Abstract
PURPOSE:
The purpose of the study is to estimate the rate of infection with carbapenem-resistant Enterobacteriaceae (CRE) in the main governmental tertiary care hospital in Bahrain.
MATERIALS AND METHODS:
All clinical samples with positive growth of CRE over 6-year period (January 2012–December 2017) were collected from the microbiology laboratory data.
RESULTS:
The CRE incidence was high in the first half of study period (2012–2014) and then decreased between 2015 and 2017, after implementation of intensified CRE control measure bundle. About 49.4% of CRE-positive samples were isolated from the elderly age group (above 65 years old), most of them were admitted in the intensive care unit (ICU). The most common isolated organisms were Klebsiella pneumoniae (87.0%), followed by Escherichia coli (7.9%). Isolates from deep tracheal aspirate and midstream urine specimens were the most common source of CRE isolates (27.3%) and (26.3%), respectively. Bacteremia was documented in 21.2% of cases. CRE isolates in the study showed high rates of resistance to aminoglycosides (72.2% resistant to amikacin and 67.3% to gentamicin). Alternatively, most isolates retained their susceptibility to colistin and tigecycline with sensitivity of 83.9% and 85.7%, respectively. Combined resistance to both colistin and tigecycline was observed in 0.06% of total isolates.
CONCLUSION:
Elderly population and ICU admission were important risk factors for CRE acquisition. Most of CRE isolates were sensitive to both colistin and tigecycline, which make them the best combination for empiric frontline therapy for suspected serious CRE infection in our facility. Implementing CRE-bundled infection control measures significantly reduced the incidence of CRE infection in our hospital.
Keywords: Bahrain, carbapenem-resistant Enterobacteriaceae, Escherichia coli, Klebsiella pneumoniae
Introduction
Antimicrobial resistance is a growing challenge worldwide. It imposes difficulties in selecting the appropriate empirical antimicrobial therapy. Since the first extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae was discovered in Western Europe in the mid-1980s, the ESBL-producing Enterobacteriaceae were progressively increasing at both community and hospital levels till it became widely prevalent,[1] and hence, the carbapenem became a preferred option in the treatment of serious Gram-negative infections.[2] Emergence of carbapenem-resistant Enterobacteriaceae (CRE) has threatened the clinical utility of carbapenem with an emerging threat for developing “extreme drug resistance” in Gram-negative bacilli.
The emergence of CRE has become a formidable public health threat as it had increased four-fold over the past 10 years worldwide,[3] particularly among K. pneumoniae and Escherichia coli, as has been reported in the global antibiotic resistance estimates published by the World Health Organization in 2014.[4]
In the coming years, CRE might evolve to cause considerable clinical problems due to their growing multidrug resistance profile that may extend to include other β-lactams, fluoroquinolones, and aminoglycosides, leaving few or, in some cases, no optimal therapeutic options. Other factors, such as late identification, subsequent delay in starting appropriate antimicrobial therapy, and the potential toxicity of their therapeutic options, such as tigecycline or polymyxins,[5] would augment the risk of high mortality, prolonged hospital stay, and huge medical expenses in patients infected with CRE.[6]
Countries of the Arabian Peninsula are more susceptible to the spread of various infectious agents, including CRE. This may be partially due to the unique feature of their population structure with high rate of resident expatriates with an average of approximately 48%,[7] added to the extensive international connections of these countries that likely facilitate the spread of CRE. Indeed, these strains have been reported from sporadic cases and from small outbreaks in almost all countries of the region.[8,9,10,11,12] However, we need to know more about the similarities and differences in the epidemiology of CRE in the different countries of the peninsula.
Bahrain has been at the center of major trade routes since antiquity, and from the extensive exposure of this country, one may predict that a considerable part of local CRE cases could be imported. However, there are no local surveillance data or published studies either to support or to oppose such predictions.
Due to the important serious clinical implication of the CRE, we designed our study, we design our study with the objective to determine CRE epidemiology in the main governmental tertiary care hospital in Bahrain over the last 6 years and to define the important risk factors for acquisition and to map the antibiotic profile of CRE in our hospital to help in the choice of appropriate empirical therapy among inpatients with suspected serious Gram-negative sepsis.
