Abstract
Background
The use of sodium polystyrene sulfonate (SPS) for the treatment of hyperkalemia lacks sufficient efficacy data in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD); however, use remains widespread. Recent evidence suggests that this population may be at risk for serious gastrointestinal adverse effects with SPS.
Methods. We conducted a single-center retrospective cohort study. Adult patients with CKD Stages 4, 5, or ESRD maintained on renal replacement therapy with serum potassium >5 mEq/L and receipt of SPS were screened for inclusion. Our primary outcome was decrease in potassium within 24 h post-30 g oral SPS suspended in 33% sorbitol. Secondary outcomes included decrease in potassium within 24 h from 15 or 30 g SPS doses and gastrointestinal adverse events.
Results
Of 596 records, 114 were included for analysis. At the first serum potassium level within 24 h post-30 g oral SPS the median potassium decrease was 0.8 mEq/L [interquartile range (IQR) 0.4–1.1; P < 0.001]. At the first potassium level within 24 h post-15 or 30 g SPS, the median potassium decrease was 0.7 mEq/L (IQR 0.4–1.0; P < 0.001]. Post-SPS potassium levels occurred 14–16 h post-SPS. Gastrointestinal side effects occurred within 30 days of SPS in 5% of patients, although only two cases were classified as possibly associated.
Conclusions
The use of single-dose SPS monotherapy resulted in a significant decrease in serum potassium levels within 24 h in patients with CKD Stage 4, 5, or ESRD. However, it remains unclear if SPS is associated with an increased risk of gastrointestinal injury in this population.
Keywords: chronic kidney disease, end-stage renal disease, hyperkalemia, sodium polystyrene sulfonate
INTRODUCTION
Chronic kidney disease (CKD) is a condition that affects more than 20 million people in the USA. In Europe, prevalence varies between 3.3% to 17.3% and in China 6.7% to 18.3% [1, 2]. As kidney function deteriorates, the body’s ability to excrete potassium begins to decline, placing those with CKD or end-stage renal disease (ESRD) at a higher risk of hyperkalemia [1, 3, 4]. Current guidelines recommend medical management directed at cardiac stabilization, intracellular shift of serum potassium and potassium removal [4]. Although not a first-line agent for treatment of hyperkalemia, multiple guidelines endorse the use of sodium polystyrene sulfonate (SPS) [5–7].
SPS is a cation exchange resin approved by the Food and Drug Administration (FDA) in the late 1950s for the treatment of hyperkalemia. This therapy functions through an exchange process where cross-linked reactive sulfonate groups exchange bound sodium for potassium in the large bowel as the resin passes through the gastrointestinal tract [8, 9]. In 2009, the FDA issued a warning recommending against the use of SPS and concomitant sorbitol due to case reports of adverse events associated with the combination [10–12]. In a later statement, the FDA further advised against the use of SPS in patients with abnormal bowel function, patients at risk for developing constipation or impaction, and patients who develop constipation after SPS use [13]. It has been suggested that patients with CKD or ESRD may be at higher risk for intestinal injury [10, 12, 14].
Since SPS’s development, many of the studies evaluating the efficacy of SPS therapy have not included patients with CKD and ESRD, did not account for confounding factors that affect potassium levels, or contained only a small number of patients [15–17]. Despite having risks and minimal data on efficacy, SPS use remains widespread. Therefore, the aim of this study is to evaluate the effects of single-dose SPS monotherapy for the treatment of acute hyperkalemia in patients with CKD or ESRD.
MATERIALS AND METHODS
Study design and setting
This study was a single-center, retrospective cohort of adult inpatients treated with SPS suspended in 33% sorbitol between 1 January 2010 and 30 September 2016. The study institution was a 664-bed large academic medical center. The protocol was approved by the study site’s Institutional Review Board (16092610-IRB03).
