Abstract
Background: The rise in serum ferritin levels among US maintenance hemodialysis patients has been attributed to higher intravenous iron administration and other changes in practice. We examined ferritin trends over time in hemodialysis patients and whether iron utilization patterns and other factors [erythropoietin-stimulating agent (ESA) prescribing patterns, inflammatory markers] were associated with ferritin trajectory.
Methods: In a 5-year (January 2007–December 2011) cohort of 81 864 incident US hemodialysis patients, we examined changes in ferritin averaged over 3-month intervals using linear mixed effects models adjusted for intravenous iron dose, malnutrition and inflammatory markers. We then examined ferritin trends across strata of baseline ferritin level, dialysis initiation year, cumulative iron and ESA use in the first dialysis year and baseline hemoglobin level.
Results: In models adjusted for iron dose, malnutrition and inflammation, mean ferritin levels increased over time in the overall cohort and across the three lower baseline ferritin strata. Among patients initiating dialysis in 2007, mean ferritin levels increased sharply in the first versus second year of dialysis and again abruptly increased in the fifth year independent of iron dose, malnutrition and inflammatory markers; similar trends were observed among patients who initiated dialysis in 2008 and 2009. In analyses stratified by cumulative iron use, mean ferritin increased among groups receiving iron, but decreased in the no iron group. In analyses stratified by cumulative ESA dose and baseline hemoglobin, mean ferritin increased over time.
Conclusions: While ferritin trends correlated with patterns of iron use, increases in ferritin over time persisted independent of intravenous iron and ESA exposure, malnutrition and inflammation.
Keywords: ferritin, hemodialysis, iron, longitudinal trends
INTRODUCTION
As the main storage molecule for iron [1, 2], serum ferritin is a protein related to both iron and oxygen metabolism [3], and it is widely used as a parameter to screen for iron deficiency and overload in chronic kidney disease (CKD) patients [4]. Intravenous (IV) iron is one of the most important pharmacotherapies used to treat anemia in CKD patients, and studies have shown that its utilization has substantially increased in most countries over time [5–7]. These trend reports have also shown that serum ferritin levels have concurrently increased over time [5, 6].
Since publication of the 2006 Kidney Disease Outcomes Quality Initiative (KDOQI) Anemia Guidelines, the practice of anemia management has substantially changed in the USA [8]. In 2012, the Kidney Disease Improving Global Outcomes (KDIGO) group issued updated guidelines on anemia management incorporating some of these changes, which includes consideration of hemoglobin levels when determining the dose of IV iron administration [4]. In parallel with these changes, the United States Renal Data System (USRDS) Annual Data Report showed that mean serum ferritin levels remained stable at ∼600 ng/mL from 2001 to 2006, but rose to 800 ng/mL in 2011 in prevalent maintenance hemodialysis (HD) patients [9, 10]. In addition, Dialysis Outcomes and Practice Patterns Study (DOPPS) data have also shown that median serum ferritin levels rose from 561 ng/mL in 2010 to 794 ng/mL in 2012 in maintenance HD patients [11]. However, to date, no studies that have examined trends in serum ferritin levels in HD patients have accounted for changes in IV iron dosing over time, nor focused specifically upon incident HD patients. To address this knowledge gap, this study aimed to investigate changes in serum ferritin levels over time in a contemporary cohort of incident HD patients who were followed for up to 5 years over the period 2007–2011. We sought to examine serum ferritin level trends over time in HD patients and whether IV iron utilization patterns, other secular changes in practice [e.g. erythropoietin-stimulating agent (ESA) administration] and patient characteristics (e.g. inflammatory status) were associated with serum ferritin trends.
MATERIALS AND METHODS
Source cohort
We conducted analyses using administrative data from all incident HD patients who initiated dialysis over the period 1 January 2007–31 December 2011 in the outpatient dialysis facilities of a large dialysis organization. From January 2007 to December 2011, 208 820 patients initiated dialysis treatment. After excluding patients <18 years of age or those who received <60 days of thrice-weekly HD treatment at the time of study entry (first quarter of serum ferritin measurement), 133 156 incident HD patients remained. After excluding patients with missing sex information and missing baseline ferritin information, the final study population consisted of 81 684 incident HD patients (Supplementary data, Appendix Figure S1). The study was approved by the International Review Committees of the University of California, Irvine, Los Angeles Biochemical Research Institute at Harbor-UCLA and the University of Washington. Given the large sample size, anonymity of the patients studied and nonintrusive nature of the research, the requirement for written consent was exempted.
Demographic and clinical measures
The construction of the cohort has previously been described [12]. Patients' follow-up time was divided into 91-day intervals (patient quarters) from the time of the patients' first dialysis treatment. To minimize measurement variability, all repeated measures for each patient during any given patient quarter (i.e. 13-week intervals) were averaged and summary estimates were used in all models. Information on race/ethnicity, primary insurance, access type and the presence of diabetes at baseline were obtained from the large dialysis organization database. The following 16 coexisting comorbidities were considered: diabetes, hypertension, cystic kidney disease, autoimmune disease, dyslipidemia, chronic obstructive pulmonary disease, liver disease, atherosclerotic heart disease, other cardiac disease (pericarditis and cardiac arrhythmia), congestive heart disease, cerebrovascular disease, malignancy, thyroid disorders, human immunodeficiency virus, substance use and alcohol dependence.
