Abstract
Background
Influenza is associated with an increase in the risk of cardiac and other vascular events. Observational data and small randomized trials suggest that influenza vaccination may reduce such adverse vascular events.
Research Design and Methods
In a randomized controlled trial patients with heart failure are randomized to receive either inactivated influenza vaccine or placebo annually for 3 years. Patients aged ≥18 years with a clinical diagnosis of heart failure and NYHA functional class II, III and IV are eligible. Five thousand patients from 10 countries where influenza vaccination is not common (Asia, the Middle East, and Africa) have been enrolled. The primary outcome is a composite of the following: cardiovascular death, non-fatal myocardial infarction, non-fatal stroke and hospitalizations for heart failure using standardized criteria. Analyses will be based on comparing event rates between influenza vaccine and control groups and will include time to event, rate comparisons using Poisson methods, and logistic regression. The analysis will be conducted by intention to treat i.e. patients will be analyzed in the group in which they were assigned. Multivariable secondary analyses to assess whether variables such as age, sex, seasonality modify the benefits of vaccination are also planned for the primary outcome.
Conclusion
This is the largest randomized trial to test if influenza vaccine compared to control reduces adverse vascular events in high risk individuals.
Trial registration number
Background
Cardiovascular disease is a leading cause of death globally estimated to be responsible for ~ 17 million deaths annually.1 Cardiovascular disease and stroke account for nearly one third of all deaths and are a major cause of hospitalization.2., 3. Patients with congestive heart failure (CHF) are at particularly high risk with 50% dying within 5 years. An additional one third of patients with CHF will experience a myocardial infarction (MI), stroke, or hospitalization for CHF annually.4., 5., 6., 7., 8.
Observational studies have established an association between influenza infection and major adverse vascular events. Mechanisms that have been postulated to explain this increased risk after an influenza infection include the precipitation of plaque rupture,9 endothelial dysfunction,10., 11. reactivation of other latent infections leading to plaque rupture,12 fever-associated tachycardia,13 and metabolic derangements related to infection, including elevation of triglycerides and serum glucose levels.14., 15. It follows that vaccinating a high-risk group such as patients with CHF against influenza may prevent adverse vascular events. Studies examining influenza vaccination and vascular events however have shown conflicting results.16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27.
We conducted an observational study using databases from two large clinical trials,27 indicating that influenza vaccination may be associated with a reduction of major adverse vascular events. Using prospectively collected data from randomized controlled trials, we performed an analysis to determine the association between influenza vaccination and major adverse vascular events. The summary OR for the four adjusted odds ratios from the influenza seasons was 0.65 (95% CI 0.58-0.74, P < .001), but there was statistically significant heterogeneity (P = .003). There have been three recent studies, all using case only observational designs, which have reported a strong association between influenza and cardiac events.28., 29., 30.
There have been four systematic reviews of randomized trials on the effect of influenza vaccination on major adverse vascular events.31., 32., 33., 34. The most recent, an updated Cochrane review, included eight randomized trials, of which four focused specially on cardiovascular outcomes in patients within known cardiovascular disease while four focused on general populations where cardiovascular outcomes were included as part of the safety analyses.34 Data from three pooled RCTs comparing influenza vaccine to placebo or control demonstrate a relative risk of 0.63 (95% CI, 0.29-01.35) with respect to major adverse coronary events cardiovascular death was reported to be significantly reduced when four cardiovascular specific trials were pooled, relative risk 0.45, 95%CI 0.26 to 0.76). Of these four trials, only two had point estimates showing a risk reduction for cardiovascular death, the other two had point estimates close to 1. The three trials that reported cardiovascular mortality as part of safety analyses did not show differences but were underpowered due to low event rates.34 None of the included trials fulfilled all criteria with respect to low risk of bias. Together these results emphasize uncertainty of the clinical trial data and the need for higher quality evidence.
Because of the strong possibility of bias, results from previous observational studies and small trials need to be independently confirmed in a prospective, randomized trial. While several guidelines endorse influenza vaccination for patients with chronic cardiac disease, these guidelines are largely based on observational data and expert opinion, with data lacking from adequately powered, prospective, randomized trials. Therefore, it is not surprising that actual vaccination rates remain low. Clinical equipoise exists as to whether influenza vaccine in fact prevents cardiovascular events in patients with CHF. Consequently, a large randomized controlled trial with the power to detect or exclude moderate treatment effects is needed to reliably address the question. Given that 80% of CVD occur in populations in low- and middle-income countries, a trial with enrolment of patients from these countries have the potential to have an important global impact.
