Abstract
Background
Multiple sclerosis (MS) is a leading cause of neurological disability in young adults. The most widely accepted hypothesis regarding its pathogenesis is that it is an immune‐mediated disease. It has been hypothesised that intraluminal defects, compression, or hypoplasia in the internal jugular or azygos veins may be important factors in the pathogenesis of MS. This condition has been named 'chronic cerebrospinal venous insufficiency' (CCSVI). It has been suggested that these intraluminal defects restrict the normal blood flow from the brain and spinal cord, causing the deposition of iron in the brain and the eventual triggering of an auto‐immune response. The proposed treatment for CCSVI is venous percutaneous transluminal angioplasty (PTA), which is claimed to improve the blood flow in the brain thereby alleviating some of the symptoms of MS. This is an update of a review first published in 2012.
Objectives
To assess the benefit and safety of venous PTA in people with MS and CCSVI.
Search methods
We searched the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group's Specialised Register up to 30 August 2018, CENTRAL (in the Cochrane Library 2018, issue 8), MEDLINE up to 30 August 2018, Embase up to 30 August 2018, metaRegister of Controlled Trials, ClinicalTrials.gov., the Australian New Zealand Clinical Trials Registry, and the World Health Organization (WHO) International Clinical Trials Registry platform. We examined the bibliographies of the included and excluded studies.
Selection criteria
We included randomised controlled trials (RCTs) in which PTA and sham interventions were compared in adults with MS and CCSVI.
Data collection and analysis
Two authors independently assessed study eligibility and risk of bias, and extracted data. We reported results as risk ratios (RR) with 95% confidence intervals (CI). We performed statistical analyses using the random‐effects model; and we assessed the certainty of the evidence using GRADE.
Main results
We included three RCTs (238 participants) in this update. One hundred and thirty‐four participants were randomised to PTA and 104 to sham treatment. We attributed low risk of bias to two (67%) studies for sequence generation and two (67%) studies for performance bias. All studies were at a low risk of detection bias, attrition bias, reporting bias and other potential sources of bias.
There was moderate‐quality evidence to suggest that venous PTA did not increase the proportion of patients who had operative or post‐operative serious adverse events compared with the sham procedure (RR 3.33, 95% CI 0.36 to 30.44; 3 studies, 238 participants); nor did it increase the proportion of patients who improved on a functional composite measure including walking control, balance, manual dexterity, postvoid residual urine volume, and visual acuity over 12‐month follow‐up (RR 0.84, 95% CI 0.55 to 1.30; 1 study, 110 participants); nor did it reduce the proportion of patients who experienced new relapses at six‐ or 12‐month follow‐up (RR 0.87, 95% CI 0.51 to 1.49; 3 studies, 235 participants). There was no effect of venous PTA on disability worsening measured by the Expanded Disability Status Scale, which was reported at follow‐up intervals of six months (one study), 11 months (one study) and 12 months (one study). Quality of life was reported in two studies with no difference between treatment groups. Moderate or severe pain during or post venography was reported in both PTA and sham‐procedure participants in all included studies. Venous PTA was not effective in restoring blood flow assessed at one‐month (one study) or 12‐month follow‐up (one study).
Authors' conclusions
This systematic review identified moderate‐quality evidence that, compared with sham procedure, venous PTA intervention did not provide benefit on patient‐centred outcomes (disability, physical or cognitive functions, relapses, quality of life) in people with MS. Venous PTA has proven to be a safe technique but in view of the available evidence of its ineffectiveness, this intervention cannot be recommended in people with MS. All ongoing trials were withdrawn or terminated and hence this updated review is conclusive. No further randomised clinical studies are needed.
Plain language summary
The technique popularly known as 'liberation procedure' for treatment of venous stenoses (CCSVI) in the brain of people with MS
What is the issue? Chronic cerebrospinal venous insufficiency (CCSVI) has been described as a vascular condition characterized by restricted venous outflow from the brain and spinal cord, mainly due to narrowing or blockage of the veins in the head and neck. It has been hypothesised that CCSVI may be an important factor in the development of MS and treatment of CCSVI by catheter venography and percutaneous transluminal angioplasty (PTA) to widen the veins might improve symptoms and quality of life in people with MS. There is uncertainty about whether PTA should be used in people with MS.
What did we do? We reviewed three studies (238 participants) which compared PTA with sham‐PTA in participants with MS and CCSVI.
What did we find? We found that venous PTA did not provide benefit on disability, physical or cognitive functions, relapses, or quality of life. No serious adverse events attributable to venography or venous PTA occurred.
Conclusions Venous PTA has proven to be a safe but ineffective intervention and cannot be recommended in patients with MS. All trials that were ongoing were either terminated or withdrawn, so this updated review is conclusive. No further randomised clinical studies are needed.
Currentness of evidence This review is up to date to August 2018.
Summary of findings
for the main comparison.
|
Patient or population: patients with multiple sclerosis and chronic cerebrospinal venous insufficiency (CCSVI) Settings: MS centres and their associated colour Doppler ultrasonography (ECD) and angiography units Intervention: venous percutaneous transluminal angioplasty (PTA) Comparison: catheter venography without venous angioplasty (sham) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Reasons for downgrading our confidence in the evidence | |
| Assumed risk with sham procedure | Corresponding risk with intervention (95%CI) | |||||
| Proportion of participants who experienced operative or postoperative serious adverse events | 0 per 100 | 0 per 100 (0 to 0) |
RR 3.33 (0.36 to 30.44) |
238 (3) |
moderate | Downgraded 1 level due to imprecision, wide CI |
| Proportion of participants who experienced improvement of composite functional endpoint over 12 months | 49 per 100 | 41 per 100 (27 to 64) |
RR 0.84, (0.55 to 1.30) |
110 (1) |
moderate | Downgraded 1 level due to imprecision, wide CI |
| Proportion of participants who experienced new relapses over 12 months | 18 per 100 | 16 per 100 (4 to 27) |
RR 0.87 (0.51 to 1.49) |
235 (3) |
moderate | Downgraded 1 level due to imprecision, wide CI |
| * The basis for the assumed risk is the sham group risk across studies included in the meta‐analysis. The corresponding risk (and its 95% CI) is based on the assumed risk with sham procedure and the relative effect of the PTA intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
Background
Multiple sclerosis (MS) is an inflammatory disease of the nervous system and the most frequent cause of neurological disability in young adults. Myelin, the material that surrounds and protects the nerves, becomes damaged and this results in the formation of scar‐like plaques. MS is considered to be an immune‐mediated disease in which the person's own immune system attacks the nervous system; and most of the current drug therapies are based on this hypothesis.
A theory has been proposed that impaired blood flow in the veins draining the central nervous system, a condition called chronic cerebrospinal venous insufficiency (CCSVI), may play a role in the cause of MS (Zamboni 2006). CCSVI is thought to be congenital and it may result in iron deposits which in turn trigger the immune system to attack the central nervous system, thus damaging the myelin (Singh 2009). The proposed treatment for CCSVI is venous balloon angioplasty, which entails the widening of narrowed (stenosed) veins (Zamboni 2009a; Zamboni 2012). This theory has gained a lot of attention via the Internet, mainly among the participants' community, and increased media interest has further enhanced the expectations of people suffering with MS. This is an update of a review first published in 2012 (van Zuuren 2012).
Unfamiliar terms are listed in the 'Glossary of terms' in Table 2.
1. Glossary of terms.
| ntigen | Substance or molecule that, when introduced into the body, triggers the production of an antibody by the immune system, which will then kill or neutralise the antigen that is recognised as a foreign and potentially harmful invader |
| Autoreactive | Immune response acting against own tissue |
| Ataxia | Neurological sign and symptom that consists of gross lack of coordination of muscle movements |
| Axon | Part of the neuron that conducts electrical impulses away from the neuron's cell body |
| Central nervous system | Part of the nervous system that integrates the information that it receives from, and coordinates the activity of, all parts of the body. It comprises the brain and the spinal cord |
| Cognitive impairment | Condition associated with forgetfulness, difficulty concentrating, or making decisions that affect everyday life. Cognitive impairment ranges from mild to severe. With mild impairment, people may begin to notice changes in cognitive functions, but still be able to do their everyday activities. Severe levels of impairment can lead to losing the ability to understand the meaning or importance of something and the ability to talk or write, resulting in the inability to live independently. |
| Congestion | Accumulation or overfilling of the blood vessels |
| Demyelination | Loss of the myelin sheath insulating the nerves |
| Dysarthria | Having a problem with articulating |
| Erythrocyte extravasation | Leakage of red blood cells into the surrounding tissue |
| Gliosis | Proliferation of astrocytes (glial cells) in damaged areas of the central nervous system |
| HLA‐DR | Major histocompatability complex (MHC) class II cell surface receptor encoded by the human leukocyte antigen complex on chromosome 6 region 6p21.31. HLA‐DR is also involved in several autoimmune conditions, disease susceptibility and disease resistance. It is also closely linked to HLA‐DQ and this linkage often makes it difficult to resolve the more causative factor in disease |
| HLA‐DQ | A cell surface receptor type protein (MHC class II type) found on antigen presenting cells. The DQ loci are in close genetic linkage to HLA‐DR. When tolerance to self‐proteins is lost, DQ may become involved in autoimmune disease |
| Immuno‐mediated disease | Conditions that result from abnormal activity of the body's immune system |
| Inflammation | Response of vascular tissues to harmful stimuli and a protective attempt to remove the injurious stimuli and to initiate the healing process. A cascade of biochemical events propagates and matures the inflammatory response, involving the local vascular system, the immune system and various cells within the injured tissue |
| Major histocompatability complex (MHC) | A large genomic region or gene family found in most vertebrates that encodes MHC molecules. MHC molecules play an important role in the immune system and autoimmunity |
| Neuron | An electrically excitable cell that processes and transmits information by electrical and chemical signalling. Chemical signalling occurs via synapses, specialised connections with other cells. Neurons connect to each other to form networks. Neurons are the core components of the nervous system |
| Pathological | Altered or caused by disease |
| Pathogenesis | The mechanism by which the disease is caused |
| Phagocytosis | Mechanism used to remove pathogens and cell debris |
| Polygenic disease | Disease controlled by several genes at once |
| Relapse | An objective new/re‐emerging neurological abnormality present for at least 24 hours in the absence of fever/infection |
| Reversible | Capable of returning to an original condition/situation |
| Stenosis | Abnormal narrowing in a blood vessel |
| Tremor | Involuntary, somewhat rhythmic, muscle contraction and relaxation involving to‐and‐fro movements of 1 or more body parts |
| Venogram | An X‐ray test that takes pictures of blood flow through the veins in a certain area of the body |
| Venotopic | Located in the veins |
| Venous angioplasty | A procedure that can be performed during a venogram to open or bypass veins. It can also be used for placement of a stent, which keeps a vessel or tissue in an open position to allow for improved blood flow |
| Venous congestion | Dilation of veins and capillaries due to impaired venous drainage |
| Vertigo | Type of dizziness, where there is a feeling of motion when one is stationary |
Description of the condition
Multiple sclerosis (MS) is a leading cause of neurological disability in young adults. The disease is characterised by focal white matter lesions, characterised by inflammation and demyelination that are associated with axonal damage (Kuhlmann 2017). Four clinical forms of MS can be distinguished: relapsing‐remitting (RRMS); secondary progressive (SPMS); primary progressive (PPMS); and progressive relapsing (PRMS) (Lublin 1996). RRMS and SPMS are the clinical forms of MS that account for approximately 80% to 85% of sufferers, and SPMS evolves from the RRMS form. MS is heterogeneous, both histopathologically and clinically (Lucchinetti 2000), and the natural history can be difficult to predict. In most cases it begins as RRMS with episodic, largely reversible neurological dysfunction. Natural history studies, which followed cohorts of MS patients not treated with any disease‐modifying drugs, have shown that, after a period of approximately 10 years, almost 50% of people with MS gradually develop permanent disability which may also include acute relapses. After a median of 15 to 28 years from disease onset, a disability milestone equivalent to the use of an assistive walking device is reached (Weinshenker 1989). Clinical features include all of the symptoms caused by the impairment of the central nervous system (e.g. loss of vision, double vision, muscle weakness, sensory disturbances, bladder dysfunction, impotence, constipation, ataxia, vertigo, tremor, spasticity, pain, cognitive impairment and dysarthria). Fatigue, anxiety and depression are also frequent occurrences. Magnetic resonance imaging (MRI) can support the clinical diagnosis, and it is integrated with clinical and other para‐clinical diagnostic methods (e.g. examining cerebrospinal fluid and evoked potentials) to facilitate the diagnosis of MS (Thompson 2018). MRI parameters are also used as surrogate markers of disease activity and progression. The disease has an adverse impact on the health‐related quality of life (HRQoL) of people with MS and their families and may also pose a financial burden, even when the disease is not physically disabling.
