Abstract
Atypical hemolytic uremic syndrome (aHUS) is a thrombotic microangiopathy (TMA) originally understood to be limited to renal and hematopoietic involvement. Whereas aberrations in complement regulatory proteins (CRPs), C3 or complement factor B (CFB) are detected in ∼60% of patients, a complement-derived pathogenesis that reflects dysregulation of the alternative pathway (AP) of complement activation is present in ∼90% of patients. aHUS remains a diagnosis of exclusion. The discovery of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) and its utility in the diagnosis of thrombotic thrombocytopenic purpura (TTP) has resulted in the appreciation that cases of aHUS have been inappropriately diagnosed as TTP. Thus there has been an evolving appreciation of clinical manifestations of aHUS that renders the appellation aHUS misleading. This article will review the pathogenesis and the evolving clinical presentations of aHUS, present a hypothesis that there can be a phenotypic expression of aHUS due to a complement storm in a disorder where direct endothelial damage occurs and discuss future areas of research to more clearly define the clinical spectrum and management of aHUS.
Keywords: aHUS; complement storm; endothelial dysfunction; immunosuppression, thrombotic microangiopathy; systematic review
INTRODUCTION
The report of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) enzyme in 1996 and its subsequently determined critical role in the pathogenesis of thrombotic thrombocytopenic purpura (TTP) has enabled biomarker confirmation for the clinical diagnosis of TTP [1–3]. The failure to detect ADAMTS13 activity (upon which the clinical diagnosis of TTP is confirmed) in individuals with absolute or relative thrombocytopenia and microangiopathic hemolysis [the classic definition for a thrombotic microangiopathy (TMA)] and in whom shiga toxin Escherichia coli (STEC) HUS has been excluded has resulted in an increasing awareness of the eclectic clinical presentations of aHUS [4–7]. It is now clear that patients with aHUS have been misdiagnosed as having TTP and that TTP is associated with more severe thrombocytopenia and less severe acute kidney injury than aHUS. Individuals with a platelet count >30 000/mL3 and creatinine >1.7 mg/dL have only about a 3% chance of having TTP [8]. In contrast, aHUS may present along the entire spectrum of platelet counts and renal function [9].
As significant as the growth of our knowledge has been over the past decade vis-à-vis distinguishing aHUS versus TTP, clinicians are now beginning to recognize that clinical scenarios of a TMA complicating another disease or disorder, in which a TMA is only infrequently seen, are associated with poor clinical outcomes. These conditions include autoimmune diseases [e.g. systemic lupus erythematosus (SLE)], bone marrow transplant complicated by graft-versus-host disease (GVHD), malignant hypertension and certain infections (e.g. Parvo B19 and human immunodeficiency virus). In these clinical settings, complement activation is often present. A TMA has rarely been seen with certain medications (e.g. calcineurin inhibitors, chemotherapeutic agents mitomycin-C and gemcitabine, platelet aggregation inhibitors clopidogrel and ticlopidine and angiogenesis inhibitor bevacizumab). These drugs are known to have the potential to directly injure endothelia [10–17]. Thus the next challenges are understanding what, if any, clinical and biomarker findings can be applied to make a diagnosis of aHUS, especially when a TMA occurs in the setting of a disease in which complement dysregulation is present, and whether it is appropriate to use the appellation aHUS for a time-limited, discrete pathophysiologic disorder, as in GVHD. Although eculizimab therapy is approved for complement-mediated aHUS [18], when the phenotypic expression of aHUS occurs in other established disorders associated with complement activation and direct endothelial injury [e.g. SLE and GVHD] and does not resolve with treatment of the underlying disease, there may be a role for a time-limited course of eculizimab.
This review will address the pathogenesis, clinical presentation, diagnosis and management of aHUS.
PATHOGENESIS OF COMPLEMENT-MEDIATED AHUS
In 1954, 58 years after the discovery of the classical pathway of complement, Louis Pillimer reported his findings on an ‘alternate pathway’ of complement activation [19]. Although initially met with fierce criticism, his data were corroborated in 1967 [20, 21]. In 1981, all the proteins in the alternative pathway (AP) were identified [22]. The AP of complement activation is now appreciated to be the most atavistic of the three activating pathways of the proximal cascade (the lectin pathway was discovered in 1976) [23–25]. Unique to the AP is that constitutively it is ‘always on’—with a default setting of attack [26].
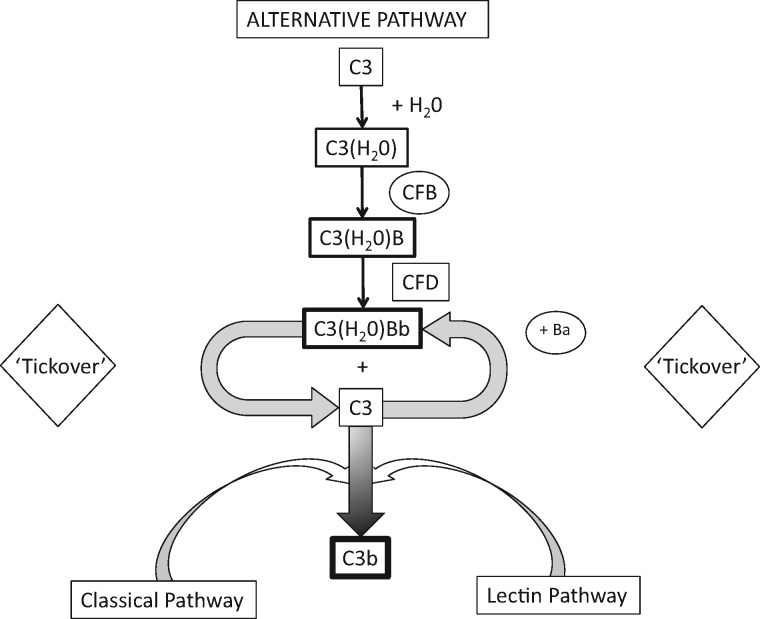
Initiation of the complement cascade, the proximal step, begins with activation of C3. Unlike the classical and lectin pathways, activation is not based on pattern recognition molecules, as it occurs with foreign antigens that gain access to the internal milieu of the organism [22, 27–29]. The ‘always on’ default setting of the AP is due to a thioester bond within C3 that in plasma water results in a conformational change of this zymogen [26]. Although existing in an ephemeral state (milliseconds), the hydrolyzed C3 allows the binding of factor B, followed by the activation of factor B by factor D. The activated fragment of factor B (Bb) remains bound to the C3 thioester hydrolyzed protein (the ‘tickover’ process). This results in a labile C3 convertase that activates adjoining C3 proteins to produce C3b, a more stable product than hydrolyzed C3, upon which identical steps of factor B and factor D activation occur to produce a membrane-bound C3 convertase (further stabilized by properdin) and C3a, a weak anaphylatoxin [19, 30]. In addition to forming C3 convertase, C3b indiscriminately opsonizes anionic surfaces—host or pathogen [30–35]. The other fragment of factor B activation, Ba, is released into the plasma. Plasma Ba can be measured and reflects proximal activation of the complement cascade [36] (Figure 1).