Materials and Methods
Setting
This study was conducted at Salmaniya Medical Complex, the main governmental hospital in the Kingdom of Bahrain with a 1100-bed capacity. Only clinical specimens with positive CRE growth during the study period (January 2012–December 2017) were identified from the microbiology database, followed by the collection of patient demographic data. For study purposes, the hospital was divided into the following divisions: adult intensive care unit (ICU), pediatric ICU, neonatal ICU, coronary care units, medical wards (14 wards), surgical wards (12 wards; including ENT, general surgery, neurosurgery, ophthalmology, and orthopedic), pediatric wards (5 wards), gynecology and obstetrics wards (4 wards), and nephrology unit (1 ward).
Collecting the data
Data of patients with positive CRE isolates were collected and entered into a centralized database. The database included the following information: patient characteristics (age, sex, and nationality), the ward that the patient was admitted, the identified Enterobacteriaceae species, the year of first isolation, the specimen type (lower respiratory tract specimens as sputum or deep tracheal aspirate, blood, urine, wound swab, or pus), and the antibiotic susceptibility results (susceptible, intermediate, or resistant).
The first isolate with positive results of CRE among inpatients was considered and included in the study. The isolates were stratified according to the time of acquisition. If the first isolate of CRE was obtained from inpatients within the first 2 days of his/her hospitalization, it was considered as community acquired; while if it was obtained from inpatients after 2 calendar days of hospitalization, it was defined as hospital acquired. Among patients with multiple hospitalizations and repeated growth of the same CRE isolate with identical sensitivity profile, only the first isolate was included in the study. The CRE phenotype was defined using the CDC criteria to identify Enterobacteriaceae as nonsusceptible to imipenem, meropenem, or ertapenem.[13]
Laboratory detection
The clinical samples from the hospitalized patients were sent to the microbiology laboratory of Salmaniya Medical Complex for testing as part of routine clinical workup. Enterobacteriaceae from the clinical samples had been isolated on MacConkey and blood agars. Identification was done using conventional biochemical tests, while susceptibility testing was done using the Kirby–Bauer disk diffusion method.[14] We used antibiotic discs for the following antibiotics: amoxicillin/clavulanate, cefuroxime, ceftriaxone, cefepime, and three carbapenems including ertapenem, meropenem, and imipenem. We tested also the following non-β-lactam agents: gentamicin, amikacin, trimethoprim-sulfamethoxazole, ciprofloxacin, and tigecycline. In most midstream urine samples, ciprofloxacin was not tested; instead, we tested norfloxacin and nitrofurantoin. Discs were obtained from Becton Dickinson (Franklin Lakes, NJ, USA). Susceptibility testing was conducted using Mueller–Hinton agar (bioMérieux, Marcy l’Étoile, France) with McFarland 0.5 from overnight cultures followed by incubation at 35°C for 16–18 h. Inhibition zone diameters were determined and interpreted using the most up-to-date resistance breakpoints as set by the Clinical Laboratory Standards Institute (CLSI) (M100-S26).[15]
Carbapenem-resistant Enterobacteriaceae screening test
Resistance to carbapenems (ertapenem, meropenem, and imipenem) was evaluated using disk diffusion method. Positive samples were confirmed using Phoenix identification and susceptibility system Minimal Inhibitory Concentration (MIC). Results of antibiotic susceptibility (including colistin) were reported according to Phoenix results. Interpretation of resistance was according to the old CLSI guideline for isolates collected prior to 2016, then according to the updated 2016 recommendations thereafter. The isolates that showed intermediate or resistant zones for imipenem or meropenem were tested for carbapenemase production by modified Hodge test (MHT).[15] Quality control of the carbapenem disks was performed according to the CLSI guidelines, running the following organisms MHT-positive K. pneumoniae ATCC1705 and MHT-negative K. pneumoniae ATCC1706 with each batch of the test.
Statistical analysis
Data were collected and tabulated using health electronic system and then analyzed using statistical software SPSS version 24 (IBM Corp., Chicago, Illinois, USA). Descriptive statistics of demographic variables were calculated including frequencies, percentages, means, and ranges. The study was approved by the Research and Ethics Committee at the Ministry of Health, Kingdom of Bahrain.
Results
Trend of carbapenem-resistant Enterobacteriaceae incidence in the hospital over the study period
CRE started to be identified in our hospital by the end of 2012 where eight CRE isolates had been recognized from six patients. After that, there was a rapid increase in the incidence of CRE to peak in 2015 reaching 45.4 cases per 10,000 patient admissions and then the incidence gradually decreased during 2016–2017, as shown in Figure 1.
Figure 1.
