Abstract
Objective. The use of multiple-level, single-injection intercostal nerve blocks for pain control following video-assisted thorascopic surgery (VATS) is limited by the analgesic duration of local anesthetics. This study examines whether the combination of perineural and intravenous (IV) dexamethasone will prolong the duration of intraoperatively placed intercostal nerve blocks following VATS compared with IV dexamethasone and a perineural saline placebo.
Design. Prospective, double-blind, randomized placebo-controlled trial.
Setting. Single level-1 academic trauma center.
Subjects. Forty patients undergoing a unilateral VATS under the care of a single surgeon.
Methods. Patients were randomly assigned to two groups and received an intercostal nerve block containing 1) 0.5% bupivacaine with epinephrine and 1 ml of 0.9% saline or 2) 0.5% bupivacaine with epinephrine and 1 ml of a 4 mg/ml dexamethasone solution. All patients received 8 mg of IV dexamethasone.
Results. Group 2 had lower NRS-11 scores at post-operative hours 8 (5.05, SD = 2.13 vs 3.50, SD = 2.50; p = 0.04), 20 (4.30, SD = 2.96 vs 2.26, SD = 2.31; p = 0.02), and 24 (4.53, SD = 1.95 vs 2.26, SD = 2.31; p = 0.02). Equianalgesic opioid requirement was decreased in group 2 at 32 hours (5.78 mg, SD = 5.77 vs 1.67 mg, SD = 3.49; p = 0.02). Group 2 also had greater FEV1 measured at 8, 12, 24, and 44 hours; greater FVC at 24 hours; greater PEF at 28 through 48 hours; and greater FEV1/FVC at 8 and 36 hours.
Conclusions. The combination of IV and perineural dexamethasone prolonged the duration of a single-injection bupivacaine intercostal nerve block as measured by NRS-11 compared with IV dexamethasone alone at 24 hours. Reduced NRS-11 at other times, reduced opioid requirements, and increased PFTs were observed in group 2.
Keywords: Acute Pain, Thoracic, Postoperative Pain, Steroids, Outcome Assessment
Introduction
VATS is a minimally invasive surgical approach utilized for the resection of pulmonary nodules, such as early stage lung cancer. Compared with open thoracotomy surgical techniques, VATS uses smaller incisions and is associated with reduced yet persistent postoperative pain [1]. Post-VATS pain management may include a combination of opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), or regional anesthesia. Thoracic incisional pain results in a restrictive breathing pattern, causing limited voluntary deep breathing and coughing. Post-VATS restrictive breathing patterns are associated with atelectasis, retention of secretions, increased pulmonary shunting, hypoxemia, and postoperative pneumonias [1]. Effective pain management will prevent or delay the development of a restrictive breathing pattern and improve effort-dependent PFTs, including forced expiratory volume over 1 second (FEV1), forced vital capacity (FVC), and peak expiratory flow (PEF) [2]. There is widespread variation on a national level for the management of postoperative pain in these patients, including the use of thoracic epidurals, intercostal and interscalene blocks, and IV analgesics. At the institution at which this study was performed, multilevel single-injection intercostal blocks in combination with opioid analgesia is the preferred method of postoperative pain control.
Dexamethasone is a soluble glucorticoid that has been evaluated as an adjunct to local anesthetics to prolong the analgesic duration of single-injection peripheral nerve blocks [3–8]. While dexamethasone’s clinical utility as a local anesthetic adjunct is common practice and supported by a strong safety profile, perineural use is not currently approved by the FDA and is considered “off-label” use [3]. When used perineurally, comparison of escalating doses of dexamethasone do not result in increased analgesic benefit at higher doses or decreased analgesic benefit at lower doses [9,10]. Comparison of the analgesic benefit of equal doses of intravenous (IV) to perineural dexamethasone to prolong peripheral nerve blocks produced mixed results, with most studies suggesting equivalency and a single prospective randomized trial suggesting superiority of perineural blocks [11–14]. In the setting of systemic IV dexamethasone therapy, it is not clear whether perineural dexamethasone will confer additional analgesic benefit to intercostal nerve blocks compared with a perineural saline placebo.
