Abstract
Background
Intraoperative MRI has been shown to improve gross-total resection of high-grade glioma. However, to the knowledge of the authors, the cost-effectiveness of intraoperative MRI has not been established.
Purpose
To construct a clinical decision analysis model for assessing intraoperative MRI in the treatment of high-grade glioma.
Materials and Methods
An integrated five-state microsimulation model was constructed to follow patients with high-grade glioma. One-hundred-thousand patients treated with intraoperative MRI were compared with 100 000 patients who were treated without intraoperative MRI from initial resection and debulking until death (median age at initial resection, 55 years). After the operation and treatment of complications, patients existed in one of three health states: progression-free survival (PFS), progressive disease, or dead. Patients with recurrence were offered up to two repeated resections. PFS, valuation of health states (utility values), probabilities, and costs were obtained from randomized controlled trials whenever possible. Otherwise, national databases, registries, and nonrandomized trials were used. Uncertainty in model inputs was assessed by using deterministic and probabilistic sensitivity analyses. A health care perspective was used for this analysis. A willingness-to-pay threshold of $100 000 per quality-adjusted life year (QALY) gained was used to determine cost efficacy.
Results
Intraoperative MRI yielded an incremental benefit of 0.18 QALYs (1.34 QALYs with intraoperative MRI vs 1.16 QALYs without) at an incremental cost of $13 447 ($176 460 with intraoperative MRI vs $163 013 without) in microsimulation modeling, resulting in an incremental cost-effectiveness ratio of $76 442 per QALY. Because of parameter distributions, probabilistic sensitivity analysis demonstrated that intraoperative MRI had a 99.5% chance of cost-effectiveness at a willingness-to-pay threshold of $100 000 per QALY.
Conclusion
Intraoperative MRI is likely to be a cost-effective modality in the treatment of high-grade glioma.
© RSNA, 2019
Online supplemental material is available for this article.
See also the editorial by Bettmann in this issue.
Summary
A microsimulation model of intraoperative MRI was constructed to record quality of life and treatment costs for patients with high-grade glioma from initial tumor resection until death. Intraoperative MRI was found to have an incremental cost-effectiveness ratio of $76 442 per quality-adjusted life year compared with neuronavigation systems.
Key Points
■ Compared with neuronavigation systems, intraoperative MRI reliably maximizes extent of glioma resection, providing patients with an average of 1.5 additional months of progression-free survival.
■ Use of intraoperative MRI resulted in an incremental cost-effectiveness ratio of $76 442 per quality-adjusted life year.
Introduction
High-grade gliomas, which include anaplastic gliomas (World Health Organization grade III) and glioblastomas (World Health Organization grade IV), are malignant tumors that rapidly proliferate. There is an incidence of 3.3–6.0 malignant tumors per 100 000 people per year (1). High-grade gliomas cause substantial morbidity and mortality. The median survival ranges from 1.13 to 15 years for World Health Organization grade III tumors, depending on molecular subtype (2), and a median survival of 10–12 months for World Health Organization grade IV tumors (3).
Diagnosis of high-grade glioma typically requires a tissue biopsy, and maximal safe resection at the time of biopsy is preferred in good surgical candidates with accessible tumors (4,5). Secondary treatments for high-grade glioma include concurrent radiation and temozolomide therapy, followed by adjuvant temozolomide in younger and otherwise healthy patients, and hypofractionated radiation and abbreviated temozolomide courses in older patients (6,7). Treatment of recurrent high-grade gliomas is less defined, although there is evidence that repeated resections can prolong survival (8,9).
