53 (38.7%) women (Table 2). The median age of the male patients was 63 years (mean 63.9±8.9 years) (95% CI, 62.3–65.4), and the median age of the female patients was 63 years (mean 62.7±10.7 years) (95% Cl, 60.4–64.9).
In the group of 137 patients treated with sorafenib, the number of deaths was 67 (48.9%), and the number of censored observations was 70 (51.1%). The median follow-up time was 17.4 months (95% CI, 14.6–22.9). The median OS was 16.9 months (95% CI, 13.7–24.3). The probability of 12-month survival was 61.9%, 24-months survival was 36.2%, and 36-month survival was 16.8% (Figure 2).
The role of gender and age in patient outcome
The influence of gender and age on OS was calculated in the proportional hazard model using Cox regression, treating age and gender as explanatory variables (Table 3). In this model, the number of censored observations was 50.38% (P<0.0001). Patient age slightly increased the risk of death in the analyzed patient population. Increase in age by one year increased the risk of death by 1.7%. Gender had a more significant impact on the risk of death, as female gender reduced the risk of death by 18.9%.
Table 3.
Result of the gender and age impact assessment in the Cox regression model.
| Analysis of the maximum credibility ratings | ||||||
|---|---|---|---|---|---|---|
| Parameter | Degrees of freedom | Assessment of parameter | Standard error | Chi-square | Pr>Chi-square | Hazard ratio (HR) |
| Sex | 1 | −0.20973 | 0.04266 | 24.1725 | <0.0001 | 0.811 |
| Age | 1 | 0.01675 | 0.00188 | 79.0834 | <0.0001 | 1.017 |
Discussion
In patients with advanced gastrointestinal stromal tumor (GIST), including patients with metastases, sequential first-line and second-line systemic therapy with imatinib and sunitinib, and third-line treatment with regorafenib, sorafenib or nilotinib is currently the recommended treatment regimen, based on results of randomized clinical trials [2–8]. The current drug regimen used in the GIST program in Poland assumes first-line treatment with imatinib 400 mg daily. In patients with tumor progression who still tolerate imatinib, the daily dose is increased to 800 mg. In the event of therapy failure, patients still eligible for further treatment are treated with sunitinib and then progress to sorafenib. The GIST drug treatment program requires specific inclusion criteria, including adequate performance status and laboratory results [1]. Therefore, the population of GIST patients treated in Poland is selected to ensure the optimal benefit from treatment and to ensure consistency of treatment regimens. Therefore, the criteria used to select the patient cohorts in the GIST program in Poland results in drug treatment populations that have similarities to patients enrolled in clinical trials.
In 2017, the results of the phase III imatinib study of patients with metastatic or locally advanced GIST were published that compared the standard dose of 400 mg per day with an increased dose of 800 mg for patients with disease progression, to a higher initial dose of 800 mg [2]. There were 946 patients who participated in this study [2]. The median age was 60 years, which was similar to the median age of patients in the present study, which was 62 years for men and 66 years for women. In the 2017 study, there was no difference in progression-free survival (PFS), as the median PFS was 1.7 and 2.0 years, respectively (HR=0.91; p=0.18) as well as in overall survival (OS), with a median of 3.9 years in both groups [2]. In the phase III study that included 746 patients in the US and Canada, there was no significant difference in PFS and OS, regardless of the initial dose of imatinib [3]. After the median duration of follow-up of 4.5 years, the median PFS was 18 and 20 months, respectively, and OS was 55 months and 51 months, respectively [3].
The results of a study that compared sunitinib as a second-line treatment for GIST with placebo, the time to progression (median, 27.3 weeks vs. 6.4 weeks) was shown, resulting in the approval of sunitinib for the treatment of patients with tumor progression during imatinib therapy [4]. In a phase III study that assessed the use of sunitinib after the failure of treatment with imatinib compared with placebo (243 and 188 patients, respectively) the median OS was 72.7 and 64.9 weeks, respectively (HR=0.88; p=0.31) [5]. However, 87% of patients who received placebo at baseline were later given sunitinib, which significantly improved prognosis in the placebo group [5]. In this previously published study, construction of the survival curve for patients receiving only placebo was performed using the rank preserving structural failure time (RPSFT) analysis, which showed that the estimated median value of OS in this group was 39 weeks [5].