Trial design and methods
This study is a multi-center, randomized, placebo controlled, trial funded by the Joint Global Health Trials Scheme which in turn is funded by the UK Department for International Development, the Medical Research Council, the National Institute for Health Research and Wellcome Trust.
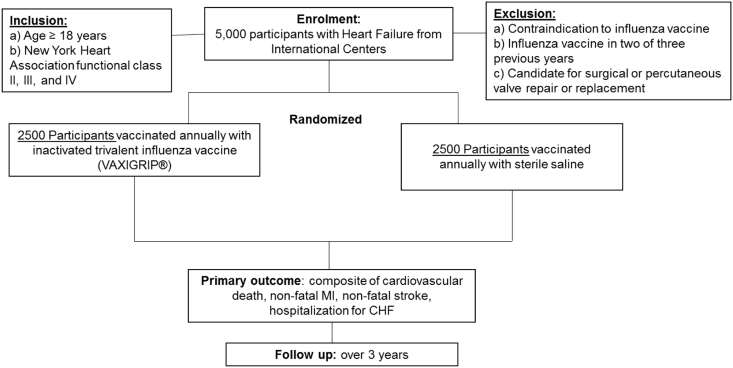
Participants at high-risk for vascular events are randomized to inactivated influenza vaccine (VAXIGRIP ®) or placebo and followed prospectively over three influenza seasons (Figure 1). The trial is being conducted by an international network of sites (Table I).
Figure 1.
Trial flow diagram.
Table I.
List of study countries and current enrollment figures by country as of January 21, 2019
| Country | Enrollment |
|---|---|
| Philippines | 708 |
| Uganda | 59 |
| Mozambique | 319 |
| Kenya | 115 |
| Zambia | 503 |
| Nigeria | 1011 |
| India | 1175 |
| China | 694 |
| Saudi Arabia | 311 |
| United Arab Emirates | 20 |
| Total | 4915 |
Although patients in the study were not randomly selected from the participating countries heart failure population, we believe that they are a reasonable representation based on study sites that include urban and rural centers as well as primary care practitioners and specialists, and sites from regions not previously well represented in the literature as we have previously established.35 The trial has been registered at Clinicaltrials.gov (NCT02762851). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Study population
The eligibility criteria are shown in Table II. Patients aged ≥18 years with a clinical diagnosis of heart failure and NYHA functional class II, III and IV are eligible. We decided to enroll heart failure patients because they area a high-risk population for heart disease as well as stroke. Moreover, this population allows for us to test whether influenza vaccination reduces hospitalization for heart failure both as part of the composite primary outcome as well as a secondary outcome alone. This is justified on the basis that it has been well documented that influenza is an important risk factor for heart failure hospitalization.36
Table II.
Eligibility criteria
| Inclusion | Exclusion |
|---|---|
| Age ≥18 years | Anaphylactic reaction to a previous dose of TIV |
| NYHA class II, III, and IV | Known IgE-mediated hypersensitivity to eggs manifested as hives, swelling of the mouth and throat, difficulty in breathing, hypotension, or shock |
| Guillain-Barré syndrome within eight weeks of a previous influenza vaccine | |
| Anaphylactic reaction to neomycin | |
| Patients who have had influenza vaccine in two of three previous years | |
| Patients who have had influenza vaccine in two of three previous years |
Patients are randomized to either influenza vaccine or placebo. Given that there is no evidence that two influenza vaccinations results in harm, participants can obtain influenza vaccination from their primary care physicians outside of the study if prescribed by them. Those with any of the following are excluded: a) anaphylactic reaction to a previous dose of influenza vaccine, b) known IgE-mediated hypersensitivity to eggs manifested as hives, swelling of the mouth and throat, difficulty in breathing, hypotension, or shock, c) Guillain-Barré syndrome within 8 weeks of a previous influenza vaccine, d) anaphylactic reaction to neomycin. We also exclude patients who have had influenza vaccine (by self-report) in two of the three previous years. This is because such patients are more likely to receive the influenza vaccine annually and will be more likely to request it outside of the trial. Patients with severe valvular disease that are candidates for surgical or percutaneous valve repair or replacement are also excluded.
Baseline characteristics of participants enrolled to date are shown in Table III.
Table III.