The importance of an upfront gross-total resection for high-grade gliomas has been demonstrated in several retrospective studies and meta-analyses (4,5). Neuronavigation systems are commonly used to enhance resection of infiltrating lesions, but do not provide surgeons with real-time intraoperative image feedback. Accordingly, prospective data regarding neuronavigation systems suggest they may not improve the extent of resection or survival in patients with solitary cerebral tumors (10). For this reason, several adjunctive intraoperative techniques, including 5-aminolevulinic acid–induced fluorescence, fluorescein image-guided surgical procedure, intraoperative US, and intraoperative MRI (Fig 1), were developed to improve real-time localization of tumor remnants (11). Intraoperative MRI was extensively studied in several randomized trials (12–17), in which it was shown to improve gross-total resection and progression-free survival (PFS) rates compared with neuronavigation technology (18). However, concerns over the cost-effectiveness of intraoperative MRI persist. We hypothesized that intraoperative MRI is cost-effective for the treatment of high-grade gliomas.
Figure 1:
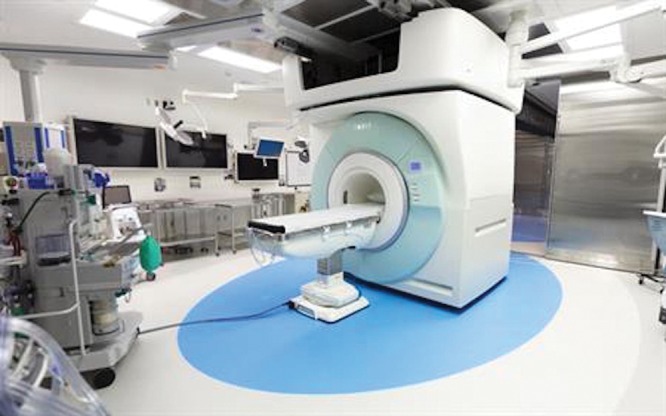
Mobile intraoperative MRI suite that allows surgeons to perform examination during surgery without moving the patient. This unit is suspended by ceiling tracks, allowing movement in two separate surgical suites. Image courtesy of University of California San Diego Health.
We used a computer modeling strategy, microsimulation, to simulate individual patients undergoing treatment for high-grade glioma from the time of initial tumor resection until death. By using available randomized clinical data, our model assigned each simulated patient a stochastically determined set of parameters. These parameters were randomly generated for each patient, but they always exist within ranges specified by randomized controlled data. By comparing patients treated by using intraoperative MRI with those not treated with it, cost-effectiveness of this modality can be assessed. Our model demonstrated the cost-effectiveness of intraoperative MRI for the treatment of high-grade gliomas, even in patients in whom multiple tumor resections are required. The purpose of our study was to construct a clinical decision analysis model for assessing intraoperative MRI in the treatment of high-grade glioma.
Materials and Methods
An integrated five-state microsimulation model was constructed by using commercially available software (TreeAge Pro 2017; TreeAge Software, Williamstown, Mass). This study was exempt from institutional review board and Health Insurance Portability and Accountability Act approval because it involved no human participants. No industry support was provided for this study and the study authors had control of the data and information submitted for publication.
Our model compared the use of intraoperative MRI to neuronavigation systems in patients with high-grade glioma. Patients entered the model at their initial debulking operation. Patients could experience prespecified events related to treatments, toxicities, survival, morbidity, and costs each month until death (1-month cycle length). Patients existed in one of a finite set of health states, but could transition between states during each cycle. Each health state has an assigned health valuation, or utility (measured in quality-adjusted life years [QALYs]), and cost. Overall prognosis depended on the number of cycles in each health state (19): Patients in health states with low utilities tend to have worse prognosis than those who transition to health states with higher utilities (20).
Patients and Interventions
The base case was a 55-year-old patient with a newly diagnosed high-grade glioma, without progression, who was eligible for resection and postoperative chemoradiotherapy.
Our model simulated 200 000 patients. These patients were similar to the base case, but parameters related to the patient’s demographics, operation, and postoperative treatment were varied within preset ranges. All patients underwent initial surgical resection followed by chemotherapy and radiation therapy, and administration of daily temozolomide (75 mg/m2 per day during 21 days of radiation) followed by adjuvant temozolomide (six 5-day cycles at 200 mg/m2 per day). Radiation courses varied by age: patients younger than 65 years were administered a 60-Gy dose in 30 fractions delivered by using intensity-modulated radiation therapy, whereas patients 65 years or older received a de-escalated course of 40 Gy in 15 fractions (21).
