Abstract
Background
Cough variant asthma in children presents with a dry nonproductive cough. This study aimed to investigate the diagnostic value of fractional exhaled nitric oxide (FeNO) combined with small airway functional parameters in cough variant asthma.
Material/Methods
Children with asthma (n=136) were divided into a cough variant asthma (CVA) group (n=57; mean age, 8.03±2.1 years) and a non-cough variant asthma (nCVA) group (n=79; mean age, 8.61±1.7 years). In both groups, FeNO and other pulmonary function parameters were measured including forced expiratory volume in one second (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF), maximum mid-expiratory flow (MMEF), forced expiratory flow (FEF), and maximum expiratory flow at 25%, 50%, and 75% expired volume (MEF25, MEF50, and MEF75). Receiver-operating characteristic (ROC) curve analysis compared the sensitivity and specificity between the diagnostic parameters.
Results
The FeNO values were significantly increased in the CVA group compared with the nCVA group (Z=6.890, p<0.001). The MMEF, MEF25, MEF50, and MEF75 values were significantly lower in the CVA group compared with the nCVA group (p=0.000, p=0.014, p=0.000, and p=0.000, respectively). The FeNO values were negatively correlated with MEF25, MEF50, and MMEF (r=−0.334, r=−0.257 and r=−0.276, respectively). FeNO was significantly more efficient diagnosing cough variant asthma comparing with pulmonary parameters (p<0.05), and was most sensitive and specific when combined with MMEF/MEF50 compared with single diagnostic parameters (p<0.05).
Conclusions
FeNO combined with pulmonary function parameters of MMEF/MEF50 showed increased sensitivity and specificity for the diagnosis of cough variant asthma.
MeSH Keywords: Airway Remodeling, Asthma, Cough, Respiratory Function Tests
Background
Cough variant asthma in children is characterized by a dry productive cough that responds to bronchodilator therapy [1,2]. Worldwide, cough variant asthma has been reported as a common cause for chronic cough in children [1–3]. Cough variant asthma has the pathophysiological characteristics of classical asthma, including atopy and chronic airway inflammation, and airway hyperreactivity [4]. Clinically, dysfunction of small airways is the most important clinical characteristic of cough variant asthma [5]. A previous study showed that approximately 30% of cases of cough variant asthma progress to classical asthma if no therapeutic intervention occurs [6].
The bronchial provocation test (BPT) is commonly used to diagnose cough variant asthma in clinical practice. However, the BPT procedure is complex and time-consuming and is associated with the risk of bronchial spasm [7]. The success of BTP requires the cooperation of the pediatric patient and there is a lack of sensitivity and specificity for this diagnostic test for cough variant asthma in clinical practice.
Fractional exhaled nitric oxide (FeNO) is a non-invasive, sensitive, and convenient biomarker for monitoring eosinophilic lower airway inflammation and is simple to use and the findings are reliable and reproducible [8]. There are several diagnostic tests for small airway function, including maximal mid-expiratory flow (MMEF), mid-expiratory flow at 25%, (MEF25), mid-expiratory flow at 50% (MEF50), and mid-expiratory flow at 75% (MEF75) of vital capacity [9]. However, there have been few studies on the clinical value of combining the evaluation of FeNO with tests of small airway function for the diagnosis and evaluation of cough variant asthma in children [10].
Therefore, this study aimed to investigate the diagnostic value of FeNO combined with small airway functional parameters in the diagnosis of cough variant asthma in pediatric patients at a single center.
Material and Methods
Patients
This study included 136 children who had symptoms of a cough more than four weeks who attended the pediatric respiratory clinic and inpatient department, between August 2014 and August 2016. The families of the patients signed written informed consent and approved the study. This study was approved by the Ethics Committee of Hospital of Nanjing Medical University, Nanjing, China.
Diagnostic criteria, study inclusion criteria, and study groups
Chronic cough and cough variant asthma were diagnosed according to the recommendations of the Chinese National Guidelines on diagnosis and management of cough [11]. Study inclusion criteria included a diagnosis of chronic cough, no abnormality on chest X-ray, no drug treatment in the previous three days, including inhaled or oral steroids, bronchodilator, oral anti-allergic drugs, no history of upper respiratory tract infection, fever, or pharyngalgia in the previous four weeks.