Participants
Patients were included if they received a single dose of oral SPS suspended in 33% sorbitol, were 18 years or older, and had CKD Stage 4 or 5 based upon Kidney Disease: Improving Global Outcomes (KDIGO) classification or ESRD maintained on renal replacement therapy (RRT) [3]. In addition, patients were required to have a baseline serum potassium level >5 mEq/L. Patients were excluded if they received any concomitant therapies for the treatment of hyperkalemia between their pre- and post-SPS serum potassium levels. These therapies included insulin, intravenous sodium bicarbonate, inhaled beta-agonists, diuretics, intravenous fluids >2 L and RRT. Patients were also excluded if laboratory values (potassium at evaluated time points or baseline serum creatinine) were missing, if prior to admission medication information was missing, they were pregnant or found to have acute kidney injury (AKI) based upon KDIGO classification [an increase in serum creatinine by ≥1.5 times baseline (50% increase within 7 days), an increase in serum creatinine by ≥0.3 mg/dL within 48 h, or urine volume <0.5 mL/kg/h for 6 h) at the time of treatment] [4].
Data sources/measurement
Electronic medical records were utilized to abstract clinical data. A patient list was generated based on patients that were prescribed oral SPS suspended in 33% sorbitol while admitted to the study institution. Variables extracted were baseline demographic data (age, gender and race), serum creatinine, location/unit in hospital where the patient received treatment with oral SPS therapy, dose of oral SPS administered, pre- and post-SPS potassium values, prior to admission and concomitant medications known to cause hyperkalemia (angiotensin-converting inhibitors, angiotensin receptor blockers, potassium sparing diuretics, sulfamethoxazole-trimethoprim, potassium supplements, cyclosporine and tacrolimus), time to post-SPS potassium values, gastrointestinal adverse events and inpatient mortality. Renal function was calculated utilizing the Modification of Diet in Renal Disease Study (MDRD) equation [18]. Gastrointestinal adverse events were extracted from provider documented notes. If an electrocardiogram (ECG) was available on the day of SPS therapy, the presence of ECG changes consistent with hyperkalemia was documented. Charlson Comorbidity Indices were calculated and reported [19]. Pre-SPS dose serum potassium levels were obtained within a 12-h period prior to SPS administration. For those with multiple serum potassium levels prior to SPS administration, the level closest to SPS administration was used. Post-SPS dose serum potassium levels were reviewed 12–24 h after the administration of SPS. For those with multiple serum potassium levels within the acceptable time frame, the level closest to 12 h post-SPS was used. For patients who received multiple doses of SPS during the same admission, only their first dose of SPS was included within the analysis.
Study endpoints
The primary endpoint of the study was decrease in serum potassium after a 30 g oral SPS dose, measured as the difference between potassium before SPS administration and the first recheck following SPS administration. Secondary endpoints included the decrease in serum potassium after 15 or 30 g oral SPS doses, incidence of normokalemia within 24 h (defined as serum potassium between 3.5 and 5 mEq/L), incidence of hypokalemia within 24 h (defined as serum potassium <3.5 mEq/L), gastrointestinal adverse events associated with SPS therapy within 30 days (defined as gastrointestinal ulceration, gastrointestinal stenosis, rectal hemorrhage, intestinal necrosis, intestinal colitis or perforation) and mortality prior to hospital discharge. Adverse effects were assessed using the World Health Organization - Uppsala Monitoring Centre (WHO-UMC) causality assessment system [20].
Statistical methods
Analyses were performed using Statistical Package for the Social Sciences (SPSS, Inc., Armonk, NY), version 23.0. Using a t-test to detect a difference between two dependent means for the primary endpoint of potassium levels at different time points with an estimated mean difference of 0.15 and a medium effect size of 0.3, a 90% power with an alpha level of 0.05 would require a sample size of 119 patients. After assessing for normality, the primary endpoint and all secondary endpoints evaluating median difference in serum potassium were analyzed using a Wilcoxon signed-rank test. Secondary incident endpoints were analyzed using descriptive statistics. A P < 0.05 was considered statistically significant.