The question we sought to answer is whether the combination of IV and perineural dexamethasone will prolong the duration of analgesia after multiple-level, single-injection intercostal nerve blocks over IV dexamethasone and perineural saline placebo as measured by an 11-point numeric rating scale (NRS-11, with 0 = no pain and 10 = the most severe pain imaginable) at 24 hours. We also aimed to examine NRS-11 at other time points, opioid requirements, and the effect on PFTs as secondary endpoints. We hypothesized that the combination of IV and perineural dexamethasone would result in lower pain scores and decreased opioid requirements and delay the onset of restrictive breathing patterns following VATS procedures compared with the IV dexamethasone and perineural saline placebo group.
Methods
Study Design and Patient Population
The study was reviewed by the IRB and registered on clinicaltrials.gov. Consecutive adult patients between the ages of 18 and 80 who were scheduled to undergo a unilateral VATS under the care of a single thoracic surgeon were recruited during the outpatient pre-operative evaluation. Exclusion criteria were as follows: patient younger than 18 or older than 80, known allergy to any of the study medications, American Society of Anesthesiologists (ASA) physical status 4 or greater, history of ipsilateral thoracoscopic surgery, uncontrolled coronary artery disease, uncontrolled hypertension, cardiac arrhythmias, chronic renal or liver disease, body mass index greater than 40 kg/m2, FEV1 or FVC less than 70% of predicted value, type 1 or type 2 diabetes mellitus, chronic neuropathic pain, chronic steroid use, or chronic opioid use. Patients were excluded from continuation with the study if the intraoperative findings required conversion to a more invasive procedure than a VATS, such as rib resection, chest wall resection, open sternotomy, or surgical re-exploration within the study period, as these surgical procedures result in more tissue trauma and higher requirements for postoperative pain. Patients requiring postoperative intubation and ventilation were also excluded due to the use of sedating medications while intubated. Finally, patients were excluded if the intraoperative analgesic protocol (see below) was deemed insufficient for safe and effective intraoperative management by an attending anesthesiologist.
Randomization and Blinding
The study was designed as a randomized, double-blind, placebo-controlled trial. Prior to recruitment, a computer was used to randomly assign consecutive patients to one of two groups. Patients were assigned a study number according to the order in which they were recruited. A nontransparent envelope was then prepared with the patient’s assigned consecutive study number on the outside and the patient’s group assignment and instructions for the intercostal nerve block solution preparation sealed within. On the day of the surgery, the operating room circulating nurse, who was not involved in the study, would open the envelope containing the instructions and prepare the intercostal nerve block solution. Using sterile technique, the circulating nurse would then pass the solution to the surgical scrub technician. The technician would draw the solution into a 30-cc syringe labeled “block solution.” Patients in group 1 received a block solution that contained 29 ml of 0.5% bupivacaine with 1:200,000 epinephrine (Hospira, Lake Park, Illinois) and 1 ml of sterile saline (Hospira, Lake Park, Illinois). Patients in group 2 received a block solution that contained 29 ml of 0.5% bupivacaine with 1:200,000 epinephrine and 1 ml of a 4 mg/ml of dexamethasone solution (Hospira, Lake Park, Illinois). All patients received 8 mg IV dexamethasone to minimize postoperative nausea and reduce airway inflammation from the use of double lumen endotracheal tubes intraoperatively for one-lung isolation.
Surgical and Anesthetic Conduct
The anesthesia team was made aware of the patient’s participation in the study and were asked to limit the amount of intraoperative IV analgesic to either 250 ug of IV fentanyl or 4 mg of IV hydromorphone, both of which are less than the usual anticipated necessary dose for minimally invasive VATS. If this opioid limitation proved to be an unsatisfactory anesthetic practice, the patient could be given additional analgesic mediations but would be excluded from analysis. Additionally, the anesthesia team was asked to refrain from using additional IV analgesics such as IV ketamine, IV ketorolac, and IV acetaminophen, as these medications can have opioid sparing effects. All patients received a dose of 8 mg of IV dexamethasone at the time of induction for the reasons listed above. The anesthesia team was not informed to which group the patient had been assigned. Oral and IV analgesic care in the post-anesthesia care unit (PACU) was at the discretion of the anesthesiologist and was not limited by participation in the study.