Baseline characteristics of 4871 participants⁎ enrolled as of January 14, 2019.
| Variable | |
|---|---|
| Mean Age (years) | 57.1 |
| 18–35 | 526/4907 (10.7%) |
| 36–64 | 2674/4907 (54.5%) |
| ≥ 65 | 1707/4907 (34.8%) |
| Sex (Female) | 2555/4907 (52.1%) |
| New York Heart Association Class for Heart Failure | |
| Class II | 3411/4907 (69.5%) |
| Class III | 1273/4907 (25.9%) |
| Class IV | 219/4907 (4.5%) |
| Prior Myocardial Infarction | 945/4878 (19.4%) |
| Depressed left ventricular Function |
3507/4641 (75.6%) |
| Prior Stroke | 391/4878 (8.0%) |
| Angiotensin converting Enzyme inhibitor |
Reduced Ejection Fraction 1736/3506 (49.5%) | Preserved Ejection Fraction 502/1376 (36.5%) |
|---|---|---|
| Beta blocker | 2232/3506 (63.7%) | 633/1376 (46.0%) |
| Diuretic | 2593/3506 (74.0%) | 581/1376 (42.2%) |
| Aldosterone inhibitor | 1954/3506 (55.7%) | 396/1376 (28.8%) |
Since data is still being entered, denominators vary.
Study objectives
The primary objective is to assess if influenza vaccination compared to placebo reduces a composite outcome of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke and hospitalizations for heart failure using standardized criteria. This is a clinically important outcome that is easily measured and one which has been used in large clinical trials of CVD and heart failure.37., 38., 39. This outcome is a composite of the most key outcomes relevant to what we are testing in the intervention.
The secondary objectives are to assess whether influenza vaccine reduces cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, hospitalizations for heart failure, all cause hospitalizations, and all cause death alone. In a sub-group of participants, the rates of influenza infection will be assessed using serology. This will be defined by ≥ 4-fold increase in influenza-specific hemagglutinin inhibition assay titer between baseline and end of season serum samples, taking into account the effect of the vaccine. The effect of influenza vaccination on Influenza-like illness (defined by cough and fever), lower respiratory tract infection (including exacerbation of chronic pulmonary disease) or pneumonia will also be assessed.
Study design
A multi-center, randomized, placebo controlled, trial. Participants at high-risk for vascular events in low to middle income countries will be randomized to inactivated influenza vaccine (VAXIGRIP ®) or placebo and followed prospectively over three influenza seasons. The primary outcome is a composite of cardiovascular (CV) death, non-fatal myocardial infarction (MI), non- fatal stroke, and hospitalization for CHF. We hypothesize that the intervention will lead to an 18 % risk reduction in the primary outcome. The study flow diagram is shown in Figure 1.
Study sites and enrollment
For each of the participating countries, one center serves as National Coordination Office (NCO). These NCOs are considered national centers of excellence in cardiovascular clinical trials and are led by experienced cardiologists who are leaders in conducting clinical trials. Each of these NCOs identified local sites for recruitment of participants in the clinical trial. The NCOs are in countries that all have a < 15% uptake of influenza vaccine, enhancing feasibility of this randomized trial which compares a strategy of routine use of the vaccine vs no routine use.
Randomization and blinding
Eligible and consenting participants are randomized to experimental and control arms using a central randomization system after patient identification and key clinical data are provided to a central web-based randomization service. The trial statistician generated a randomization list, blocked by site prior to the start of the study such that participants are assigned at random to one of the two study groups (influenza vaccine or placebo), in a 1:1 ratio. A web-based system for allocation of the vaccine was set up and shipping of the study vaccine (influenza vaccine or placebo) to study sites is coordinated by working with the vaccine manufacturer.
Concealment of allocation is done using a centralized web-based system. To maintain blinding of the participants, those allocated to placebo receive a 0.5ml sterile saline vaccine to mimic the influenza vaccine. Investigators, study coordinators, outcome adjudicators, and the data and safety monitoring board are blinded. The study vaccines are administered by an unblinded study nurse. The primary outcome of the trial is assessed by research staff at study centers blinded to allocation by direct communication with the participant (or next of kin) and review of medical records.
Study interventions
Experimental (inactivated influenza vaccine): Participants at high risk for adverse vascular events are immunized with inactivated trivalent influenza vaccine (VAXIGRIP® vaccine) recommended for the influenza season. A 0.5 ml dose of the vaccine is administered intramuscularly annually for three consecutive influenza seasons. The inactivated influenza vaccine is administered world-wide to prevent influenza and therefore was selected as the intervention. We selected VAXIGRIP® vaccine specifically because it is licensed in the countries selected as study sites. The use of the trivalent vaccine is pragmatic, that is, trivalent Vaxigrip is the most widely licensed influenza vaccine in participating countries. The quadrivalent vaccine adds a second lineage of B influenza. It is notable that there are no randomized trials of a head to head comparison between trivalent and quadrivalent vaccines. Furthermore, the extent to which influenza B contributes to cardiovascular events is unknown. The impact of the quadrivalent vaccine would only be during a year in which the B strain that is circulating is of a different lineage that the one in the trivalent vaccine.