The age of onset of MS is usually between 20 and 40 years. Incidence is low in childhood and is rarer at the age of 50 years or older. Female‐to‐male ratios vary between 1.5:1 and 2.5:1 in most populations (Sellner 2011). The incidence and prevalence of MS varies geographically (Simpson 2011): high‐frequency areas (prevalence in excess of 60 per 100,000 people) include all of Europe in addition to southern Canada, northern USA, New Zealand, and south‐east Australia. In many of these areas the prevalence is more than 100 per 100,000 people. This geographic variance may be explained in part by racial differences: white populations, especially those from northern Europe, appear to be most susceptible.
The most widely accepted hypothesis on the pathogenesis of MS is that it is an immune‐mediated disease characterised by infiltration of blood‐derived monocytes, microglia, and lymphocytes leading to damage of myelin and axons. Although the aetiology is largely indeterminate, a large proportion of the scientific community considers that MS develops in genetically predisposed subjects and that environmental factors play a central role in its pathogenesis, based on immune‐mediated mechanisms. It is thought that aberrant immune responses to self or foreign antigens cause and perpetuate inflammation (Wu 2011). The inflammation leads to demyelination and subsequent axonal damage. The role of inflammation is considered to be complex, however, and may include both beneficial and detrimental effects (Martino 2002).
The hypothesis suggesting that chronic venous congestion may be a factor in the pathogenesis of MS became a focus in multiple sclerosis research when public participation emphasised interest in the procedure to correct it. (Zamboni 2006;Zamboni 2011) The predominantly venotopic location of MS lesions in the CNS is postulated to be a consequence of local erythrocyte extravasation owing to elevated transmural venous pressure, followed by erythrocyte degradation and iron‐driven phagocytosis and subsequent lymphocytic infiltration (Singh 2009). This condition has been named 'chronic cerebrospinal venous insufficiency' (CCSVI) and is characterised by stenoses of the internal jugular veins or azygos veins, or both, which restrict the normal blood flow from the brain, along with the appearance of small collateral veins that may have developed to reduce the impact of the stenoses. In his initial study, Zamboni found CCSVI in all subjects in the study group that were diagnosed with MS, and none in the healthy controls (Zamboni 2011). CCSVI, as defined by Zamboni and colleagues, is diagnosed with combined extracranial and transcranial echo colour Doppler (ECD) radiography when two or more of five established parameters are present (Zamboni 2009b).
There has been some criticism of several of the limitations in the ultrasound‐based investigation used to measure the rather complex and dynamic (i.e. postural dependent) cerebrospinal venous outflow. These include the wide individual variability, operator dependence and intra‐ and inter‐rater bias, the difficulty of standardising values for diagnostic criteria and the necessity of venography as a gold standard (Doepp 2010; Hojnacki 2010; Zivadinov 2011b). A high degree of correlation between CCSVI and MS was found in a number of studies (Al‐Omari 2010; Bavera 2011; Hojnacki 2010; Simka 2010); but this has been contested by other studies (Baracchini 2011; Centonze 2011; Comi 2013; Doepp 2010; Krogias 2010; Marder 2011; Sundström 2010; Tsivgoulis 2011; Wattjes 2011; Yamout 2010). Several reviews have reported that the incidence of CCSVI varies in people with MS, ranging from 0% to 100% and from 0% to 23% in healthy controls (Ghezzi 2011; Zivadinov 2011b). One study of 499 people with MS found an increased prevalence of CCSVI but with a modest sensitivity and specificity, and suggestive of a less likely primary causative role for CCSVI in the development of MS (Zivadinov 2011a). A further study found no relationship between CCSVI and HLA DRB1*1501, a genetic variation that has been consistently linked to MS (Weinstock‐Guttman 2011). Attempts have been made to correlate CCSVI with specific symptoms of MS: in particular, an association with fatigue (which often severely affects people with MS) (Malagoni 2010).
The hypothesised association between CCSVI and MS implicates CCSVI as a treatable cause of MS and hence it has formed the basis for the so‐called 'liberation procedure' which is based on the technique of venous balloon angioplasty (Zamboni 2009a; Zamboni 2012). Venous stent placement has also been used to treat CCSVI in people with MS but this treatment has been associated with a small number of serious adverse events (Anon 2010; Burton 2011). Much of the research on this topic has generated major interest and continuing debate in the scientific community on the definition of CCSVI as a pathological entity; the correlation between CCSVI and MS; the proposed etiopathogenetic mechanisms; and, as a consequence, on the utility of its treatment (Bagert 2011; D'haeseleer 2011; Dorne 2010; Ghezzi 2011; Khan 2010; Lazzaro 2011; Reekers 2011; van Rensburg 2010; Waschbisch 2011; Zivadinov 2011b).
The narrative of scientific research underwent a revolutionary change as the participation of the MS community and social media became the mobilizing factors for conduct of research in this field to help the scientific community to provide evidence for or against this 'liberation procedure' (Benjaminy 2018; Driedger 2017).
Description of the intervention
Percutaneous transluminal angioplasty (PTA) involves the insertion of a small catheter with a balloon attachment via percutaneous access to the left femoral vein. Initially a venogram is performed so that images can be obtained to identify the narrowed sections of the veins. The catheter is then inserted and advanced into the azygos and internal jugular veins. The balloon is inflated at the narrowed section of the vein, thereby increasing its diameter and improving the flow of blood. The procedure is performed with venographic control. These procedures are performed generally as day surgery — overnight hospital stay is not required. Patients receive prophylactic low‐molecular‐weight heparin during subsequent weeks, to lower the thrombotic risk.
How the intervention might work
Were the CCSVI hypothesis tenable, repairing venous stenosis and re‐establishing correct venous flow from the brain toward the heart could have therapeutic effects.
Why it is important to do this review
The original review published in 2012 did not find studies meeting inclusion criteria (van Zuuren 2012). There have been several studies done over the last six years which have looked at the benefit and safety of PTA intervention in people with MS. CCSVI is characterized by restricted venous outflow from the brain and spinal cord. Whether this condition is associated with MS and whether venous PTA is beneficial in persons with MS and CCSVI is controversial. We felt it was important to update the 2012 review to provide up‐to‐date evidence on the effects of PTA in in people with MS.
The MS‐CCSVI hypothesis has generated both enthusiasm and skepticism among people with MS and the specialists who treat them (Paul 2014). The 'liberation procedure' has attracted considerable attention among people with MS as well as the media and on the Internet (Driedger 2017; Piga 2014). Consumers have been frequently exposed to media hyperbole with exaggerated claims that have led to unrealistic expectations. As a consequence, CCSVI treatment has been offered to MS participants in many countries, mostly not at conventional MS centres, in spite of the lack of confirmation of early results from Zamboni's pivotal trials (Zamboni 2009a; Zamboni 2012). This review update attempts to highlight possible methodological issues in available clinical trials in order to provide an evidence‐based review of the effect of treating CCSVI in people with MS. Our aim in this update is to contribute so that the expectations of people with MS stay within the boundaries of the evidence‐based medicine paradigm.
Objectives
To assess the benefit and safety of venous PTA in people with MS and CCSVI.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We did not exclude trials on the basis of duration of follow‐up.
Types of participants
Participants of both genders, 17 years of age or older, with a diagnosis of MS according to the original or the revised McDonald criteria (McDonald 2001;Polman 2005;Polman 2011; Thompson 2018), and a diagnosis of CCSVI according to Zamboni's criteria (Zamboni 2009a) or other internationally recognised and validated criteria (Zivadinov 2014; Traboulsee 2014).
Types of interventions
PTA alone or in combination with MS pharmacological treatments, versus sham intervention alone or in combination with MS pharmacological treatments. We did not consider PTA associated with stenting in this review.
Types of outcome measures
Primary outcomes
Safety
The total number of operative or post‐operative serious adverse events (SAEs) or adverse events (AEs).
The total number of SAEs or AEs reported during the follow‐up.
If not enough studies reported the total number of SAEs or AEs and person‐years, we used the number of participants with at least one SAE or AE as defined in the study.
Benefit
Clinical measured outcomes, including disability worsening measured by Expanded Disability Status Scale (EDSS) (Kurtzke 1983); or any other functional outcome as reported by the authors of included studies.
Patient‐reported outcomes (PROs), including quality of life (QoL) assessed by any validated disease‐specific instrument (e.g. MSQOL‐54 (Vickrey 1995), MSQLI (Fischer 1999), MusiQoL (Simeoni 2008), or generic instrument, e.g. Short Form 36 (SF‐36) (Rudick 2007)); well‐being as measured with any visual analogue scale (VAS); fatigue measured by Modified Fatigue Impact Scale (MFIS) (Kos 2005), or other recognised and validated MS‐fatigue scale; and any other PRO as reported by the authors of included studies.
Secondary outcomes
The number of participants experiencing at least one relapse during follow‐up. We accepted definitions of relapse as reported in the original studies.
Mean change in cognitive functions' assessment through validated battery in MS (e.g. Brief Repeatable Battery of Neuropsychological Tests (BRBNT) (Rao 1991).
Restored blood flow primary patency. Primary patency is the interval following the initial angioplasty procedure until a re‐intervention is performed to preserve patency. Secondary patency is defined as the interval following the initial angioplasty procedure until treatment of the vein is abandoned due to an inability to treat the original lesion (Diehm 2007).
Search methods for identification of studies
This review is an update of a previously published review (van Zuuren 2012). We conducted a systematic search with no restrictions to identify all relevant published and unpublished RCTs. We searched trials published in any language.
Electronic searches
We searched the following databases.
The Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group's Specialised Register up to 30 August 2018 (Appendix 1)
The Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Library 2018, Issue 8) (Appendix 2)
MEDLINE (PubMed) up to 30 August 2018 (Appendix 3)
Embase (embase.com) up to 30 August 2018 (Appendix 4)
Searching other resources
References from published studies
We examined the bibliographies of the included and excluded studies for further references to potentially eligible RCTs.