Simulating Clinical Courses
All patients underwent a debulking operation with maximal safe resection after randomization to resection with or without intraoperative MRI. Patients who experienced a major surgical adverse event (defined as serious hemorrhage) returned to the operating department for hematoma evacuation. After evacuation (if necessary), patients existed in one of three health states: PFS after radiation therapy and temozolomide, progressive disease, or dead. Second and third resections were permitted in patients with progressive or recurrent disease on the basis of the data from Chaichana et al (8), which demonstrated a significant survival advantage for patients with three resections compared with one or two resections. Repeated operations returned patients to the initial point of treatment in the model. Patients who underwent initial resection with intraoperative MRI underwent all subsequent resections with intraoperative MRI (Fig 2).
Figure 2:
Microsimulation model flowchart. Simulated patients were initially randomized to resection with or without intraoperative MRI. Initial resection may be complicated by intraoperative hemorrhage, which could require return to the operating department. Greater extent of resection (gross-total resection > subtotal resection) indicates higher probability of progression-free survival, although all patients are at some risk of cancer progression after operation. Patients that have persistent progressive disease (ie, recurrences) are offered up to two additional resections (three total). Patient death can occur at any time throughout the simulation. HGG = high-grade glioma, iMRI = intraoperative MRI, QALY = quality-adjusted life year.
Because of the high probability of grade 3 or higher hematologic toxicity in older adults by using temozolomide (22), hematologic toxicity could occur during the PFS and/or progressive disease states. Serious radiation therapy–related neurotoxicity was modeled as neurocognitive decline and could occur in the PFS state.
Probabilities, Costs, and Health Utilities
The base case values, ranges, and references for model parameters including probabilities, costs, and health utilities are shown in Table E1 (online).
Parameters were calculated as weighted averages of values in randomized trials. Ranges of the reported values were included for each parameter. Parameters without randomized data were based on meta-analyses, reviews of the literature, and national databases. Age-specific mortality was estimated by using the U.S. Centers for Disease Control National Vital Statistics System (23). Health utilities were based on prospective trials and previous cost-effectiveness research. Costs were converted to 2017 U.S. dollars by accounting for inflation. Cost of intraoperative MRI was modeled as 15%–25% of up-front and ongoing costs of an intraoperative MRI unit according to meta-analysis data (11). Costs for temozolomide, radiation therapy, and medical care during PFS were estimated from the U.S. Department of Veterans Affairs Federal Supply Schedule as 121% of costs listed (24,25). Because of variations in reported costs for both treatment of recurrent glioma and radiation therapy, ranges were estimated as ±30% of the base cost. A 3% annual discount was applied to all health utilities and costs.
Statistical Analysis
The incremental cost-effectiveness ratio was the chief assessment of cost-effectiveness in this analysis. A willingness-to-pay threshold of $100 000 per QALY gained was set (26). After the incremental cost-effectiveness ratio for the base case was calculated, an assessment of the models’ sensitivity to each model parameter was conducted by varying one input at a time (one-way deterministic sensitivity analysis). This analysis demonstrated which parameter values made intraoperative MRI cost ineffective at our willingness-to-pay threshold and accounted for uncertainty associated with the model parameters.
A probabilistic sensitivity analysis with 1000 Monte Carlo simulations was then used to vary multiple parameters simultaneously within the ranges specified in Table E1 (online). For 1000 simulated patients transitioning through the model until death, each parameter range was sampled 5000 independent times. By plotting the proportion of simulations that were cost-effective at our willingness-to-pay threshold, a cost-acceptability curve was produced.
Results
Our model closely approximated real-world survival, surgical outcome, and toxicity data. A comparison of model estimates and trial data are shown in Table 1. Overall survival is shown in Figure 3.
Table 1:
Microsimulation Model Accurately Approximates Trial Data

*The majority of patients with immediate postoperative aphasia recover most verbal functionality by 3 months, but some recent data suggest glioma resection near language areas can lead to long-term aphasia lasting greater than 6 months (12). Patients in this study experienced a mean of 3 months of aphasia (modeled as a range from 0 to 6 months).