Control (Saline): Participants at high risk for adverse vascular events will be immunized with sterile saline. A 0.5 ml dose is administered intramuscularly annually for three consecutive influenza seasons.
Safety monitoring
Reactogenicity events at the injection site (soreness at the site of injection, redness >2 cm, swelling >2 cm, and limitation of movement) are recorded. Each adverse event is graded for severity on a five-point scale and its relationship to study vaccine (associated or not associated) assessed by the study nurses and the investigators. We assess for immediate hypersensitivity allergic reactions by having the vaccination nurse monitor participants for 15 minutes following immunization, as is the general practice in public health units. Additionally, each participant that is vaccinated receives a symptom list to be checked daily for 5 days following vaccination. The major risk of influenza vaccine, although rare (1 per 200,000 doses), is an anaphylactic reaction, characterized by hives, swelling of mouth and throat, difficulty breathing, and low blood pressure. Such a rare event occurs immediately after injection. However, these adverse events are known to occur with influenza vaccination and would not be a problem that would interfere with implementing the trial.
Timing of vaccination
Participants are recruited and randomized prior to influenza season. There is considerable variability in the circulation of the influenza between and within the countries (e.g. China and India) participating in this trial.40., 41. We use data from WHO and from within the country to determine optimal timing of influenza vaccination (i.e. November/December or April/May). In some African countries, influenza circulates throughout the year. In these countries, participants are enrolled either in October to November (a “fall” cohort) and from April to May (a “spring” cohort). The start of vaccination is determined by the availability of the influenza vaccine. Formulations for either the Northern or Southern hemispheres are used.
Follow-up
Follow-up occurs every six months over the course of the study. Site investigators schedule clinic visits with participants to determine whether events occurred in the previous six months. When this is not feasible, a telephone call is scheduled. Where a clinic visit or telephone call with the participant is not feasible (e.g. death or hospitalization), next of kin are contacted.
Outcome measures
The primary outcome is a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke and hospitalizations for heart failure using standardized criteria. This is a clinically important outcome that is easily measured and one which we have extensive experience using in clinical trials. Heart failure diagnosis is captured by physician report. Cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, hospitalizations for heart failure, all cause hospitalizations, and all cause death, are secondary outcomes. In a sub-study, a sub-group of participants in selected countries are assessed for influenza infection using serology. There will be specimens from 2,000 participants in the serological sub-study. Using standardized criteria, all study outcomes will be adjudicated within each country/site by independent adjudicators who are blinded to treatment assignment.
Sample size and statistical analyses
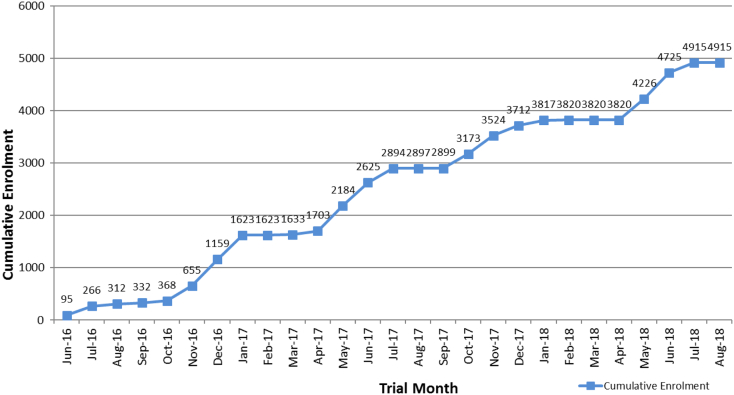
Event rates for this study are based on our clinical trial experience using similar composite primary outcome. In fact, the ONTARGET and TRANSCEND randomized controlled trials which provided observational data to support this proposed trial used adverse vascular events as a composite outcome. We estimate that using our primary outcome, which includes hospitalization for heart failure, 5000 participants will need to be enrolled and followed over 3 years. This is based on assumptions that consider the prevention of influenza by the vaccine. We first assume that 10% of adverse vascular events will be independent of influenza (250 events in group), leaving 2250 in each group if we conservatively count only first events. A 25% attack rate of influenza in the control group (based on clinical and serologically defined infection) equates to 562 cases and a similarly defined 15% attack rate in the vaccine group, conservatively assuming an influenza risk reduction of 40% with the vaccine, equates to 338 influenza cases in vaccine group. Assuming that 30% of these influenza infections result in adverse vascular events results in 168 and 101 adverse vascular events, respectively. Adding back the 250 adverse vascular events independent of influenza vaccination to each group leads to 418 adverse vascular events in the control group (418/2500 or 17%) and 351 adverse vascular events (351/2500 or 14%) in the vaccine group – for 83% power to detect this difference. Two