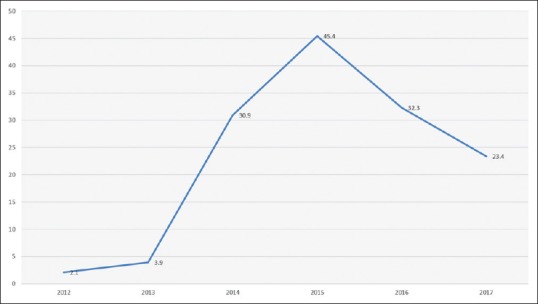
Incidence of carbapenem-resistant Enterobacteriaceae over the study period (cases per 10,000 patient admission)
Demographic data of patients with positive carbapenem-resistant Enterobacteriaceae
During the period from 2012 to 2017, 631 isolates corresponding to pathogens of interest had been isolated. Majority of the cases were among Bahrainis (89%). Hospital-acquired infection was present in 87% of cases. There was nearly equal sex distribution; 316 isolates (50.1%) were isolated from males and 315 isolates (49.9%) from females [Table 1]. When stratifying all CRE isolates by age group, 312 isolates (49.4%) were isolated from senior age group (patients >65 years old), and only 17 isolates (2.6%) had been isolated from pediatric patients (<14 years old). The remaining isolates (48%) were smoothly distributed over the age group from 14 to 64 years [Table 1]. When stratifying CRE, cases according to the location in the hospital showed that most of the positive CRE isolates were from the medical wards (417 of 631 isolates, 66.09%), as shown in Table 1. The highest incidence was observed in the adult ICU, followed by pediatric ICU, and the nephrology unit [Figure 2].
Table 1.
Demographic data of the age, gender, nationality, area, and sample-wise distributions of carbapenem-resistant Enterobacteriaceae isolates
| Parameter | Percent |
|---|---|
| Age (years) | |
| Pediatric (0-13) | 2.60 |
| Adult (14-65) | 48 |
| Elderly>65 | 49.40 |
| Gender | |
| Male | 50.10 |
| Female | 49.90 |
| Nationality | |
| Bahraini | 89 |
| Non-Bahraini | 11 |
| Source of infection | |
| Hospital acquired | 86.80 |
| Community acquired | 13.20 |
| Area-wise distribution of CRE isolates | |
| Medical wards | 66.09 |
| Surgical wards | 13.95 |
| Adult ICU | 8.87 |
| Nephrology unit | 6.49 |
| Cardiac ICU | 1.74 |
| Pediatric wards | 1.28 |
| Neonatal ICU | 0.79 |
| Gynecology and obstetrics | 0.63 |
| Pediatric ICU | 0.16 |
| Sample-wise distribution of CRE isolates by specimen type | |
| Sputum | 3.0 |
| Blood | 21.2 |
| Deep tracheal aspirate | 27.3 |
| Wound swab | 19.2 |
| Pus | 2.8 |
| Midstream urine | 26.3 |
| Endotracheal tube secretion | 0.2 |
CRE=Carbapenem-resistant Enterobacteriaceae, ICU=Intensive care unit
Figure 2.
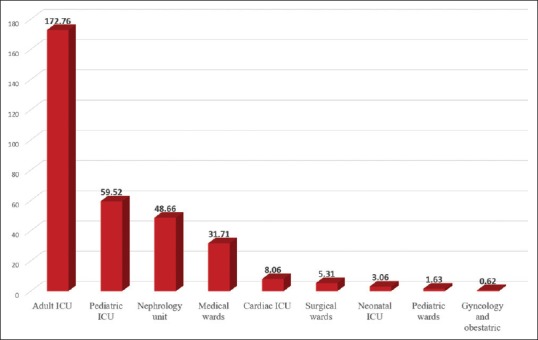
Incidence of carbapenem-resistant Enterobacteriaceae by location (carbapenem-resistant Enterobacteriaceae cases per 10,000 patient admissions in each location)
Microbiology and specimen type of carbapenem-resistant Enterobacteriaceae isolates
The types of CRE isolates as well as the specimen types were shown in Tables 1 and 2. K. pneumoniae was the most common isolated CRE organisms in the current study (549 of 631 isolates, 87.0%), followed by E. coli (50 of 631 isolates, 7.9%) and then Enterobacter cloacae, Klebsiella oxytoca, Serratia marcescens, Enterobacter aerogenes, Proteus vulgaris, Citrobacter farmeri, and Citrobacter braakii. The most common specimen with CRE isolates was deep tracheal aspirate (27.3%), followed by midstream urine specimens (26.3%) and blood culture (21.2%).