Surgery was conducted utilizing a standardized general anesthetic technique and one-lung ventilation in the lateral position. A standardized minimally invasive technique was employed. Masses were identified with palpation and removed with endoscopic staple devices. After masses were excised, they were sent for formal pathologic analysis. A chest tube was placed at a site chosen by the surgeon between T3 and T8 prior to skin closure. No patient underwent additional pain control regimens such as a thoracic epidural or repeated intercostal nerve blocks. The chest tubes were maintained for the duration of the study.
At the conclusion of the surgical procedure but prior to dermal closure, multiple-level, single-injection, unilateral intercostal nerve blocks of T3 through T8 were performed by the surgical team under direct thoracoscopic visualization. The T3 through T8 region included all surgical incisions for trochar and chest tube insertion. The exact site of surgical incision was at the discretion of the surgical team within the T3 to T8 region. A 1.5-inch 22-gauge quinke point needle was inserted under the inferior aspect of the ribs as posterior in the chest as possible and away from the visualized sympathetic trunk. After negative aspiration for blood, 5 ml of block solution was introduced when the needle could be seen just below the pleura to create a subpleural wheel. The contents of the block solution were determined by randomization into one of the two treatment groups. The surgeon was blinded to the assignment.
The standardized postoperative pain regimen included 1000 mg of intravenous acetaminophen every 8 hours as a standing order, and oral oxycodone was ordered pro re nata according to the patient’s individual pain needs. Additional IV hydromorphone was also utilized for severe pain not effectively managed with oral medication. No patient enrolled in the study required additional pain medications such as methadone or patient-controlled analgesia (PCA) pumps. Early mobilization, incentive spirometry, and ambulation with aggressive physical therapy was encouraged in all patients.
Outcomes
The primary outcome measure is pain assessed by NRS-11 pain scores at 24 hours postoperatively. Measurements of pain were assessed every 4 hours. Pain was determined by integrating the NRS-11 score over the 4-hour interval to minimize reporting bias.
The secondary outcome measures of the study included NRS-11 at other time points; opioid requirements; and PFTs, including FEV1, FVC, FEV1/FVC as measured at 4-hour intervals, starting 4 hours after discontinuation of anesthesia and continuing for 48 hours postoperatively. Gender, age, race, and height determined normal predicted values of PFTs. Spirometry readings were taken with the patient sitting upright and at rest. All PFT measurements were taken using a spiroUSB spirometer connected to a portable laptop computer running SPCS Spirometry software (CareFusion, Hoechberg, Germany). The spirometer was calibrated prior to each spirometric reading. For analysis, spirometry data was divided into 4-hour blocks starting from the end of surgery.
Sample Size Estimation
At the time of initiation of this study, no published material was available concerning the effect of dexamethasone on intercostal nerve blocks with bupivacaine. Based on institutional experience, at 24 hours postoperatively, the addition of dexamethasone to an intercostal nerve block resulted in a 50% reduction in NRS-11 pain. A power calculation was performed prior to the recruitment of subjects. Using an alpha value of 0.05 and a beta value of 0.02, a sample size estimation determined that 20 patients per group for a total of 40 patients would have 85% power to a 50% difference in postoperative pain between groups at 24 hours after placement of the nerve block, assuming a standard deviation of 2.5. To correct for patient dropout, 25 patients per group were included in the study.
Statistical Analysis
All data were coded in an Excel file (Microsoft, Redmond, WA) and imported into SAS version 9.3 statistical software package (SAS Institute Inc., Cary, NC) and R software for analysis. Continuous variables are reported as means and standard deviations, while categorical variables are presented as frequencies and proportions. A Shapiro-Wilk test was used to determine normalcy of data distribution. Continuous variables were compared using the T test if normally distributed and the Wilcoxon-Mann-Whitney U test if not normally distributed. Categorical variables were compared using a Chi-square test. A P value less than or equal to 0.05, which corresponds to a 95% confidence interval or greater, was deemed statistically significant.
Spirometric data was compared between the two treatment groups at each of the equivalent postsurgical blocks of time using the Wilcoxon-Mann-Whitney test. In other words, the mean at 8 hours for group 1 was compared against the mean for group 2 at 8 hours.