Figure 3:
Overall survival for patients who did and did not undergo intraoperative MRI. Median survival for patients who underwent intraoperative MRI was 26 months compared with 22 months for patients who did not undergo intraoperative MRI. A nonuniform scale on the horizontal axis was used to more easily compare the model’s monthly survival rates with survival rates at monthly and yearly intervals in the literature.
Base Case Outputs and Accounting for Uncertainty
When restricted to base case values, intraoperative MRI yielded an incremental benefit of 0.17 QALYs (1.39 QALY with intraoperative MRI vs 1.21 QALYs without intraoperative MRI) at an incremental cost of $11 374 ($183 478 with intraoperative MRI vs $172 105 without intraoperative MRI), resulting in an incremental cost-effectiveness ratio of $65 064 per QALY.
To account for uncertainty in our model inputs, we performed sampling of parameter reference ranges to maximize the likelihood of capturing probabilities, costs, and utilities resembling clinical data. Within this sampling, intraoperative MRI yielded an incremental benefit of 0.18 QALYs (1.34 QALYs with intraoperative MRI vs 1.16 QALY without intraoperative MRI) at an incremental cost of $13 447 ($176 460 with intraoperative MRI vs $163 013 without intraoperative MRI), which resulted in an incremental cost-effectiveness ratio of $76 442 per QALY.
Sensitivity Analyses
One-way sensitivity analyses demonstrated that the cost-effectiveness of intraoperative MRI was durable within almost all parameter reference ranges. Low probability of aphasia and high gross-total resection rate in the group without intraoperative MRI made intraoperative MRI cost-ineffective, whereas duration of postoperative aphasia greatly reduced the incremental cost-effectiveness ratio (Fig 4). Factors such as patient age, probability of survival, and operative time did not diminish intraoperative MRI cost-effectiveness (plots not shown). A deterministic sensitivity analysis is shown in Table 2.
Figure 4:
Selected one-way sensitivity analyses. Progression-free survival advantage, A, less than about 1 month and, D, overall survival advantage less than 0.5 months; B, probability of aphasia less than 9.36%, and, C, gross-total resection rate greater than 60% in the group that did not undergo intraoperative MRI made intraoperative MRI cost-ineffective at a willingness-to-pay threshold of $100 000. E, Duration of postoperative aphasia (maximum 84 months) can considerably reduce the incremental cost-effectiveness ratio (ICER; as low as $42 131 per quality-adjusted life year [QALY]), though intraoperative MRI is cost-effective regardless of the number of months of postoperative aphasia. F, Intraoperative MRI ceases to be cost-effective at a cost greater than $3500 per operation, which was about 1.5 times our upper-limit cost.
Table 2:
Deterministic Sensitivity Analysis of Parameters That Most Affect the Incremental Cost-effectiveness Ratio
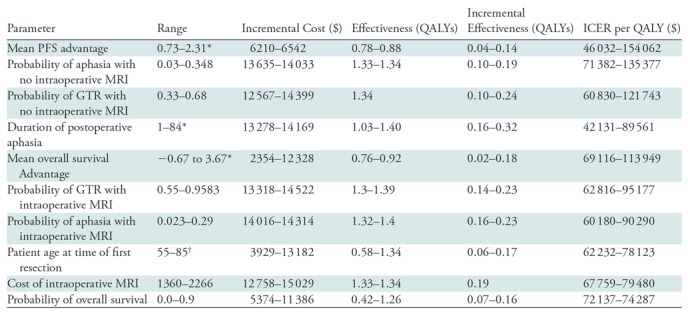
Note.—GTR = gross-total resection, ICER = incremental cost-effectiveness ratio, PFS = progression-free survival, QALY = quality-adjusted life year.
*Data are months.
†Data are years.
Probabilistic Sensitivity Analysis
Probabilistic sensitivity analysis demonstrated intraoperative MRI had a 99.5% chance of being cost-effective at a willingness-to-pay threshold of $100 000 per QALY. A cost-acceptability curve demonstrated that operation with intraoperative MRI overtakes operation without intraoperative MRI as the more cost-effective modality at an incremental cost-effectiveness ratio of $77 000 per QALY (Fig 5).