FIGURE 1.
Initiation of complement cascade begins with activation of C3. Activation occurs via the ‘tickover’ process of the alternative pathway, the classical pathway and lectin pathway. Enzymatic cleavage of CFB bound to C3(H20) or C3b by complement Factor D results in activation of CFB to C3(H20)Bb or C3bBb, and Ba (a marker of proximal complement activation). C3bBb is stabilized by Properdin protein.
The downstream activation of the complement cascade—the terminal pathway—commences following the binding of an additional C3b protein to C3 convertase (C3bBb) to form C5 convertase (C3bBbC3b). C5 convertase activates C5 by cleaving it into C5a and C5b. C5a is a potent anaphylatoxin, with pro-thrombotic and pro-inflammatory properties, and also activates endothelial cells and leukocytes. C5b is the nidus upon which C6, C7, C8 and multimers of C9 attach to produce C5b-9, the membrane attack complex (MAC) [19, 37]. In addition to pro-inflammatory and pro-thrombotic properties, MAC activates leukocytes, endothelial cells and platelets and destroys the cell it is nondiscriminately attached to—host or pathogen—as a consequence of its forming a channel through the cell membrane. Cell lysis ensues as extracellular calcium entry disrupts intracellular homeostasis, resulting in mitochondrial failure. Cell lysis occurs as early as 30 min following assembly of the MAC [19].
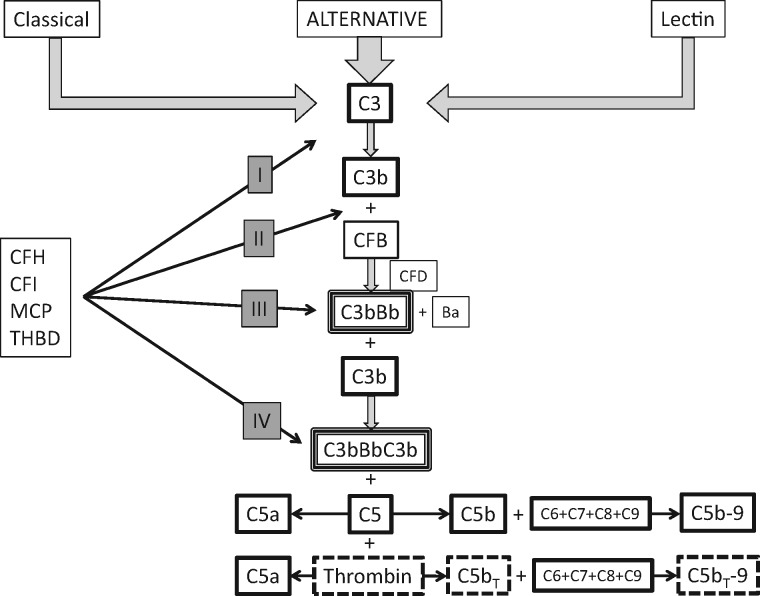
Crosstalk between the coagulation system and the complement cascade has been recognized with the demonstration of a thrombin-dependent activation of C5 that is both independent of and dependent upon C5 convertase [38] (Figure 2).
FIGURE 2.
Complement cascade and complement regulatory proteins (CRP). CRP modulate the ‘tickover’ process of alternative pathway activation and protect host cells from activated complement proteins generated via all three pathways. Thrombin independently activates C5 in conjunction with C5 convertase. C3b – activated C3; CFB – complement factor B; CFD – complement factor D; C3bBb – C3 convertase; C3bBbC3b – C5 convertase; CRP may inactivate C3b, prevent binding of CFB to C3b, or accelerate decay of C3 convertase and C5 convertase. CRP regulation: I – inactivation C3 to C3i; II – inhibit binding of CFB to C3b; III – accelerate decay of C3 convertase; IV – accelerate decay of C5 convertase. CFH (complement factor H) – regulatory functions I, II, III, IV; CFI (complement factor I) – regulatory function I as cofactor for membrane cofactor protein (MCP) and thrombomodulin (TBMD).
Complement regulatory proteins (CRPs) were first discovered in 1981 [22, 31]. They comprise approximately half of all proteins in the complement cascade. They have two roles. The first is to modulate the ‘always on’ feature of the AP to keep the system primed (‘tickover process’) but not fully activated (<1% of total plasma C3 is activated hourly) [26, 29, 39]. By permitting the proximal activation of complement to be primed, the regulatory proteins allow near instantaneous amplification of the AP once a perturbation in the internal milieu (by any of the three activating processes) is detected. The second important function of the regulatory proteins is to ensure that once the complement cascade is amplified, the potent end products (C5a and C5b-9) are limited to interacting with pathogens and nonhealthy host cells [40]. The regulatory proteins form an overlapping protective matrix for host endothelia. The regulatory proteins currently recognized as important in the pathogenesis of aHUS are complement factor H (CFH), complement factor I (CFI), membrane cofactor protein (MCP or CD46) and thrombomodulin [4, 29, 33, 41]. Loss-of-function mutations of these proteins, polymorphisms in CFH, fusion proteins of CFH, antibodies against CFH and deletions of CFH-related proteins have been associated with aHUS [5, 6, 42, 43]. Dysregulation of the AP can also arise from gain-of-function mutations in C3 and complement factor B (CFB) and are also associated with aHUS [6]. In this pathogenic setting, CRPs are normal but ineffective in regulating the complement cascade due to the formation of ‘super’ C3 and C5 convertases (Figure 2).