Measures of both primary and secondary outcomes were repeated in individual subjects over time. Mixed effect analyses were used to isolate and characterize intersubject variability and to analyze repeated outcomes, including pain relief over time [15]. Six separate nonparametric mixed effect modeling methods were used to analyze the time and group and their interaction effects for each of the primary and secondary endpoints individually using the R software package.
Results
A total of 49 patients were consecutively enrolled and randomized from December 2013 until February 2015. Nine patients were excluded; two were excluded due to a strong desire not to participate in the study on the day of surgery, one was excluded due to the need for intubation on postoperative day 1 for reasons unrelated to the study, and six were excluded due to the need for extensive chest wall resection in order to offer therapeutic benefit. Figure 1 details patient selection for the study. All patients received single-injection, multiple-level intercostal nerve blocks during surgery. Patients were randomized to group 1, which received IV dexamethasone and perineural saline placebo, or group 2, which received IV and perineural dexamethasone. All patients who met inclusion criteria completed the study, and no major adverse events were observed. No symptoms attributable to systemic doses of either bupivacaine or dexamethasone were observed. Baseline characteristics and anesthetic factors were balanced between the groups (Table 1).
Figure 1.

Study design and patient recruitment.
Table 1.
Characteristics of 40 VATS patients treated with intercostal nerve blocks
| Bupivacaine + Saline (n = 20) | Bupivacaine + Dexamethasone (n = 20) | P values | |
|---|---|---|---|
| Age (years) | 67.1 (12.12) | 68.75 (9.34) | 0.63 |
| Gender | |||
| Male | 8 (40%) | 13 (65%) | |
| Female | 12 (60%) | 7 (35%) | |
| BMI | 25.9 (5.42) | 23.23 (3.27) | 0.07 |
| ASA status | |||
| ASA 2 | 7 (35%) | 2 (10%) | |
| ASA 3 | 13 (65%) | 18 (90%) | |
| Comorbidities | 15 (30.6) | 5 (14.7) | |
| Congestive heart failure | 0 (0%) | 0 (0%) | |
| Coronary artery disease | 6 (30%) | 4 (20%) | |
| Hypertension | 10 (50%) | 11 (55%) | |
| Cerebrovascular accident | 0 (0%) | 1 (5%) | |
| Myocardial infarction | 0 (0%) | 1 (5%) | |
| Asthma | 4 (20%) | 2 (10%) | |
| Preoperative glucose (mg/dL) | 103.3 (17.7) | 105.1 (18.7) | 0.8 |
| Social history | |||
| Prior smoker | 14 (70%) | 13 (65%) | |
| Smoker at preoperative visit | 4 (20%) | 2 (10%) | |
| Pack-years smoked | 32.26 (26.27) | 25.58 (37.32) | 0.41 |
| Lung tumor pathology | |||
| Stage 1adenocarcinoma | 5 (25%) | 7 (35%) | |
| Stage 2 adenocarcinoma | 3 (15%) | 5 (25%) | |
| Stage 3 adenocarcinoma | 5 (25%) | 0 (0%) | |
| Stage 4 adenocarcinoma | 2 (10%) | 2 (10%) | |
| Other pathology | 5 (25%) | 6 (30%) | |
| Tumor longest dimension (cm) | 2.25 (1.3) | 2.18 (1.43) | 0.87 |
| Mass of removed tissue (g) | 97.07 (80.39) | 124.67 (86.38) | 0.32 |
| Duration of anesthesia (minutes) | 149.1 (27.86) | 133.65 (32.69) | 0.16 |
| Intraoperative equianalgesic opioid dose (mg of PO MSO4) | 48.68 (21.42) | 45 (20.64) | 0.58 |
| Time in PACU (minutes) | 159.42 (82.31) | 196.4 (92.4) | 0.2 |
Values are expressed as mean (standard deviation) for continuous data or count (percentage) for ordinal data. ASA = American Society of Anesthesiologists physical status. Mg = milligrams. dL= deciliter. Cm= centimeters. G = grams. PO= per os. MSO4= Morphine sulfate. Intraoperative opioid dose is reported in milligrams equianalgesic dose of oral morphine. PACU = postanesthesia care unit.