Ongoing trials registers
We searched the following ongoing trial registers.
metaRegister of Controlled Trials www.controlled‐trials.com
US National Institutes of Health Ongoing Trials Register www.ClinicalTrials.gov
Australian New Zealand Clinical Trials Registry www.anzctr.org.au
World Health Organization (WHO) International Clinical Trials Registry platform www.who.int/trialsearch
Data collection and analysis
Selection of studies
We used the search strategy described above to obtain titles and abstracts of studies that were relevant to the review. Two review authors (VJ and EP) independently screened the titles and abstracts and discarded studies that were not applicable. Two review authors (VJ and EP) independently assessed the retrieved abstracts, and when necessary the full text of these studies, to determine which studies satisfied the inclusion criteria. We resolved any disagreements through discussion and consensus.
Data extraction and management
Two authors (VJ and GVA) independently extracted data using a predefined data extraction form. We checked data for consistency and resolved disagreements by discussion.
We extracted from each included study the following data.
Study: first author or acronym, setting, number of centres, year of publication, years that the study was conducted (recruitment and follow‐up), publication (full‐text publication, abstract publication, unpublished data).
Study design: inclusion criteria, number of randomised participants, duration of follow‐up, sequence generation, allocation, blinding of participants and outcomes assessors, selective outcome reporting, early termination of trial.
Participants: age, gender, inclusion and exclusion criteria, number of participants excluded after randomisation and number of losses at follow‐up.
Intervention and comparison: type and intervention details.
Outcomes — primary and secondary outcomes.
Notes: other comments.
Assessment of risk of bias in included studies
The review authors (VJ and GVA) independently assessed the risk of bias using the Cochrane tool for assessing risk of bias as described in Chapter 8, Section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We rated the following domains separately for any included study as 'low risk of bias', 'high risk of bias' and 'unclear' if the risk of bias was uncertain or unknown.
Sequence generation
Allocation concealment
Blinding of participants, personnel
Blinding of outcomes assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We judged incomplete outcome data at low risk of bias when numbers and causes of dropouts were balanced (i.e. in the absence of a significant difference) between arms and appeared to be unrelated to the studied outcomes. We assessed selective outcome reporting bias by comparing outcomes reported in the study protocol along with published outcome results.
To summarise the quality of the evidence, we used the following criteria.
Low risk of bias (plausible bias unlikely to seriously alter the results) when we judged all the criteria as at low risk of bias.
Unclear risk of bias (plausible bias that raises some doubt about the results) when we judged one or more criteria as at unclear risk of bias.
High risk of bias (plausible bias that seriously weakens confidence in the results) when we judged one or more criteria as at high risk of bias.
Measures of treatment effect
We planned presenting continuous outcomes where possible on the original scale as reported in each individual study, and dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (95% CI) at each time point, using the Mantel‐Haenszel test, unless stated otherwise.
Unit of analysis issues
Cluster and cross‐over trials or studies with multiple treatment groups have not been carried out to evaluate PTA intervention for MS.
Dealing with missing data
We planned to assess the effect of missing outcome data, analysing data according to a likely scenario (i.e. assuming that treated and control group participants who contributed to missing outcome data both had an unfavourable outcome).
Assessment of heterogeneity
We planned to assess clinical heterogeneity within treatment comparisons by examining characteristics of study participants (i.e. differences in age, disease duration, and baseline EDSS scores, and characteristics of interventions across the trials using information reported in the Characteristics of included studies table).
Assessment of reporting biases
Considering that it is not mandatory to publish results of clinical trials, it is difficult to have an estimate of the number of unpublished trials of PTA in MS. We planned to evaluate the possibility of reporting bias by means of a funnel plot, if a sufficient number of trials were identified for inclusion in this review (Egger 1997).
Data synthesis
We planned to perform pairwise meta‐analyses for each primary outcome using a random‐effects model for each treatment comparison with at least two studies (DerSimonian 1986). Two review authors (VJ and GVA) analysed the data in Review Manager 5 (RevMan 5) (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analysis if a sufficient number of studies (> 10) with moderate to substantial heterogeneity were included. Although we did not identify a sufficient number of studies at this time, we planned to consider carrying out subgroup analysis based on the different subtypes of MS, disease duration and baseline EDSS level. We statistically assessed the presence of heterogeneity for all pairwise comparisons using the Chi² test and I² statistic. We considered heterogeneity as important if it was at least moderate to substantial (an I² statistic greater than 50%) (Higgins 2011).
Sensitivity analysis
We planned to conduct sensitivity analysis to assess the robustness of our review results if a sufficient number of studies were included. We planned to perform the following sensitivity analyses.
Including only trials with low risk of bias.
Excluding studies that did not provide complete and clear reporting of dropout data.
'Summary of findings' table
We present the main results of the review in a 'Summary of findings' table, as recommended by Cochrane (Schünemann 2011). The 'Summary of findings' table provides an overall grading of the quality of evidence related to each outcome based on GRADEpro GDT (www.gradepro.org; GRADEproGDT 2015). We graded the quality of evidence as high, moderate, low, or very low considering within‐study risk of bias, directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias. We based the grading of the evidence related to the study limitations on allocation concealment, blinding of participants and outcome assessor, and incomplete outcome data.
We included an overall grading of the evidence for the following outcomes.
Proportion of participants who experienced operative or postoperative serious adverse events.
Proportion of participants who experienced improvement of composite functional endpoint over 12 months.
Proportion of participants who experienced new relapses over 12 months.
Results
Description of studies
Results of the search
The initial review resulted in a total of 159 study reports from the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System's Specialised Register to June 2012, CENTRAL (in the Cochrane Library Issue 5, 2012), MEDLINE (to June 2012) and Embase (to June 2012). From these 159 reports, no studies were included in the systematic review; there were six ongoing studies.
For the 2019 update of this review, we identified 58 new reports. We excluded four duplicate references and 40 articles on the basis of abstracts that we considered not pertinent. We identified three new included studies (Siddiqui 2014; Traboulsee 2018; Zamboni 2018); and 11 new excluded studies. Of the three new included studies, two were publications of trials identified as ongoing trials in the 2012 review (Siddiqui 2014; Zamboni 2018). Of the 11 new excluded studies, four were trials identified as ongoing in the 2012 review which were terminated because of challenges with enrolment and no data reported (ACTRN12612000302853; NCT01089686; NCT01201707; NCT01555684). Figure 1 shows the results of the search.
1.
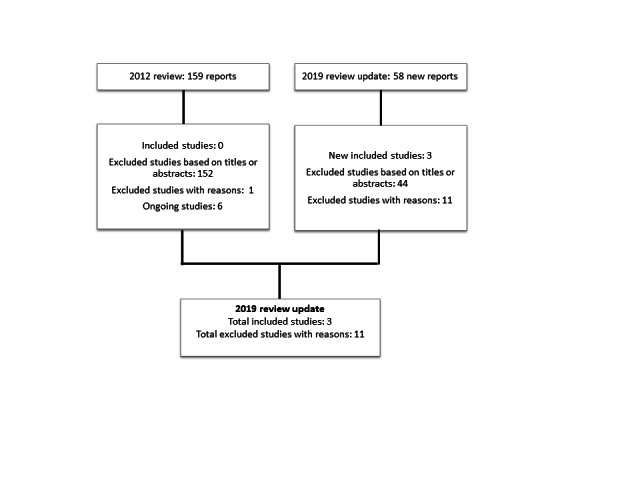
Flow diagram of studies included in the systematic review
Included studies
We identified three new studies conducted between 2012 and 2016 in the USA, Italy and Canada (Siddiqui 2014; Traboulsee 2018; Zamboni 2018). The studies included 238 participants of ages 18 to 65 years with MS, of whom 134 were randomised to PTA and 104 to sham treatment. Follow‐up was six months (Siddiqui 2014), 11 months (Traboulsee 2018) and 12 months (Zamboni 2018 ). The table 'Characteristics of included studies' provides details of included studies.
Excluded studies
From the 2012 review, 155 reports were excluded based on titles and abstracts; one study was excluded after full‐text review because participants were not randomised. We excluded 11 studies identified in the search for the 2019 update. Seven studies included non‐randomised patients (Alroughani 2013; De Pasquale 2014; Ghezzi 2013; Hubbard 2012; Radak 2014; Zagaglia 2013; Zivadinov 2013); and four studies, available only as protocols from ClinicalTrials.gov, were terminated due to inability to enrol adequate number of participants — no data were available (ACTRN12612000302853; NCT01089686; NCT01201707; NCT01555684). The table 'Characteristics of excluded studies' provides details of excluded studies.
Risk of bias in included studies
We have summarised the risks of bias of the included studies in Figure 2 and Figure 3. Considering our predefined criteria for assessing the overall risk of bias of a study (Assessment of risk of bias in included studies), we judged two trials at unclear risk of bias (Siddiqui 2014; Traboulsee 2018).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
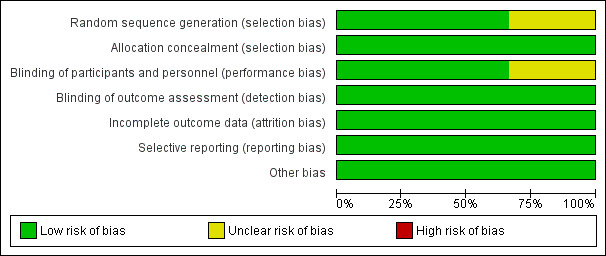
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation was at low risk of bias in two studies (Traboulsee 2018; Zamboni 2018); and was not reported in Siddiqui 2014. Allocation concealment was at low risk of bias in all studies.
Blinding
Two studies blinded either participants or personnel so we considered them to be at low risk of bias (Siddiqui 2014; Zamboni 2018). Traboulsee 2018 reported blinding of participants incompletely so we judged this study as having an unclear risk of bias. As all studies used assessors who were blinded to intervention assignment, we judged all studies as having a low risk of bias for outcome assessment.
Incomplete outcome data
We considered the data reporting of outcomes to be complete, with a low risk of bias in all studies.
Selective reporting
We considered that all included studies have reported all outcomes based on the detailed published protocols or described in the trial methods.
Other potential sources of bias
In Zamboni 2018, participants in the sham group had longer disease duration. We considered that this baseline imbalance did not cause bias in the intervention effect estimate. We did not find any other potential source of bias in Siddiqui 2014 and Traboulsee 2018.
Effects of interventions
See: Table 1
See Table 1 for the main comparison.
Primary outcomes
Safety
Operative or post‐operative serious adverse events (SAEs)
The PTA intervention probably does not increase the risk of SAEs compared with the sham procedure (risk ratio (RR) versus sham 3.33, 95% confidence interval (CI) 0.36 to 30.44; I² = 0%; 3 studies, 238 participants; moderate‐quality evidence; Analysis 1.1 ). Siddiqui 2014 reported that one patient in the PTA arm presented with an SAE at 24 hours. It was an episode of symptomatic bradycardia that was confirmed by telemetry; consequently, a cardiac consultation recommended pacemaker installation. Traboulsee 2018 reported one asymptomatic internal jugular dissection in the PTA group that did not require intervention or hospitalization. Zamboni 2018 reported that no SAEs attributable to catheter venography or venous PTA or sham occurred within 24 hours from the PTA intervention.