Laboratory measures
Blood samples were drawn using standardized techniques in all dialysis clinics and were transported to a central laboratory in Deland, FL, USA, typically within 24 h. Most laboratory values were measured monthly, including serum creatinine, albumin, hemoglobin, platelet count, peripheral white blood cell count, lymphocyte percentage, total iron binding capacity (TIBC), calcium, phosphorus, bicarbonate and alkaline phosphatase. Serum intact parathyroid hormone (PTH) and ferritin levels were usually measured at least once during each calendar quarter. Most blood samples were collected before dialysis, except for postdialysis blood urea nitrogen to calculate urea kinetics. The normalized protein catabolic rate was measured monthly as an indicator of daily protein intake. Dialysis dose was estimated by single-pool Kt/V using the urea kinetic model. The 3-month-averaged values during the first quarter of dialysis treatment were used as baseline values to address measurement variability in laboratory parameters. We categorized patients into four strata according to baseline serum ferritin level: <200, 200–<500, 500–<800 and ≥800 ng/mL.
Statistical methods
Baseline characteristics across the four strata of baseline ferritin were summarized as proportions, means ± SD or medians (interquartile ranges). Changes in ferritin over time (over a period of up to 20 patient-quarters) were examined in 81 684 patients who had serum ferritin measurements from at least 3 patient-quarters. To estimate predictor variables associated with predicted serum ferritin levels versus time, we used linear mixed effects models [13]. We included fixed effect terms for patient calendar quarter of dialysis initiation, as well as case-mix and malnutrition-inflammation-cachexia syndrome (MICS) covariates. Case-mix covariates included age, sex, race/ethnicity, diabetes mellitus, primary insurance, vascular access type and single-pool Kt/V as well as the 15 underlying comorbidities described above (see Demographic and clinical measures). MICS variables included 12 surrogates of nutritional and inflammatory status: serum creatinine, hemoglobin, albumin, peripheral white blood cell, lymphocyte percentage, TIBC, calcium, phosphorus, bicarbonate, intact PTH, body mass index and normalized protein catabolic rate. In sensitivity analyses, we incrementally adjusted for IV iron dose as well as ESA dose. To account for the within-patient correlation of these laboratory measurements over time, we included a random effects term of patient quarter. The models were fit using MIXED in STATA MP version 13.1 (StataCorp, College Station, TX, USA). To address potential biases due to right censoring, ferritin slopes over patient time (Δ ng/mL/quarter) were estimated in the overall cohort as well as among a subcohort of patients who survived the first year of dialysis. Trends in serum ferritin were examined across strata of year of dialysis initiation, cumulative IV iron and ESA utilization in the first year of dialysis and baseline hemoglobin level.
RESULTS
Study population
Table 1 shows the baseline characteristics of the overall cohort and across strata of baseline serum ferritin category (<200, 200–<500, 500–<800 and ≥800 ng/mL). Patients whose baseline ferritin was in the highest category (≥800 ng/mL) were more likely to be female and were less likely to be white or Hispanic; had a lower prevalence of diabetes, congestive heart failure, hypertension, atherosclerotic heart disease, cystic kidney disease and dyslipidemia; had higher PTH and serum phosphorus levels and had lower serum calcium, hemoglobin and TIBC levels. Patients whose baseline ferritin was in the lowest category (<200 ng/mL) tended to have more favorable markers of nutritional and inflammatory status (e.g. higher serum albumin, creatinine and normalized protein catabolic rate).
Table 1.
Baseline characteristics stratified by baseline serum ferritin in 81 684 incident hemodialysis patients
| Characteristics | Total (n = 81 684) | Baseline serum ferritin |
P-value | |||
|---|---|---|---|---|---|---|