The primary finding of this study is that patients in group 1 reported significantly greater NRS-11 pain scores postoperatively compared with group 2 at 24 hours (4.53, SD = 1.95 vs 2.26, SD = 2.31; P = 0.02). Additionally, patients in group 1 reported higher pain scores at hours 8 (5.05, SD = 2.13 vs 3.50 SD = 2.50; P = 0.04) and 20 (4.30, SD = 2.96 vs 2.26, SD = 2.31; P = 0.02; Figure 2). Mixed effect analysis demonstrated that group 2 was significantly negatively related to NRS-11 scores (–1.83, t= –3.41, P = 0.01). The mixed effect model also demonstrated a decrease in NRS-11 with time in both groups, which is expected as patients recover from surgery (–0.06, t = –5.57, P = 0.01).
Figure 2.

NRS-11 pain scores.
*indicates P values less than 0.05. Solid blue line represents group 1. Dashed black line indicates group 2. NRS-11 pain scores were measured every 4 hours
Group 1 required more postoperative opioids as measured in equianalgesic milligrams of morphine at 32 hours (5.78 mg, SD = 5.77 vs 1.67 mg, SD = 3.49; P = 0.02; Figure 3). Mixed effect analysis demonstrated a statistical decrease in mean opioid requirement by time, which is also expected as patients recover from surgery (–0.22, t = –5.04, P = 0.01). The interaction of time and group assignment was significantly related to the mean postoperative opioid requirement, with group 1 having a steeper time slope compared with that of group 2 (–1.51, t = –2.33, P = 0.02).
Figure 3.
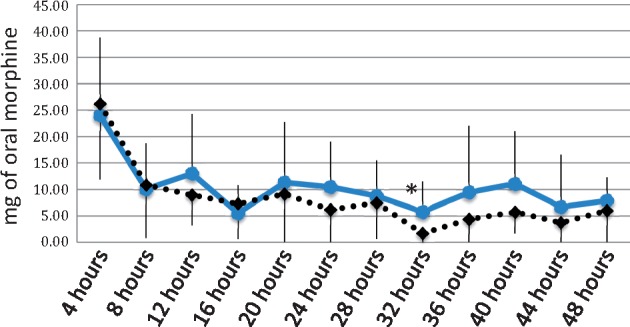
Postoperative opioid requirements.
*indicates P values less than 0.05. Solid blue line represents group 1. Dashed black line indicates group 2. Postoperative opioid requirement is reported in equivalent milligrams of oral morphine.
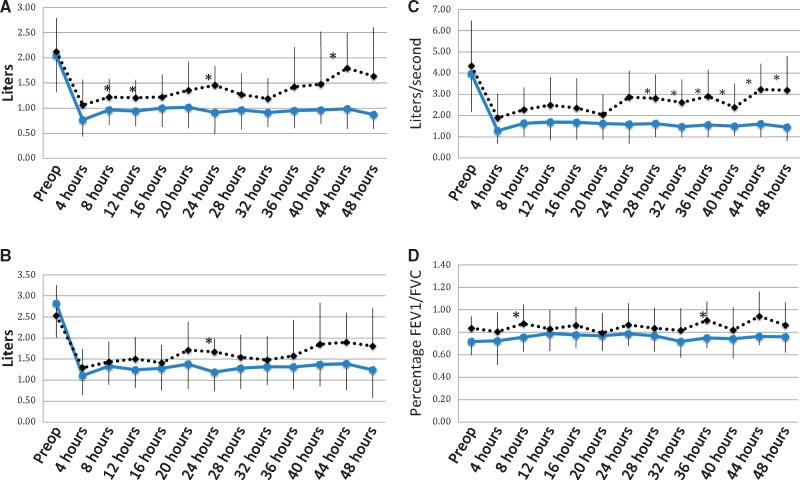
Immediately following the operation, the absolute values of the PFTs were mostly equivalent for the two groups. However, group 2 demonstrated significantly greater FEV1 at 8 hours (0.97 L, SD = 0.30 vs 1.22 L, SD = 0.36; P = 0.02), 12 hours (0.94 L, SD = 0.29 vs 1.21 L, SD = 0.36; P = 0.03), 24 hours (0.91 L, SD = 0.43 vs 1.46 L, SD = 0.39; P = 0.01), and 44 hours (0.99 L, SD = 0.40 vs 1.80 L, SD = 0.70; P = 0.03; Figure 4A). Mixed effect modeling demonstrated that group 2 was significantly positively related to FEV1 (0.21, t = 2.11, P = 0.04). FVC was significantly improved in group 2 at 24 hours postoperatively (1.19 L, SD = 0.45 vs 1.67 L, SD = 0.32; P = 0.01; Figure 4B). Mixed effect modeling demonstrated that group 2 was significantly positively related to FVC (0.12, t = 2.18, P = 0.04) The absolute PEFs were significantly greater in group 2 at 28 hours (1.63 L/s, SD = 0.64 vs 2.81 L/s, SD = 1.14; P = 0.01), and remained significantly greater at 48 hours postoperatively (Figure 4C). Mixed effect modeling demonstrated that group 2 was significantly positively related to PEF (0.19, t = 2.65, P = 0.01). FEV1/FVC was greater in group 2 at 8 hours (0.76, SD = 0.17 vs 0.88, SD = 0.13; P = 0.02) and 36 hours (0.75, SD = 0.17 vs 0.91, SD 0.09, P = 0.02; Figure 4D). Mixed effect modeling did not demonstrate any significant relationships.