RESULTS
Participants
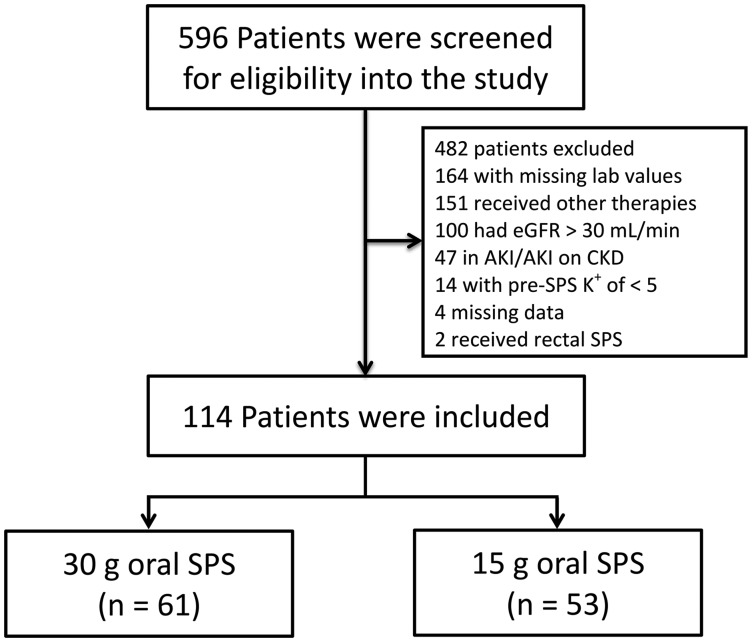
A total of 596 patients were reviewed for inclusion into the study. After screening for eligibility (Figure 1), 61 patients received 30 g oral SPS and 53 patients received 15 g oral SPS. No other SPS doses were utilized during the pre-specified time frame that met inclusion requirements. The majority of patients were African American (62%), female (52%) and classified as having ESRD requiring RRT (56%) (Table 1). Eighty-six (75%) patients were admitted through the emergency department with 61 (54%) of these patients going on to receive treatment on a medicine or surgical unit. Of the 48 patients with documented ECGs on the day of SPS therapy, 17 (15%) had ECG changes. Thirty-six (32%) patients were prescribed a medication prior to admission that can cause hyperkalemia, 30 (83%) of which were angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Fifteen patients (13%) continued medications that can cause hyperkalemia between the pre- and post-SPS potassium.
FIGURE 1.
Patient screening. eGFR, estimated glomerular filtration rate; K+, potassium.
Table 1.
Baseline characteristics
| Characteristic | Patients (n = 114) |
|---|---|
| Age (years) | 66 (56–77) |
| Female | 59 (52) |
| Race or ethnic group | |
| Caucasian | 16 (14) |
| African American | 71 (62) |
| Asian American | 2 (1.8) |
| Hispanic/Latino | 25 (22) |
| CKD staging | |
| Stage 4 (eGFR 15–29 mL/min) | 34 (30) |
| Stage 5 (eGFR <15 mL/min) | 16 (14) |
| ESRD on RRT | 64 (56) |
| Charlson Comorbidity Index | 7 (5–8) |
| ECG changesa | 17 (15) |
| Location/unit in hospital | |
| Medicine/surgery | 61 (54) |
| ICU | 17 (15) |
| Emergency department | 27 (24) |
| Hematology/oncology | 3 (2.6) |
| Rehabilitation | 5 (4.4) |
| Psychiatry | 1 (0.9) |
| Medications prior to admission | |
| Medications causing hyperkalemiab | 36 (32) |
| Angiotensin-converting enzyme inhibitors | 17 (15) |
| Angiotensin receptor blockers | 13 (11) |
| Otherc | 13 (11) |
| Medications given between pre- and post-potassium | |
| Medications causing hyperkalemia | 15 (13) |
| Angiotensin-converting enzyme inhibitors | 4 (4) |
| Angiotensin receptor blockers | 6 (5) |
| Tacrolimus | 5 (4) |
eGFR, estimated glomerular filtration rate; ICU, intensive care unit.
All values reported as median (IQR) or n (%).
Sixty-eight patients did not have an ECG done at the time of treatment.
Patients may have had multiple medications (percentages are not additive).
Other included sulfamethoxazole–trimethoprim (n = 1), cyclosporine or tacrolimus (n = 8), potassium supplement (n = 1) and potassium-sparing diuretics (n = 4).