| <200 (n = 29 067) | 200–<500 (n = 35 012) | 500–<800 (n = 11 158) | ≥800 (n = 6447) | |||
| Age (years) | 62 ± 15 | 60 ± 15 | 63 ± 15 | 64 ± 14 | 64 ± 15 | <0.001 |
| Female (%) | 44 | 42 | 43 | 46 | 49 | <0.001 |
| Diabetes mellitus (%) | 63 | 65 | 64 | 62 | 56 | <0.001 |
| Race/ethnicity (%) | ||||||
| White | 44 | 45 | 44 | 42 | 40 | <0.001 |
| African American | 33 | 31 | 33 | 35 | 39 | <0.001 |
| Hispanic | 16 | 18 | 16 | 14 | 12 | <0.001 |
| Asian | 3.4 | 2.5 | 3.4 | 4.5 | 5.4 | <0.001 |
| Others | 3.8 | 3.7 | 3.8 | 4 | 4.3 | 0.069 |
| Primary insurance (%) | ||||||
| Medicare | 53 | 52 | 53 | 55 | 57 | <0.001 |
| Medicaid | 7.2 | 7.8 | 6.9 | 7 | 7 | <0.001 |
| Others | 40 | 41 | 40 | 38 | 36 | <0.001 |
| Access type (%) | ||||||
| CVC | 73 | 70 | 74 | 76 | 79 | <0.001 |
| AVF | 16 | 20 | 15 | 12 | 9 | <0.001 |
| AVG | 4.4 | 4.5 | 4.5 | 4.3 | 3.7 | 0.003 |
| Others | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.689 |
| Unknown | 6.6 | 6.5 | 6 | 7.2 | 8.8 | <0.001 |
| KRU (mL/min) | 3.44 (1.93–5.56) | 3.84 (2.23–6.06) | 3.42 (1.95–5.47) | 2.98 (1.58–5.04) | 2.70 (1.34–4.53) | <0.001 |
| spKt/V | 1.47 ± 0.32 | 1.44 ± 0.32 | 1.47 ± 0.31 | 1.51 ± 0.32 | 1.52 ± 0.33 | <0.001 |
| Comorbidities (%) | ||||||
| Hypertension | 52 | 53 | 53 | 51 | 49 | <0.001 |
| Cystic kidney disease | 2.5 | 2.9 | 2.4 | 1.9 | 1.6 | <0.001 |
| CHF | 42 | 43 | 41 | 39 | 39 | <0.001 |
| ASHD | 16 | 16 | 16 | 15 | 14 | 0.036 |
| Other cardiac disease | 16 | 16 | 16 | 16 | 16 | 0.294 |
| CVD | 1.8 | 1.7 | 1.9 | 1.8 | 1.7 | 0.158 |
| COPD | 5.1 | 5 | 5 | 5.3 | 5.4 | 0.387 |
| Liver disease | 1.4 | 1.3 | 1.4 | 1.5 | 2 | <0.001 |
| Thyroid disease | 11 | 11 | 10 | 11 | 10 | 0.032 |
| Dyslipidemia | 28 | 28 | 28 | 27 | 26 | 0.001 |
| Autoimmune disease | 2 | 1.6 | 1.9 | 2.4 | 3.6 | <0.001 |
| Malignancy | 2.1 | 1.6 | 2 | 2.2 | 4 | <0.001 |
| HIV | 0.5 | 0.3 | 0.5 | 0.7 | 1.2 | <0.001 |
| Substance abuse | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | 0.824 |
| Alcohol abuse | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.032 |
| BMI (kg/m2) | 28.1 ± 7.3 | 29.0 ± 7.5 | 28.3 ± 7.3 | 27.2 ± 6.8 | 26.3 ± 6.7 | <0.001 |
| Serum Alb (g/dL) | 3.53 ± 0.46 | 3.60 ± 0.43 | 3.54 ± 0.45 | 3.45 ± 0.48 | 3.32 ± 0.52 | <0.001 |
| Serum Cr (mg/dL) | 6.02 ± 2.36 | 6.07 ± 2.30 | 6.06 ± 2.40 | 5.89 ± 2.38 | 5.80 ± 2.37 | <0.001 |
| Bicarbonate (mEq/L) | 23.48 ± 2.67 | 23.32 ± 2.67 | 23.52 ± 2.63 | 23.65 ± 2.69 | 23.67 ± 2.76 | <0.001 |
| Hb (g/dL) | 11.24 ± 1.15 | 11.35 ± 1.10 | 11.31 ± 1.11 | 11.09 ± 1.18 | 10.67 ± 1.32 | <0.001 |
| WBC (mm3) | 7.74 ± 2.54 | 7.56 ± 2.51 | 7.73 ± 2.41 | 7.99 ± 2.65 | 8.20 ± 3.06 | <0.001 |
| Lymphocyte (% of WBC) | 21.03 ± 7.36 | 21.46 ± 7.18 | 21.08 ± 7.28 | 20.35 ± 7.44 | 19.96 ± 8.15 | <0.001 |
| Platelet (×109/L) | 251.68 ± 85.90 | 253.88 ± 83.59 | 250.97 ± 82.68 | 252.18 ± 85.56 | 247.74 ± 106.53 | <0.001 |
| iPTH (pg/mL) | 322 (206–494) | 341 (223–522) | 323 (208–493) | 298 (188–456) | 272 (160–434) | <0.001 |
| ALP (µ/L) | 87 (69–113) | 85 (68–110) | 86 (69–113) | 87 (70–118) | 94 (72–128) | <0.001 |
| Calcium (mg/dL) | 9.09 ± 0.56 | 9.07 ± 0.56 | 9.09 ± 0.55 | 9.12 ± 0.56 | 9.15 ± 0.60 | <0.001 |
| Phosphorus (mg/dL) | 4.99 ± 1.14 | 5.14 ± 1.14 | 4.98 ± 1.13 | 4.81 ± 1.11 | 4.67 ± 1.13 | <0.001 |
| Ferritin (ng/mL) | 268 (156–458) | 126 (86–163) | 309 (249–388) | 611 (550–689) | 1035 (895–1312) | <0.001 |
| TIBC (mg/dL) | 226.81 ± 47.36 | 247.59 ± 44.34 | 223.03 ± 42.77 | 206.08 ± 44.02 | 189.61 ± 45.99 | <0.001 |
| ISAT (%) | 22.91 ± 8.50 | 20.68 ± 7.07 | 23.08 ± 7.47 | 24.59 ± 8.85 | 29.12 ± 13.67 | <0.001 |
| nPCR (g/kg/day) | 0.80 ± 0.21 | 0.81 ± 0.21 | 0.81 ± 0.21 | 0.80 ± 0.22 | 0.77 ± 0.24 | <0.001 |
| ESA dose (U/week)a | 0 (−3274–7471) | 115 (−3252–7837) | −1 (−3273–7287) | −123 (−3325–7239) | −163 (−3346–7287) | 0.016 |
| Iron dose (mg/month)b | 0 (−183–317) | +34 (−100–200) | 0 (−150–167) | −66 (−258–100) | −300 (−333 to −33) | <0.001 |
| Iron use (%) | 89 | 94 | 93 | 86 | 56 | <0.001 |
Continuous values are expressed as mean ± SD if normally distributed or median (interquartile range) if skewed.