CLINICAL PRESENTATION
In contrast to the pentad descriptors of TTP (TMA—thrombocytopenia and Coombs negative hemolytic anemia, neurologic and renal findings and fever), until recently the clinical picture ascribed to aHUS was TMA and renal involvement [44–48]. Data over the last 10–15 years have upended this decades-long misconception and have also resulted in our appreciation of the equally long misconception that aHUS is primarily a disease of children [4, 7, 44, 49–52].
As befits a syndrome that reflects disruption of the modulators and proteins of complement activation and host cell protection, the clinical manifestations of aHUS may be protean and involve, with different frequencies, all organ systems. These presentations include:
Hematologic—Absolute or relative thrombocytopenia along with a microangiopathic hemolytic anemia [schistocytes, increased lactate dehydrogenase (LDH), decreased haptoglobin and decreased hemoglobin] are frequently, but not invariably, observed at the time of presentation. Further complicating the diagnosis is the recognition that not all the findings of a microangiopathic anemia need be observed at presentation or throughout the clinical presentation (e.g. no schistocytes). It is appreciated that a renal-limited form of aHUS can occur [4, 6, 7].
Renal—Although acute kidney injury is the most common finding at initial presentation, isolated proteinuria or hematuria may occur [4, 6, 7]. Patients may present along the entire spectrum of kidney injury, including the need for renal replacement therapy. Nephrotic range proteinuria is not infrequent. Commonly ascribed in pediatric patients with aHUS arising from mutations in diacyl glycerol kinase epsilon (DGKE) that presents within the first year of life, nephrotic range proteinuria is found across the age spectrum [53–56]. Although the pathogenesis of DGKE was initially described as a complement-independent process, complement involvement, albeit rare, has subsequently been reported [55]. Complement-mediated podocyte injury has recently been reported in patients with and without DGKE aHUS and offers a pathogenic mechanism for nephrotic range proteinuria [54]. The classic histologic findings seen in aHUS include platelet and fibrin deposition in capillaries, endothelial cell injury, proliferation of the myocyte layer, electron lucent subendothelial space widening, double contouring of glomerular capillary walls, endotheliosis and negative immunofluorescence findings for immunoglobulins. Histologic findings of aHUS have also been reported in patients with previously diagnosed other forms of glomerulonephritides who subsequently develop nephrotic range proteinuria [54].
Gastrointestinal—Gastrointestinal involvement most commonly manifests with diarrhea, which may be bloody, and occurs in up to 30% of individuals. Additional findings include nausea and vomiting, pancreatitis and hepatic and colonic involvement [5–7, 44, 51].
Central nervous system (CNS)—CNS involvement, long thought to be solely within the purview of TTP, is now a well-recognized occurrence in aHUS. Presentations include confusion and encephalopathy, stroke and seizures [6, 7, 51, 52].
Respiratory tract—Next to diarrhea and gastroenteritis, upper respiratory tract infection is the most common condition on presentation of aHUS [6]. Lower respiratory tract involvement, including pulmonary hypertension, has been reported [51]. In contrast to TTP, pulmonary hemorrhage occurs in aHUS [57].
Cardiovascular (CV)—CV involvement occurs in ∼3–10% of patients and manifests with myocardial infarction, myocarditis and dilated or ischemic cardiomyopathy. Peripheral gangrene and large vessel disease involving the carotid, cerebral, subclavian and pulmonary arteries have been reported in the pediatric population. Hypertension is frequently seen in patients with aHUS [58, 59]. Malignant hypertension occurs in aHUS [14–16, 60].
Skin—Dermatologic involvement in aHUS is rare. Purpuric and ulcerative or necrotic skin lesions have been reported [61].
Ocular—Ophthalmologic involvement is another rare manifestation of aHUS. Retinal artery occlusion and serous retinal detachment have been reported [62–64].
DIAGNOSIS AND DEFINITION
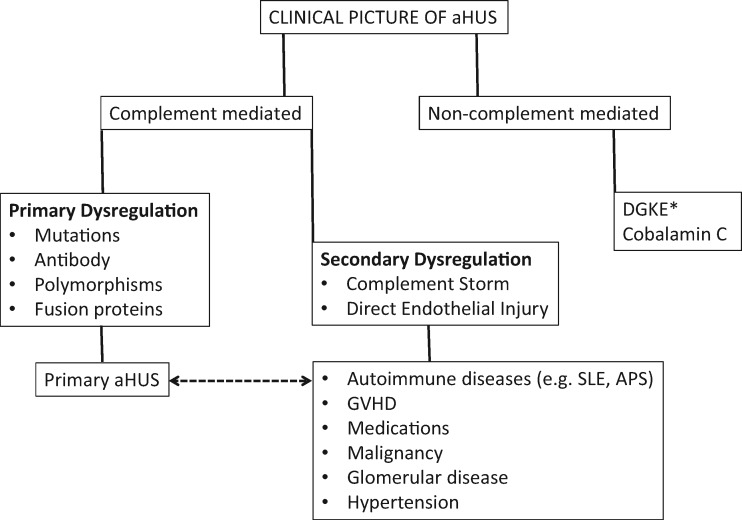
In the absence of a specific test, at present, aHUS is a clinical diagnosis of a complement-mediated TMA that rests upon the exclusion of TTP and STEC infection. The recent appreciation of the protean organ system involvement that can occur in aHUS stands in distinct contrast to earlier beliefs. In this context, ADAMTS13 testing in aHUS reveals an activity percentage that is more than the lowest detectable value for the reference lab performing the analysis (>5–10%) [6, 7, 44]. Indeed in aHUS, ADAMTS13 activity percentages can range from normal values to markedly reduced levels [65, 66]. Antiphospholipid antibody syndrome (APS) is also in the differential diagnosis of a complement-mediated TMA [67]. Figure 3 offers an approach to the diagnosis.