Figure 4.
(A) Postoperative FEV1. (B) Post Operative FVC. (C) Post Operative PEF and (D) Post Operative FEV1/FVC.
* indicates P values less than 0.05. Solid blue line represents group 1. Dashed black line indicates group 2.
Conclusions
The primary finding of this study is that the combination of IV and perineural dexamethasone resulted in lower NRS-11 pain scores compared with IV dexamethasone alone at 24 hours. Secondary outcomes of this study are that the combination of IV and systemic dexamethasone resulted in lower NRS-11 pain scores at other time points, lower opioid requirements in the postoperative phase of care, and improved postoperative PFTs. The study was not statistically designed to explore differences in the secondary outcomes. As such, the data can only suggest an improvement in pulmonary mechanics. Improvements in pulmonary mechanics could translate into improved outcomes such as decreased pneumonias and atelectasis and shorter recovery times. However, these were not specifically examined in this study. No adverse outcomes were reported in any of the subjects. Inter- and intrasubject variability was accounted for using mixed effect modeling to determine significance.
Prior published work evaluating the management of thoracic incisional pain has concluded that multiple-level, single-injection, intraoperative intercostal nerve blocks with bupivacaine reduce patient-reported pain scores [16]. However, the same review noted that a reduction in postoperative opioid requirements was not observed after single-injection bupivacaine nerve blocks and that repeat injections or catheters were required for such an observation [16]. This is possibly a reflection on the limited analgesic duration of bupivacaine and other local anesthetics. Augmentation of the duration of analgesia of bupivacaine with adjuvant dexamethasone could improve the efficacy of single-injection regional analgesia relative to multiple-injection or continuous catheter regional analgesia. The efficacy of dexamethasone to prolong the analgesic effect of bupivacaine microspheres for intercostal nerve blocks in animal and healthy volunteers has been demonstrated [17,18]. However, the prolongation of regular bupivacaine intercostal nerve blocks by dexamethasone in a surgical patient population has not been fully elucidated.
Randomized controlled trials and meta-analyses have consistently demonstrated a prolongation of analgesia with the addition of dexamethasone to local anesthetics for both upper and lower extremity nerve blocks [3–10]. These studies report sparse occurrence of significant adverse effects from perineural use of dexamethasone. The most commonly observed side effects include hyperglycemia and flushing, indicating a relatively high therapeutic safety index. In animal models, high doses of perineural dexamethasone have been associated with neuronal cell death [19]. Conversely, also in animal models, dexamethasone has been associated with decreased bupivacaine-induced neuronal injury [20]. The exact risk or benefit that perineural dexamethasone presents with human use has not been established. The utilization of perineural vs systemic dexamethasone for prolongation of analgesia has been studied [13,14]. IV dexamethasone has been demonstrated to produce lower subjective pain scores following surgery compared with a placebo [21]. A limited role of perineural dexamethasone has been postulated based on several studies reporting equivalent observed analgesia of equal doses of perineural dexamethasone compared with IV dexamethasone [11,13]. However, a single study suggests superiority of perineural dexamethasone compared with IV dexamethasone at equal doses [12].