Table 2.
Identified species of carbapenem-resistant Enterobacteriaceae isolates
| Organism | Number of total samples (%) | Isolates resistant to colistin (%) | Isolates resistant to tigecycline (%) |
|---|---|---|---|
| Klebsiella pneumoniae | 549 (87) | 93 (16.94) | 21 (4) |
| Escherichia coli | 50 (7.9) | 2 (4) | 3 (6) |
| Enterobacter cloacae | 17 (2.6) | 1 (5.88) | 1 (5.88) |
| Klebsiella oxytoca | 4 (0.6) | 0 | 0 |
| Serratia marcescens | 3 (0.4) | 3 (100) | 1 (33.3) |
| Enterobacter aerogenes | 3 (0.4 | 1 (33.3) | 1 (33.3) |
| Citrobacter freundii | 2 (0.3) | 0 | 0 |
| Proteus vulgaris | 1 (0.1) | 1 (100) | 1 (100) |
| Citrobacter farmeri | 1 (0.1) | 0 | 1 (100) |
| Citrobacter braakii | 1 (0.1) | 0 | 0 |
| Total Specimens | 631 (100) | 101 (16.1) | 29 (4.6) |
Antibiotic susceptibility of carbapenem-resistant Enterobacteriaceae isolates
Most CRE isolates had resistance to the tested non-β-lactam antibiotics including quinolones, aminoglycosides, and trimethoprim-sulfamethoxazole for all specimen types and to nitrofurantoin for urine specimens. The resistance rates in all isolates were 67.3% (425 isolates), 72.2% (456 isolates), and 79% (499 isolates) for gentamicin, amikacin, and trimethoprim-sulfamethoxazole, respectively. Ciprofloxacin was tested in 378 isolates (nonurine specimen), with a resistance rate of 94% (358 isolates). The resistance rates among the 163 urine specimens were 98.75% for norfloxacin (158 isolates) and 84% for nitrofurantoin (137 isolates).
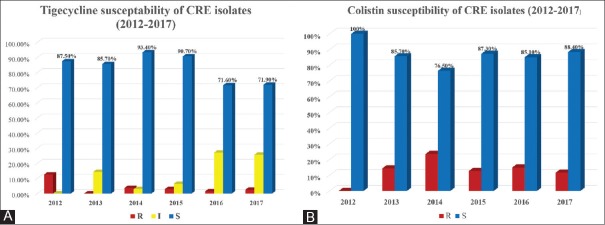
However, almost CRE isolates in our study retain their sensitivity to colistin and tigecycline among all 631 isolates, with susceptibility to colistin and tigecycline of 83.9% and 85.7%, respectively. Resistance rates of 16% (101 isolates) to colistin and 4.6% (29 isolates) to tigecycline were observed [Table 2]. There were 61 (9.7%) isolates showing intermediate resistance to tigecycline. Only four isolates (0.6%) demonstrated combined resistance to both colistin and tigecycline, in which two of them were K. pneumoniae, one was P. vulgaris, and one was S. marcescens (which is intrinsically resistant to colistin). Figure 3 shows the susceptibility and resistance patterns of CRE isolates to tigecycline and colistin. There was 20% decrease in the susceptibility percentage to tigecycline over the past 2 years (2016–2017), while colistin susceptibility remained the same [Figures 3A and B].
Figure 3.