Figure 5:
Cost-effectiveness acceptability curve demonstrates the result of 1000 simulated patients transitioning through the model. To determine the effect of varying multiple model parameters simultaneously on the incremental cost-effectiveness ratio, distributions in Table E1 (online) were sampled 5000 times per patient. Operation with intraoperative MRI overtakes operation without intraoperative MRI as the more cost-effective modality at an incremental cost-effectiveness ratio of $77 000 per quality-adjusted life year (QALY; before the willingness-to-pay threshold of $100 000 per QALY).
Discussion
Despite wide variation in published values, microsimulation modeling demonstrated that intraoperative MRI was a cost-effective adjunct for high-grade glioma resection in patients of all ages undergoing up to three resections, regardless of prognosis. Intraoperative MRI was cost-effective in 99.5% of simulated patients, and increased progression-free suvival (PFS), higher gross-total resection rates, and lower postoperative aphasia rates affected the incremental cost-effectiveness ratio most dramatically. Our data suggested that surgeons should not be deterred from using intraoperative MRI in patients at advanced age or at the end of life: Intraoperative MRI remained cost-effective for patients with a probability of 1-month survival close to zero. Intraoperative MRI is especially cost-effective in patients with preoperative language deficits because of infiltration of language-associated structures and should be seriously considered in young patients with such tumors.
The use of intraoperative MRI in glioma resection was investigated in multiple randomized and nonrandomized studies (12–14,17), and meta-analysis data demonstrated that intraoperative MRI can improve gross-total resection rates by 25% and PFS by at least 1.5 months (11,18) while extending surgical time by about 60 minutes. Our model accounted for these published differences and found that patients who were treated with intraoperative MRI experienced a 4-month median survival advantage, a 5-month overall survival advantage, and a 2.3-month PFS advantage compared with patients treated without intraoperative MRI. Zhang et al (12) published data that showed new postoperative aphasia at 6 months was significantly lower in patients who underwent intraoperative MRI than in those who did not (2.3% vs 34.8%). We modeled this discrepancy conservatively, but if we had modeled a 15-times-greater risk of aphasia in the nonintraoperative MRI group, the incremental cost-effectiveness ratio would have more emphatically demonstrated the cost-effectiveness of intraoperative MRI.
Our incremental cost-effectiveness ratio was over twice that estimated by Eljamel et al (11); Eljamel et al estimated $32 955 per QALY. However, their estimated value was not obtained by using microsimulation modeling. Instead, a 0.11-QALY difference was assumed from previous studies, and only the cost of intraoperative MRI was considered. Eljamel et al did not consider the following: toxicities related to radiation, chemotherapy, or operation; cost differences related to longer surgical time (60 minutes in their study); and treatment of toxicities. Toxicity treatment is important because patients treated with intraoperative MRI experience toxicities at a higher rate because of improved survival. Our model accounted for these differences and found a difference of 0.18 QALYs.
The choice of a willingness-to-pay threshold for patients with glioblastoma is complex; median survival is less than 1 year. We chose a willingness-to-pay threshold of $100 000 per QALY, in accordance with previous oncologic cost-effectiveness research (27,28). Qian et al (27) stated that many commonly used oncologic interventions (eg, tumor-treating fields in glioblastoma) have incremental cost-effectiveness ratios that vastly exceed the $100 000 per QALY threshold but continue to be regarded as essential. Similarly, Ubel et al (29) stated that societal willingness-to- pay is dynamic over time and thresholds around $200 000 per QALY are more appropriate.