Outcomes
Primary endpoint
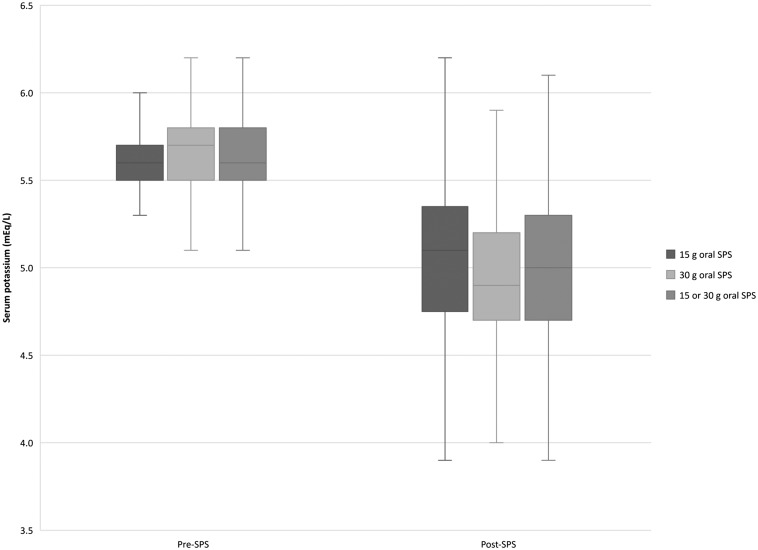
The median decrease in serum potassium after a 30 g oral SPS dose (n = 61) was 0.8 mEq/L [interquartile range (IQR 0.4–1.1 mEq/L; P < 0.001] (Table 2, Figure 2). Post-SPS serum potassium levels were reviewed 14 h (IQR 10–18 h) post-30 g oral SPS therapy.
Table 2.
Efficacy outcomes by SPS dose
| Pre-SPS potassium (mEq/L) | Post-SPS potassium (mEq/L) | Difference (mEq/L) | Time to post-SPS potassium (h) | P | |
|---|---|---|---|---|---|
| CKD 4, 5, and ESRD 30 g oral SPS (n = 61) | 5.7 (5.5–5.8) | 4.9 (4.7–5.2) | 0.8 (0.4–1.1) | 14 (10–18) | <0.001 |
| 15 g oral SPS (n = 53) | 5.6 (5.5–5.7) | 5.1 (4.8–5.4) | 0.5 (0.2–0.9) | 16 (12–19) | <0.001 |
| 15 or 30 g oral SPS (n = 114) | 5.6 (5.5–5.8) | 5.0 (4.7–5.3) | 0.7 (0.4–1.0) | 15 (11–19) | <0.001 |
| ESRD only | |||||
| 15 or 30 g oral SPS (n = 64) | 5.7 (5.5–5.8) | 5.1 (4.8–5.4) | 0.5 (0.1–0.9) | 15 (10–18) | <0.001 |
All values reported as median (IQR).
FIGURE 2.
Efficacy. Serum potassium pre- and post-SPS therapy by dose. Data are presented as box-and-whisker plots, in which the horizontal lines within the rectangles indicate the 50th percentile. The top and bottom of the rectangles indicate the 75th and 25th percentiles, respectively. The lines above and below the rectangles indicate Q1 or Q3 + (1.5 × IQR), respectively.
Secondary endpoints
The median decrease in serum potassium after a 15 g oral SPS dose (n = 53) was 0.5 mEq/L (IQR 0.2—0.9 mEq/L; P < 0.001), and after a 15 or 30 g oral SPS dose (n = 114) was 0.7 mEq/L (IQR 0.4—1.0 mEq/L; P < 0.001). The decrease in potassium remained significant [0.5 mEq/L (IQR 0.1–0.9 mEq/L); P < 0.001] when examining only ESRD patients (n = 64). Normokalemia occurred post-SPS therapy in 64 (56%) patients. Hypokalemia was not seen in any patients after SPS. Serious gastrointestinal adverse events occurred within 30 days of SPS therapy in six patients (5%), five patients (4%) experienced gastrointestinal ulceration and one patient (0.9%) experienced rectal hemorrhaging (Table 3), however, only two patients had the WHO-UMC causality assessment of possibility related, with the other four being unlikely related to SPS. Inpatient mortality occurred in five patients (4%), although none of the cases was thought to be related to hyperkalemia or the adverse effects of SPS.
Table 3.