Tigecycline (A) and colistin (B) susceptibility of carbapenem-resistant Enterobacteriaceae isolates by year (2012–2017)
Discussion
Carbapenem-resistant Enterobacteriaceae incidence trend and intensified infection control bundle in high-risk areas
The present study shows a high incidence of CRE in our hospital, with an average incidence rate of approximately 23/10,000 admissions over the study period. It is a relatively high incidence compared to other international figures and previous studies.[16,17] However, when analyzing the trend of CRE incidence over the study period, there was a significant decrease of CRE incidence in the second half of the study period (2015–2017) compared to the rise during the first half (2013–2015). The latter decline was related to the intense CRE control program through development and strict implementation of new CRE policy. This policy was developed at the end of 2014 through collaborative efforts from the infection control unit and the microbiology laboratory in our hospital. This local policy was adopted from the international guidelines,[18,19,20] with some modification according to the available facilities in terms of staffing, structural, or financial resources in our hospital. This policy included initial CRE screening (by conventional culture) for patients admitted to high-risk areas (e.g., adult ICU) and for any admitted patients with documented previous colonization or infection with CRE (as this will appear as a flag in the patient electronic record system). Preemptive contact precaution for such cases under the process of screening till the surveillance culture of CRE is ready is another important item in the policy. A timely verbal notification (phone call) from microbiology staff to the concerned clinical areas upon getting any positive culture for CRE is the routine in our policy. At the same time, daily e-mail notification from microbiology staff to infection control staff about all cases of positive CRE was used to be done to ensure efficient follow-up of all cases in clinical areas. On the other hand, proper implementation of contact precautions (including isolated room) for all CRE cases during the hospital stay, as well as intensification of educational programs for health-care workers, was cornerstones in our policy. Strengthening of environmental cleaning through training of cleaning staff who work in high-risk areas (medical floor and ICUs) with monitoring of cleaning performance through checklist to ensure consistent cleaning and disinfection of surfaces in close proximity to the patient and those likely to be touched by the patient and health-care workers (e.g., bedrails, carts, bedside commodes, doorknobs, and faucet handles) was also emphasized.
Species identification and antimicrobial susceptibility
Increasing the prevalence of CRE infections is of paramount importance to the public health due to the increasing threat to the most vulnerable patient populations including older age group with a substantial economic burden. In the present study, K. pneumoniae accounts for the largest proportion of CRE (87.0%), followed by E. coli (only 7.9%). This agrees with another study done in a network of community hospitals in the Southeastern United States where K. pneumoniae was the most prevalent species (91%).[21] Predominance of K. pneumoniae (62%) and E. coli (29%) was observed in 23 community hospitals participating in the Duke Infection Control Outreach Network from 2008 to 2012[22] as well. This growing rate of antimicrobial resistance among the most common uropathogens (Klebsiella and Escherichia coli) in addition to their resistance to B-lactam usually showed higher levels of coresistance to the other tested antimicrobial agents (such as aminoglycosides and quinolones) which is considered as a threat to the favorable outcome of urinary tract infection that is considered as one of the most common infections in human.
Previously, aminoglycosides were brought to the frontline as a combination therapy for infections caused by CRE since it may be the only antimicrobial to which CRE isolates show in vitro susceptibility.[23,24] However, increasing resistance to aminoglycosides was observed in most recent studies. Capone et al.[25] showed that only 20.4% of CRE isolates were susceptible to gentamicin. At the same time, Shanghai study showed also that only 10.4 % and 13.0 % of their isolates were susceptible to amikacin and gentamicin, respectively.[26] Accordingly, aminoglycosides are not considered anymore as frontline therapy for CRE. A similar resistance pattern was observed in our study, where 72.2% of our CRE isolates were resistant to amikacin and 67.3% were resistant to gentamicin.
Colistin and tigecycline were found to be the most effective agents in our study with a sensitivity rate of 83.9% and 85.7% of isolates, respectively. Similar high susceptibility to tigecycline had been reported previously in a study conducted in a tertiary care center in North India where tigecycline resistance was demonstrated only in 13.9% of CRE isolates.[27] A recent study by Chew et al., 2017, has demonstrated the retained activity of colistin against CRE, so that it remains as a part of the last-line antibiotics for multidrug-resistant Gram-negative bacteria, such as CRE.[28] This low-resistance pattern observed in the current study for both colistin and tigecycline is most probably related to the restricted use of both antibiotics in our facility.
CRE is of a significant concern to public health – both for the community and for the health-care facilities. Infections caused by these organisms can be serious and even deadly. Extension of this organism outside the hospital setting could have deleterious effects. Every effort should be done to prevent the spread of CRE through good hand washing, proper cleaning and disinfection of surfaces with hospital-grade disinfectants, detecting patients who carry CRE as soon as possible, proper use of gloves and gowns, clearly communicating with other health-care facilities when a patient with CRE is transferred from one facility to another, and very importantly, using the antibiotics appropriately. The epidemic spread of CRE since 2008 (due to rapid gene transfer between species) indicates the importance of international collaboration to limit its spread.[29]
Limitation of the study
The results of rectal screening cultures (for detection of asymptomatic gastrointestinal colonization among ICU-admitted patients and readmitted positive cases) were not included in the analysis. This could be a reason for underestimation of the true burden of CRE infection. We did not conduct a genomic study of the CRE isolates. Understanding the mechanism of resistance in the isolates that showed combined resistance to both colistin and tigecycline is also warranted. Follow-up of the positive cases in the study was not done. Data about the clinical status, drug treatment, and prognostic details of these cases were not included. It will be good to conduct another study including these data which give some light about the clinical aspects of those patients as well as genomic studies.