FIGURE 3.
Proposed algorithm to distinguish primary from secondary aHUS. This algorithm will require validation of markers of proximal and terminal complement activation. Secondary aHUS includes autoimmune disease, GVHD, medications, malignant hypertension, malignancy and glomerular disease.
Occasionally the clinician will care for a patient where the diagnosis of aHUS is not clear-cut [44, 68].
An ever-increasing, albeit small number of series and case reports have illustrated that treating a TMA component in systemic diseases not usually associated with a TMA (e.g. lupus nephritis, GVHD complication of stem cell transplant and pregnancy) as aHUS (when the differential diagnoses have been excluded) can result in significantly improved outcomes when treating the primary disease fails to resolve the TMA [11–13, 69–72]. This has recently been described as a phenotypic expression of aHUS without the risk of recurrence as occurs in ‘classic’ or ‘primary’ aHUS as it is presently understood (vida infra) [73]. Current opinion does not view the finding of a TMA (without supporting evidence for STEC, TTP or APLS) in these complement-dysregulated conditions as aHUS [44]. Yet this opinion does not take into account either the otherwise poor prognosis of these patients without aHUS-specific therapy or the beneficial reports of patients who are treated for the presumed diagnosis of aHUS. It is in this context that a narrowly defined diagnosis of aHUS limits treatment options and is potentially injurious to the patient. Illustrative of the uncertainty regarding the application of the appellation, secondary aHUS is found in a paper that examined potential genetic risk factors in aHUS relapse following discontinuation of eculizimab therapy (vida infra) [74].
In the interim, utilizing our understanding of the pathogenesis of complement-mediated aHUS and the response to eculizimab therapy these complicated patients manifest may better guide the clinician to a diagnosis of an aHUS component to the underlying disease state.
Paramount to the diagnosis of aHUS is the dysregulation of the AP of complement activation. However, detection of genetic abnormalities or antibodies to CFH is not a universal finding in aHUS—and has been reported in 50–70% of patients [4, 6, 7, 44]. Thus does the inability to detect a genetic mutation reflect our present diagnostic limitations to detect all disruptors of complement homeostasis? An affirmative answer was demonstrated in a recent report that described intronic mutations in the DGKE gene, when standard genomic testing failed to do so [75]. In addition, data have also demonstrated that patient outcomes are comparable whether or not genetic mutations are detected [6, 50]. These observations are consistent with the belief that there exist undiagnosed genetic predispositions to the pathogenesis of complement-mediated aHUS [76].
However, I believe that aHUS due to dysregulation of the AP of complement can also manifest phenotypically without a genetic predisposition [73]. The clinical scenarios described (e.g. lupus nephritis and GVHD following stem cell transplant) provide the basis for this hypothesis. These disorders can be associated with marked complement activation and are associated with direct endothelial damage [11–13, 69, 72, 77]. Importantly, direct endothelial damage is a primary and additional activator of the AP of complement [22, 78, 79]. This further amplifies complement activation, resulting in a complement storm that overwhelms otherwise intact CRPs. In this setting, therapy directed solely at the underlying disease process may be insufficient to break the cycle of ongoing endothelial cell damage. Thus a two-prong therapeutic approach may offer a better treatment regimen. When this approach has been reported, the results have been encouraging [11–13, 69–72] (Figure 3). As described in the next section, the utility of known biomarkers to indicate activation of proximal and terminal complement activation remains a nascent field of inquiry.
As will be discussed in the last section of this review, this methodological approach to treatment need not obligate an individual to long-term eculizimab therapy.
NATURAL HISTORY, BIOMARKER TESTING AND TREATMENT
In 2013, eculizimab (Soliris) received expedited approval from the US Food and Drug Administration (FDA) for the treatment of aHUS to inhibit complement-mediated TMA [18]. Following additional required prospective studies in pediatric and adult populations, in 2015 the FDA gave standard approval for eculizimab therapy for aHUS. It is presently the only FDA-approved therapy for aHUS. Eculizimab is a humanized monoclonal antibody that inhibits the formation of terminal complement proteins C5a and C5b-9. Eculizimab binds with high affinity to C5 complement protein, thus preventing downstream complement activation [80].
Prior to the availability of eculizimab therapy, outcomes were poor in patients with aHUS treated with plasma-based therapy [4]. Despite the historical use of plasmapheresis in the treatment of aHUS, there has never been a prospective trial to evaluate the usefulness of expensive plasma-based therapies (plasma infusion and plasmapheresis) [4, 81]. Although reports have demonstrated short-term benefits (months) primarily in thrombocytopenia and anemia, there are no data that show a long-term renal benefit of plasma-based therapy in aHUS [4].
During the first presentation of aHUS, death or end-stage renal disease was reported in up to 40% of patients. Nearly 80% of patients with aHUS died, required renal replacement therapy or had chronic kidney disease within 3 years after diagnosis. These poor short- and long-term outcomes occurred on plasma-based therapy. Patients in whom a genetic mutation was not detected were reported to have short-term (months) and long-term (5- to 10-year) outcomes comparable with patients with a genetic mutation. Historical outcomes for children who develop aHUS from an MCP mutation have revealed an ∼20% rate of death or end-stage renal disease at 5–10 years [4].
The introduction of eculizimab has transformed the outcome of this disease and the quality of life of those afflicted [80–84]. In contrast to plasma-based therapies, the TMA response to eculizimab therapy has reflected the dramatic inhibition of terminal complement activation. Following initiation of eculizimab therapy in adults, the median time for the platelet count and LDH levels to reach normal range was 7 and 28 days, respectively [80, 81, 83], and in children it was 7 and 48 days, respectively [84]. Hematological normalization occurred in ∼75% of adult patients at week 26 of therapy and in nearly 90% of adult patients at 52 and 104 weeks [80, 83]. In a pediatric trial, hematologic normalization occurred in 82% of children after a median of 55 days [84].