Adverse events
| Patient | Adverse event summary | WHO-UMC classification20 |
|---|---|---|
| Patient #1 | GI ulceration occurred beyond 30 days | Unlikely |
| Patient #2 | Presented with nausea, vomiting and abdominal pain prior to SPS therapy. Developed BRBPR 2 days after. EGD revealed large old ulcer and colonoscopy revealed ischemic colitis and possible mesenteric ischemia | Possible |
| Patient #3 | Developed perforated duodenal ulcer within 14 days of SPS. Other risk factors included recent admission for DAH, possible vasculitis and treatment with high-dose steroids | Possible |
| Patient #4 | Presented with dark stools and hematemesis within 14 days of SPS. EGD showed AVMs. Colonoscopy showed moderate to severe diverticulosis | Unlikely |
| Patient #5 | Had abdominal pain prior to SPS. EGD two days after SPS revealed gastric ulcer | Unlikely |
| Patient #6 | Had abdominal pain prior to SPS. Had BRBPR within 30 days. No colonoscopy performed | Unlikely |
GI, gastrointestinal; EGD, esophagogastroduodenoscopy; BRBPR, bright red blood per rectum; AVM, arteriovenous malformation; DAH, diffuse alveolar hemorrhage.
DISCUSSION
This was the largest study to date evaluating the efficacy and safety of single-dose SPS monotherapy for the treatment of hyperkalemia in patients with CKD Stages 4, 5 or ESRD on RRT. The results of this study demonstrated that SPS therapy was effective at lowering serum potassium levels in patients with severe renal insufficiency with a median reduction of 0.8 mEq/L when using a 30-g oral dose suspended in 33% sorbitol.
Many of the prior studies evaluating the efficacy of SPS therapy excluded those with renal insufficiency and ESRD, or did not account for confounding factors (such as concomitant therapies) that affect potassium levels [7, 15, 16, 21, 22]. A study published by Mistry et al. in 2016 found a dose-dependent reduction in serum potassium following single-dose SPS monotherapy in hospitalized patients with renal insufficiency [15]. A mean difference of 0.69 mEq/L following 30 g oral SPS and 0.91 mEq/L following 60 g oral SPS (P < 0.05) was found 12 h post-SPS therapy. However, ESRD patients who required chronic hemodialysis (HD) were excluded from the study and the remainder of the patients included had a mean creatinine clearance (CrCl) classified as CKD Grade 3 or less [15]. Similarly, a large retrospective study by Hagan et al. evaluated single-dose SPS (average dose 32.4 g) and found a potassium decrease of 0.93 mEq/L. Although this study did include patients with CKD and ESRD, they also included patients with normal renal function and a high percentage of patients received other therapies for hyperkalemia known to cause a reduction in potassium such as insulin, albuterol, sodium bicarbonate and diuretics [16].
Of prospective randomized controlled trials including patients with CKD, a prior study involving 33 outpatients with hyperkalemia found a mean serum potassium decrease of 1.25 ± 0.56 mEq/L (P < 0.001) compared with placebo in patients who were given oral SPS therapy for seven consecutive days [17]. Although this study was well designed and included patients with CKD (12.5% CKD Stage 3, 62.5% CKD Stage 4 and 25% CKD Stage 5), the results were after 7 days of SPS and patients on RRT were excluded. Our study evaluated only patients with CKD Stage 4, 5, or ESRD on RRT and limited confounders by excluding patients that had received other therapies for hyperkalemia, making our observed potassium lowering effect more specific to therapy with only single-dose SPS.
Despite minimizing confounders in our study, there is evidence that colonic potassium secretion is already increased in patients with CKD and ESRD without the use of medications [23, 24]. Although this process is currently poorly understood, it could account for some of the decreases in serum potassium when resins or resins plus cathartics are utilized. Studies have evaluated the effectiveness of laxatives for decreasing serum potassium levels in patients with ESRD in an attempt to prove this hypothesis, but there have been mixed results depending on the laxative used [25, 26]. Our preparation of SPS included a cathartic (sorbitol) and this should be taken into account when evaluating the results of our study.