Study exclusion criteria included patients with the chronic respiratory disorders, such as cystic fibrosis, tuberculosis, and congenital bronchopulmonary dysplasia, classical asthma, and patients with other complications, including epilepsy, congenital heart disease, congenital respiratory disease, and thoracic deformity.
According to the above diagnostic criteria and study inclusion and exclusion criteria, children with asthma (n=136) were divided into a cough variant asthma (CVA) group (n=57) with a mean age of 8.03±2.1 years, and a non-cough variant asthma (nCVA) group (n=79) with a mean age of 8.61±1.7 years. In the nCVA group, there were 46 cases with upper airway cough syndrome (UACS), 21 cases with cough following infection, five cases with cough associated with gastroesophageal reflux, four cases with Tourette’s syndrome, and three cases with eosinophilic bronchitis.
Measurement of fractional exhaled nitric oxide (FeNO)
Fractional exhaled nitric oxide (FeNO) was measured according to the Guidelines of American Thoracic Society and used the electrochemical method [12] and the NIOX MINO® FeNO detection system (Aerocrine, Solna, Sweden) according to the manufacturer’s instructions. The FeNO measurement was conducted using a mouth pressure of 16 cm H2O with the 50 ml/s expiratory flow for 10 seconds. To obtain three nitric oxide (NO) values that achieved 5% level, exhalation was repeated in this study. The main detection levels for FeNO were measured in ppb mol/l, where 1 ppb=1×10−9 mol/l.
Small airway pulmonary function testing
Small airway pulmonary function testing was performed using the MasterScreen™ PAED pulmonary function analyzer, and the MasterScreen™ Pneumo von JAEGER™ (CareFusion, Würzburg, Germany), as previously described [13]. The forced expiratory volume in one second (FEV1), the forced vital capacity (FVC), the peak expiratory flow (PEF), the maximum mid-expiratory flow (MMEF), the forced expiratory flow (FEF), the maximum expiratory flow (MEF), and the maximum expiratory flow at 25%, 50%, and 75% expired volume (MEF25, MEF50, and MEF75) were measured. The FEV1/FVC ratios were calculated. For the FEV1 analysis, the percentage of predicted FEV1 was calculated as FEV1%.
Statistical analysis
Data were analyzed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). The categorical data were described as the percentage (%) and the continuous data were described as the mean ± standard deviation (SD). Differences in the continuous data between the two groups were analyzed with Student’s t-test. Differences in categorical data between groups were analyzed using a chi-squared (χ2) test. Pearson’s correlation analysis was used for data with a normal distribution and Spearman’s correlation analysis was used for data with a non-normal distribution. The receiver-operating characteristic (ROC) curve and area under the curve (AUC) were used to analyze the diagnostic sensitivity and specificity of FeNO and small airway parameters for cough variant asthma. A P-value <0.05 was considered to be statistically significant.
Results
Comparison of the fractional exhaled nitric oxide (FeNO) and pulmonary function testing parameters between the cough variant asthma (CVA) group and the non-cough variant asthma (nCVA) group
The fractional exhaled nitric oxide (FeNO) values were evaluated in both cough variant asthma (CVA) group and the non-cough variant asthma (nCVA) group, The results showed that the FeNO value in the CVA group was significantly increased when compared with the nCVA group (Table 1) (Z=6.890; p<0.001). For the small airway pulmonary function test parameters, the maximum mid-expiratory flow (MMEF), maximum expiratory flow at 25%, 50%, and 75% expired volume (MEF25, MEF50, and MEF75) values in the CVA group were significantly lower compared with the nCVA group (p=0.000, p=0.014, p=0.000, and p=0.000, respectively) (Table 1). However, there were no significant differences for the FVC% predicted value, FEV1% predicted value, EFV1/FVC ratio, and peak expiratory flow (PEF)% predicted value between the CVA group and the nCVA group (p>0.05) (Table 1).
Table 1.