In the pediatric and adult trials, ∼80% of patients who required renal replacement therapy at presentation and who began eculizimab treatment within a month following diagnosis of aHUS were able to come off dialysis. Pediatric patients experienced a mean improvement in eGFR from baseline of 64 mL/min/1.73 m2 and in adult patients the mean change from baseline was +30 mL/min/1.73 m2 at 27 weeks of eculizimab therapy. The 2-year follow-up of aHUS adult patients on eculizimab has demonstrated ongoing improvement in renal function (eGFR +33 mL/min/1.73 m2) [80, 83, 84].
The duration of treatment is unclear [83].
Withdrawal of eculizimab has been reported. In the largest series reported to date, where 108 patients enrolled in the French aHUS registry between 2010 and 2014 who received eculizimab and in whom eculizimab was discontinued were followed [74]. At the time of this report, 11 patients were receiving renal replacement therapy and two patients had died. Renal transplant recipients and patients with secondary aHUS (autoimmune disease, drugs, infection or cancer) were excluded from consideration to have eculizimab withdrawn. The decision to stop eculizimab was made by the treating physician. Six patients were lost to follow-up. Of the 38 patients in whom eculizimab was withdrawn, CFH and MCP mutations were discovered in 11 (29%) and 8 (21%) of patients, respectively. C3 and a CFI variant were each noted in one (2.5%) patient. No complement gene variants were detected in 16 (42.5%) patients. Relapse was noted in 8 of 11 (72%) patients with CFH mutations and in 4 of 8 (50%) patients with MCP mutations. Relapse was not observed in the 16 patients with no identified gene variants. The median time to relapse was 7.5 months (range 3–29). Eculizimab was restarted within 48 h in all patients with return of renal function to pre-eculizimab discontinuation values. No biomarker data were obtained. The second paper reported on the discontinuation of eculizimab in 10 of 22 patients with aHUS who were treated with eculizimab. All 10 patients had an identified complement abnormality. Relapse occurred in 3 of the 10 patients. Each patient had a CFH mutation. In each patient, relapse occurred within 6 weeks of the last dose of eculizimab. Eculizimab was immediately resumed in all three patients and all patients completely recovered [85]. Data reported from the clinical trials of eculizimab reveal a recurrence rate following discontinuation of therapy of 20% (12/61 patients) [86]. This paper also reported on patients registered in the global aHUS registry. In patients <18 years of age, 28 patients (24%) had eculizimab discontinued and recurrence resulted in reinitiation of eculizimab in 7 patients (25%), whereas 48 adult patients (27%) discontinued therapy, with resumption of eculizimab in 5 patients (10%) [86]. Recurrence occurred in 16 of 52 patients (31%) in published case reports reviewed by the authors. The authors note that cessation of eculizimab can increase TMA recurrence and that the timing and severity are unforeseeable.
At present, there are no biomarker data to permit the identification of patients who are at risk to recur. Additional clinical data are needed to assess recurrence risk in patients in whom no complement gene mutation is detected since historical data reveal morbidity and mortality outcomes comparable with patients with complement gene mutations [4]. There are no data that examine withdrawal of eculizimab in secondary aHUS or in the renal transplant recipient who develops aHUS.
In addition to the clinical profile and genetic profile of the patient, judicious withdrawal of eculizumab will be aided when biomarker and complement data in controlled studies become available.
A recent exploratory study evaluated complement biomarker data in adult patients with aHUS treated with short-term plasmapheresis [36]. Of note, irrespective as to whether LDH, haptoglobin and/or platelet counts improved with plasmapheresis or remained unchanged, biomarker evaluation of baseline elevated proximal (plasma Ba) and terminal (urine C5a and urine C5b-9 excretion) complement activation remained unchanged. It is important to highlight that any improvement seen in platelet count, haptoglobin and LDH when patients with aHUS were treated short term with plasmapheresis was not associated with an improvement in the pathogenic mechanism, that is, dysregulation of the AP of complement activation, of aHUS. Until biomarker and complement testing become routinely available to diagnose and assess treatment response to eculizumab, the use of LDH, haptoglobin and platelet count levels and renal response (creatinine and urinalysis) remain the sole means to evaluate treatment response.
In this article, eculizumab was initiated following short-term plasmapheresis therapy. Immediately following the first dose of eculizumab significant improvements in urine C5a and urine sC5b-9 values were noted in all patients. Levels of these terminal complement biomarkers were comparable with healthy normal volunteers by 2.5 weeks and remained so for the duration of the study (52 weeks). Consistent with the mechanism of action of eculizumab, plasma Ba levels did not normalize. By 6 weeks after the first dose of eculizumab, proximal complement activation was reduced by 30% and persisted over the year time frame. That plasma Ba levels did decrease with eculizumab is a reflection of the abrogation of the positive feedback of endothelial damage on AP activation, the underlying pathogenesis of aHUS with ongoing disruption of complement regulation and presumably improvement in renal function (data were not provided to determine the extent of this factor) [22, 36, 78]. At present, reliable and sensitive markers of proximal and terminal activation and direct comparison of serum and urine markers are not available [87]. aHUS is a clinical diagnosis. Once TTP and shiga toxin HUS are excluded (which should be possible by 48 h), therapy with eculizimab is begun. Genetic testing is neither required nor indicated to initiate therapy.
Since terminal complement activation is required for encapsulated organisms (i.e. meningococcal), all patients must be vaccinated with meningococcal vaccinations. In addition to the quadrivalent vaccine that protects against serogroups A, C, W and Y, the FDA has recently approved a meningococcal vaccine for serogroup B [88]. In light of the risks associated with aHUS if therapy with eculizimab is delayed, the standard of practice is to administer the vaccination as soon as the decision is made to begin eculizimab therapy. Patients receive penicillin or ciprofloxacin prophylaxis for the subsequent 2 weeks it takes for antibody production and protection to be generated. Since immunization against meningococcal infection is not 100%, some physicians maintain antibiotic prophylaxis. Advisory Committee on Immunization Practices vaccination guidelines regarding pneumococcal vaccination and Haemophilus influenzae must be followed.