The results in our study were not without safety concerns. Out of the 114 patients evaluated, six patients (5%) experienced a severe gastrointestinal adverse event within 30 days of SPS therapy. However, only two cases were classified as possibly associated with SPS use and in these two cases, the events could have also been reasonably explained by other causes. Historically, the incidence of SPS-mediated gastrointestinal injury is estimated to be between 0.14% and 1.8%; however, a systematic review by Harel et al. identified 58 cases of SPS bowel-related gastrointestinal adverse events and 91% of the cases included in the review had a history of AKI, CKD or ESRD, concluding that patients with renal insufficiency may be at higher risk than previously described [10, 27, 28]. Despite these cases, many countries still continue to use potassium resins like SPS in a significant percentage of ESRD patients (e.g. up to 42% of ESRD patients in France) with no clear association on mortality or gastrointestinal injury [29].
This study also had limitations. Patients within this study, on average, had mild hyperkalemia with a median pre-SPS serum potassium level of 5.7 mEq/L. With the exclusion of any concomitant hyperkalemia treatments, many patients with more severe hyperkalemia were inherently excluded. Although this leads to less generalizability for application to more severe hyperkalemia cases, this study was the largest to date that did not include any hyperkalemia therapies other than single-dose SPS. Furthermore, a large number of patients who were screened for eligibility were excluded due to receiving SPS therapy and being started on RRT prior to any post-SPS potassium values. This also may have led to a bias towards less severe cases of hyperkalemia, but will be useful in the future for evaluating the need for SPS therapy in patients at our institution who are already scheduled to receive RRT.
Additional limitations included the single-center retrospective study design and absence of a placebo control group. By lacking a placebo for comparator, the decrease in potassium cannot be solely attributed to SPS and despite excluding patients who received medications that may decrease serum potassium, a portion of the observed decrease may be attributable to natural patient elimination. In addition, not all factors known to affect potassium balance could be reliably collected and factors such as diet, receipt of dextrose-containing fluids and blood glucose levels could have affected the results. There were also 36 patients that were prescribed medications known to cause hyperkalemia prior to admission and 21 of these patients did not receive these medications during the study time points, and holding these medications during the time between pre- and post-SPS potassium may have confounded the results in a percentage (∼18%) of the patients. However, ESRD patients with reduced intrinsic potassium elimination also experienced a significant decrease in potassium after SPS monotherapy, supporting the hypothesis that the decrease in potassium is at least partially related to SPS. In addition, given the emergent need for treatment in patients with hyperkalemia, most medical centers would be unlikely to withhold treatment by giving placebo and our results represent usual treatment. Next, adverse events that occurred could only be reported if patients presented to our health care system for follow-up care, potentially leading to underreported safety data. Finally, there are no known racial disparities in SPS effectiveness; however, this study included a large proportion of African American patients (62%) and may not be generalizable to other populations.
In conclusion, the use of single-dose SPS monotherapy resulted in a significant decrease in serum potassium levels within 24 h in patients with CKD Stages 4, 5, or ESRD, irrespective of dose. However, it remains unclear if SPS is associated with an increased risk of gastrointestinal injury in this population.
CONFLICT OF INTEREST STATEMENT
The authors received no financial support for the research, authorship, and/or publication of this article and have no actual or potential conflicts of interest to declare.