meta-analyses are consistent in establishing the vaccine efficacy of the influenza vaccine as 60%, that is, a 40% risk reduction in preventing influenza.42., 43. Although there are no direct data that support the assumption that 30% of influenza infections in high risk patients lead to adverse vascular events, this figure is in keeping with what would be expected if the attributable effect of the influenza vaccine is due to prevention of influenza. Using the assumptions we present in the paper, the trial is powered to detect adverse vascular events of 17% in the control group to 14% in the influenza group. This is less than the risk reductions which have been predicted by meta-analyses of previously published randomized controlled trials.42., 43. Our experience to date suggests that receipt of vaccine outside of the study is negligible so would be expected to have no impact on control event rates or on sample size. Enrollment is shown in Figure 2.
Figure 2.
Cumulative enrollment as of September 1, 2018.
Analyses will be based on comparing event rates between influenza vaccine and control groups and will include time to event, rate comparisons using Poisson methods, and logistic regression. The analysis will be conducted by intention to treat i.e. patients will be analyzed in the group in which they were assigned. Multivariable secondary analyses where variables such as age, sex, seasonality are assessed independently as potential risk factors for the primary outcome and adjusted for in the analysis of the main effects (study vaccine) will be conducted. Influenza vaccines contain three antigens which change annually. Although there typically is a predominant strain that circulates, often there are co-circulating strains. Because the trial will be conducted over several seasons it will help ensure that the effect of a match between the vaccine strain and a circulating strain can be assessed. The analysis will be conducted at the end of the study period.
Ethical considerations
There is uncertainty about the benefit of influenza vaccination to prevent adverse vascular events. Observational studies cannot adequately address the question because of confounding. There is also uncertainty about the non-cardiovascular benefits of the influenza vaccine. A Cochrane review of influenza vaccine in persons >65 years provided evidence that although the vaccine could reduce influenza and influenza-like illness, there was insufficient data to make conclusions about death, pneumonia, or hospitalization.44 In order to reduce the possibility of randomizing participants who are routinely vaccinated against influenza, we excluded participants who received the influenza vaccine in two of the three previous years. We also allowed participants to receive influenza vaccine outside of the trial if they chose to do so. This way not only is the uptake of influenza vaccine increased in patients who otherwise would not be vaccinated, but in fact no one in the trial is denied vaccination. Ethics approval was obtained from the research ethics boards of all participating centers prior to the start of the study.
Data collection, management, and monitoring
Recruitment is based at centers in Asia, Africa and the Middle East, where it is not common to vaccinate routinely. Simple data collection forms are completed and submitted by the site using the internet version of the DataFax software. Data management for data control and completeness checks is performed centrally by the PHRI coordinating center by trained personnel. All data are kept secure and confidentiality of all study participants is carefully protected.The quality control process has been integrated into the overall data management process. Quality assurance or audit process is performed by staff at the coordinating center. A sample of participant records (10%) is audited quarterly using our participant record audit tool. This retrospective review will focus on the following indicators: consent forms; eligibility; vaccine administration and reactogenicity; adverse event/serious adverse event reporting; study endpoints; missed vaccinations and blood draws; signatures, as required; and study discontinuation. Regulatory records are audited annually using a regulatory file audit tool. The following indicators are included during this process: ethics approvals, safety reports, protocol and consent, sample CRFs, and monitoring reports.
Oversight for the trial is being conducted by a steering committee composed of the Principal Investigator and the National Leaders. A DSMB committee is responsible for safety oversight of the study (including monitoring of adverse reactions). The DSMB, composed of a cardiologist, an infectious disease physician and a biostatistician is responsible for making recommendations on safety issues, premature trial termination, and unblinding of study groups.