Comparison of the fractional exhaled nitric oxide (FeNO) and parameters of lung function between the cough variant asthma (CVA) group (n=57) and non-cough variant asthma (nCVA) group of pediatric patients.
| Items | CVA (n=57) | nCVA (n=79) | t/Z values | P values |
|---|---|---|---|---|
| FeNO (ppb) | 37.8 (13.7) | 19.1 (9.8) | Z=6.890 | <0.001 |
| FEV1% predicted value (%, x±s) | 96.6±12.6 | 99.9±11.0 | t=1.891 | 0.093 |
| FVC% predicted value (%, x_±s) | 98.8±13.4 | 100.3±14.2 | t=0.898 | 0.372 |
| FEV1/FVC (%, x±s) | 103.5±8.7 | 103.1±14.2 | t=0.126 | 0.900 |
| PEF% predicted value (%, x±s) | 98.2±14.6 | 96.6±11.8 | t=0.857 | 0.392 |
| MMEF (%, x±s) | 73.7±17.7 | 91.9±18.4 | t=4.521 | 0.000 |
| MEF75 (%, x±s) | 84.4±15.3 | 91.8±16.4 | t=1.989 | 0.014 |
| MEF50 (%, x±s) | 73.4±18.1 | 93.5±18.1 | t=5.253 | 0.000 |
| MEF25 (%, x±s) | 68.0±24.1 | 94.8±15.4 | t=3.976 | 0.000 |
The FeNO values were negatively correlated with MMEF, MEF25, and MEF50 values
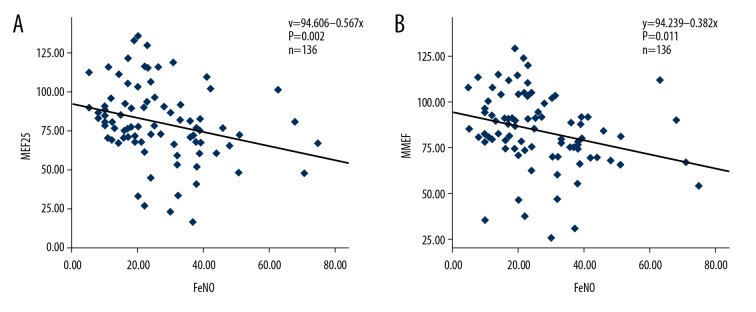
To determine the diagnostic value of FeNO and pulmonary function test parameters for the diagnosis of cough variant asthma, these parameters were compared. FeNO was negatively correlated with the MEF25 values (r=−0.334, p=0.002) (Figure 1A, Table 2). Also, the FeNO values were negatively correlated with the MMEF values (r=−0.276, p=0.011) (Figure 1B) (Table 2), and the MEF50 values (r=−0.257, p=0.018) (Table 2). However, there were no significant correlations between FeNO and the other pulmonary function test parameters (p>0.05) (Table 2).
Figure 1.
Correlation analysis between fractional exhaled nitric oxide (FeNO) and small airway functional parameters. (A) Correlation between fractional exhaled nitric oxide (FeNO) and maximum expiratory flow at 25% (MEF25). (B) Correlation between FeNO and maximum mid-expiratory flow (MMEF).
Table 2.
Correlation analysis between the fractional exhaled nitric oxide (FeNO) and large and small airway lung function.