CVC, central venous catheter; AVF, arteriovenous fistula; AVG, arteriovenous graft; KRU, kidney residual urine; spKt/V, single-pool Kt/V; CHF, congestive heart failure; ASHD, atherosclerotic heart disease; CVD, cerebrovascular disease; COPD, chronic obstructive pulmonary disease; BMI, body mass index; Alb, albumin; Hb, hemoglobin; WBC, white blood cell; iPTH, intact parathyroid hormone; ALP, alkaline phosphatase; TIBC, total iron binding capacity; ISAT, iron saturation; nPCR, normalized protein catabolic rate; ESA, erythropoietin-stimulating agent.
aDenotes the ESA dose relative to the median dose of the overall cohort.
bDenotes the iron dose relative to the median dose of the overall cohort.
Ferritin trends according to baseline ferritin strata
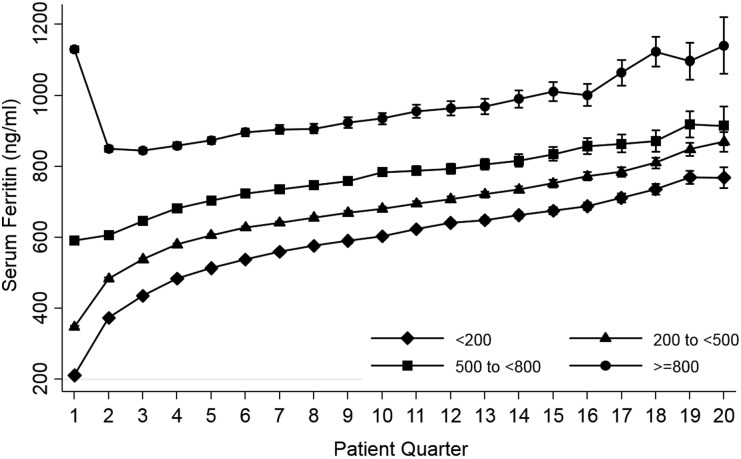
Across the four categories of baseline serum ferritin, ferritin levels increased in parallel over time in models adjusted for case-mix + MICS covariates as well as those incrementally adjusted for IV iron (Figure 1) and ESA dose (Supplementary data, Appendix Figure S2). The mean change in serum ferritin level per quarter following dialysis initiation (designated as ‘patient-quarter’) was 29.7 [95% confidence interval (CI) 29.2–30.2], 32.9 (95% CI 32.4–33.5) and 19.4 (95% CI 18.4–20.5) ng/mL/patient-quarter among patients whose baseline ferritin levels were <200, 200–<500 and 500–<800 ng/mL, respectively. However, among patients whose baseline ferritin was ≥800 ng/mL, mean ferritin levels initially decreased over time: −7.5 (95% CI −9.6 to −5.3) ng/mL/patient-quarter. Among these patients, the mean serum ferritin level decreased from 1133.8 (95% CI 1125.6–1141.9) ng/mL in the first patient-quarter to 847.0 (95% CI 838.6–855.4) ng/mL in the third patient-quarter. From the fourth patient-quarter onward, serum ferritin levels increased over time as observed in the rest of the baseline ferritin strata. Overall slopes of mean ferritin level changes per patient-quarter were similar in the overall cohort versus those who survived the first year of dialysis (24.4 versus 24.0 ng/mL/quarter; Supplementary data, Appendix Table S1).
FIGURE 1.
Mean serum ferritin per patient-quarter over 5 years according to four categories of baseline ferritin in case-mix + MICS– and IV iron–adjusted model.