Our analysis had limitations. First, our model used several fixed probabilities: The probability of gross-total resection, PFS, and complications were assumed to be equal each month. Time-dependent probabilities more accurately model the hazard of death over time, but these values were available only for overall survival. Research into changes in these probabilities over time could improve future cost-effectiveness models. Second, our model sought to understand the immediate effect of surgical procedure on PFS and health utility. Thus, recurrent disease was treated with repeated operation followed by chemotherapu and radiation therapy rather than immediate reirradiation or repeated chemotherapy, though these approaches are appropriate for some patients (30). This decision is justifiable because intraoperative MRI would not be expected to affect QALYs at chemotherapy and radiation therapy. Despite this, differences in QALYs and costs that could have resulted from other treatments for recurrent disease were not captured in this simulation. Third, our consideration of postoperative treatment effects was limited to aphasia, grade 3 or higher hematologic toxicity from temozolomide, and neurocognitive deficits associated with radiation therapy. Radiation therapy toxicity was modeled by using stereotactic radiosurgery data, but further research into the health utility changes associated with de-escalated radiation therapy regimens in older populations might demonstrate different rates.
This analysis was conducted from the perspective of the health care payer, which correctly assumes that the burden of the up-front costs of the intraoperative MRI unit belongs to health care organizations, such as hospitals, and purchasers of health care, such as health plan sponsors and insurance carriers. Although the up-front costs of an intraoperative MRI unit may seem prohibitive, intraoperative MRI offers surgeons high-spatial-resolution images in real time at tumor resection and other stereotactic procedures. Because intraoperative MRI improves outcomes in a growing array of procedures, intraoperative MRI units will become exceedingly cost-effective. The use of intraoperative MRI to improve the extent of resection in less infiltrative tumors, such as low-grade gliomas, could prove to be cost-effective because these lesions typically demonstrate well-circumscribed borders and patients survive many years after gross-total resection. Mobile intraoperative MRI units could also prove to be cost-effective if used as ancillary outpatient MRI units when not in the operating department.
Our study adds to the growing body of evidence that demonstrates the cost effectiveness of intraoperative MRI. Further study of health-related quality of life may improve the ability to predict which interventions improve quality of life in patients with high-grade gliomas.
SUPPLEMENTAL TABLES
P.A. supported by Radiological Society of North America (RMS1801). P.A., M.G.B., J.P., and R.S. supported by National Institutes of Health (TL1TR001443).
Disclosures of Conflicts of Interest: P.A. disclosed no relevant relationships. R.S. disclosed no relevant relationships. M.G.B. disclosed no relevant relationships. A.R.W. disclosed no relevant relationships. R.C.R. disclosed no relevant relationships. C.L.R. disclosed no relevant relationships. J.P. disclosed no relevant relationships. J.A.S. disclosed no relevant relationships. D.R.S.D. disclosed no relevant relationships. V.C. disclosed no relevant relationships. J.S.P. disclosed no relevant relationships. J.D.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author’s institution for consultancy with Boston Consulting Group. Other relationships: disclosed no relevant relationships. A.A.K. disclosed no relevant relationships.
Abbreviations:
- PFS
- progression-free survival
- QALY
- quality-adjusted life year
References
- 1.Johannesen TB, Langmark F, Lote K. Cause of death and long-term survival in patients with neuro-epithelial brain tumours: a population-based study. Eur J Cancer 2003;39(16):2355–2363. [DOI] [PubMed] [Google Scholar]