| Items | ρ values | P values |
|---|---|---|
| FeNO/FEV1% predicted value | −0.125 | 0.257 |
| FeNO/PEF% predicted value | −0.189 | 0.185 |
| FeNO/FVC% predicted value | −0.067 | 0.715 |
| FeNO/MMEF% predicted value | −0.276 | 0.011 |
| FeNO/MEF75% predicted value | −0.195 | 0.075 |
| FeNO/MEF50% predicted value | −0.257 | 0.018 |
| FeNO/MEF25% predicted value | −0.334 | 0.002 |
The FeNO test showed increased sensitivity and specificity for the diagnosis of cough variant asthma when compared with other small airway pulmonary function tests
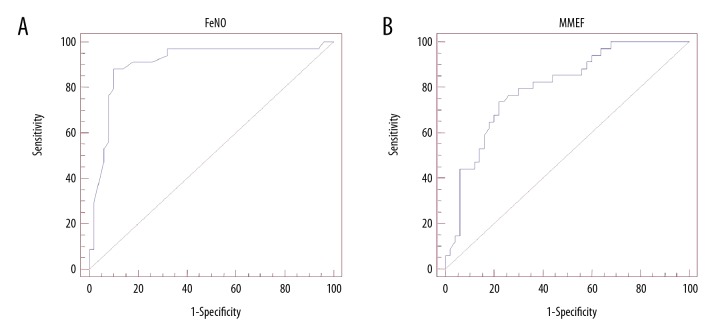
For FeNO and the diagnosis of cough variant asthma, the result of the area under the curve (AUC) was 0.905 and the optimal critical value was 25.5 ppb (Table 3). The sensitivity, specificity, positive predictive value, and negative predictive value for FeNO were 82.2%, 90.0%, 91.8%, and 85.7%, respectively (all, p<0.05) (Table 3). For the diagnosis of cough variant asthma using the pulmonary function test parameters, MEF50 and MMEF, the area under the curve (AUC) were 0.792 and 0.800, respectively (Table 3). The sensitivity and specificity for the diagnosis of cough variant asthma were significantly increased for FeNO testing compared with the MMEF, MEF25, MEF50 and MEF75 tests (all, p<0.05) (Table 3). Also, the FeNO test resulted in a maximal AUC comparing with the other groups, with FeNO >MMEF >MEF50 >MEF25 >MEF75 (Table 3). In particular, the FeNO test (Figure 2A) and the MMEF test (Figure 2B) demonstrated significantly improved sensitivity and specificity for the diagnosis of cough variant asthma in children compared with the other pulmonary function test parameters.
Table 3.
Diagnostic efficacy of fractional exhaled nitric oxide (FeNO) and lung function for the cough variant asthma (CVA) group.
| Items | AUC (95% CI) | Cutoff value | Sensitivity (%) | Specificity (%) | P value |
|---|---|---|---|---|---|
| FeNO (ppb) | 0.905 (0.821–0.958) | 25.5 | 82.2 | 90.0 | <0.01 |
| MEF75 (%) | 0.726 (0.618–0.818) | 90.9 | 80.2 | 50.0 | <0.01 |
| MEF50 (%) | 0.792 (0.690–0.873) | 78.0 | 64.7 | 82.0 | <0.01 |
| MEF25 (%) | 0.777 (0.673–0.861) | 70.6 | 70.6 | 76.0 | <0.01 |
| MMEF (%) | 0.800 (0.698–0.879) | 80.5 | 73.5 | 78.0 | <0.01 |
Figure 2.
Receiver-operating characteristic (ROC) curve analysis of the diagnostic sensitivity and specificity of fractional exhaled nitric oxide (FeNO) and maximum mid-expiratory flow (MMEF) for the diagnosis of cough variant asthma. (A) Diagnostic efficacy of fractional exhaled nitric oxide (FeNO). (B) Diagnostic efficacy of maximum mid-expiratory flow (MMEF).
The FeNO test combined with the MMEF/MEF50 were associated with increased sensitivity and specificity for the diagnosis of cough variant asthma
The diagnostic sensitivity of the single use of the FeNO test, and the combined use of the FeNO test combined with the MEF50 test, and the FeNO test combined with the MMEF test were 82.2%, 84.6%, and 85.4%, respectively (Table 4). The diagnostic specificity of the single use of the FeNO test, and the combined use of the FeNO test combined with the MEF50 test, and the FeNO test combined with the MMEF test were 90.0%, 94.0%, and 96.0%, respectively (Table 4). These results indicated that the combination of the FeNO test and the tests for small airway function increased the accuracy of the diagnosis of cough variant asthma in children (Table 4).
Table 4.
Diagnostic effects of fractional exhaled nitric oxide (FeNO) combined with small airway function in children with cough variant asthma.
| Items | AUC (95% CI) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| FeNO | 0.905 (0.821–0.958) | 82.2 | 90.0 |
| FeNO+MEF50 (%) | 0.919 (0.839–0.968) | 84.6 | 94.0 |
| FeNO+MMEF (%) | 0.928 (0.850–0.973) | 85.4 | 96.0 |
Combination 1: Fractional exhaled nitric oxide (FeNO) + maximum mid-expiratory flow (MMEF) (%). Combination 2: Fractional exhaled nitric oxide (FeNO) + maximum expiratory flow at 50% (MEF50) (%).