Ferritin trends according to year of dialysis initiation
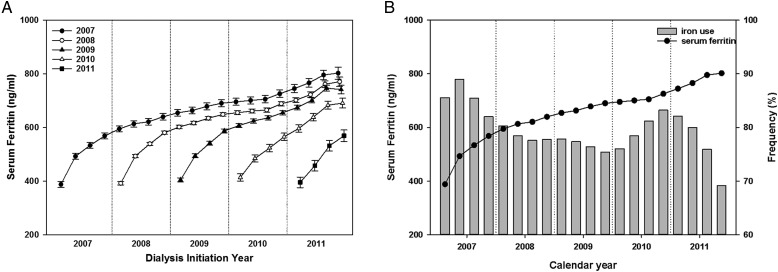
To further evaluate secular changes in ferritin level over time, all patients were then stratified according to their year of dialysis initiation. The baseline characteristics of patients according to dialysis initiation year are shown in Supplementary data, Appendix Table S2. Among these subgroups there were no significant differences in age, sex or race/ethnicity, except in the proportion of Asians. Baseline mean ferritin levels were incrementally higher with each subsequent year of dialysis initiation: 387.8 (95% CI 376.4–399.2), 391.8 (95% CI 385.7–397.9), 405.0 (95% CI 397.7–412.3) and 413.9 (95% CI 400.4–427.4) ng/mL among patients who initiated dialysis in 2007, 2008, 2009 and 2010, respectively (Figure 2A). Among patients who started dialysis in 2007, serum ferritin levels increased sharply in the first year of HD [mean slope 56.4 (95% CI 54.1–58.7) ng/mL/year] compared with the second year of HD [mean slope 13.7 (95% CI 11.4–15.9) ng/mL/year] in case-mix + MICS– and IV iron–adjusted linear mixed models (Supplementary data, Appendix Table S3). The ferritin slope again sharply increased in the fifth year of HD [mean slope 20.1 (95% CI 14.9–25.3) ng/mL/year] (Supplementary data, Appendix Table S3). Changes in ferritin level over time among patients who started dialysis in 2008 and 2009 were similar in pattern to those who started dialysis in 2007. Figure 2B concomitantly shows the trajectory of ferritin level and the prevalence of IV iron use among patients who initiated HD in 2007. Among these patients, the prevalence of IV iron use exceeded 80% in the first year of HD and fell to <80% from the second year to the first half of their fourth year of HD. From the second half of their fourth year of HD, the frequency of IV iron use again rose to >80% and remained elevated for 1 year. During periods of higher IV iron use, there was a steeper rise in mean ferritin level over time except in the fifth year of HD.
FIGURE 2.
(A) Mean serum ferritin in each year from 2007 to 2011 in case-mix + MICS– and IV iron–adjusted models. (B) Ferritin trajectory and the prevalence of IV iron use in patients who started HD in 2007.
Ferritin trends according to IV iron and ESA utilization
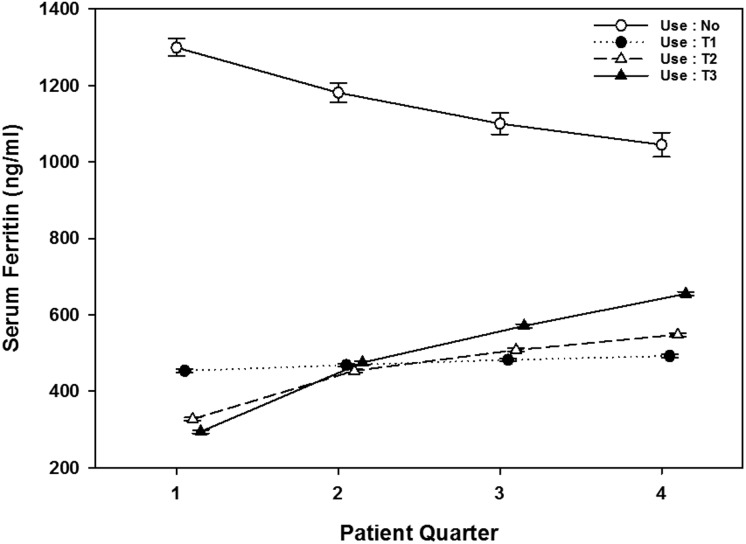
We examined the trajectory of ferritin levels over time among patients divided into four strata of cumulative IV iron utilization during their first year of dialysis, defined as no IV iron use versus the lowest, middle and highest tertiles of cumulative IV iron use (T1, T2 and T3, respectively). Figure 3 shows that the mean serum ferritin levels increased over time across all three groups prescribed IV iron, with steeper rises observed in those with higher IV iron utilization. In contrast, mean serum ferritin levels gradually decreased over time in the no iron group: −78 (95% CI −108.1 to −49.1) ng/mL/patient-quarter after adjustment for case-mix + MICS covariates.
FIGURE 3.
Mean serum ferritin by iron use (by tertile) versus no use during the first dialysis year in case-mix + MICS–adjusted models. No, no iron use; T1, lowest iron dose; T2, middle iron dose; T3, highest iron dose.
We also evaluated changes in serum ferritin over time among patients divided into four strata of cumulative ESA dose during the first year of dialysis, divided into quartiles. Notably, we observed that only 94 patients in the cohort had not received ESA in the first year of dialysis. Given the small sample size, the non-ESA users were included in the lowest quartile of ESA use. We observed that serum ferritin levels increased over time in the first year of dialysis across all ESA strata (Supplementary data, Appendix Figure S3). However, compared with the highest ESA quartile, there was a sharper rise in ferritin during the first to second quarter in the three lowest ESA quartiles.
Ferritin trends according to baseline hemoglobin level
We also examined serum ferritin trends over time among patients divided by baseline hemoglobin levels (<9, 9–<10, 10–<11, 11–<12 and ≥12 g/dL). In models adjusted for case-mix + MICS covariates as well as IV iron and ESA dose, we observed that patients with baseline hemoglobin levels <9 g/dL had comparatively higher baseline serum ferritin levels compared with all other baseline hemoglobin categories (600 versus ∼350–400 ng/mL, respectively) (Supplementary data, Appendix Figure S4). However, serum ferritin levels increased over time at similar rates across all baseline hemoglobin strata.