While the value of imatinib and sunitinib as first-line and second-line treatment of patients with GIST has been established and is evidence-based, treatment regimens in cases of GIST that are resistant to these drugs remain unclear, but there is data to support the use of regorafenib therapy [6]. There are also reports suggesting the effectiveness of sorafenib and nilotinib. Regorafenib in the phase III trial on 133 patients with at least imatinib and sunitinib failure was associated with a significant increase in PFS compared with placebo (median, 4.8 months vs. 0.7 months), but this study did not achieve median OS at the time of publication [7]. According to a later report presented at the American Society of Clinical Oncology (ASCO) conference in 2016, the median OS was 17.4 months [8].
In a phase II study that included 33 patients treated with regorafenib, the median OS was 25 months and PFS was 13.2 months [9]. In a retrospective review of the results of treatment of 20 patients with GIST at the Royal Marsden Hospital and University College Hospital, the median OS was 12.2 months and the median PFS was 9.4 months [10]. In a phase III study that included 248 patients previously treated with imatinib and sunitinib, a significantly increased PFS was found in patients treated with nilotinib compared with placebo when evaluated by the investigators, but the findings were not confirmed in a blinded radiological evaluation (median PFS, 119 days vs. 70 days; p=0.0007) and a similar OS were found (median, 332 days vs. 280 days) [11]. In a phase II study conducted in Korea that evaluated the efficacy of sorafenib therapy in 31 patients with advanced GIST after failure of imatinib and sunitinib treatment, median PFS and OS of 4.9 and 9.7 months were achieved, respectively [12]. In a phase II study that included six centers in the US and 38 patients, the median PFS and OS were 5.2 and 11.6 months, respectively [13].
There have been several retrospective studies that have evaluated the efficacy of sorafenib therapy in third-line and fourth-line treatment of advanced GIST. The median PFS and OS were shown to be 7.2 months and 15.2 months, respectively in a study that included 25 patients [14], 20 weeks and 42 weeks, respectively in a study that included 32 patients undergoing fourth-line treatment after nilotinib [15], 7.7 months and 13.5 months, respectively in a study that included 60 patients [16] and 6.4 months and 13.5 months, respectively in a study that included 124 patients [17]. In some studies, a significantly increased PFS was found in patients with improved performance status [17]. In a study that evaluated the results of treatment with nilotinib (n=67), sorafenib (n=55), and imatinib (n=40), in patients with GIST after two courses of standard treatment, the median PFS and OS were 3.6 months and 9.2 months, respectively [18].
In the present retrospective study, conducted on patients in Poland with advanced GIST, treatment in patients with advanced or nonoperative GIST included a large patient population of 1,047 patients given first-line treatment with imatinib, 547 patients given second-line treatment with sunitinib, and 137 patients given third-line treatment with sorafenib. This patient study sample size significantly exceeded those of previously published studies. Accurate epidemiological data for Poland are not available but adopting epidemiological indicators from other countries, with an incidence of approximately 1.0–1.5 per 100,000 per year and the percentage of patients with advanced GIST of 20–40%, it is possible that 150–200 people per year have diagnosis of advanced GIST in Poland [19]. Therefore, more than 1,000 people who started therapy with imatinib within 7 years, between January 2007 to March 2014, appear to be a representative number for the entire population of patients with advanced or metastatic GIST in Poland. This is understandable because the GIST drug treatment program is the only form of public reimbursement for imatinib, sunitinib, and sorafenib treatment in patients with GIST.