Conclusion
The worldwide global increase in infections with CRE is of great concern due to the association of infections with these highly virulent bacteria with high morbidity and mortality rates. Regular surveillance and timely approaches in prevention through implementation of bundled infection control measures, education and training programs, as well as other interventions were successful in our hospital to mitigate the health-care-associated risk factors for infection and helped to decrease the incidence rate of CRE over the past 2 years (2015–2017).
Updated mapping of local antibiotic susceptibility of CRE isolates in each health-care facility and defining the resistance pattern are of paramount importance to optimize the empiric antimicrobial therapy for patients with serious Gram-negative sepsis and high-risk factors for Multi drug resistance (MDR). Knowing the local resistance pattern, as defined in the current study, would favor combination therapy with colistin and tigecycline as the best combination for empiric frontline therapy for suspected serious CRE infection at present.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shin S, Jeong SH, Lee H, Hong JS, Park MJ, Song W. Emergence of multidrug-resistant Providencia rettgeri isolates co-producing NDM-1 carbapenemase and PER-1 extended-spectrum β-lactamase causing a first outbreak in Korea. Ann Clin Microbiol Antimicrob. 2018;17:20. doi: 10.1186/s12941-018-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Y, Gu B, Huang M, Liu H, Xu T, Xia W, et al. Epidemiology of carbapenem resistant Enterobacteriaceae (CRE) during 2000-2012 in Asia. J Thorac Dis. 2015;7:376–85. doi: 10.3978/j.issn.2072-1439.2014.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37:1288–301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson DL, Doi Y. A step closer to extreme drug resistance (XDR) in Gram-negative Bacilli. Clin Infect Dis. 2007;45:1179–81. doi: 10.1086/522287. [DOI] [PubMed] [Google Scholar]
- 5.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. 2013;19:E23–E30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 6.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18:54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 7.Sonnevend Á, Ghazawi AA, Hashmey R, Jamal W, Rotimi VO, Shibl AM, et al. Characterization of carbapenem-resistant Enterobacteriaceae with high rate of autochthonous transmission in the Arabian Peninsula. PLoS One. 2015;10:e0131372. doi: 10.1371/journal.pone.0131372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Qadheeb NS, Althawadi S, Alkhalaf A, Hosaini S, Alrajhi AA. Evolution of tigecycline resistance in Klebsiella pneumoniae in a single patient. Ann Saudi Med. 2010;30:404–7. doi: 10.4103/0256-4947.67087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balkhy HH, El-Saed A, Al Johani SM, Francis C, Al-Qahtani AA, Al-Ahdal MN, et al. The epidemiology of the first described carbapenem-resistant Klebsiella pneumoniae outbreak in a tertiary care hospital in Saudi Arabia: How far do we go? Eur J Clin Microbiol Infect Dis. 2012;31:1901–9. doi: 10.1007/s10096-011-1519-0. [DOI] [PubMed] [Google Scholar]
- 10.Dortet L, Poirel L, Al Yaqoubi F, Nordmann P. NDM-1, OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in sultanate of Oman. Clin Microbiol Infect. 2012;18:E144–8. doi: 10.1111/j.1469-0691.2012.03796.x. [DOI] [PubMed] [Google Scholar]
- 11.Poirel L, Al Maskari Z, Al Rashdi F, Bernabeu S, Nordmann P. NDM-1-producing Klebsiella pneumoniae isolated in the sultanate of Oman. J Antimicrob Chemother. 2011;66:304–6. doi: 10.1093/jac/dkq428. [DOI] [PubMed] [Google Scholar]
- 12.Sonnevend A, Al Baloushi A, Ghazawi A, Hashmey R, Girgis S, Hamadeh MB, et al. Emergence and spread of NDM-1 producer Enterobacteriaceae with contribution of IncX3 plasmids in the United Arab Emirates. J Med Microbiol. 2013;62:1044–50. doi: 10.1099/jmm.0.059014-0. [DOI] [PubMed] [Google Scholar]
- 13.Facility Guideline for Control of Carbapenem-Resistant Enterobacteriaceae (CRE): CDC Update – CRE Toolkit. 2015. Nov, [Last accessed on 2018 May 28]. Available from: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf .
- 14.Bonev B, Hooper J, Parisot J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J Antimicrob Chemother. 2008;61:1295–301. doi: 10.1093/jac/dkn090. [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 26nd Informational Supplement M100-S26. Wayne, PA: Clinical and Laboratory Standards Institute; 2016. [Last accessed on 2018 May 28]. Available from: http://www.facm.ucl.ac.be/intranet/CLSI/CLSI-2017-M100-S27.pdf . [Google Scholar]
- 16.Mataseje LF, Abdesselam K, Vachon J, Mitchel R, Bryce E, Roscoe D, et al. Results from the Canadian nosocomial infection surveillance program on carbapenemase-producing Enterobacteriaceae, 2010 to 2014. Antimicrob Agents Chemother. 2016;60:6787–94. doi: 10.1128/AAC.01359-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: Report from the China CRE network. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01882-17. pii: e01882-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Government of Western Australia, Department of Public Health, Infection Prevention and Control of Carbapenem-resistant Enterobacteriaceae (CRE) in Western Australian Healthcare Facilities. 2012. [Last accessed on 2018 May 02]. Available from: http://www.health.wa.gov. au/circularsnew/attachments/712.pdf .
- 19.Health Protection Agency. Department of Health Advisory Committee on Antimicrobial Resistance and Healthcare Associated Infection. Advice on Carbapenemase Producers: Recognition, Infection Control and Treatment. United Kingdom: Health Protection Agency; 2012. [Last accessed on 2018 May 28]. Available from: https://www.assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/707165/ARHAI_annual_report_2014_to_2015.pdf . [Google Scholar]
- 20.Magiorakos AP, Burns K, Rodríguez Baño J, Borg M, Daikos G, Dumpis U, et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European centre for disease prevention and control. Antimicrob Resist Infect Control. 2017;6:113. doi: 10.1186/s13756-017-0259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thaden JT, Lewis SS, Hazen KC, Huslage K, Fowler VG, Jr, Moehring RW, et al. Rising rates of carbapenem-resistant Enterobacteriaceae in community hospitals: A mixed-methods review of epidemiology and microbiology practices in a network of community hospitals in the Southeastern United States. Infect Control Hosp Epidemiol. 2014;35:978–83. doi: 10.1086/677157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livorsi DJ, Chorazy ML, Schweizer ML, Balkenende EC, Blevins AE, Nair R, et al. A systematic review of the epidemiology of carbapenem-resistant Enterobacteriaceae in the United States. Antimicrob Resist Infect Control. 2018;7:55. doi: 10.1186/s13756-018-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benenson S, Navon-Venezia S, Carmeli Y, Adler A, Strahilevitz J, Moses AE, et al. Carbapenem-resistant Klebsiella pneumoniae endocarditis in a young adult. Successful treatment with gentamicin and colistin. Int J Infect Dis. 2009;13:e295–8. doi: 10.1016/j.ijid.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Lee GC, Burgess DS. Treatment of Klebsiella pneumoniae carbapenemase (KPC) infections: A review of published case series and case reports. Ann Clin Microbiol Antimicrob. 2012;11:32. doi: 10.1186/1476-0711-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. 2013;19:E23–30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 26.Hu F, Chen S, Xu X, Guo Y, Liu Y, Zhu D. Emergence of carbapenem-resistant clinical Enterobacteriaceae isolates from a teaching hospital in Shanghai, China. J Med Microbiol. 2012;61:132–6. doi: 10.1099/jmm.0.036483-0. [DOI] [PubMed] [Google Scholar]
- 27.Khare V, Gupta P, Haider F, Begum R. Study on MICs of tigecycline in clinical isolates of carbapenem resistant Enterobacteriaceae (CRE) at a tertiary care centre in North India. J Clin Diagn Res. 2017;11:DC18–DC21. doi: 10.7860/JCDR/2017/24594.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew KL, La MV, Lin RT, Teo JW. Colistin and polymyxin B susceptibility testing for carbapenem-resistant and mcr-positive Enterobacteriaceae: Comparison of sensititre, microscan, vitek 2, and etest with broth microdilution. J Clin Microbiol. 2017;55:2609–16. doi: 10.1128/JCM.00268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J Infect Dis. 2017;215:S28–36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]