DISCUSSION
Among a contemporary cohort of >80 000 incident HD patients who were followed for up to 5 years, we found that serum ferritin levels increased sharply in the first year of dialysis treatment followed by more gradual increases from the second year of HD onwards. In analyses stratified by baseline ferritin level, serum ferritin levels rose over time across all subgroups except for those whose ferritin levels were ≥800 ng/mL. In analyses stratified according to year of dialysis initiation, those who started dialysis in 2007 experienced a particularly sharp increase in mean serum ferritin over the course of the first year of HD (56.4 ng/mL/year) compared with the second year of HD (13.7 ng/mL/year) in case-mix + MICS– and IV iron–adjusted models. We additionally observed that serum ferritin levels rose over time across strata of IV iron use, ESA dose and baseline hemoglobin levels.
Epidemiologic data have shown that from 2001 to 2006, mean serum ferritin levels remained stable at ∼600 ng/mL in US patients on maintenance HD [10, 14]. In 2006, KDOQI anemia guidelines recommended that serum ferritin and iron saturation levels be maintained at >200 ng/mL (with previous KDOQI guidelines recommending a threshold >100 ng/mL) and >20% (similar to prior KDOQI guidelines), respectively, in maintenance HD patients, with the stipulation that there is insufficient evidence for routine IV iron if ferritin is ≥500 ng/mL [8]. However, following publication of these guidelines, mean serum ferritin levels among prevalent HD patients in the USA rose from 586 ng/mL in 2007 to ∼850 ng/mL in 2012 [14]. In our study, we observed comparatively lower mean serum ferritin and iron saturation levels in our cohort measured at baseline: 268 ng/mL and 22.9%, respectively. Our study also showed that serum ferritin levels began to increase sharply during the first year of HD treatment compared with subsequent years of HD. This increase in ferritin occurred in parallel with the higher prevalence of IV iron use observed during the first versus second year of HD treatment among patients who initiated dialysis in 2007. The increase in serum ferritin over time from the start of maintenance HD therapy did not attenuate over the 5 years of follow-up.
Compared with the KDOQI guidelines, the 2012 KDIGO anemia guidelines recommended a higher threshold of iron saturation (iron saturation <30%) for initiation of iron supplementation as well as an upper ferritin bound (serum ferritin <500 ng/mL) for iron supplementation in HD patients [4]. The Dialysis Patients' Response to IV Iron with Elevated Ferritin I and II trials have previously demonstrated the efficacy of IV iron in reducing ESA dose and improving hemoglobin response in HD patients with ferritin levels >500 ng/mL and iron saturation ≤25% [15]. However, given the select population of randomized controlled participants, it is unclear whether these findings are generalizable to the greater US HD population. With regards to safety considerations, a recent observational study observed that although IV iron was not related to all-cause and cardiovascular mortality, it was indeed associated with a trend towards higher infection-related death risk [16]. In another large cohort study, IV iron administration >400 mg/month was associated with higher mortality in maintenance HD patients [17]. In our cohort, we observed that IV iron was administered in 86 and 56% of patients with baseline serum ferritin levels of 500–<800 ng/mL and >800 ng/mL at the start of dialysis. At this time, further studies are needed to determine the target serum ferritin ranges and IV iron doses associated with optimal survival in well-characterized observational cohorts of maintenance HD patients with long-term follow-up.
In our study, among patients who initiated dialysis in 2007, serum ferritin levels sharply rose in the first year of HD and thereafter exhibited a more gradual increase in subsequent years, although serum ferritin levels increased somewhat more sharply again in the fifth year of dialysis. It has been suggested that upward trends in serum ferritin levels may be due to more conservative hemoglobin targets and ESA prescribing patterns and disincentives to administer expensive intradialytic medications, leading to greater IV iron administration [18]. It has also been suggested that lower hemoglobin targets may result in a greater proportion of iron being transferred from the erythrocyte to storage tissues. Without any changes in IV iron, it would have been expected that storage iron would have increased and serum ferritin would have increased. For example, following publication of the CREATE, CHOIR and TREAT studies demonstrating the toxicities of higher hemoglobin targets with ESA administration [19–21], the US Food and Drug Administration issued warnings in June 2011 of the risks of ESA overuse and recommended that ESAs only be initiated when hemoglobin is <10 g/dL in CKD patients [22]. In January 2011, the Centers for Medicare and Medicaid Services implemented the ESRD Prospective Payment System, a ‘bundled’ case-payment method to reduce utilization of high-cost medication like ESAs in outpatient dialysis centers [23]. These changes may have contributed to greater use of IV iron use in lieu of ESAs, from 61% in August 2010 to 73% in April 2011 [24]. However, since August 2011, IV iron use has declined steadily to 62% by the second half of 2012, as in prior years [9]. International data show similar practice pattern changes, and in a Swedish study of dialysis patients, it was shown that the frequency of ESA administration and mean ESA dose declined over the period of 2006–12 (coincident with release of the KDOQI and KDIGO anemia guidelines, respectively) [18]. In addition, patients without ESA use had greater iron utilization over time, and those who received the lowest ESA doses tended to have the highest serum ferritin levels in the first year of dialysis. Notably, our study demonstrated that serum ferritin levels increased over time in dialysis patients even after accounting for ESA dose through multivariable adjustment and stratification. However, in the ESA-stratified analyses, we did observe a sharper rise in ferritin during the first to second quarter in the three lowest ESA versus highest ESA strata. In analyses stratified by baseline hemoglobin level, while baseline ferritin levels were higher in the lowest hemoglobin versus higher hemoglobin categories, serum ferritin levels increased at similar rates across all hemoglobin strata independent of case-mix + MICS covariates, IV iron and ESA dose.