Funding source
The Joint Global Health Trials Scheme which is funded by the UK Department for International Development, the Medical Research Council, the National Institute for Health Research and Wellcome Trust. Sanofi Pasteur is providing the influenza vaccine for the trial.
Discussion
This randomized trial has important implications for the management of patients at high risk for major adverse vascular events. Although the influenza vaccine is recommended annually for groups with diabetes and cardiovascular disease in many counties, uptake of these recommendations is relatively low, perhaps because the current recommendations are not based on robust results from large well conducted RCTs. Cardiologists in most jurisdictions do not routinely recommend annual influenza vaccine for their patients as a strategy to reduce future adverse vascular events such as acute coronary syndrome or stroke. Apart from lack of evidence, cost, lack of physician reminders, and insurance coverage may be other reasons for this. Rigorous demonstration of influenza vaccine leading to a reduction in major adverse vascular events would represent a landmark study.
We anticipate that this trial has the potential to influence management decisions by physicians for patients at high risk for major vascular events. The effect size we are testing is comparable to secondary prevention strategies available and given the fact that a vaccine is given once annually it is simple and inexpensive. Given the large burden of disease, the possibility to reduce cardiovascular and stroke related death is a compelling argument for this trial. If influenza vaccine is shown to reduce adverse vascular events, it will represent an important change in how prevention of adverse vascular events is thought about. The fact that our primary outcome is a composite, including various forms of vascular disease, will potentially increase health benefit.
A unique feature of this randomized trial is that it is being conducted such in a broad and diverse population. The study is being conducted in regions where the epidemiology of influenza is highly variable and this has been taken into account with respect to timing of the influenza vaccine. There are two other large randomized trials assessing the effect of influenza vaccine on cardiovascular outcomes.45., 46. The INVESTED trial is comparing the effect of a high dose vaccine to a standard vaccine while the IAMI trial is randomizing patients with STEMI or non-STEMI undergoing coronary angiography to influenza vaccine or placebo. Both of these studies are being conducted in Western countries. Our trial is the largest placebo-controlled trial conducted to date and is unique in that it is being conducted in diverse geographical locations including Asia, Africa, and the Middle East. A recent study published in the Lancet on the global burden of influenza estimated that the greatest burden of death is in southeast Asia and Africa,47 both represented in our study. In many low and middle- income countries, the cost of influenza vaccine is prohibitive and as a result the vaccine is not made publicly available. Despite data suggesting that the greatest burden of illness is in southeast Asia and Africa, uptake of influenza vaccine remains low. The existence of competing risks for death, such as malaria or tuberculosis in Africa, emphasize that a significant effect of the influenza vaccine in reducing adverse vascular events will be compelling to policy-makers to enable access to influenza vaccine. Our findings will therefore have important implications for global health since the results will be generated in low- and middle-income countries and not extrapolated from highly developed countries. We recognize that we are not doing surveillance for influenza in this study as we do not have the resources. However, we will capture influenza-like illness that is self-reported, and we are doing serological sub-study to assess influenza illness.
Declaration of interest
Sanofi Pasteur has provided influenza vaccine in-kind for this study. Dr. Loeb has received research funding from Seqirus for an influenza vaccination trial. Dr. Loeb has received personal funding for consultation from both Sanofi and Seqirus as well as from WHO.
The other authors have no conflicts of interest to declare.
Acknowledgments
This trial is funded by the Joint Global Health Trials Scheme which in turn is funded by the UK Department for International Development, the Medical Research Council, and the National Institute for Health Research and Wellcome Trust.
Dr. Khalid Alhabib: Saudi Heart Association. The Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia (Research group number: RG -1436-013).