1.1. Analysis.
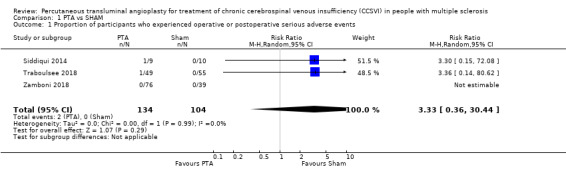
Comparison 1 PTA vs SHAM, Outcome 1 Proportion of participants who experienced operative or postoperative serious adverse events.
Operative or post‐operative adverse events
Siddiqui 2014 reported that one patient in the PTA arm presented with swelling and soreness at the left side of the neck and no treatment was required. Traboulsee 2018 reported that three (5%) of 54 sham and three (6%) of 49 PTA participants had moderate or severe pain during the intervention (P = 0.88); six (11%) sham and four (8%) PTA participants had post‐procedure pain (P = 0.62). Twenty (36%) sham participants and 17 (35%) PTA participants reported 37 and 22 AEs respectively within 48 hours post intervention. The most commonly reported periprocedural AEs were groin pain (7.7%), haematoma (8.6%), and neck pain (5.7%). Zamboni 2018 reported two AEs (1.7%): one vagal reaction and one episode of transient neck pain.
Serious adverse events reported during the follow‐up
Siddiqui 2014 reported that one patient in the sham arm presented with an SAE at 6 months after the venography. The event was a viral infection causing immune thrombocytopenic purpura and the authors judged it as unrelated to the intervention. Traboulsee 2018 reported SAEs in 2% and 10% of sham and PTA participants respectively. None of the reported SAEs were judged as related to the PTA by the blinded physician. The SAEs were generalized seizure (1 sham, week 17), sepsis (2 venoplasty, weeks 20 and 25), bleeding of a previously undiagnosed cerebral aneurysm (1 venoplasty, week 46), myocardial infarction (1 venoplasty, week 28), and pulmonary embolism (1 venoplasty, week 17). Zamboni 2018 did not provide information about SAEs during the 12 months' follow‐up.
Adverse events reported during the follow‐up
Siddiqui 2014 reported AEs over six months: one bladder infection and one shingles event in the sham arm and one hospitalization for scheduled transobturator sling procedure in the PTA arm. Traboulsee 2018 reported that the number of participants with any AEs reported from baseline to week 48 was 42% (23/55) for sham and 43% (21/49) for venoplasty (P = 1). The most commonly reported AEs were gastrointestinal reflux or discomfort, paraesthesia and/or lightheadedness, arthralgia, and general malaise. There were no cases of venous thrombosis up to week 48. Zamboni 2018 did not provide information about AEs during the 12 months' follow‐up.
Benefit
Clinical measured outcomes, including disability worsening measured by Expanded Disability Status Scale (EDSS) (Kurtzke 1983); or any other functional outcome as reported by the authors of included studies
Clinical outcomes
Siddiqui 2014 reported no significant within‐ or between‐group changes in the EDSS at six months' follow‐up. Traboulsee 2018 reported that there was little change in median EDSS score at 11 months' follow‐up in either group. Zamboni 2018 reported that the median (interquartile range) EDSS score was 2.0 (1.5 to 3.0) in the PTA group and 2.0 (1.5 to 2.5) in the sham group (P = 0.49) at 12 months' follow up.
A composite functional outcome including walking control, balance, manual dexterity, postvoid residual urine volume, and visual acuity was evaluated in Zamboni 2018 at 12 months. A total of 30 of 73 participants (41%) in the PTA group and 18 of 37 (49%) in the sham group improved on the functional outcome — a difference of −7% (95% CI −26.7 to 10.1) in favour of the sham group (RR 0.84, 95% CI 0.55 to 1.30; 1 study, 110 participants; low‐quality evidence; Analysis 1.2). Worsening occurred in nine participants (12%) in the PTA group versus seven (19%) in the sham group; functional stability was maintained in 17 (23%) in the PTA group and 8 (22%) in the sham group. A fluctuant outcome (improvement in one or more functions and worsening in one or more) occurred in 16 participants (22%) in the PTA group and 4 (11%) in the sham group.
1.2. Analysis.
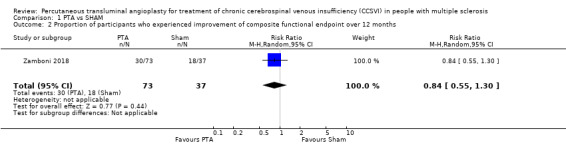
Comparison 1 PTA vs SHAM, Outcome 2 Proportion of participants who experienced improvement of composite functional endpoint over 12 months.
Patient‐reported outcomes (PROs)
Siddiqui 2014 reported that no significant between‐group changes in QoL outcomes were detected in participants. Traboulsee 2018 reported a transient increase in MSQOL scores within 72 hours (mental scores) and 2 weeks (physical scores) in both groups. The mean improvement from baseline to week 48 for MSQOL physical score was 1.3 and 1.4 (sham vs venoplasty P = 0.95); MSQOL mental score 1.2 and −0.8 (sham vs venoplasty P = 0.55); fatigue score was 0.2 and 0.1 (sham vs venoplasty P = 0.65); pain score was 0.1 and −0.2 (sham vs venoplasty P = 0.19). There was no significant difference in the proportion of sham and venoplasty participants who had an improvement in all the other PROs from baseline to week 48. Zamboni 2018 did not report any PRO outcome.
Secondary outcomes
Proportion of participants who experienced new relapses over 12 months
PTA compared with sham probably makes no difference to the risk of new relapses (RR 0.87, 95% CI 0.51 to 1.49; I² = 0%; 3 studies, 235 participants; moderate‐quality evidence; Analysis 1.3). Siddiqui 2014 reported that there were four relapses in the treated arm (among 3 participants) and one in the sham arm. The relapses occurred at 1, 3 (2 relapses), and 6 months in the treated arm and at 5 months in sham group. Traboulsee 2018 reported relapses in eleven participants (6 in sham, 5 in venoplasty) over 48 weeks. Zamboni 2018 reported that seventeen of 73 participants (23%) in the PTA group had one relapse over the 12 months compared with 12 of 39 (31%) in the sham group.
1.3. Analysis.
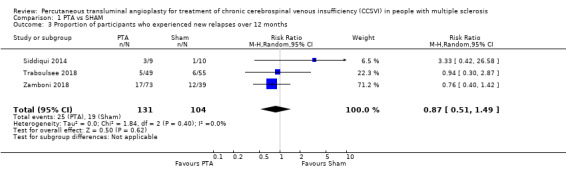
Comparison 1 PTA vs SHAM, Outcome 3 Proportion of participants who experienced new relapses over 12 months.
Mean change in cognitive functions assessment
Siddiqui 2014 reported that no significant between‐group changes in cognitive outcomes were detected.
Proportion of participants who experienced post‐intervention restored venous flow
Siddiqui 2014 reported that improvement of venous haemodynamic insufficiency severity score (VHISS) was observed in treatment arm (P = 0.02) and sham arm (P = 0.04) at month 1 post intervention but did not reach more than 75% restoration of venous outflow compared to baseline. No differences in VHISS improvement were detected between treated and sham groups (P = 0.89). Zamboni 2018 reported that blinded flow assessment at 12 months revealed restored flow in 38 of 71 patients (54%) in the PTA group and 14 of 37 (38%) in the sham group. Traboulsee 2018 did not report the outcome.
Discussion
Summary of main results
The aim of this updated review was to assess the effects of percutaneous transluminal angioplasty (PTA) for the treatment of CCSVI in people with MS. We added three new studies to the original review; four studies previously identified as ongoing had been terminated and provided no outcome data. The three studies we included comprised 238 people of ages 18 to 65 years with mainly relapsing‐remitting MS; 134 were randomised to PTA and 104 to sham treatment. Durations of studies ranged from six to 12 months.
Serious adverse events (SAEs) were reported in all studies and the results showed that the PTA intervention probably did not increase the risk of operative or post‐operative SAEs compared with the sham procedure. Our confidence in the long‐term safety of the PTA intervention is low because information on SAEs during follow‐up was poorly reported. Moderate or severe pain during or post venography was reported in the PTA and sham participants in all studies.
Patient‐centred outcomes such as disability worsening — measured by the Expanded Disability Status Scale (three studies), a functional outcome including walking control, balance, manual dexterity, postvoid residual urine volume, and visual acuity (one study), and relapses over 12 months post intervention (three studies) — were available to evaluate benefit of the PTA intervention. We detected no differences overall in these outcomes between treatment groups and there was no heterogeneity between studies. Quality of life was reported in two studies with no difference between treatment groups. While the data available were limited they were of moderate quality (GRADE), so we have moderate‐certainty evidence that PTA compared with sham makes no difference to all these outcomes.
Venous PTA was not effective in restoring blood flow assessed at one month (one study) or 12 months (one study) post intervention.
Overall completeness and applicability of evidence
The review includes representation from people with MS of age range 18 to 65 years, of mainly the RRMS subtype and evaluated in populations in Italy, Canada and USA. Two were multicentric studies and hence overall applicable. The evidence required had to provide information about the safety of the procedure, improvement in disability, relapses and patient‐reported outcomes, as well on the effectiveness of the procedure and long‐term safety and effectiveness. There were data available on safety and primary effectiveness of the procedure regarding patency but the outcomes reflecting clinical improvement were addressed only partially in the trials of the review.
The applicability of the available evidence needs to be considered in light of the fact that some of the non‐randomised excluded studies have been well‐documented studies of either poor association of CCSVI with MS or ineffectiveness of PTA for CCSVI; and they were undertaken during a period similar to that of the studies included in the review. It should also be noted that the most recent international practice of countries outside the aforementioned countries excludes this form of treatment.
Quality of the evidence
Our review included three studies, which involved 238 participants. We considered the quality of the evidence for safety and benefit outcomes to be moderate because of small patient numbers included in these studies; and imprecision. We further downgraded the quality of evidence for patient‐reported outcomes due to incomplete outcomes data.
Potential biases in the review process
We made every attempt to limit bias in the review process by ensuring a comprehensive search of potentially eligible studies (up to August 2018) since the publication of the original review in 2012, to reduce the possibility that we might overlook additional studies eligible for inclusion. The authors' independent assessments of eligibility of studies for inclusion in this review minimised the potential for additional bias.
Agreements and disagreements with other studies or reviews
To our knowledge, no systematic reviews are available to compare with our review.
Authors' conclusions
Implications for practice.
This systematic review identified moderate‐quality evidence that, compared with sham procedure, the PTA intervention did not provide benefit on patient‐centred outcomes (disability, physical or cognitive functions, relapse, quality of life) in people with MS. Moreover, the fact that results for restored blood flow were similar for treated and sham groups suggested that PTA was not effective in restoring venous outflow. Venous PTA has proven to be a safe technique but this intervention cannot be recommended in people with MS in view of the available evidence that it is largely ineffective.
Implications for research.
No further randomised clinical studies are needed.
Feedback
Alessandro Rasman, 5 July 2019
Summary
Comment: I think the studies examined are too few to draw definitive conclusions on this topic
Do you have any affiliation with or involvement in any organisation with a financial interest in the subject matter of your comment?:
Reply
The review authors ensured a comprehensive search of potentially eligible randomized clinical trials up to August 2018. They judged the quality of the evidence for safety and benefit outcomes to be moderate according to GRADE. This means that the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
One of the four review authors has declared to have COI. This is reported in the section “Declarations of interest” in the published review.