Ferritin is the representative protein that stores and releases iron [25]. While lower serum ferritin levels typically indicate iron deficiency, higher levels may represent both iron overload as well as the presence of malnutrition and inflammation complex syndrome [21, 26]. In a recently published randomized trial, 75% of patients randomized to the high ferritin group maintained target ferritin levels of 400–600 ng/mL throughout the 56-week study period after receiving only four doses of IV iron [27]. DOPPS data have also shown that IV iron use increased from 57 to 73% and that median serum ferritin increased from 561 to 794 ng/mL from August 2010 to December 2012 [11]. However, there were interval differences between the increase in IV iron use and increases in serum ferritin. Even if IV iron doses rose sharply at the end of 2010 and remained elevated for 1 year, serum ferritin levels had already begun to rise sharply in mid-2010 before increases in IV iron dose, and remained high >1 year after reduction in IV iron doses [28]. This discrepancy between iron dose and serum ferritin levels was observed in national data collected in England as well [29]. There are limitations in accurately determining the distribution of iron and the degree of iron overload in dialysis patients [14]. To assess iron load in dialysis patients, liver biopsy, bone marrow staining or liver iron measurement using magnetic resonance imaging may be considered, but their use remains limited in routine clinical practice [1, 28]. The 2012 KDIGO Anemia Guidelines recommend considering the patients' clinical status as well as serum ferritin and iron saturation levels when iron treatment is prescribed [4]. In our study, we observed that trends in serum ferritin level correlated with patterns of IV iron use over time. However, we also observed that serum ferritin levels rose over time after adjusting for IV iron use and MICS covariates and that there was an upward trend in ferritin levels even during periods of reduced IV iron use. Taken together, these data suggest that serum ferritin trends are not purely influenced by the load of administrated IV iron or malnutrition/inflammatory markers, and further studies of the determinants of iron parameter trends in HD patients are needed.
There are several strengths of our study. To our knowledge, this is the first study examining trends in serum ferritin and IV iron administration over time using time-dependent multilevel linear mixed models. In addition, this study was conducted in a contemporary cohort of incident HD patients, allowing us to examine changes in serum ferritin during the first several years after dialysis initiation.
Our study also has several limitations. First, although many potential confounders were accounted for, C-reactive protein as an inflammatory marker used to better ascertain functional iron deficiency was not included. However, we did account for a number of inflammatory markers, including serum albumin, peripheral white blood cell count and lymphocyte percentage [29–31], and adjusted for IV iron dose as a more direct modulator of iron stores. Second, we lacked data on certain factors that may have influenced the serum ferritin trajectory, such as the timing of IV iron administration relative to the timing of serum ferritin measurement, as well as receipt of blood transfusions. Third, while the bundled case-payment method was implemented in January 2011, our study examines data collected over the period of 1 January 2007–31 December 2011 and thus examines only 1 year of data in the postbundling era. While it is possible that there were changes in the practice patterns involving ESA dosing and hemoglobin targets prior to January 2011 in anticipation of implementation of the bundling policy, further studies examining serum ferritin trends in more contemporary cohorts are needed. In addition, the number of patients who were followed decreased over time due to events such as kidney transplantation, transfer to a nonaffiliated dialysis center, regain of renal function and death. However, our comparison of ferritin trajectories among patients in the overall cohort versus those who survived at least 1 year after dialysis showed similar slopes over time.
In conclusion, our study showed that serum ferritin levels increased in parallel among all patients irrespective of year of dialysis initiation, suggesting that these trends may be independent of changes in practice patterns over time. Serum ferritin levels increased sharply during the first year of HD treatment compared with subsequent years of HD independent of treatment-related factors such as IV iron utilization, and these trends were robust even after adjustment for sociodemographics, comorbidities and markers of malnutrition and inflammation. At this time, further observational studies are warranted to comprehensively determine the factors that influence trends in other anemia markers and iron parameters over time and their implications on outcomes in HD patients.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
We thank DaVita Clinical Research for providing the clinical data, analysis and review for this research project and for advancing the knowledge and practice of kidney care. Portions of these data have been presented at the 52nd European Renal Association–European Dialysis Transplant Association Congress, 28–31 May, 2015, London, UK, and have been accepted for an oral abstract presentation at the American Society of Nephrology Kidney Week Conference, 3–8 November 2015, San Diego, CA, USA. The study was supported by research grants (to K.K.-Z.) from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (K24-DK09141 and R01-DK078106) and philanthropic grants from Mr Harold Simmons, Mr Louis Chang and AVEO.