The FeNO test combined with the MMEF/MEF50 ratio showed the optimal diagnostic sensitivity and specificity for cough variant asthma in children
According to the AUC values, the sensitivity and specificity for the diagnosis of cough variant asthma for the FeNO test combined with the MMEF test and the FeNO test combined with the MEF50 test were significantly increased when compared with the FeNO test alone (Figure 3).
Figure 3.
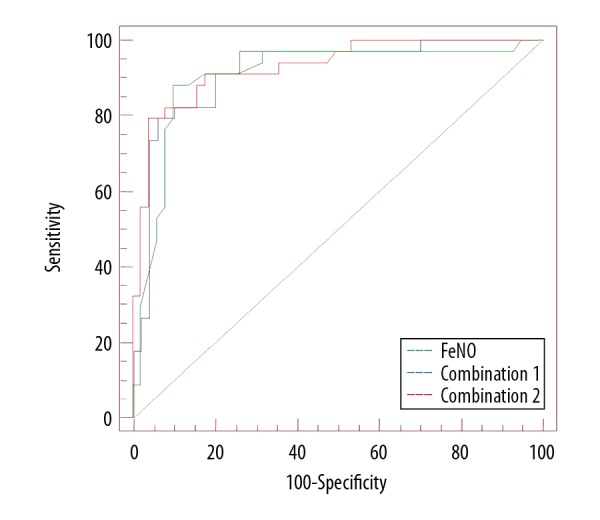
Receiver-operating characteristic (ROC) curve analysis to compare the diagnostic sensitivity and specificity between fractional exhaled nitric oxide (FeNO) alone or in combination with maximum mid-expiratory flow/maximum expiratory flow at 25% (MMEF/MEF25) for the diagnosis of cough variant asthma.
Discussion
Several recently published studies have shown that cough variant asthma, upper airway cough syndrome (UACS), respiratory tract infection, and gastroesophageal reflux are the main causes for chronic cough in children [2,14]. A study from China reported that the three leading causes of chronic cough were cough variant asthma (41.95%), UACS (24.71%), and respiratory tract infection (21.73%) [15]. In the present study, there were 57 cases of cough variant asthma (41.9%).
The pathogenesis of cough variant asthma is similar to typical asthma, with the pathogenesis being atopy, airway hyperresponsiveness, and chronic inflammation [16,17]. Fractional exhaled nitric oxide (FeNO) is a sensitive marker for monitoring eosinophilic airway inflammation of the central airway and surrounding small airways [18]. Clinically, the FeNO test can be used to diagnose asthma and chronic cough. Previously published studies have reported that levels of FeNO in patients with cough variant asthma were significantly increased when compared with other patients with chronic cough [1,19]. However, there have been some controversial findings for the diagnostic role of FeNO for cough variant asthma due to the differences in the optimal threshold for FeNO and the diagnostic sensitivity and specificity [1,20–22]. The findings of the present study showed that the levels of FeNO in the patients with cough variant asthma were significantly increased when compared with the other patients with chronic cough, including patients with UACS. The results of this study also showed that the optimal diagnostic critical value for FeNO was 25.5 ppb, which might be of value clinically for the diagnosis of cough variant asthma. Also, both of the sensitivity and specificity of the FeNO test for the diagnosis of cough variant asthma were significantly greater, which suggests that FeNo might have a clinical role in screening and diagnosing cough variant asthma in clinical practice, which is consistent with the findings from a previous study [23].
Routine spirometry is an important method for evaluating and diagnosing the cause of chronic cough in children [24,25]. A previously published study also reported that patients with asthma had dysfunction of the small airways, although the forced expiratory volume in one second (FEV1) was normal [26]. Similar to the inflammation of the central airway, the inflammation in small airway causes the thickening of the airway wall, stenosis of the lumen, and hyperresponsiveness of airway [27]. In this study, small airways were defined as having a diameter of <2 mm, with airway obstruction previously reported to occur between the first and fifth bronchial branch [28,29]. Previously published studies have shown that the most common characteristic of cough variant asthma is airway dysfunction, especially dysfunction of small airways [5,30]. The routine pulmonary function parameters for evaluating small airways include the maximum mid-expiratory flow (MMEF), maximum expiratory flow at 25%, and 50% (MEF25, MEF50) [31,32]. The findings of the present study showed that the values for MMEF, MEF50, and MEF25 were significantly lower in patients with cough variant asthma compared with patients with classical asthma, but there were no differences for large airway function. These results suggest that cough variant asthma is mainly characterized by dysfunctions of the small airways. Feng-Jia et al. [33] retrospectively analyzed 150 patients with cough variant asthma and found that small airway function was significantly reduced when compared with patients without non-cough variant asthma. Therefore, the findings of the present study were consistent with previously published findings [33].