Contributors
Vanitha A Jagannath
What's new
| Date | Event | Description |
|---|---|---|
| 29 July 2019 | Feedback has been incorporated | We have replied to feedback from Alessandro Rasman |
History
Protocol first published: Issue 6, 2012 Review first published: Issue 12, 2012
| Date | Event | Description |
|---|---|---|
| 30 August 2018 | New citation required and conclusions have changed | Three new studies added. Conclusion changed. GRADE used to assess the evidence. |
| 30 August 2018 | New search has been performed | Full searches were performed for this review in August 2018. New studies were identified for inclusion in the review. |
Acknowledgements
The review authors would like to thank the Editorial Base of the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group and the peer reviewers for their help in updating this review.
Appendices
Appendix 1. Keywords for searching the Cochrane MS Group Specialised Register
{cerebral vein\*} OR {cerebrospinal fluid} OR {jugular vein\*} OR {regional blood flow} OR {venous insufficiency} OR {vertebral artery} OR {chronic cerebrospinal venous insufficiency} OR {chronic vein insufficiency} OR {vein} OR {veins} OR {CCSVI} OR {vascular disorder}
AND
{angioplasty} OR {venoplasty} OR {percutaneous transluminal angioplasty} OR {endovascular} OR {angioplasties} OR {transluminal angioplasty} OR {endoluminal repair\*} OR {percutaneous transluminal artery dilatation} OR {balloon} OR {transluminal artery dilatation} OR {stent} OR {stenting} OR {surgery} OR {catheter venography}
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Multiple Sclerosis, this term only
#2 MeSH descriptor Multiple Sclerosis, Chronic Progressive, this term only
#3 MeSH descriptor Multiple Sclerosis, Relapsing‐Remitting, this term only
#4 MeSH descriptor Myelitis, Transverse explode trees 3, 5 and 7
#5 MeSH descriptor Optic Neuritis explode all trees
#6 MeSH descriptor Encephalomyelitis, Acute Disseminated, this term only
#7 MeSH descriptor Demyelinating Autoimmune Diseases, CNS, this term only
#8 MeSH descriptor Demyelinating Diseases, this term only
#9 "multiple sclerosis":ti,ab,kw or "chronic progressive multiple sclerosis":ti,ab,kw or "progressive relapsing multiple sclerosis":ti,ab,kw or "secondary progressive multiple sclerosis":ti,ab,kw or "primary progressive multiple sclerosis":ti,ab,kw or "relapsing remitting multiple sclerosis":ti,ab,kw or "remitting‐relapsing multiple sclerosis":ti,ab,kw or "acute relapsing multiple sclerosis":ti,ab,kw or "neuromyelitis optica":ti,ab,kw or "optic neuritis":ti,ab,kw or "devic disease":ti,ab,kw or "demyelinating disease":ti,ab,kw or (adem):ti,ab,kw or "demyelinating disorder":ti,ab,kw or "clinically isolated syndrome":ti,ab,kw or "transverse myelitis":ti,ab,kw or "acute disseminated encephalomyelitis":ti,ab,kw or (encephalomyelitis):ti,ab,kw
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Constriction, Pathologic explode all trees
#12 MeSH descriptor Cerebrovascular Disorders, this term only
#13 MeSH descriptor Venous Insufficiency, this term only
#14 MeSH descriptor Endothelium, Vascular explode all trees
#15 "cerebral vein*":ti,ab,kw or "chronic cerebrospinal venous insufficiency":ti,ab,kw or (CCSVI):ti,ab,kw or "cerebrospinal fluid":ti,ab,kw
#16 (#11 OR #12 OR #13 OR #14 OR #15)
#17 MeSH descriptor Angioplasty, this term only
#18 MeSH descriptor Angioplasty, Balloon explode all trees
#19 MeSH descriptor Catheterization, this term only
#20 MeSH descriptor Catheterization, Central Venous, this term only
#21 MeSH descriptor Ambulatory Surgical Procedures explode all trees
#22 (angioplast*):ti,ab,kw or "percutaneous transluminal angioplasty":ti,ab,kw or "transluminal angioplasty":ti,ab,kw or (venoplasty):ti,ab,kw or (percutaneous AND transluminal):ti,ab,kw
#23 (endoluminal AND repair):ti,ab,kw or "catheter venography":ti,ab,kw or "endoluminal repair":ti,ab,kw or (endovascular):ti,ab,kw or (stent*):ti,ab,kw
#24 (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23)
#25 (#16 AND #24)
#26 (#10 AND #25)
Appendix 3. MEDLINE (PubMed) search strategy
((((("jugular vein"[Title/Abstract]) OR "regional blood flow"[Title/Abstract]) OR "vascular disorder"[Title/Abstract]) OR vein*[Title/Abstract]) OR "venous insufficiency"[Title/Abstract]) OR "vertebral artery"[Title/Abstract] OR pathologic constriction[MeSH Terms] OR cerebrovascular disorder[MeSH Terms] OR (("Venous Insufficiency"[Mesh:noexp]) OR ("Endothelium, Vascular"[Mesh]) OR ((((("cerebral vein*"[Title/Abstract]) OR "chronic cerebrospinal venous insufficiency"[Title/Abstract]) OR "chronic vein insufficiency"[Title/Abstract]) OR CCSVI[Title/Abstract]) OR "cerebrospinal fluid"[Title/Abstract])) AND ((((("Angioplasty"[Mesh:noexp]) OR "Angioplasty, Balloon"[Mesh:noexp]) OR "Catheterization"[Mesh:noexp]) OR (((angioplast*[Title/Abstract]) OR "percutaneous transluminal angioplasty"[Title/Abstract]) OR "transluminal angioplasty"[Title/Abstract]) OR (venoplasty[Title/Abstract]) OR (((percutaneous[Title/Abstract]) AND transluminal[Title/Abstract]) OR ((endoluminal[Title/Abstract]) AND repair*[Title/Abstract])) OR ("Ambulatory Surgical Procedures"[Mesh]))) OR (((("catheter venography"[Title/Abstract]) OR "endoluminal repair"[Title/Abstract]) OR endovascular[Title/Abstract]) OR stent*[Title/Abstract]) AND (((((((("Multiple Sclerosis"[Mesh:noexp] OR "Multiple Sclerosis, Chronic Progressive"[Mesh]) OR "Multiple Sclerosis, Relapsing‐Remitting"[Mesh]) OR "Demyelinating Diseases"[Mesh:noexp]) OR "Optic Neuritis"[Mesh]) OR "Demyelinating Autoimmune Diseases, CNS"[Mesh:noexp]) OR "Encephalomyelitis, Acute Disseminated"[Mesh]) OR "Myelitis, Transverse"[Mesh]) OR ((((((((((((((((("multiple sclerosis"[Title/Abstract]) OR "chronic progressive multiple sclerosis"[Title/Abstract]) OR "progressive relapsing multiple sclerosis"[Title/Abstract]) OR "secondary progressive multiple sclerosis"[Title/Abstract]) OR "primary progressive multiple sclerosis"[Title/Abstract]) OR "relapsing remitting multiple sclerosis"[Title/Abstract]) OR "remitting‐relapsing multiple sclerosis"[Title/Abstract]) OR "acute relapsing multiple sclerosis"[Title/Abstract]) OR "neuromyelitis optica"[Title/Abstract]) OR "optic neuritis"[Title/Abstract]) OR "devic disease"[Title/Abstract]) OR "demyelinating disease"[Title/Abstract]) OR adem[Title/Abstract]) OR "demyelinating disorder"[Title/Abstract]) OR "clinically isolated syndrome"[Title/Abstract]) OR "transverse myelitis"[Title/Abstract]) OR "acute disseminated encephalomyelitis"[Title/Abstract] OR ("encephalomyelitis"[Title/Abstract])))
Appendix 4. EMBASE (embase.com)
'multiple sclerosis'/exp OR 'demyelinating disease'/exp OR 'optic neuritis'/exp OR 'acute disseminated encephalomyelitis'/exp OR 'myelooptic neuropathy'/exp OR 'myelitis'/exp OR 'multiple sclerosis':ab,ti OR 'chronic progressive multiple sclerosis':ab,ti OR 'progressive relapsing multiple sclerosis':ab,ti OR 'secondary progressive multiple sclerosis':ab,ti OR 'primary progressive multiple sclerosis':ab,ti OR 'relapsing remitting multiple sclerosis':ab,ti OR 'remitting‐relapsing multiple sclerosis':ab,ti OR 'acute relapsing multiple sclerosis':ab,ti OR 'optic neurities':ab,ti OR 'neuromyelitis optica':ab,ti OR encephalomyelitis:ab,ti OR 'clinically isolated syndrome':ab,ti OR 'transverse myelitis':ab,ti OR 'devic disease':ab,ti OR 'demyelinating disease':ab,ti OR 'demyelinating disorder':ab,ti OR 'acute disseminated encephalomyelitis':ab,ti OR adem:ab,ti AND (percutaneous AND transluminal:ab,ti OR (endoluminal AND repair*:ab,ti) OR 'ambulatory surgery'/exp OR 'angioplasty'/exp OR 'percutaneous transluminal angioplasty'/exp OR 'catheterization'/de OR angioplast*:ab,ti OR 'percutaneous transluminal angioplasty':ab,ti OR 'transluminal angioplasty':ab,ti OR venoplasty:ab,ti OR 'catheter venography':ab,ti OR 'endoluminal repair':ab,ti OR endovascular*:ab,ti) AND ('stenosis, occlusion and obstruction'/exp OR 'cerebrovascular disease'/de OR 'vein insufficiency'/de OR 'chronic cerebrospinal venous insufficiency'/exp OR 'chronic vein insufficiency'/exp OR 'vascular endothelium'/exp OR ccsvi:ab,ti OR 'cerebral vein':ab,ti OR 'chronic cerebrospinal venous insufficiency':ab,ti OR 'chronic vein insufficiency':ab,ti OR 'cerebrospinal fluid':ab,ti OR 'vascular disorder':ab,ti OR vein*:ab,ti OR 'venous insufficiency':ab,ti) AND [embase]/lim NOT [medline]/lim
Data and analyses
Comparison 1. PTA vs SHAM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants who experienced operative or postoperative serious adverse events | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 3.33 [0.36, 30.44] |
| 2 Proportion of participants who experienced improvement of composite functional endpoint over 12 months | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.55, 1.30] |
| 3 Proportion of participants who experienced new relapses over 12 months | 3 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.51, 1.49] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Siddiqui 2014.