Eculizimab is a category C drug [18].
Notwithstanding the aforementioned lack of controlled trials in the use of plasmapheresis in aHUS, aHUS arising from anti-CFH antibodies has been reported to respond to plasmapheresis and immunosuppressive therapy [89]. As seen in the eculizimab trials, renal recovery was associated with earlier initiation of therapy—in this article—plasmapheresis. However, renal recovery was noted in 71% of children versus 82% of children in the eculizimab trial [83, 89]. It is not known whether the use of eculizimab would have resulted in outcomes different than seen with plasmapheresis and immunosuppressive therapy.
In those cases where eculizimab is unavailable or not tolerated, liver transplantation, with the attendant risks, appears curative in aHUS when it arises from soluble CRP or complement protein mutations [4].
The treatment of this ultrarare disease is expensive. Dosing is weight based. In adults, the standard annual maintenance cost approaches $600 000 [85].
TMA IN SLE, GVHD, MALIGNANT HYPERTENSION, OTHER DISEASES AND PREGNANCY
As previously stated, TMA has been infrequently or rarely associated with or ascribed to other diseases, including SLE, GVHD complicating bone marrow transplant, malignant hypertension, pregnancy (intra- and postpartum), sepsis and certain medications. These observations have been known for decades. In addition, recent basic research and clinical reports of patients with APS and catastrophic APS (CAPS) lend further support to the role of complement activation and direct endothelial damage (as opposed to cell membrane injury caused by the MAC) in the pathogenesis of TMA when present in these disorders [90–97]. These case reports illustrate the potential efficacy of eculizimab when plasmapheresis and other immunosuppressive therapies are not effective. Until additional means of diagnosing aHUS become available, the significance of a concomitant TMA is a conundrum. Nevertheless, when a TMA coexists in a disease or disorder where direct endothelial damage is present and marked complement activation is felt to be present that is unresponsive to conventional therapy, especially when there is ongoing or new renal, cardiac or CNS events, it behooves the clinician to consider the diagnosis of secondary aHUS.
If genetic testing reveals a complement protein mutation or antibody then, in retrospect, primary aHUS was unmasked by the presenting disorder. But, as noted earlier, since this takes time and only ∼60% of patients have a detectable gene variant, the clinician’s ultimate decision to consider secondary aHUS must be based on the clinical picture and the best interest of the patient.
In addition to the examples noted above, complement is involved in a wide range of inflammatory and infectious disorders. Complement is part of our innate immune system [19]. Thus a common link between aHUS and these other conditions is complement activation. When complement activation is marked—that is, a complement storm—and endothelial damage is present as a consequence of the underlying condition, a pathogenic picture occurs that parallels that seen in primary aHUS. In this construct, the phenotypic expression of aHUS can occur in the absence of genetic mutations of proteins and regulatory proteins. Undoubtedly other modifying factors are present to account for this uncommon phenotypic expression of secondary aHUS. Another difference from primary aHUS would be the chronicity and duration of therapy required. ‘Classical’ or primary aHUS is defined as a primary dysregulation of AP activation. Although this suggests the need for long-term therapy in these patients, as previously described, there are presently limited data to assess in whom eculizimab therapy may be stopped [74, 85, 86]. In contrast, the therapy for secondary aHUS should be required only until the etiology for the direct endothelial cell damage and marked complement activation has resolved. In this unique clinical setting a two-prong therapeutic approach should be efficacious: (i) treating the underlying disorder (e.g. SLE, GVHD and malignant hypertension) in order to halt direct endothelial cell damage that directly activates AP of complement and (ii) treating the secondary aHUS component to abrogate the profound complement amplification that further injures endothelial cells. After sufficient time has passed on eculizimab treatment (most likely months) to allow for endothelial cell healing to take place and the patient has clinically improved, the availability of biomarker data and proximal and terminal complement functional testing will allow the clinician to distinguish between a primary aHUS unmasked by the underlying disease and a secondary aHUS. Until these tests become available the distinction between primary and secondary aHUS remains a clinical judgment.
Our present understanding of aHUS would predict that if the aHUS associated with another disease or disorder was due to an unmasking of a genetic predisposition to aHUS, that is, primary aHUS, there would be evidence of ongoing evidence of AP activation, that is, demonstration of continued proximal complement activation [7]. Whether other laboratories corroborate the exploratory data that show abnormal plasma Ba levels to be highly sensitive in patients with ‘primary’ aHUS and whether this can be meaningfully interpreted in the setting of renal injury remains to be seen [7]. Direct endothelial cell evaluation (i.e. biopsy) of proximal and terminal complement biomarker activation may be necessary to assess ongoing complement activity [98].
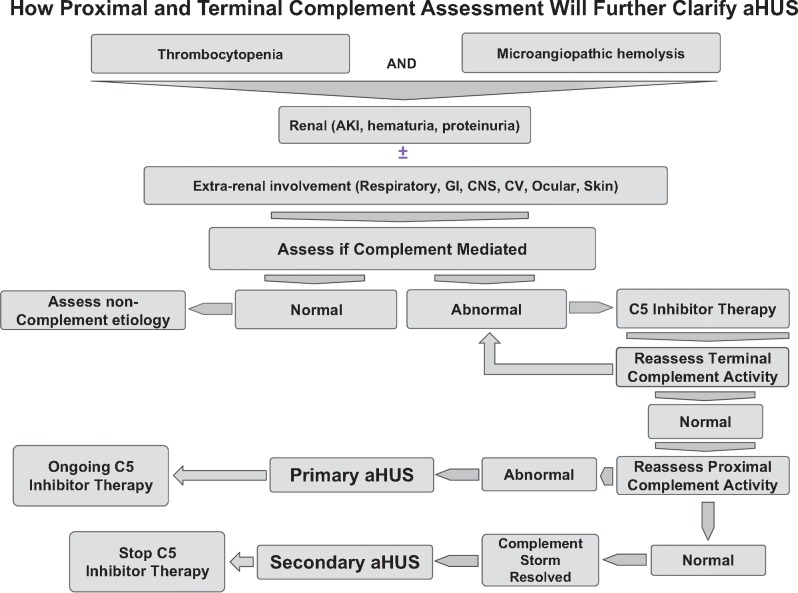
In contrast, if the ‘two-prong’ approach results in no further detection of proximal complement activation, then a genetic predisposition to the development of aHUS did not exist and a diagnosis of secondary aHUS can be made. Cessation of eculizimab therapy should be permissible without concern for recurrence (Figures 3 and 4).