Appendix. List of Study Centers
| Study Center |
|---|
| Philippines |
| University of Philippines Manila-Philippine General Hospital |
| De La Salle Health Sciences Institute |
| East Avenue Medical Center |
| Ospital ng Makati |
| Uganda |
| Mulago Hospital |
| Mozambique |
| Department of Medicine, Faculty of Medicine, Eduardo Mondlane University |
| Instituto Nacional de Saúde |
| Kenya |
| Aga Khan University |
| Zambia |
| University of Zambia |
| Nigeria |
| Bayero University and Aminu Kano Teaching Hospital |
| Murtala Muhammed Specialist Hospital |
| Obafemi Awolowo University (OAU) and OAU Teaching Hospitals Complex |
| University College Hospital |
| India |
| All India Institute of Medical Sciences Dayanand Medical College and Hospital Eternal Heart Care Centre & Research Institute Govind Ballabh Pant Institute of Postgraduate |
| Indira Gandhi Medical College (IGMC) King George's Medical College |
| Medical Education and Research |
| China |
| FuWai Hospital |
| Shandong Academy of Medical Sciences |
| Hongqi Hospital of Mudanjiang Medical College |
| The Second Affiliated Hospital of Zhengzhou University |
| People's Hospital of Zunhua |
| Shenyang Dadong Wanghua Hospital |
| Saudi Arabia |
| King Fahad Cardiac Center |
| King Abdulaziz University Hospital |
| King Fahad Medical City |
| Madinah Cardiac Center |
| United Arab Emirates |
| Sheikh Khalifa Medical City |
References
- 1.Causes of death. World Health Organization; Geneva: 2008. http://www.who.int/healthinfo/global_burden_disease/cod_2008_sources_methods.pdf [Google Scholar]
- 2.Statistics Canada . 2008. Morality, Summary List of Causes. [Released October 18, 2011] [Google Scholar]
- 3.Tracking Heart Disease and Stroke in Canada. Released June 2009.
- 4.Remme W.J., Torp-Pedersen C., Cleland J.G. Carvedilol protects better against vascular events than metoprolol in heart failure: results from COMET. J Am Coll Cardiol. 2007;49(9):963–971. doi: 10.1016/j.jacc.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M., Böhm M., Greene S.J., ASTRONAUT Investigators and Coordinators Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 6.Massie B.M., Carson P.E., McMurray J.J. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 7.Swedberg K., Komajda M., Bohm M. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 8.Homma S., Thompson J.L., Pullicino P.M. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinlay S., Ganz P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am J Cardiol. 1997;80:11I–16I. doi: 10.1016/s0002-9149(97)00793-5. [DOI] [PubMed] [Google Scholar]
- 10.Chan N.N., Colhoun H.M., Vallance P. Cardiovascular risk factors as determinants of endothelium-dependent and endotheliumindependent vascular reactivity in the general population. J Am Coll Cardiol. 2001;38:1814-2. doi: 10.1016/s0735-1097(01)01669-2. [DOI] [PubMed] [Google Scholar]
- 11.Davies M.J. The composition of coronary-artery plaques. N Engl J Med. 1997;336:1312–1314. doi: 10.1056/NEJM199705013361809. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y.F., Wanishsawad C., Epstein S.E. Chlamydia pneumonia-induced transactivation of cytomegalovirus: potential synergy of infectious agents in the pathogenesis of atherosclerosis. J Am Coll Cardiol. 1999;33(suppl. A):260A. doi: 10.1086/315235. [DOI] [PubMed] [Google Scholar]
- 13.Conti C.R. Vascular events responsible for thrombotic occlusion of a blood vessel. Clin Cardiol. 1993;16:761–762. doi: 10.1002/clc.4960161103. [DOI] [PubMed] [Google Scholar]
- 14.Lundman P., Eriksson M., Schenck-Gustafsson K. Transient triglyceridemia decreases vascular reactivity in young, healthy men without risk factors for coronary heart disease. Circulation. 1997;96:3266–3268. doi: 10.1161/01.cir.96.10.3266. [DOI] [PubMed] [Google Scholar]
- 15.Kawano H., Motoyama T., Hirai N. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999;34:146–154. doi: 10.1016/s0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 16.Gwini S.M., Coupland C.A., Siriwardena A.N. The effect of influenza vaccination on risk of acute myocardial infarction: self-controlled case-series study. Vaccine. 2011;29(6):1145–1149. doi: 10.1016/j.vaccine.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Naghavi M., Barlas Z., Siadaty S. Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation. 2000;102:3039–3045. doi: 10.1161/01.cir.102.25.3039. [DOI] [PubMed] [Google Scholar]
- 18.Meyers D., Beahm D., Jurisich P. Influenza and pneumococcal vaccinations fail to prevent myocardial infarction. Heart Drug. 2004;4:96–100. [Google Scholar]
- 19.Lavellee P., Perchaud V., Gautier-Bertrand M. Association between influenza vaccination and reduced risk of brain infarction. Stroke. 2002;33:513–518. doi: 10.1161/hs0202.102328. [DOI] [PubMed] [Google Scholar]
- 20.Siscovick D., Raghunathan T., Lin D. Influenza vaccination and the risk of primary cardiac arrest. Am J Epidemiol. 2000;152:674–677. doi: 10.1093/aje/152.7.674. [DOI] [PubMed] [Google Scholar]