| Methods | Randomised controlled study of 6 months' duration. Participants were enrolled between June 2010 and March 2012 at the University of Buffalo, NY. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
The mean age was 43.3 years in the treatment group and 44.8 years in the sham group. The median disease duration was 9 years in the treatment group and 10 years in the sham group. Females were 56% (5/9) and 80% (8/10) respectively in the treatment and the sham group; and all participants were on disease‐modifying drugs. The 2 groups were similar for baseline characteristics. |
|
| Interventions | Venous angioplasty: 9 participants; Sham: 10 participants Venous angioplasty. Patients were heparinized to confirm an activated clotting time of at least 250 seconds. A noncompliant balloon with nominal diameter of at least 80% of the proximal vein (of interest) was placed across the stenosis over a 0.035‐inch glide wire. Inflation proceeded slowly at a rate of 1 atmosphere per 30s until nominal pressure was reached (8 to 12 atmospheres). The dilated balloon was left in place for 5 minutes and then deflated at a rate of 1 atmosphere per 15s. Once the balloon was completely deflated, it was withdrawn and the diagnostic catheter reintroduced over an exchange wire to perform a post‐procedure selective venogram and assess residual stenosis. The goal of the angioplasty was to restore the venous outflow stricture to > 50% of normal proximal venous diameter. Additional angioplasty was performed if > 50% residual stenosis remained. Once adequate angioplasty had been performed, the catheter, wire, and sheath were removed and the venous access site at the level of the common femoral vein was compressed using manual compression for 20 minutes followed by placement of a non‐occlusive vascular clamp for 1 hour. Post‐procedurally, patients were admitted to a monitored (constant apnea and cardiac monitors) unit for observation of any immediate adverse events (AEs). The groin was inspected every 4 hours until discharge. At 4 hours post procedure, if there was no evidence of a growing groin haematoma, the patients received the first dose of enoxaparin sodium, 30 mg subcutaneously, which was continued on a once‐daily basis for 3 weeks (drug provided at discharge). In addition, a daily dose of aspirin, 81 mg, was given starting on the day of the procedure for a total of 3 weeks. Patients were observed overnight and discharged the next morning if there were no AEs. Sham procedure. As above, except the balloon was inserted but not inflated in the sham‐procedure group. |
|
| Outcomes |
Primary outcomes: (i) percentage of patients presenting with severe AEs at 24 hours (immediate) and 1 month (short‐term) post‐endovascular procedure; (ii) number of relapses over the 6 months. Secondary outcomes: restoration of venous outflow > 75% at 1 month compared to baseline; new MRI‐based lesion activity; clinical relapse rate over 6 months. Additional endpoints included changes in EDSS, brain volume, cognitive test, 6‐minute walk, quality of life, and MS functional composite scores. |
|
| Notes | Funding. Kaleida Health (New York) in the form of provision of diagnostic and interventional services at no cost for the study; Direct MS Foundation (Canada), Volcano Corp (California), Covidien/ev3 Corp (California), and Jacquemin Family Foundation (Virginia) in the form of unrestricted educational grants or donations to Kaleida Health or to the State University of New York at Buffalo. This was an investigator‐initiated study and there was no involvement from any of the sponsoring organizations in the design, collection, analysis, interpretation of data, writing of the report, or submission for publication. ClinicalTrials.gov: NCT01450072. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Randomization was performed by an independent statistician in 1:1 fashion using sealed and numbered envelopes with predetermined treatments (Appendix e‐3). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No staff member from the safety, imaging, or clinical evaluation arms of this study was present during the treatment procedure. The operating room staff received training about the blinding requirements and avoided any loud procedure‐related conversation. Relatively loud music of the patient's choice was played to further distract sedated patients so that procedural conversation was inaudible. X‐ray shields were covered with opaque sterile covers and monitors were angled away from patients to prevent them from observing images of their procedure. All patients received a rigorous sternal rub (painful stimulus) upon insertion of the angioplasty balloon, regardless of inflation. A blindness assessment survey was administered the following morning prior to discharge. The survey results showed that 90% of patients confirmed that they did not know whether they received angioplasty or the sham endovascular procedure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel, with the exception of the interventional neurosurgeons, were blind to the assigned procedure (p442). No staff member from the safety, imaging, or clinical evaluation arms of this study was present during the treatment procedure (Appendix e‐3). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were followed up till the end of the study (6 months) and were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | A priori protocol mentioned and the outcomes indicated reported. |
| Other bias | Low risk | |
Traboulsee 2018.
| Methods | Randomised controlled study of 11 months' duration. The study was conducted at 4 Canadian academic centres with MS clinics and interventional radiology expertise (University of British Columbia [UBC] Hospital, Vancouver; Health Sciences Centre, Winnipeg; CHUM, Hôpital Notre‐Dame, Montreal; Hôpital Enfant‐Jesus, Quebec). Participants were recruited between 29 May 2013, and 19 August 2015. | |
| Participants | Participants with relapsing‐remitting MS (RRMS), secondary progressive MS, and primary progressive MS. Inclusion criteria:
Participants on standard disease‐modifying therapies (DMTs) were permitted to continue on medication, and changes were allowed for on‐study relapses after randomization. Exclusion criteria:
274 participants screened, 104 randomized The mean age was 50.5 years (range 33 to 65) with a mean disease duration of 17 years; 65% (68/104) of participants were women and 62% (64/104) had RRMS. 69% percent (44/64) of participants with RRMS were on disease‐modifying drugs. Characteristics were similar between treatment groups and centres. On baseline venography, 50% of participants (32/64) with RRMS and 72% (29/40) with secondary progressive or primary progressive MS had multiple vessels with > 50% narrowing. |
|
| Interventions | Venous angioplasty: 49 participants; Sham: 55 participants. Intervention was either sham or active balloon venoplasty of all narrowed veins. The venoplasty participants were treated with an angioplasty balloon 2 mm greater than the nominal vein diameter, which was inflated for 60 seconds. A repeat venoplasty was performed for persistent narrowing > 50%. The participants of sham had a catheter that was advanced across the stenosis and left for 60 seconds. |
|
| Outcomes |
Primary outcomes:
Secondary outcomes were the changes in MSQOL‐54 physical and mental composite from baseline to 72 hours. Fatigue Severity Scale, North American Research Committee on MS pain scale, and CCSVI symptom scale from baseline to 72 hours and week 48, and change in EDSS (median) and MSFC (mean) from baseline to week 48, relapses. Combined unique MRI active lesions, defined as a contrast‐enhancing lesion on T1‐weighted scan or a non‐T1 enhancing, new/enlarging T2 lesions, measured at baseline, weeks 24 and 48. |
|
| Notes | The study was primarily supported by cooperative agreements from the Canadian Institutes of Health Research (CIHR), MS Society of Canada, Michael Smith Foundation for Health Research, Research Manitoba, and Ministère de la Santé et des Services Sociaux du Quebec. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified treatment randomization (RRMS vs progressive MS course) at each site was completed by a permuted block size of 6 to reduce the likelihood of obtaining unbalanced groups. The randomization table was generated by an independent statistician (e1662). |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were sealed in individual envelopes, only opened after eligibility was confirmed, and resealed after the procedure (e1662). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All participants were blinded to intervention assignment (e1662). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessors were blinded to intervention assignment. The interventional team was not involved in any outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 49 participants in the treatment group and all 55 participants in the sham group were analysed. 1 participant randomized to the sham group withdrew at week 24 because of a time conflict. 1 participant randomized to sham only completed MRI and blood work at week 48 (e1663). |
| Selective reporting (reporting bias) | Low risk | Sufficient information to judge low risk in view of a priori protocol published and outcomes reported as mentioned. |
| Other bias | Low risk | |
Zamboni 2018.
| Methods | Randomised controlled trial of 12 months' duration. The study was conducted at 6 MS centres in Italy and their associated colour Doppler ultrasonography (ECD) and angiography units. The trial began in August 2012 and concluded in March 2016; data were analysed from April 2016 to September 2016. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
177 participants with relapsing‐remitting MS assessed for eligibility; 62 excluded; 115 participants enrolled and randomised. The mean age was 40.0 years in the PTA group and 37.5 years in the sham group. The median disease duration was 4.3 years in the PTA group and 6.1 years in the sham group. Females were 59% (45/76) and 74% (29/39) respectively in the PTA and the sham group, and 41% (31/76) and 46% (18/39) were on disease‐modifying drugs. The 2 groups were similar for baseline characteristics except that participants in the sham group had more women and longer disease duration. |
|
| Interventions | Venous percutaneous transluminal angioplasty (PTA): 76 participants. Sham: 39 participants. PTA. Participants underwent catheter venography without venous angioplasty of the azygos and internal jugular veins, with percutaneous access via the left femoral vein. If venography was positive for CCSVI, participants randomized to the PTA group received venous PTA during the venography session. If CCSVI was absent, those assigned to the PTA group received catheter venography without venous angioplasty. Sham. Those allocated to the sham group received catheter venography without venous angioplasty. These procedures were performed via day surgery. Overnight hospital stay was never required in this trial. All patients received prophylactic low‐molecular‐weight heparin during the 3 subsequent weeks. |
|
| Outcomes |
2 primary outcomes:
Secondary outcomes at 12 months were the proportion of participants with CCSVI diagnosed by EchoDoppler but not confirmed by venography; annualized relapse rate; change in EDSS score; proportion of participants with relapses; and proportion of participants who had venous PTA with restored flow on EchoDoppler at 12 months. |
|
| Notes | This article was supported by the Directorate‐General for Health and Welfare of the Italian Region of Emilia Romagna and by contributing charities. ClinicalTrials.gov: NCT01371760. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The data coordinating centre set up an Internet‐based computerized central randomisation protocol stratified by participating centre with variable length blocks, which assigned participants to the PTA or sham group in a 2:1 ratio (p36). |
| Allocation concealment (selection bias) | Low risk | Treatment assignment was made known to the treating surgeon (via the electronic case report form) only on the day of the operation. Patients and operating room and hospital personnel were blinded to assignment (p36). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To maintain patient blinding, surgeons were trained to deliver a catheter venography intervention that simulated venous PTA. This involved sudden acceleration of the catheter as it passed through the internal jugular vein together with a comment from the radiologist suggesting that venous PTA had been performed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study investigators were blinded to assignment (p36). The MRI scans were assessed by an experienced specialist blinded to treatment assignment (p37). Flow assessment at 12 months was blinded (p39). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72 (95%) of 76 participants in the PTA group and 37 (95%) of 39 in the sham group were analysed for the combined clinical end point. |
| Selective reporting (reporting bias) | Low risk | Sufficient information to judge low risk in view of a priori protocol published and outcomes reported as mentioned. |
| Other bias | Low risk | Participants in the sham group had more women and longer disease duration. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12612000302853 | Terminated in 2012 (www.amzctr.org.au). Unpublished results |
| Alroughani 2013 | Retrospective study of people with MS who had undergone PTA procedure |
| De Pasquale 2014 | An uncontrolled study |
| Ghezzi 2013 | Non‐randomised study |
| Hubbard 2012 | Non‐randomised study |
| NCT01089686 | Terminated on 7 March 2012. 2 participants enrolled (ClinicalTrials.gov). Unpublished results |
| NCT01201707 | Terminated on 26 November 2013. Inability to enrol adequate number of participants (ClinicalTrials.gov). Unpublished results |
| NCT01555684 | Withdrawn on 2 December 2015 (ClinicalTrials.gov). Unpublished results |
| Radak 2014 | Non‐randomised study |
| Zagaglia 2013 | Non‐randomised study |
| Zivadinov 2013 | Non‐randomised study |
Differences between protocol and review
There are no differences between the protocol and the review.
Contributions of authors
The trial search coordinator of the Cochrane MSRDCNS Group was responsible for running the search.
VAJ was responsible for organising the retrieval of papers, writing to authors of papers for additional information, screening search results, screening retrieved papers against inclusion criteria, appraising the quality of papers, and obtaining and screening data on unpublished studies.
GVA and VAJ were responsible for reviewing the studies and extracting outcome data, assessing risk of bias from the papers and entering it into RevMan 5.
VAJ, GVA, and EP were responsible for writing the effects of intervention, analysis and interpretation of the data.
All review authors contributed to writing the review.
VAJ, GVA and EWR conceived the idea for the review and are the guarantors for the review.