FIGURE 4.
Clinical approach to a patient with the phenotypic picture of aHUS in whom TTP and STEC-E. Coli has been excluded. There are limited data to ascertain if the presence of aHUS in a complement amplifying disease is from an unmasking of a primary aHUS or from a complement storm in the setting of direct endothelial damage. *DGKE aHUS has been reported to be both dependent and independent of the complement system.
This construct will require the determination and validation of specific and sensitive markers for proximal and terminal complement activation. In this context, a recently described modified Hams test to distinguish between TTP and aHUS has been reported [99]. Patients with aHUS have a positive modified Hams test. In theory, patients who have a phenotypic expression of aHUS arising from a complement storm and direct noncomplement-initiated damage to endothelia would have a negative modified Hams test when evaluated after a two-prong therapeutic approach, resulting in resolution of the inciting event and the attendant secondary complement activation. The functional assays reported by different laboratories all rely on serum or plasma and therefore are only able to evaluate CRPs such as CFH, CFI or mutations in C3 and CFB [36, 85, 98]. As such, they are limited to evaluating fluid phase activators and inhibitors of complement. Further, highlighting the difficulty in ascertaining sensitive and interpretable serum, plasma or urine biomarkers of complement activation is the recognition that the pathogenesis of aHUS is ascribed to membrane (i.e. endothelial cell) activation and amplification of complement. Thus serum, blood and urine biomarkers of complement activity may not reflect what is occurring in the microenvironment on the cell membrane.
For now, however, in the absence of a specific biomarker for aHUS, and pending commercial availability and reliability of proximal and terminal complement activation testing, evaluation of the response of complement activation can only be obtained through university research testing.
CONCLUSION
Complement-mediated aHUS is understood to result from dysregulation of membrane-bound activation of the AP of complement. Mutations in complement proteins are identified in ∼50–70% of patients. Whether refinements of present genetic testing procedures will identify new classes of mutations remain to be determined.
On occasion, the clinician encounters a patient with a TMA, consistent with a complement-mediated pathogenesis, in the setting of another established disease (e.g. GVHD and SLE). When the clinical picture fails to resolve or worsens despite ongoing aggressive therapy directed at the presenting illness or withdrawal of the suspected offending drug, consideration should be given to the diagnosis of a secondary aHUS or an unmasking of a primary aHUS. The addition of eculizimab to the treatment of the underlying disease has been reported to be effective in many but not all such patients. This two-prong therapeutic approach to the patient with a clinical picture of secondary aHUS is consistent with an abrogation of ongoing complement dysregulation arising from direct endothelial damage and profound complement activation induced by the underlying disease or disorder.
In contrast to primary aHUS, where primary dysregulation of the AP of complement exists and the duration of therapy is uncertain, a defined duration of anticomplement therapy in secondary aHUS should be sufficient.
The clinical paradigm presented in this review can allow the appropriate use of eculizimab in select patients with secondary aHUS while remaining sensitive and cognizant of the cost of this drug.
CONFLICT OF INTEREST STATEMENT
B.B. reports personal fees from Alexion, outside the submitted work, and is on the speakers bureau of Alexion.
REFERENCES
- 1. Furlan M, Robles R, Lämmle B.. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood 1996; 87: 4223–4234 [PubMed] [Google Scholar]
- 2. Tsai H-M. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood 1996; 87: 4235–4244 [PubMed] [Google Scholar]
- 3. Tsai H-M, Lian EC.. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med 1998; 339: 1585–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noris M, Remuzzi G.. Atypical hemolytic-uremic syndrome. N Engl J Med 2009; 361: 1676–1687 [DOI] [PubMed] [Google Scholar]
- 5. Dragon-Durey M-A, Sethi SK, Bagga A. et al. Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. J Am Soc Nephrol 2010; 21: 2180–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noris M, Caprioli J, Bresin E. et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 2010; 5: 1844–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nester CM, Barbour T, de Cordoba SR. et al. Atypical aHUS: state of the art. Mol Immunol 2015; 67: 31–42 [DOI] [PubMed] [Google Scholar]
- 8. Coppo P, Schwarzinger M, Buffet M.. Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: the French TMA reference center experience. PLoS One 2010; 5: e10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sellier-Leclerc A-L, Fremeaux-Bacchi V, Dragon-Durey M-A. et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol 2007; 18: 2392–2400 [DOI] [PubMed] [Google Scholar]
- 10. Song D, Wu L-H, Wang F-M. et al. The spectrum of renal thrombotic microangiopathy in lupus nephritis. Arthritis Res Ther 2013; 15: R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coppo R, Peruzzi L, Amore A. et al. Dramatic effects of eculizimab in a child with diffuse proliferative lupus nephritis resistant to conventional therapy. Pediatr Nephrol 2015; 30: 167–172 [DOI] [PubMed] [Google Scholar]
- 12. Jodele S, Fukuda T, Vinks A. et al. Eculizimab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant 2014; 20: 518–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jodele S, Licht C, Goebel J. et al. Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood 2013; 122: 2003–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nzerue C, Oluwole K, Adejorin D. et al. Malignant hypertension with thrombotic microangiopathy and persistent acute kidney injury (AKI). Clin Kidney J 2014; 7: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van den Born BJH, Honnebier UPF, Koopmans RP. et al. Microangiopathic hemolysis and renal failure in malignant hypertension. Hypertension 2005; 45: 246–251 [DOI] [PubMed] [Google Scholar]