CONFLICT OF INTEREST STATEMENT
K.K.Z. has received honoraria from Genzyme/Sanofi and Shire and was the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA, USA, during 2007–12. All other authors have declared no conflicts of interest.
REFERENCES
- 1. Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH. The fascinating but deceptive ferritin: to measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol 2006; 1(Suppl. 1): S9–S18 [DOI] [PubMed] [Google Scholar]
- 2. Fishbane S, Kalantar-Zadeh K, Nissenson AR. Serum ferritin in chronic kidney disease: reconsidering the upper limit for iron treatment. Semin Dial 2004; 17: 336–341 [DOI] [PubMed] [Google Scholar]
- 3. Theil EC. Ferritin: at the crossroads of iron and oxygen metabolism. J Nutr 2003; 133(5 Suppl 1): 1549s–1553s [DOI] [PubMed] [Google Scholar]
- 4. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
- 5. Bailie GR, Larkina M, Goodkin DA et al. . Variation in intravenous iron use internationally and over time: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2013; 28: 2570–2579 [DOI] [PubMed] [Google Scholar]
- 6. Miskulin DC, Zhou J, Tangri N et al. . Trends in anemia management in US hemodialysis patients 2004-2010. BMC Nephrol 2013; 14: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brookhart MA, Schneeweiss S, Avorn J et al. . Comparative mortality risk of anemia management practices in incident hemodialysis patients. JAMA 2010; 303: 857–864 [DOI] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes, National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 2006; 47(5 Suppl 3): S11–S145 [DOI] [PubMed] [Google Scholar]
- 9. Collins AJ, Foley RN, Chavers B et al. . US Renal Data System 2013 annual data report. Am J Kidney Dis 2014; 63(1 Suppl): A7. [DOI] [PubMed] [Google Scholar]
- 10. Fishbane S, Mathew AT, Wanchoo R. Intravenous iron exposure and outcomes in patients on hemodialysis. Clin J Am Soc Nephrol 2014; 9: 1837–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuller DS, Pisoni RL, Bieber BA et al. . The DOPPS practice monitor for U.S. dialysis care: update on trends in anemia management 2 years into the bundle. Am J Kidney Dis 2013; 62: 1213–1216 [DOI] [PubMed] [Google Scholar]
- 12. Kuttykrishnan S, Kalantar-Zadeh K, Arah OA et al. . Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant 2015; 30: 1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sophia Rabe-Hesketh AS. Mutilevel and Longitudinal Modeling Using Stata. Vol. 1, Continuous Responses, 3rd edn STATA Press, College Station, TX: 2012 [Google Scholar]
- 14. Charytan DM, Pai AB, Chan CT et al. . Considerations and challenges in defining optimal iron utilization in hemodialysis. J Am Soc Nephrol 2015; 26: 1238–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coyne DW, Kapoian T, Suki W et al. . Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol 2007; 18: 975–984 [DOI] [PubMed] [Google Scholar]
- 16. Miskulin DC, Tangri N, Bandeen-Roche K et al. . Intravenous iron exposure and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 2014; 9: 1930–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalantar-Zadeh K, Regidor DL, McAllister CJ et al. . Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol 2005; 16: 3070–3080 [DOI] [PubMed] [Google Scholar]
- 18. Evans M, Suttorp MM, Bellocco R et al. . Trends in haemoglobin, erythropoietin-stimulating agents and iron use in Swedish chronic kidney disease patients between 2008 and 2013. Nephrol Dial Transplant 2015Aug 4. pii: gfv298. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19. Drueke TB, Locatelli F, Clyne N et al. . Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 20. Singh AK, Szczech L, Tang KL et al. . Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 21. Pfeffer MA, Burdmann EA, Chen CY et al. . A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 22.2011. FDA Drug Safety Communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. (20 September 2015, date last accessed)
- 23. Department of Health and Human Services, Centers for Medicare & Medicaid Services. End Stage Renal Disease Prospective Payment System: Final Rule and Proposed Rule, CMS-1418-F, RIN 0938-AP57, 42 CFR Parts 410, 413 and 414. 2010. https://www.gpo.gov/fdsys/pkg/FR-2010-08-12/pdf/2010-18466.pdf. (20 September 2015, date last accessed)
- 24. United States Renal Data System. 2014 annual data report: an overview of the epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 25. Streja E, Kovesdy CP, Molnar MZ et al. . Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis 2011; 57: 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalantar-Zadeh K, Rodriguez RA, Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant 2004; 19: 141–149 [DOI] [PubMed] [Google Scholar]
- 27. Macdougall IC, Bock AH, Carrera F et al. . FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 2014; 29: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrari P, Kulkarni H, Dheda S et al. . Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kovesdy CP, George SM, Anderson JE et al. . Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr 2009; 90: 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuwae N, Kopple JD, Kalantar-Zadeh K. A low lymphocyte percentage is a predictor of mortality and hospitalization in hemodialysis patients. Clin Nephrol 2005; 63: 22–34 [DOI] [PubMed] [Google Scholar]
- 31. Reddan DN, Klassen PS, Szczech LA et al. . White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant 2003; 18: 1167–1173 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.