This study demonstrates improved analgesia at 24 hours postoperatively and suggests prolongation at other time points of the effects of bupivacaine with regards to both subjective and objective outcomes. The use of subjective pain assessment tools to access peripheral nerve block effectiveness or duration are valid, but have several potentially confounding shortcomings, such as a lack of ratio qualities, reliance on individual patients’ pain experiences, and over-reporting of pain relief [22–24]. This study attempted to utilize both subjective measurements of pain and objective pulmonary function testing in order to minimize these limitations.
This study has several weaknesses. The major weakness of this study is that it compares 8 mg of IV dexamethasone to a total of 12 mg dexamethasone by two separate routes. Multiple prior studies have failed to demonstrate that there is an observed analgesic benefit with dose escalation of dexamethasone given by the same route [9,10]. This could indicate that the observed analgesic benefit seen in this study is not the result of simply a higher dose of dexamethasone but rather the result of two separate analgesic processes. The positive result of this study may be reflective of different routes of dexamethasone administration producing analgesia via different mechanisms. However, further studies with multiple dosing and administration route arms are needed to optimize this theory for clinical application.
Additionally, in an effort to reduce confounding surgical management variables, patients of a single thoracic surgeon undergoing similar procedures at a level-1 academic medical center were evaluated; this could result in difficulty broadly applying the findings to other settings, such as hospitals that do not practice regional anesthesia with VATS cases due to different operative conditions. Also, as no suitable retrospective or pilot intercostal nerve block studies were available for power analysis, a conservative calculation was performed based on institutional experience. This led to a study design with a specific primary endpoint. Other reported results can only suggest differences between the two groups but will need further validation in future studies.
Despite widespread use as an analgesic adjunct to local anesthetics for regional anesthesia, dexamethasone is not currently approved as an adjuvant to local anesthetics by the United States Food and Drug Administration or European Union [3]. Dexamethasone’s regulatory status was made clear to patients participating in this study. The mechanism of action of perineural dexamethasone is thought to be due to a systemic anti-inflammatory effect, decreased activity of nociceptive C-fibers, and up-regulation of inhibitory potassium channels [8]. The long-term application of steroids to peripheral nerve blocks in animal models does not induce changes in the electrical properties or structure of nerves [25]. Current research does not establish whether systemic steroids have the same analgesic mechanism of action as perineural steroids. Further preclinical study is needed to establish why dose escalation of dexamethasone via the same route does not result in increased analgesia, but dose escalation by two different routes, IV and perineural, dose result in increased analgesia. Different mechanisms of action could account for the observed analgesic benefit when dexamethasone is administered by two distinct routes. Additionally, optimization of the exact ratio of perineural and IV steroid and the choice of steroid needs to be optimized in future studies. Comparative effectiveness and cost-benefit evaluations against other local anesthetic formulations, such as liposomal bupivacaine, will further clarify optimal patient management.
In conclusion, the combination of systemic and perineural dexamethasone increases the duration of intercostal nerve block analgesia, decreases postoperative opioid requirements, and delays the development of restrictive breathing patterns following VATS. Additional studies are needed to conclusively optimize the ratio of systemic vs perineural dexamethasone for pain control. The observed delay in restrictive breathing patterns as measured by postoperative pulmonary function tests in the combined systemic and perineural group compared with the saline control group has the potential to not only improve analgesia but also improve postoperative pulmonary mechanics following VATS.
Acknowledgments
The authors would like to acknowledge Donato Kusuanco for his assistance coordinating this study.