Further published studies have shown that the FeNO was associated with the pulmonary function parameters of small airway [2,34,35]. The present study showed that the small airway functional parameters of MMEF, MEF50, and MEF25 were significantly correlated with the levels of FeNO in the patients with cough variant asthma, which suggests that the dysfunction of small airway might be correlated with airway inflammation. Liu et al. reported that the FeNO test combined with impulse oscillometry (IOS) testing resulted in increased diagnostic sensitivity and specificity for dysfunction of small airways in patients with asthma [34]. Feng-Jia et al. also reported that the increased levels of FeNO indicated dysfunction of small airways and was diagnostic for cough variant asthma [33].
The findings of the present study showed that the combination of testing for FeNO and small airway parameters resulted in increased diagnostic sensitivity when compared with testing for FeNO alone in patients with cough variant asthma. Among the combined test strategies, the FeNO test combined with the MMEF resulted in an increased area under the curve (AUC) for receiver-operating characteristic (ROC) curve analysis (0.928), which demonstrated the increased sensitivity and specificity for the diagnosis of cough variant asthma. These findings indicate that FeNO reflects the degree of eosinophilic inflammation of the airway rather than small airway obstruction [35,36], and that combining parameters for testing small airway function, such as MMEF, reflects small airway obstruction. However, the findings from a previously published study did not support a similar role for MMEF for the clinical diagnosis of cough variant asthma [37].
This study had several limitations. The allergic parameters of the children included in the study were not investigated, including the levels of total IgE and the radioallergosorbent test (RAST) that detects allergen-specific IgE in the blood. Tests for allergy should be performed in future studies that include patients with cough variant asthma. The study sample size was limited by the findings from a single center, and future multicenter studies should be performed to increase the study size.
Conclusions
In pediatric patients, measurement of the fractional exhaled nitric oxide (FeNO) combined with tests of small airway function could distinguish between cough variant asthma and classical asthma, due to dysfunction of small airways in patients with cough variant asthma. The FeNO test combined with tests of small airway function that included the maximum mid-expiratory flow/maximum expiratory flow at 50%, (MMEF/MEF50) resulted in improved diagnostic sensitivity and specificity for cough variant asthma. These findings might have clinical application in screening for cough variant asthma in children with chronic cough.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Tajiri T, Niimi A, Matsumoto H, et al. Prevalence and clinical relevance of allerigic rhinitis in patients with classic asthma and cough variant asthma. Respiration. 2014;87:211–18. doi: 10.1159/000355706. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, Lin SH, Zhu D, et al. WeChart public account use improves clinical control of cough-variant asthma: A randomized controlled trial. Med Sci Monit. 2018;24:1524–32. doi: 10.12659/MSM.907284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corrao WM, Braman SS, Irwin RS. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med. 1979;300:633–37. doi: 10.1056/NEJM197903223001201. [DOI] [PubMed] [Google Scholar]
- 4.Niimi A, Matsumoto H, Mishima M. Eosinophilic airway disorders associated with chronic cough. Pulm Pharmacol Ther. 2009;22:114–20. doi: 10.1016/j.pupt.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Lougheed MD, Turcotte SE, Fisher T. Cough variant asthma: Lessons learned from deep inspirations. Lung. 2012;190:17–22. doi: 10.1007/s00408-011-9348-6. [DOI] [PubMed] [Google Scholar]