In the present study, the median OS observed in patients treated with imatinib was 4.74 years (56.8 months) and was similar to that found in the phase III study published in 2008, which was 55 months and 51 months, depending on the initial dose of imatinib [3], and was 10 months more than the results of the long-term findings in the study from 2017, which was 3.9 years (46.8 months) [2]. In the case of sunitinib, the OS found in the present study was also longer than in the previously published phase III study (22.8 months vs. 18.2 months) [5]. Also, in this study, the median OS of patients treated with sorafenib was 16.9 months, which was also higher than that reported by other authors (median OS between 9.7–15.2 months) [12–17]. It is difficult to compare the results of treatment with sorafenib as third-line treatment with the results obtained on regorafenib because previous studies in which the OS of patients treated with regorafenib has been reported gave divergent values for the median OS of between 12.2 months and 25 months [9,10]. It is likely that the numbers of subjects in the study groups assessed, which were 20 and 33, respectively may have influenced the findings.
However, a long-term observational study that included 133 patients in a phase III study of regorafenib showed a median OS of 17.4 months [8]. The findings of this previous study were similar to the results of the present study regarding sorafenib, which showed a median OS of 16.9 months in the 137 patients studied. Therefore, the findings of the present study supported the value of sorafenib as a third-line treatment for advanced GIST. This finding is particularly important given the lack of previous studies on sorafenib in large groups of patients with advanced GIST, probably because sorafenib has no formal approval for the treatment of advanced GIST.
The findings of the present study also allowed the estimation of the percentage of patients who received second-line and third-line treatment in the GIST program in Poland. In the ten-year period analyzed, the average number of patients who began imatinib treatment was 146 per year, the average number of patients who began sunitinib treatment was 57 per year, and the average number of patients who began sorafenib treatment was 46 per year. Therefore, approximately 40% of patients with tumor progression, despite an increase in the dose of imatinib up to 800 mg, started treatment with sunitinib as second-line treatment, and sorafenib therapy was initiated in most patients with tumor progression in the course of treatment with sunitinib.
This study had several limitations. This was a retrospective study that relied on the accuracy of available clinical data and that relied on patient selection as determined by the GIST program in Poland. The requirement for starting drug treatment, including third-line treatment, was good performance status as well as blood, liver, and renal parameters similar to these required in most clinical trials. The advantage of the requirement for inclusion in the GIST treatment program meant that the analysis of patient outcome data was likely to be more representative, or real-world, when compared with the results of some observational studies, in which inclusion criteria were less restrictive. The strict inclusion criteria required for the GIST program might explain the finding of the improved results for patients treated with imatinib when compared with the findings from previously reported studies. A further limitation of the present study was the lack of data on progression-free survival (PFS). However, an advantage of the present study was access to the data of a large group of patients, as well as the relatively long duration of follow-up.
Conclusions
Analysis of ten years of real-world evidence (RWE) on overall survival (OS) following treatment of advanced gastrointestinal stromal tumor (GIST) with imatinib, sunitinib, and sorafenib using data from the Polish National Health Fund, included 1,641 patients. The findings confirmed the effectiveness of imatinib, sunitinib, or sorafenib for the treatment of advanced GIST and the findings were similar to the findings from clinical trials. Imatinib, sunitinib, and sorafenib treatment for GIST are financed in Poland based on the results of clinical trials. However, most clinical studies do not have long-term follow-up periods. Therefore, real-world data collected in the large population during a ten-year period confirm benefits from the use of imatinib, sunitinib, or sorafenib for the treatment of advanced GIST.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Polish Ministry of Health. URL: http://www.mz.gov.pl/leki/refundacja/programy-lekowe/ [in Polish]