References
- 1. Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin 2008;26:355–67. vii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andreetti C, Menna C, Ibrahim M, et al. Postoperative pain control: Videothoracoscopic versus conservative mini-thoracotomic approach. Eur J Cardiothorac Surg 2014;46:907–12. [DOI] [PubMed] [Google Scholar]
- 3. Choi S, Rodseth R, McCartney CJ.. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: A systematic review and meta-analysis of randomized trials. Br J Anaesth 2014;112:427–39. [DOI] [PubMed] [Google Scholar]
- 4. De Oliveira GS Jr., Castro Alves LJ, Nader A, et al. Perineural dexamethasone to improve postoperative analgesia with peripheral nerve blocks: A meta-analysis of randomized controlled trials. Pain Res Treat 2014;2014:179029.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huynh TM, Marret E, Bonnet F.. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: A meta-analysis of randomised controlled trials. Eur J Anaesthesiol 2015;32:751–8. [DOI] [PubMed] [Google Scholar]
- 6. Kumar S, Palaria U, Sinha AK, Punera DC, Pandey V.. Comparative evaluation of ropivacaine and ropivacaine with dexamethasone in supraclavicular brachial plexus block for postoperative analgesia. Anesth Essays Res 2014;8:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim YJ, Lee GY, Kim DY, et al. Dexamathasone added to levobupivacaine improves postoperative analgesia in ultrasound guided interscalene brachial plexus blockade for arthroscopic shoulder surgery. Korean J Anesthesiol 2012;62:130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albrecht E, Kern C, Kirkham KR.. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia 2015;70:71–83. [DOI] [PubMed] [Google Scholar]
- 9. Tandoc MN, Fan L, Kolesnikov S, Kruglov A, Nader ND.. Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: A prospective randomized trial. J Anesth 2011;25:704–9. [DOI] [PubMed] [Google Scholar]
- 10. Liu J, Richman KA, Grodofsky SR, et al. Is there a dose response of dexamethasone as adjuvant for supraclavicular brachial plexus nerve block? A prospective randomized double-blinded clinical study. J Clin Anesth 2015;27:237–42. [DOI] [PubMed] [Google Scholar]
- 11. Desmet M, Braems H, Reynvoet M, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: A prospective, randomized, placebo-controlled study. Br J Anaesth 2013;111:445–52. [DOI] [PubMed] [Google Scholar]
- 12. Kawanishi R, Yamamoto K, Tobetto Y, et al. Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: A prospective randomized trial. Local Reg Anesth 2014;7:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rahangdale R, Kendall MC, McCarthy RJ, et al. The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: A randomized, double-blind, placebo-controlled study. Anesth Analg 2014;118:1113–9. [DOI] [PubMed] [Google Scholar]
- 14. Abdallah FW, Johnson J, Chan V, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: A randomized, triple-arm, double-blind, placebo-controlled trial. Reg Anesth Pain Med 2015;40:125–32. [DOI] [PubMed] [Google Scholar]
- 15. Shafer SL, Struys MM.. Mixed effect modeling in analgesia trials. Anesth Analg 2008;9–10. [DOI] [PubMed] [Google Scholar]
- 16. Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;1026–40. [DOI] [PubMed] [Google Scholar]
- 17. Kopacz DJ, Lacouture PG, Wu D, et al. The dose response and effects of dexamethasone on bupivacaine microcapsules for intercostal blockade (T9 to T11) in healthy volunteers. Anesth Analg 2003;96:576–82. [DOI] [PubMed] [Google Scholar]
- 18. Drager C, Benziger D, Gao F, Berde CB.. Prolonged intercostal nerve blockade in sheep using controlled-release of bupivacaine and dexamethasone from polymer microspheres. Anesthesiology 1998;89:969–79. [DOI] [PubMed] [Google Scholar]
- 19. Williams BA, Hough KA, Tsui BY, et al. Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med 2011;36:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma R, Wang X, Lu C, et al. Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience 2010;167:329–42. [DOI] [PubMed] [Google Scholar]
- 21. De Oliveira GS Jr., Almeida MD, Benzon HT, McCarthy RJ.. Perioperative single dose systemic dexamethasone for postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology 2011;115:575–88. [DOI] [PubMed] [Google Scholar]
- 22. Feine JS, Lavigne GJ, Dao TT, Morin C, Lund JP.. Memories of chronic pain and perceptions of relief. Pain 1998;77:137–41. [DOI] [PubMed] [Google Scholar]
- 23. Dannecker EA, George SZ, Robinson ME.. Influence and stability of pain scale anchors for an investigation of cold pressor pain tolerance. J Pain 2007;8:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price DD, Bush FM, Long S, Harkins SW.. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 1994;56:217–26. [DOI] [PubMed] [Google Scholar]
- 25. Johansson A, Dahlin L, Kerns JM.. Long-term local corticosteroid application does not influence nerve transmission or structure. Acta Anaesthesiol Scand 1995;39:364–9. [DOI] [PubMed] [Google Scholar]