- 6.Dicpinigaitis PV. Chronic cough due to asthma: ACCP evidence based clinical practice guidelines. Chest. 2006;129:75S–79S. doi: 10.1378/chest.129.1_suppl.75S. [DOI] [PubMed] [Google Scholar]
- 7.Guan W, Zheng J, Gao Y, et al. Leukotriene D4 bronchial provocation test: Methodology and diagnostic value. Curr Med Res Opin. 2012;28:797–803. doi: 10.1185/03007995.2012.678936. [DOI] [PubMed] [Google Scholar]
- 8.Roos AB, Mori M, Gronneberg R, et al. Elevated exhaled nitric oxide in allergen-provoked asthma is associated with airway epithelia iNOS. PLoS One. 2014;9:e90018. doi: 10.1371/journal.pone.0090018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang HQ, Zhang HQ, Zhang JJ, et al. [Application of pulmonary function and fractional exhaled nitric oxide tests in the standardized management of bronchial asthma in children]. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:419–24. doi: 10.7499/j.issn.1008-8830.2017.04.012. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang S, Chen SY, He X, et al. [Evaluating the efficacy of fractional exhaled nitric oxide and impulse oscillometry in screening out cough variant asthma form patients with subacute cough]. Zhonghua Yi Xue Za Zhi. 2017;97:2338–43. doi: 10.3760/cma.j.issn.0376-2491.2017.30.005. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 11.Clinical Research Coordination Group of Chronic Cough, The Subspecialty Group of Respiratory Diseases, The Society of Pediatrics, Chinese Medical Association, Editorial Board, Chinese Journal of Pediatrics. [Guideline for diagnosis and treatment of chronic cough in Chinese children]. Zhonghua Er Ke Za Zhi. 2014;52:184–88. [in Chinese] [PubMed] [Google Scholar]
- 12.Smilkoff PE. Recommendation for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric and nasal nitric oxide in adults and children, 1999. Am J Respir Crit Care Med. 1999;160:2104–17. doi: 10.1164/ajrccm.160.6.ats8-99. [DOI] [PubMed] [Google Scholar]
- 13.Ko FW, Diba C, Roth M, et al. A comparison of airway and serum matrix metalloproteinase-9 activity among normal subjects, asthmatic patients, and patients with asthmatic mucus hypersecretion. Chest. 2005;127:1919–27. doi: 10.1378/chest.127.6.1919. [DOI] [PubMed] [Google Scholar]
- 14.Xu K, Li X. Risk factors for depression in patients with chronic obstructive pulmonary disease. Med Sci Monit. 2018;24:1417–23. doi: 10.12659/MSM.904969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Research Coordination Group of the Causes Constituents Ratio of Chronic in Chinese Children. [Prospective multicenter clinical study on the causes constituents ratio of chronic cough in Chinese children]. Zhonghua Er Ke Za Zhi. 2012;50:83–92. [in Chinese] [PubMed] [Google Scholar]
- 16.Zhang C, Zhang H, Wu YF, et al. Suhuang antitussive capsule at lower doses attenuates airway hyperresponsiveness, inflammation, and remodeling in a murine model of chronic asthma. Sci Rep. 2016;6:21515. doi: 10.1038/srep21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Li S. Transient receptor potential ankyrin 1 (TRPA1) channel and neurogenic inflammation in pathogenesis of Asthma. Med Sci Monit. 2016;22:2917–23. doi: 10.12659/MSM.896557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T, Wu J, Zhao J, et al. Type 2 innate lymphoid cells: a novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma. Respir Med. 2015;109:1391–96. doi: 10.1016/j.rmed.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Song WJ, Kim HJ, Shim JS, et al. Diagnostic accuracy of fractional exhaled nitric oxide measurement in predicting cough-variatn asthma and eosinophilic bronchitis in adults with chronic cough: A systematic review and meta-analysis. J Allergy Clin Immunol. 2017;140:701–9. doi: 10.1016/j.jaci.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Tang W, Zhou J, Miao L, et al. Clinical features in patients of cough variant asthma with normal and high level of exhaled fractional nitric oxide. Clin Respir J. 2018;12:595–600. doi: 10.1111/crj.12568. [DOI] [PubMed] [Google Scholar]