- 2.Casali PG, Zalcberg J, Le Cesne A, et al. Ten-year progression-free and overall survival in patients with unresectable or metastatic GI stromal tumors: Long-term analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III randomized trial on imatinib at two dose levels. J Clin Oncol. 2017;35(15):1713–20. doi: 10.1200/JCO.2016.71.0228. [DOI] [PubMed] [Google Scholar]
- 3.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–32. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 4.Goodman VL, Rock EP, Dagher R, et al. Approval summary: Sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13(5):1367–73. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 5.Demetri GD, Garrett CR, Schöffski P, et al. Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res. 2012;18(11):3170–79. doi: 10.1158/1078-0432.CCR-11-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishida T, Doi T, Naito Y. Tyrosine kinase inhibitors in the treatment of unresectable or metastatic gastrointestinal stromal tumors. Expert Opin Pharmacother. 2014;15(14):1979–89. doi: 10.1517/14656566.2014.937707. [DOI] [PubMed] [Google Scholar]
- 7.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demetri GD, Reichardt P, Kang Y-K, et al. Final overall survival (OS) analysis with modeling of crossover impact in the phase III GRID trial of regorafenib vs. placebo in advanced gastrointestinal stromal tumors (GIST) J Clin Oncol. 2017 Abstract 156. [Google Scholar]
- 9.Ben-Ami E, Barysauskas CM, von Mehren M, et al. Long-term follow-up results of the multicenter phase II trial of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of standard tyrosine kinase inhibitor therapy. Ann Oncol. 2016;27(9):1794–99. doi: 10.1093/annonc/mdw228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollàr A, Maruzzo M, Messiou C, et al. Regorafenib treatment for advanced, refractory gastrointestinal stromal tumor: A report of the UK managed access program. Clin Sarcoma Res. 2014;4:17. doi: 10.1186/2045-3329-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichardt P, Blay JY, Gelderblom H, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol. 2012;23(7):1680–87. doi: 10.1093/annonc/mdr598. [DOI] [PubMed] [Google Scholar]
- 12.Park SH, Ryu MH, Ryoo BY, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: A phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs. 2012;30(6):2377–83. doi: 10.1007/s10637-012-9795-9. [DOI] [PubMed] [Google Scholar]
- 13.Kindler HL, Campbell NP, Wroblewski K, et al. Sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): Final results of a University of Chicago Phase II Consortium trial. J Clin Oncol (ASCO Meeting Proceedings) 2011;29:10009. [Google Scholar]
- 14.Kefeli U, Benekli M, Sevinc A, et al. Efficacy of sorafenib in patients with gastrointestinal stromal tumors in the third- or fourth-line treatment: A retrospective multicenter experience. Oncol Lett. 2013;6(2):605–11. doi: 10.3892/ol.2013.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reichardt P, Montemurro M, Gelderblom H, et al. Sorafenib fourth-line treatment in imatinib-, sunitinib-, and nilotinib-resistant metastatic GIST: A retrospective analysis. J Clin Oncol (2009 ASCO Annual Meeting) 2009;27(15 Suppl) Abstract 10564. [Google Scholar]
- 16.Rutkowski P, Jagielska B, Andrzejuk J, et al. The analysis of the long-term outcomes of sorafenib therapy in routine practice in imatinib and sunitinib resistant gastrointestinal stromal tumors (GIST) Contemp Oncol (Pozn) 2017;21(4):285–89. doi: 10.5114/wo.2017.72393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montemurro M, Gelderblom H, Bitz U, et al. Sorafenib as third- or fourth-line treatment of advanced gastrointestinal stromal tumour and pretreatment including both imatinib and sunitinib, and nilotinib: A retrospective analysis. Eur J Cancer. 2013;49(5):1027–31. doi: 10.1016/j.ejca.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Italiano A, Cioffi A, Coco P, et al. Patterns of care, prognosis, and survival in patients with metastatic gastrointestinal stromal tumors (GIST) refractory to first-line imatinib and second-line sunitinib. Ann Surg Oncol. 2012;19(5):1551–59. doi: 10.1245/s10434-011-2120-6. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the pre-imatinib mesylate era – a population-based study in western Sweden. Cancer. 2005;103(4):821–29. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]