VAJ and GVA updated the review.
Sources of support
Internal sources
No sources of support, Netherlands.
External sources
No sources of support, Netherlands.
Declarations of interest
EP has received funds from a non‐profit association, the "Associazione Marchigiana sclerosi multipla e altre malattie neurologiche"; this association has received donations from Biogen Dompé, Merck‐Serono and Bayer‐Schering. In the period 2007 to 2012, EP also received honoraria, reimbursement for attending congresses, and grant support for organising scientific activities from the above‐mentioned drug industries and from Aventis, UCB, Lundbeck and Novartis. The other review authors have no financial conflicts of interest and they do not have any associations with any parties who may have vested interests in the results of this review. One of the authors (ER) has undergone the procedure under consideration in this review.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Siddiqui 2014 {published data only}
- Siddiqui AH, Zivadinov R, Benedict RH, Karmon Y, Yu J, Hartney ML, et al. Prospective randomized trial of venous angioplasty in MS (PREMiSe). Neurology 2014;83(5):441‐9. [PUBMED: 24975855] [DOI] [PMC free article] [PubMed] [Google Scholar]
Traboulsee 2018 {published data only}
- Traboulsee AL, Machan L, Girard JM, Raymond J, Vosoughi R, Hardy BW, et al. Safety and efficacy of venoplasty in MS: A randomized, double‐blind, sham‐controlled phase II trial. Neurology 2018;91(18):e1660‐8. [PUBMED: 30266886] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamboni 2018 {published data only}
- Zamboni P, Tesio L, Galimberti S, Massacesi L, Salvi F, D'Alessandro R, et al. Efficacy and safety of extracranial vein angioplasty in multiple sclerosis: a randomized clinical trial. JAMA Neurology 2018;75(1):35‐43. [PUBMED: 29150995] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12612000302853 {published and unpublished data}
- ACTRN12612000302853. A randomised, blinded, controlled study of percutaneous transluminal angioplasty (PTA) for extracranial vein stenoses in patients with multiple sclerosis (MS). www.anzctr.org.au.
Alroughani 2013 {published data only}
- Alroughani R, Lamdhade S, Thussu A. Endovascular treatment of chronic cerebrospinal venous insufficiency in multiple sclerosis: a retrospective study. International Journal of Neuroscience 2013;123(5):324‐8. [PUBMED: 23301864] [DOI] [PubMed] [Google Scholar]
De Pasquale 2014 {published data only}
- Pasquale C, Pistorio ML, Veroux M, Giaquinta A, Veroux P, Fornaro M. Cognitive functioning and subjective quality of life in relapsing‐remitting multiple sclerosis patients before and after percutaneous transluminal angioplasty: a preliminary report. Neuropsychiatric Disease and Treatment 2014;10:1039‐44. [PUBMED: 24959079] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghezzi 2013 {published data only}
- Ghezzi A, Annovazzi P, Cocco E, Coarelli G, Lugaresi A, Rovaris M, et al. Endovascular treatment of CCSVI in patients with multiple sclerosis: clinical outcome of 462 cases. Neurological Sciences 2013;34(9):1633‐7. [DOI] [PubMed] [Google Scholar]
Hubbard 2012 {published data only}
- Hubbard D, Ponec D, Gooding J, Saxon R, Sauder H, Haacke M. Clinical improvement after extracranial venoplasty in multiple sclerosis. Journal of Vascular and Interventional Radiology : JVIR 2012;23(10):1302‐8. [PUBMED: 22951366] [DOI] [PubMed] [Google Scholar]
NCT01089686 {unpublished data only}
- NCT01089686. Study to evaluate treating chronic cerebrospinal venous insufficiency (CCSVI) in multiple sclerosis patients. www.clinicaltrials.gov.
NCT01201707 {unpublished data only}
- NCT01201707. Evaluation of angioplasty in the treatment of chronic cerebrospinal venous insufficiency (CCSVI) in multiple sclerosis. www.clinicaltrials.gov.
NCT01555684 {unpublished data only}
- NCT01555684. Functional changes following percutaneous venoplasty in multiple sclerosis patients. www.clinicaltrials.gov.
Radak 2014 {published data only}
- Radak D, Kolar J, Sagic D, Ilijevski N, Tanaskovic S, Aleksic N, et al. Percutaneous angioplasty of internal jugular and azygous veins in patients with chronic cerebrospinal venous insufficiency and multiple sclerosis: early and mid‐term results. Phlebology 2014;29(6):367‐75. [PUBMED: 23563645] [DOI] [PubMed] [Google Scholar]
Zagaglia 2013 {published data only}
- Zagaglia S, Balestrini S, Perticaroli E, Danni MC, Luzzi S, Silvestrini M, et al. Percutaneous transluminal angioplasty for chronic cerebrospinal venous insufficiency in multiple sclerosis: dichotomy between subjective and objective outcome scores. Neurological Sciences 2013;34(12):2205‐10. [PUBMED: 23660636] [DOI] [PubMed] [Google Scholar]
Zivadinov 2013 {published data only}
- Zivadinov R, Magnano C, Galeotti R, Schirda C, Menegatti E, Weinstock‐Guttman B, et al. Changes of cine cerebrospinal fluid dynamics in patients with multiple sclerosis treated with percutaneous transluminal angioplasty: a case‐control study. Journal of Vascular and Interventional Radiology : JVIR 2013;24(6):829‐38. [PUBMED: 23523158] [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Omari 2010
- Al‐Omari MH, Rousan LA. Internal jugular vein morphology and hemodynamics in patients with multiple sclerosis. International Angiology 2010;29(2):115‐20. [PUBMED: PMID: 20351667] [PubMed] [Google Scholar]
Anon 2010
- Anon. Experimental multiple sclerosis vascular shunting procedure halted at Stanford. Annals of Neurology 2010;67(1):A13‐5. [PUBMED: PMID: 20186848] [DOI] [PubMed] [Google Scholar]
Bagert 2011
- Bagert BA, Marder E, Stüve O. Chronic cerebrospinal venous insufficiency and multiple sclerosis. Archives of Neurology 2011;68(11):1379‐84. [PUBMED: PMID: 21747006] [DOI] [PubMed] [Google Scholar]
Baracchini 2011
- Baracchini C, Perini P, Calabrese M, Causin F, Rinaldi F, Gallo P. No evidence of chronic cerebrospinal venous insufficiency at multiple sclerosis onset. Annals of Neurology 2011;69(1):90‐9. [PUBMED: PMID: 21280079] [DOI] [PubMed] [Google Scholar]
Bavera 2011
- Bavera PM, Mendozzi L, Cavarretta R, Agus GB. Venous extracranial Duplex ultrasound and possible correlations between multiple sclerosis and CCSVI: an observational study after 560 exams. Acta Phlebologica 2011;12(2):109‐13. [Google Scholar]
Benjaminy 2018
- Benjaminy S, Schepmyer A, Illes J, Traboulsee A. Resilience, trust, and civic engagement in the post‐CCSVI era. BMC Health Services Research 2018;18(1):366. [PUBMED: 29769084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 2011
- Burton JM, Alikhani K, Goyal M, Costello F, White C, Patry D, et al. Complications in MS patients after CCSVI procedures abroad (Calgary, AB). Canadian Journal of Neurological Sciences [Journal Canadien des Sciences Neurologiques] 2011;38(5):741‐6. [PUBMED: PMID: 21856578] [DOI] [PubMed] [Google Scholar]
Centonze 2011
- Centonze D, Floris R, Stefanini M, Rossi S, Fabiano S, Castelli M, et al. Proposed chronic cerebrospinal venous insufficiency criteria do not predict multiple sclerosis risk or severity. Annals of Neurology 2011;70(1):51‐8. [PUBMED: PMID: 21786298] [DOI] [PubMed] [Google Scholar]
Comi 2013
- Comi G, Battaglia MA, Bertolotto A, Sette M, Ghezzi A, Malferrari G, et al. Observational case‐control study of the prevalence of chronic cerebrospinal venous insufficiency inmultiple sclerosis: results from the CoSMo study. Multiple Sclerosis (Houndmills, Basingstoke, England) 2013;19(11):1508‐17. [PUBMED: 24014572] [DOI] [PubMed] [Google Scholar]
D'haeseleer 2011
- D'haeseleer M, Cambron M, Vanopdenbosch L, Keyser J. Vascular aspects of multiple sclerosis. Lancet Neurology 2011;10(7):657–66. [PUBMED: PMID: 21683931] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
Diehm 2007
- Diehm N, Baumgartner I, Jaff M, Do DD, Minar E, Schmidli J, et al. A call for uniform reporting standards in studies assessing endovascular treatment for chronic ischaemia of lower limb arteries. European Heart Journal 2007;28(7):798‐805. [PUBMED: PMID: 17317699] [DOI] [PubMed] [Google Scholar]
Doepp 2010
- Doepp F, Paul F, Valdueza JM, Schmierer K, Schreiber SJ. No cerebrocervical venous congestion in patients with multiple sclerosis. Annals of Neurology 2010;68(2):173‐83. [PUBMED: PMID: 20695010] [DOI] [PubMed] [Google Scholar]
Dorne 2010
- Dorne H, Zaidat OO, Fiorella D, Hirsh J, Prestigiacomo C, Albuquerque F, et al. Chronic cerebrospinal venous insufficiency and the doubtful promise of an endovascular treatment for multiple sclerosis. AACN Clinical Issues in Critical Care Nursing 2010;2(4):309‐11. [DOI] [PubMed] [Google Scholar]
Driedger 2017
- Driedger SM, Maier R, Marrie RA, Brouwers M. Caught in a no‐win situation: discussions about CCSVI between persons with multiple sclerosis and their neurologists ‐ a qualitative study. BMC Neurology 2017;17(1):176. [PUBMED: 28882115] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: PMID: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 1999
- Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Multiple Sclerosis 1999;5(4):251‐9. [PUBMED: PMID:10467384] [DOI] [PubMed] [Google Scholar]
Ghezzi 2011
- Ghezzi A, Comi G, Federico A. Chronic cerebro‐spinal venous insufficiency (CCSVI) and multiple sclerosis. Neurological Sciences 2011;32(1):17‐21. [PUBMED: PMID: 21161309] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hojnacki 2010
- Hojnacki D, Zamboni P, Lopez‐Soriano A, Galleotti R, Menegatti E, Weinstock‐Guttman B, et al. Use of neck magnetic resonance venography, Doppler sonography and selective venography for diagnosis of chronic cerebrospinal venous insufficiency: a pilot study in multiple sclerosis patients and healthy controls. International Angiology 2010;29(2):127‐39. [PUBMED: PMID: 20351669] [PubMed] [Google Scholar]
Khan 2010
- Khan O, Filippi M, Freedman MS, Barkhof F, Dore‐Duffy P, Lassmann H, et al. Chronic cerebrospinal venous insufficiency and multiple sclerosis. Annals of Neurology 2010;67(3):286‐90. [PUBMED: PMID: 20373339] [DOI] [PubMed] [Google Scholar]
Kos 2005
- Kos D, Kerckhofs E, Carrea I, Verza R, Ramos M, Jansa J. Evaluation of the Modified Fatigue Impact Scale in four different European countries. Multiple Sclerosis (Houndmills, Basingstoke, England) 2005;11(1):76‐80. [DOI] [PubMed] [Google Scholar]
Krogias 2010