REFERENCES
- 1.Centers for Disease Control and Prevention. Chronic kidney disease surveillance system – United States. https://nccd.cdc.gov/ckd/ (4 April 2018, date last accessed)
- 2. Bruck K, Stel VS, Gambaro G. et al. CKD prevalence varies across the European general population. J Am Soc Nephrol 2016; 27: 2135–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 91–111 [DOI] [PubMed] [Google Scholar]
- 4. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 6 [Google Scholar]
- 5. Alfonzo A, Soar J, MacTier R. Clinical practice guidelines: treatment of acute hyperkalaemia in adults. UK Renal Association, 2014. https://renal.org/wp-content/uploads/2017/06/hyperkalaemia-guideline-1.pdf (10 August 2016, date last accessed)
- 6. Hollander-Rodriguez JC, Calvert JF Jr.. Hyperkalemia. Am Fam Physician 2006; 73: 283–290 [PubMed] [Google Scholar]
- 7. Fordjour KN, Walton T, Doran JJ.. Management of hyperkalemia in hospitalized patients. Am J Med Sci 2014; 347: 93–100 [DOI] [PubMed] [Google Scholar]
- 8. Ahee P, Crowe AV.. The management of hyperkalaemia in the emergency department. J Accid Emerg Med 2000; 17: 188–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayexalate [package insert]. Laval, Quebec: Sanofi-Aventis. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/011287s022lbl.pdf (10 August 2016, date last accessed)
- 10. Harel Z, Harel S, Shah PS. et al. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med 2013; 126: 264.e9–e24 [DOI] [PubMed] [Google Scholar]
- 11. Jacob SS, Parameswaran A, Parameswaran SA. et al. Colitis induced by sodium polystyrene sulfonate in sorbitol: a report of six cases. Indian J Gastroenterol 2016; 35: 139–142 [DOI] [PubMed] [Google Scholar]
- 12. McGowan CE, Saha S, Chu G. et al. Intestinal necrosis due to sodium polystyrene sulfonate (kayexalate) in sorbitol. South Med J 2009; 102: 493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. Safety: Kayexalate (sodium polystyrene sulfonate) powder. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm186845.htm9/12/2016 (12 August 2016, date last accessed)
- 14. Chelcun JL, Sable RA, Friedman K.. Colonic ulceration in a patient with renal disease and hyperkalemia. JAAPA 2012; 25: 34, 37–38 [DOI] [PubMed] [Google Scholar]
- 15. Mistry M, Shea A, Giguere P. et al. Evaluation of sodium polystyrene sulfonate dosing strategies in the inpatient management of hyperkalemia. Ann Pharmacother 2016; 50: 455–462 [DOI] [PubMed] [Google Scholar]
- 16. Hagan AE, Farrington CA, Wall GC. et al. Sodium polystyrene sulfonate for the treatment of acute hyperkalemia: a retrospective study. Clin Nephrol 2016; 85: 38–43 [DOI] [PubMed] [Google Scholar]
- 17. Lepage L, Dufour A-C, Doiron J. et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol 2015; 10: 2136–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS, Bosch JP, Lewis JB. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL. et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383 [DOI] [PubMed] [Google Scholar]
- 20.The use of the WHO–UMC system for standardised case causality assessment. http://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf (24 April 2018, date last accessed)
- 21. Joshi P, Beaulieu J, Shemin D.. The effect of a single dose of sodium polystyrene sulfonate (SPS) and sorbitol in hyperkalemic patients with kidney disease. J Am Soc Nephrol 2008; 19: 355A [Google Scholar]
- 22. Kessler C, Ng J, Valdez K. et al. The use of sodium polysterene sulfonate in the inpatient management of hyperkalemia. J Hosp Med 2011; 6: 136–140 [DOI] [PubMed] [Google Scholar]
- 23. Mathialahan T, Maclennan KA, Sandle LN. et al. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol 2005; 206: 46–51 [DOI] [PubMed] [Google Scholar]
- 24. Sorensen MV, Matos JE, Praetorius HA. et al. Colonic potassium handling. Pflugers Arch 2010; 459: 645–656 [DOI] [PubMed] [Google Scholar]
- 25. Mathialahan T, Sandle G.. Dietary potassium and laxatives and regulators of colonic potassium secretion in end-stage renal disease. Nephrol Dial Transplant 2003; 18: 341–347 [DOI] [PubMed] [Google Scholar]
- 26. Emmett M, Hootkins R, Fine K. et al. Effects of three laxatives and a cation exchange resin on fecal sodium and potassium excretion. Gastroenterology 1995; 108: 752–760 [DOI] [PubMed] [Google Scholar]
- 27. Gerstman BB, Kirkman R, Platt R.. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis 1992; 20: 159–161 [DOI] [PubMed] [Google Scholar]
- 28. Watson MA, Baker TP, Nguyen A. et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis 2012; 60: 409–416 [DOI] [PubMed] [Google Scholar]
- 29. Jadoul M, Karaboyas A, Goodkin DA. et al. Potassium-binding resins: associations with serum chemistries and interdialytic weight gain in hemodialysis patients. Am J Nephrol 2014; 39: 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]