- 2.van den Bent MJ, Weller M, Wen PY, Kros JM, Aldape K, Chang S. A clinical perspective on the 2016 WHO brain tumor classification and routine molecular diagnostics. Neuro Oncol 2017;19(5):614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol 2016;18(suppl_5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol 2016;2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trifiletti DM, Alonso C, Grover S, Fadul CE, Sheehan JP, Showalter TN. Prognostic implications of extent of resection in glioblastoma: analysis from a large database. World Neurosurg 2017;103:330–340. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 7.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 2004;22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 8.Chaichana KL, Zadnik P, Weingart JD, et al. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg 2013;118(4):812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helseth R, Helseth E, Johannesen TB, et al. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand 2010;122(3):159–167. [DOI] [PubMed] [Google Scholar]
- 10.Willems PW, Taphoorn MJ, Burger H, Berkelbach van der Sprenkel JW, Tulleken CA. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg 2006;104(3):360–368. [DOI] [PubMed] [Google Scholar]
- 11.Eljamel MS, Mahboob SO. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagn Photodyn Ther 2016;16:35–43. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Chen X, Zhao Y, Wang F, Li F, Xu B. Impact of intraoperative magnetic resonance imaging and functional neuronavigation on surgical outcome in patients with gliomas involving language areas. Neurosurg Rev 2015;38(2):319–330; discussion 330. [DOI] [PubMed] [Google Scholar]
- 13.Roder C, Bisdas S, Ebner FH, et al. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 2014;40(3):297–304. [DOI] [PubMed] [Google Scholar]
- 14.Napolitano M, Vaz G, Lawson TM, et al. Glioblastoma surgery with and without intraoperative MRI at 3.0T. Neurochirurgie 2014;60(4):143–150. [DOI] [PubMed] [Google Scholar]
- 15.Senft C, Bink A, Heckelmann M, Gasser T, Seifert V. Glioma extent of resection and ultra-low-field iMRI: interim analysis of a prospective randomized trial. Acta Neurochir Suppl (Wien) 2011;109:49–53. [DOI] [PubMed] [Google Scholar]
- 16.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 2011;12(11):997–1003. [DOI] [PubMed] [Google Scholar]
- 17.Tsugu A, Ishizaka H, Mizokami Y, et al. Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurg 2011;76(1-2):120–127. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Qian R, Niu C, Fu X. Impact of intraoperative MRI-guided resection on resection and survival in patient with gliomas: a meta-analysis. Curr Med Res Opin 2017;33(4):621–630. [DOI] [PubMed] [Google Scholar]
- 19.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13(4):322–338. [DOI] [PubMed] [Google Scholar]
- 20.Wali AR, Brandel MG, Santiago-Dieppa DR, et al. Markov modeling for the neurosurgeon: a review of the literature and an introduction to cost-effectiveness research. Neurosurg Focus 2018;44(5):E20. [DOI] [PubMed] [Google Scholar]
- 21.Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 22.Minniti G, De Sanctis V, Muni R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol 2008;88(1):97–103. [DOI] [PubMed] [Google Scholar]
- 23.NVSS - Mortality Tables - Life Expectancy - LEWK3 . Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Published 2017.
- 24.Barnett P. HERC: Determining the Cost of Pharmaceuticals for a Cost-Effectiveness Analysis. https://www.herc.research.va.gov/include/page.asp?id=pharmaceutical-costs. Published 2016. Updated April 25, 2016.
- 25.Office of Acquisition and Logistics . Pharmaceutical Prices. Washington, DC: Office of Acquisition and Logistics. Pharmaceutical Prices, 2017. [Google Scholar]
- 26.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 27.Qian Y, Maruyama S, Kim H, et al. Cost-effectiveness of radiation and chemotherapy for high-risk low-grade glioma. Neuro Oncol 2017;19(12):1651–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovic B, Xie F. Economic evaluation of bevacizumab for the first-line treatment of newly diagnosed glioblastoma multiforme. J Clin Oncol 2015;33(20):2296–2302. [DOI] [PubMed] [Google Scholar]
- 29.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med 2003;163(14):1637–1641. [DOI] [PubMed] [Google Scholar]
- 30.NCCN Clinical Practice Guidelines in Oncology . https://www.nccn.org/professionals/physician_gls/default.aspx. Published 2017.