- Krogias C, Schröder A, Wiendl H, Hohlfeld R, Gold R. "Chronic cerebrospinal venous insufficiency" and multiple sclerosis: critical analysis and first observation in an unselected cohort of MS patients [in German]. Der Nervenarzt 2010;81(6):740‐6. [PUBMED: PMID: 20386873] [DOI] [PubMed] [Google Scholar]
Kuhlmann 2017
- Kuhlmann T, Ludwin S, Prat A, Antel J, Bruck W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathologica 2017;133(1):13‐24. [DOI] [PubMed] [Google Scholar]
Kurtzke 1983
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33(11):1444‐52. [PUBMED: PMID: 6685237] [DOI] [PubMed] [Google Scholar]
Lazzaro 2011
- Lazzaro MA, Zaidat OO, Mueller‐Kronast N, Taqi MA, Woo D. Endovascular therapy for chronic cerebrospinal venous insufficiency in multiple sclerosis. Frontiers in Neurology 2011;2:44. [PUBMED: PMID: 21808631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lublin 1996
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46(4):907‐11. [PUBMED: PMID: 8780061] [DOI] [PubMed] [Google Scholar]
Lucchinetti 2000
- Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions:implications for the pathogenesis of demyelination. Annals of Neurology 2000;47(6):707‐17. [DOI] [PubMed] [Google Scholar]
Malagoni 2010
- Malagoni AM, Galeotti R, Menegatti E, Manfredini F, Basaglia N, Salvi F, et al. Is chronic fatigue the symptom of venous insufficiency associated with multiple sclerosis? A longitudinal pilot study. International Angiology 2010;29(2):176‐82. [PUBMED: PMID: 20351673] [PubMed] [Google Scholar]
Marder 2011
Martino 2002
- Martino G, Adorini L, Rieckmann P, Hillert J, Kallmann B, Comi G, et al. Inflammation in multiple sclerosis: the good, the bad, and the complex. Lancet Neurology 2002;1(8):499‐509. [PUBMED: 12849335] [DOI] [PubMed] [Google Scholar]
McDonald 2001
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. [PUBMED: PMID: 11456302] [DOI] [PubMed] [Google Scholar]
Paul 2014
- Paul F, Wattjes MP. Chronic cerebrospinal venous insufficiency in multiple sclerosis: the final curtain. Lancet 2014;383(9912):106‐8. [PUBMED: 24119383] [DOI] [PubMed] [Google Scholar]
Piga 2014
- Piga MA. About patients, "inventors", journalists, scientists and IRBS (to say nothing of the institutions): CCSVI and MS. Medicine and Law 2014;33(4):177‐87. [PUBMED: 27351054] [PubMed] [Google Scholar]
Polman 2005
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Annals of Neurology 2005;58(6):840‐6. [PUBMED: PMID: 16283615] [DOI] [PubMed] [Google Scholar]
Polman 2011
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology 2011;69(2):292‐302. [PUBMED: PMID: 21387374] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 1991
- Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 1991;41(5):692‐6. [PUBMED: PMID: 1823781] [DOI] [PubMed] [Google Scholar]
Reekers 2011
- Reekers JA, Lee MJ, Belli AM, Barkhof F. Cardiovascular and Interventional Radiological Society of Europe commentary on the treatment of chronic cerebrospinal venous insufficiency. Cardiovascular and Interventional Radiology 2011;34(1):1‐2. [PUBMED: PMID: 21136256] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rudick 2007
- Rudick RA, Miller D, Hass S, Hutchinson M, Calabresi PA, Confavreux C, et al. Health‐related quality of life in multiple sclerosis: effects of natalizumab. Annals of Neurology 2007;62(4):335‐46. [PUBMED: PMID: 17696126] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sellner 2011
- Sellner J, Kraus J, Awad A, Milo R, Hemmer B, Stüve O. The increasing incidence and prevalence of female multiple sclerosis ‐ a critical analysis of potential environmental factors. Autoimmunity Reviews 2011;10(8):495‐502. [PUBMED: PMID: 21354338] [DOI] [PubMed] [Google Scholar]
Simeoni 2008
- Simeoni M, Auquier P, Fernandez O, Flachenecker P, Stecchi S, Constantinescu C. Validation of the Multiple Sclerosis International Quality of Life questionnaire. Multiple Sclerosis (Houndmills, Basingstoke, England) 2008;14(2):219‐30. [PUBMED: PMID:17942521] [DOI] [PubMed] [Google Scholar]
Simka 2010
- Simka M, Kostecki J, Zaniewski M, Majewski E, Hartel M. Extracranial Doppler sonographic criteria of chronic cerebrospinal venous insufficiency in the patients with multiple sclerosis. International Angiology 2010;29(2):109‐14. [PUBMED: PMID: 20351666] [PubMed] [Google Scholar]
Simpson 2011
- Simpson S Jr, Blizzard L, Otahal P, Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta‐analysis. Journal of Neurology, Neurosurgery, and Psychiatry 2011;82(10):1132‐41. [PUBMED: PMID: 21478203] [DOI] [PubMed] [Google Scholar]
Singh 2009
- Singh AV, Zamboni P. Anomalous venous blood flow and iron deposition in multiple sclerosis. Journal of Cerebral Blood Flow and Metabolism 2009;29(12):1867‐78. [PUBMED: PMID: 19724286] [DOI] [PubMed] [Google Scholar]
Sundström 2010
- Sundström P, Wåhlin A, Ambarki K, Birgander R, Eklund A, Malm J. Venous and cerebrospinal fluid flow in multiple sclerosis: a case‐control study. Annals of Neurology 2010;68(2):255‐9. [PUBMED: PMID: 20695018] [DOI] [PubMed] [Google Scholar]
Thompson 2018
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, at al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurology 2018;17(2):162‐73. [DOI] [PubMed] [Google Scholar]
Traboulsee 2014
- Traboulsee AL, Knox KB, Machan L, Zhao Y, Yee I, Rauscher A, et al. Prevalence of extracranial venous narrowing on catheter venography in people with multiple sclerosis, their siblings, and unrelated healthy controls: a blinded, case‐control study. Lancet (London, England) 2014;383(9912):138‐45. [PUBMED: 24119384] [DOI] [PubMed] [Google Scholar]
Tsivgoulis 2011
- Tsivgoulis G, Mantatzis M, Bogiatzi C, Vadikolias K, Voumvourakis K, Prassopoulos P, et al. Extracranial venous hemodynamics in multiple sclerosis: a case‐control study. Neurology 2011;77(13):1241‐5. [PUBMED: PMID: 21849653] [DOI] [PubMed] [Google Scholar]
van Rensburg 2010
- Rensburg SJ, Toorn R. The controversy of CCSVI and iron in multiple sclerosis: is ferritin the key?. Neurology 2010;75(18):1581‐2. [PUBMED: PMID: 20881276] [DOI] [PubMed] [Google Scholar]
Vickrey 1995
- Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health‐related quality of life measure for multiple sclerosis. Quality of Life Research 1995;4(3):187‐206. [PUBMED: PMID: 7613530] [DOI] [PubMed] [Google Scholar]
Waschbisch 2011
- Waschbisch A, Manzel A, Linker RA, Lee D. Vascular pathology in multiple sclerosis: mind boosting or myth busting?. Experimental & Translational Stroke Medicine 2011;3(1):7. [PUBMED: PMID: 21756314] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wattjes 2011
- Wattjes MP, Oosten BW, Graaf WL, Seewann A, Bot JC, Berg R, et al. No association of abnormal cranial venous drainage with multiple sclerosis: a magnetic resonance venography and flow‐quantification study. Journal of Neurology, Neurosurgery, and Psychiatry 2011;82(4):429‐35. [PUBMED: PMID: 20980483] [DOI] [PubMed] [Google Scholar]
Weinshenker 1989
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain 1989;112(Pt 6):1419‐28. [PUBMED: PMID: 2597989] [DOI] [PubMed] [Google Scholar]
Weinstock‐Guttman 2011
- Weinstock‐Guttman B, Zivadinov R, Cutter G, Tamaño‐Blanco M, Marr K, Badgett D, et al. Chronic cerebrospinal vascular insufficiency is not associated with HLA DRB1*1501 status in multiple sclerosis patients. PLoS One 2011;6(2):e16802. [PUBMED: PMID: 21340025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2011
- Wu GF, Alvarez E. The immuno‐pathophysiology of multiple sclerosis. Neurologic Clinics 2011;29(2):257‐78. [PUBMED: 21439440] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamout 2010
- Yamout B, Herlopian A, Issa Z, Habib RH, Fawaz A, Salame J, et al. Extracranial venous stenosis is an unlikely cause of multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2010;16(11):1341‐8. [PUBMED: PMID: 21041329] [DOI] [PubMed] [Google Scholar]
Zamboni 2006
- Zamboni P. The Big Idea: iron‐dependent inflammation in venous disease and proposed parallels in multiple sclerosis. Journal of the Royal Society of Medicine 2006;99(11):589‐93. [PUBMED: PMID: 18045150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamboni 2009a
- Zamboni P, Galeotti R, Menegatti E, Malagoni AM, Gianesini S, Bartolomei I, et al. A prospective open‐label study of endovascular treatment of chronic cerebrospinal venous insufficiency. Journal of Vascular Surgery 2009;50(6):1348‐58. [PUBMED: PMID: 19958985] [DOI] [PubMed] [Google Scholar]
Zamboni 2009b
- Zamboni P, Galeotti R, Menegatti E, Malagoni AM, Tacconi G, Dall’Ara S, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry 2009;80(4):392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamboni 2011
- Zamboni P, Menegatti E, Weinstock‐Guttman B, Dwyer MG, Schirda CV, Malagoni AM, et al. Hypoperfusion of brain parenchyma is associated with the severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: a cross‐sectional preliminary report. BMC Medicine 2011;9:22. [PUBMED: PMID: 21385345] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamboni 2012
Zivadinov 2011a
- Zivadinov R, Marr K, Cutter G, Ramanathan M, Benedict RH, Kennedy C, et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology 2011;77(2):138‐44. [PUBMED: PMID: 21490322] [DOI] [PubMed] [Google Scholar]
Zivadinov 2011b
- Zivadinov R, Ramanathan M, Dolic K, Marr K, Karmon Y, Siddiqui AH, et al. Chronic cerebrospinal venous insufficiency in multiple sclerosis: diagnostic, pathogenetic, clinical and treatment perspectives. Expert Review of Neurotherapeutics 2011;11(9):1277‐94. [PUBMED: 21864074] [DOI] [PubMed] [Google Scholar]
Zivadinov 2014
- Zivadinov R, Bastianello S, Dake MD, Ferral H, Haacke EM, Haskal ZJ, et al. International Society for Neurovascular Disease. Recommendations for multimodal non invasive and invasive screening for detection of extracranial venous abnormalities indicative of chronic cerebrospinal venous insufficiency: a position statement of the International Society for Neurovascular Disease. Journal of Vascular and Interventional Radiology : JVIR 2014;25(11):1785‐1794.e17. [PUBMED: 25255703] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
van Zuuren 2012
- Zuuren EJ, Fedorowicz Z, Pucci E, Jagannath VA, Robak EW. Percutaneous transluminal angioplasty for treatment of chronic cerebrospinal venous insufficiency (CCSVI) in multiple sclerosis patients. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD009903.pub2] [DOI] [PubMed] [Google Scholar]