- 21.Siriwardena A., Gwini S., Coupland C. Influenza vaccination, pneumococcal vaccination and risk of acute myocardial infarction: matched case-control study. CMAJ. 2010;182:1617–1623. doi: 10.1503/cmaj.091891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heffelfinger J., Heckbert S., Psaty B. Influenza vaccination and risk of incident myocardial infarction. Hum Vaccin. 2006;2:161–166. doi: 10.4161/hv.2.4.2943. [DOI] [PubMed] [Google Scholar]
- 23.Nichol K., Nordin J., Mullooly J. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 24.Jackson L., Yu O., Heckbert S. Influenza vaccination is not associated with a reduction in the risk of recurrent coronary events. Am J Epidemiol. 2002;156:634–640. doi: 10.1093/aje/kwf073. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong B., Mangtani P., Fletcher A. Effect of influenza vaccination on excess deaths occurring during periods of high circulation of influenza: cohort study in elderly people. BMJ. 2004;329:660–663. doi: 10.1136/bmj.38198.594109.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C., Wang S., Lai C. Impact of influenza vaccination on major cause-specific mortality. Vaccine. 2007;25:1196–1203. doi: 10.1016/j.vaccine.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone J., Loeb M., Teo K.K. Influenza vaccination and major adverse vascular events in high-risk patients. Circulation. 2012;126(3):278–286. doi: 10.1161/CIRCULATIONAHA.111.071100. [DOI] [PubMed] [Google Scholar]
- 28.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N Engl J Med. 2018;378(26):2540–2541. doi: 10.1056/NEJMc1805679. [DOI] [PubMed] [Google Scholar]
- 29.Barnes M., Heywood A.E., Mahimbo A. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101(21):1738–1747. doi: 10.1136/heartjnl-2015-307691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warren-Gash C., Blackburn R., Whitaker H. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51(3) doi: 10.1183/13993003.01794-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller T., Weeds V., van Dongen C. Influenza vaccines for preventing coronary heart disease. Cochrane Database Syst Rev. 2008;3 doi: 10.1002/14651858.CD005050.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Warren-Gash C., Smeeth L., Hayward A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9:602–610. doi: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 33.Udell J.A., Zawi R., Bhatt D.L. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310:1711–1720. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 34.Clar C., Oseni Z., Flowers N. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst Rev. 2015;5 doi: 10.1002/14651858.CD005050.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dokainish H., Teo K., Zhu J. Heart failure in low- and middle-income countries: background, rationale, and design of the INTERnational Congestive Heart Failure Study (INTER-CHF) Am Heart J. 2015;170(4):627-634.e1. doi: 10.1016/j.ahj.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Fukuta H., Goto T., Wakami K. The effect of influenza vaccination on mortality and hospitalization in patients with heart failure: a systematic review and meta-analysis. Heart Fail Rev. 2018 doi: 10.1007/s10741-018-9736-6. [DOI] [PubMed] [Google Scholar]
- 37.ONTARGET Investigators, Yusuf S., Teo K.K. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 38.Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators, Yusuf S., Teo K. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372(9644):1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 39.Yusuf S., Pfeffer M.A., Swedberg K. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 40.Yu H., Alonso W.J., Feng L. Characterization of regional influenza seasonality patterns in China and implications for vaccination strategies: spatio-temporal modeling of surveillance data. PLoS Med. 2013;10(11) doi: 10.1371/journal.pmed.1001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chadha M.S., Potdar V.A., Saha S. Dynamics of influenza seasonality at sub-regional levels in india and implications for vaccination timing. PLoS ONE. 2015;10(5) doi: 10.1371/journal.pone.0124122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osterholm M.T., Kelley N.S., Sommer A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 43.Belongia E.A., Simpson M.D., King J.P. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–951. doi: 10.1016/S1473-3099(16)00129-8. [DOI] [PubMed] [Google Scholar]
- 44.Demicheli V., Jefferson T., Di Pietrantonj C. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2018;2 doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Vardeny O., Udell J.A., Joseph J. High-dose influenza vaccine to reduce clinical outcomes in high-risk cardiovascular patients: Rationale and design of the INVESTED trial. Am Heart J. 2018;202:97–103. doi: 10.1016/j.ahj.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fröbert O., Götberg M., Angerås O. Design and rationale for the Influenza vaccination After Myocardial Infarction (IAMI) trial. A registry-based randomized clinical trial. Am Heart J. 2017;189:94–102. doi: 10.1016/j.ahj.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 47.GBD 2017 Influenza Collaborators Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;7(1):69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]