- 31.Nuño M, Ly D, Mukherjee D, Ortega A, Black KL, Patil CG. Quality of surgical care and readmission in elderly glioblastoma patients. Neurooncol Pract 2014;1(2):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg 2016;124(4):977–988. [DOI] [PubMed] [Google Scholar]
- 33.Ahmadi R, Campos B, Haux D, Rieke J, Beigel B, Unterberg A. Assessing perioperative complications associated with use of intraoperative magnetic resonance imaging during glioma surgery - a single centre experience with 516 cases. Br J Neurosurg 2016;30(4):397–400. [DOI] [PubMed] [Google Scholar]
- 34.Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg 2011;115(6):1115–1125. [DOI] [PubMed] [Google Scholar]
- 35.Gupta DK, Chandra PS, Ojha BK, Sharma BS, Mahapatra AK, Mehta VS. Awake craniotomy versus surgery under general anesthesia for resection of intrinsic lesions of eloquent cortex--a prospective randomised study. Clin Neurol Neurosurg 2007;109(4):335–343. [DOI] [PubMed] [Google Scholar]
- 36.Meyer FB, Bates LM, Goerss SJ, et al. Awake craniotomy for aggressive resection of primary gliomas located in eloquent brain. Mayo Clin Proc 2001;76(7):677–687. [DOI] [PubMed] [Google Scholar]
- 37.Rusconi A, Sangiorgi S, Bifone L, Balbi S. Infrequent hemorrhagic complications following surgical drainage of chronic subdural hematomas. J Korean Neurosurg Soc 2015;57(5):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014;15(4):387–395. [DOI] [PubMed] [Google Scholar]
- 39.Gerber DE, Grossman SA, Zeltzman M, Parisi MA, Kleinberg L. The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas. Neuro Oncol 2007;9(1):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteves S, Alves M, Castel-Branco M, Stummer W. A pilot cost-effectiveness analysis of treatments in newly diagnosed high-grade gliomas: the example of 5-aminolevulinic Acid compared with white-light surgery. Neurosurgery 2015;76(5):552–562; discussion 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka S, Meyer FB, Buckner JC, Uhm JH, Yan ES, Parney IF. Presentation, management, and outcome of newly diagnosed glioblastoma in elderly patients. J Neurosurg 2013;118(4):786–798. [DOI] [PubMed] [Google Scholar]
- 42.Grossman R, Mukherjee D, Chang DC, et al. Predictors of inpatient death and complications among postoperative elderly patients with metastatic brain tumors. Ann Surg Oncol 2011;18(2):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernard-Arnoux F, Lamure M, Ducray F, Aulagner G, Honnorat J, Armoiry X. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol 2016;18(8): 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garside R, Pitt M, Anderson R, et al. The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation. Health Technol Assess 2007;11(45):iii–iv, ix-221. [DOI] [PubMed] [Google Scholar]
- 45.Rogers G, Garside R, Mealing S, et al. Carmustine implants for the treatment of newly diagnosed high-grade gliomas: a cost-utility analysis. Pharmacoeconomics 2008;26(1):33–44. [DOI] [PubMed] [Google Scholar]
- 46.Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes 2008;6(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latimer NR, Dixon S, Palmer R. Cost-utility of self-managed computer therapy for people with aphasia. Int J Technol Assess Health Care 2013;29(4):402–409. [DOI] [PubMed] [Google Scholar]
- 48.Lester-Coll NH, Dosoretz AP, Hayman JA, Yu JB. Health state utilities for patients with brain metastases. Cureus 2016;8(7):e667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eseonu CI, Rincon-Torroella J, ReFaey K, Quiñones-Hinojosa A. The cost of brain surgery: awake vs asleep craniotomy for perirolandic region tumors. Neurosurgery 2017;81(2):307–314. [DOI] [PubMed] [Google Scholar]
- 50.Regan JM, Worley E, Shelburne C, Pullarkat R, Watson JC. Burr hole washout versus craniotomy for chronic subdural hematoma: patient outcome and cost analysis. PLoS One 2015;10(1):e0115085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellis C, Simpson AN, Bonilha H, Mauldin PD, Simpson KN. The one-year attributable cost of poststroke aphasia. Stroke 2012;43(5):1429–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wasserfallen JB, Ostermann S, Leyvraz S, Stupp R. Cost of temozolomide therapy and global care for recurrent malignant gliomas followed until death. Neuro Oncol 2005;7(2):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 2005;103(9):1916–1924. [DOI] [PubMed] [Google Scholar]
- 54.Liou SY, Stephens JM, Carpiuc KT, Feng W, Botteman MF, Hay JW. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig 2007;27(6):381–396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