- 21.Shimoda T, Obase Y, Kishikawa R, et al. The fractional exhaled intric oxide and serum high sensitivity C-reactive protein protein levels in cough variant asthma and typical bronchial asthma. Allergol Int. 2013;62:251–57. doi: 10.2332/allergolint.12-OA-0515. [DOI] [PubMed] [Google Scholar]
- 22.Maniscalco M, Faraone S, Sofia M, et al. Extended analysis of exhaled and nasal nitric oxide for the evaluation of chronic cough. Respir Med. 2015;109:970–74. doi: 10.1016/j.rmed.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Chen SY, Fang ZK, Fang S, et al. [Comparison of functional parameters of small airways between patients with typical asthma and cough-variant asthma]. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37:330–36. doi: 10.3969/j.issn.1673-4254.2017.03.09. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miric M, Turkalj M, Nogalo B, et al. Lung diffusion capacity in children with respiratory symptoms and untreated GERD. Med Sci Monit. 2014;20:774–81. doi: 10.12659/MSM.890336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentry S, Gentry B. Chronic obstructive pulmonary disease: Diagnosis and management. Am Fam Physician. 2017;95:433–41. [PubMed] [Google Scholar]
- 26.Pisi R, Tzani P, Aiello M, et al. Small airway dysfunction by impulse oscillometry in asthmatic patients with normal forced expiratory volume in the 1st second values. Allergy Asthma Proc. 2013;34:e14–20. doi: 10.2500/aap.2013.34.3641. [DOI] [PubMed] [Google Scholar]
- 27.Tulic MK, Christodoulopoulos P, Hamid Q. Small airway inflammation in asthma. Respir Res. 2001;2:333–39. doi: 10.1186/rr83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen MS, de Wolf MWP, Rasmussen LS. Ventilation via the 2.4 mm internal diameter Tritube with cuff-new possibilities in airway management. Acta Anaesthesiol Scand. 2017;61:580–89. doi: 10.1111/aas.12894. [DOI] [PubMed] [Google Scholar]
- 29.Weibel ER. Lung morphometry: The link between structure and function. Cell Tissue Res. 2017;367:413–26. doi: 10.1007/s00441-016-2541-4. [DOI] [PubMed] [Google Scholar]
- 30.Ando A, Farrell MJ, Mazzone SB. Cough-related neural processing in the brain: A roadmap for cough dysfunction? Neurosci Biobehav Res. 2014;47:457–68. doi: 10.1016/j.neubiorev.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Marseglia GL, Cirillo I, Vizzaccaro A, et al. Role of forced expiratory flow at 25–75% as an early marker of small airways impairment in subjects with allergic rhinitis. Allergy Asthma Proc. 2007;28:74–78. doi: 10.2500/aap.2007.28.2920. [DOI] [PubMed] [Google Scholar]
- 32.Cirillo I, Klersy C, Marseglia GL, et al. Role of FEF 25%–75% as a predictor of bronchial hyperreactivity in allergic patients. Ann Allergy Asthma Immunol. 2006;96:692–700. doi: 10.1016/S1081-1206(10)61067-8. [DOI] [PubMed] [Google Scholar]
- 33.Feng-Jia C, Xin-Yan H, Geng-Peng L, et al. Validity of fractional exhaled nitric oxide and small airway function indices in diagnosis of cough-variant asthma. J Asthma. 2018;55:750–55. doi: 10.1080/02770903.2017.1366509. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Liu W, Liu C, et al. Study on small airway function in asthmatics with fractional exhaled nitric oxide and impulse oscillometry. Clin Respir J. 2018;12:483–90. doi: 10.1111/crj.12548. [DOI] [PubMed] [Google Scholar]
- 35.Verbanck S, Schuermans D, Vincken W. Inflammation and airway function in the lung periphery of patients with stable asthma. J Allergy Clin Immunol. 2010;125:611–16. doi: 10.1016/j.jaci.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 36.Rytila R, Rehn T, Ilumets H, et al. Increased oxidative stress in asymptomatic current chronic smokers and GOLD stage 0 COPD. Respir Res. 2006;7:69. doi: 10.1186/1465-9921-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, Yan X, Xue C, et al. Association between increased small airway obstruction and asbestos exposure in patients with asbestosis. Clin Respir J. 2018;12:1676–84. doi: 10.1111/crj.12728. [DOI] [PubMed] [Google Scholar]