- 16. Zhang B, Xing C, Yu X. et al. Renal thrombotic microangiopathies induced by severe hypertension. Hypertens Res 2008: 31; 479–483 [DOI] [PubMed] [Google Scholar]
- 17. Markowitz GS, Bomback AS, Perazella MA.. Drug-induced glomerular disease: direct cellular injury. Clin J Am Soc Nephrol 2015; 10: 1291–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prescribing information for Soliris. 2007. www.accessdata.fda.gov/drugsatfda_docs/label/2011/125166s172lbl.pdf
- 19. Dunkelberger JR, Song W-C.. Complement and its role in innate and adaptive immune responses. Cell Res 2010; 20: 34–50 [DOI] [PubMed] [Google Scholar]
- 20. Ratnoff WD. A war with the molecules: Louis Pillemer and the history of properdin. Perspect Biol Med 1980; 100: 638–658 [DOI] [PubMed] [Google Scholar]
- 21. Ensky J, Hinz CF, Todd EW. et al. Properties of highly purified human properdin. J Immunol 1968; 100: 142–158 [PubMed] [Google Scholar]
- 22. Thurman JM, Holers VM.. The central role of the alternative complement pathway in human disease. J Immunol 2006; 176: 1305–1310 [DOI] [PubMed] [Google Scholar]
- 23. Nonaka M, Kimura A.. Genomic view of the evolution of the complement system. Immunogenetics 2006; 58: 701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nonaka M. The complement C3 protein family in invertebrates. Invert Surviv J 2011; 8: 21–32 [Google Scholar]
- 25. Soothill JF, Harvey BAM.. Defective opsonization: a common immunity deficiency. Arch Dis Child 1976; 51: 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pangburn MK, Schreiber RD, Müller-Eberhard HJ.. Formation of the initial C3 convertase of the alternative complement pathway. J Exp Med 1981; 154: 856–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Degn SE, Jensenius JC, Thiel S.. Disease-causing mutations in genes of the complement system. Am J Hum Genet 2011; 88: 689–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dodds AW, Law SKA.. The phylogeny and evolution of the thioester bond-containing proteins C3, C4 and α2-macroglobulin. Immunol Rev 1998; 166: 15–26 [DOI] [PubMed] [Google Scholar]
- 29. Kopp A, Hebecker M, Svobodová E. et al. Factor H: a complement regulator in health and disease, and a mediator of cellular interactions. Biomolecules 2012; 2: 46–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hourcade DE. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J Biol Chem 2006; 281: 2128–2132 [DOI] [PubMed] [Google Scholar]
- 31. Ricklin D. Manipulating the mediator: modulation of the alternative complement pathway C3 convertase in health, disease and therapy. Immunobiology 2012; 217: 1057–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ollert MW, Kadlec JV, David K. et al. Antibody-mediated complement activation on nucleated cells: a quantitative analysis of the individual reaction steps. J Immunol 1994; 153: 2213–2221 [PubMed] [Google Scholar]
- 33. Atkinson JP, Goodship THJ.. Complement factor H and the hemolytic uremic syndrome. J Exp Med 2007; 204: 1245–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fearon DT, Austen KF.. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med 1975; 142: 856–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spitzer D, Mitchell LM, Atkinson JP. et al. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol 2007; 179: 2600–2608 [DOI] [PubMed] [Google Scholar]
- 36. Cofiell R, Kukreja A, Bedard K. et al. Eculizimab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood 2015; 125: 3253–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kemper C, Pangburn MK, Fishelson Z.. Complement nomenclature 2014. Mol Immunol 2014; 61: 56–58 [DOI] [PubMed] [Google Scholar]
- 38. Krisinger MJ, Goebeler V, Lu Z. et al. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood 2012; 120: 1717–1725 [DOI] [PubMed] [Google Scholar]
- 39. Weiler JM, Daha MR, Austen KF. et al. Control of the amplification convertase by the plasma protein β1H. Proc Natl Acad Sci USA 1976; 73: 3268–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim DD, Song W-C.. Membrane complement regulatory proteins. Clin Immunol 2006; 118: 127–136 [DOI] [PubMed] [Google Scholar]
- 41. Warwicker P, Goodship TH, Donne RL. et al. Genetics studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int 1998; 53: 836–844 [DOI] [PubMed] [Google Scholar]
- 42. Bu F, Maga T, Meyer NC. et al. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J Am Soc Nephrol 2014; 25: 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Valoti E, Alberti M, Tortajada A. et al. A novel atypical hemolytic uremic syndrome-associated hybrid CFHR1/CFH gene encoding a fusion protein that antagonizes factor H-dependent complement regulation. J Am Soc Nephrol 2015; 26: 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. George JN, Nester CM.. Syndromes of thrombotic microangiopathy. N Engl J Med 2014; 371: 654–666 [DOI] [PubMed] [Google Scholar]
- 45. Moschcowitz E. An acute febrile pleiochromic anemia with hyaline thrombosis of the terminal arterioles and capillaries: an undescribed disease. Proc NY Pathol Sci 1924; 24: 21–24 [PubMed] [Google Scholar]
- 46. Edelsten AD, Tuck S.. Familial haemolytic syndrome. Arch Dis Child 1978; 53: 255–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ariceta G, Besbas N, Johnson S. et al. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol 2009; 24: 687–696 [DOI] [PubMed] [Google Scholar]
- 48. Kavanagh D, Goodship THJ, Richards A.. Atypical haemolytic uraemic syndrome. Br Med Bull 2006; 77–78: 5–22 [DOI] [PubMed] [Google Scholar]
- 49. Loirat C, Frémeaux-Bacchi V.. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis 2011; 6: 60–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caprioli J, Noris M, Brioschi S. et al. Genetics of HUS: the impact of MCP, CFH and IF mutations on clinical presentation, response to treatment, and outcome. Blood 2006; 108: 1267–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hofer J, Rosales A, Fischer C. et al. Extra-renal manifestations complement-mediated thrombotic microangiopathy. Front Pediatr 2014; 2: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fremeaux-Bacchi V, Fakhouri F, Garnier A. et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol 2013; 8: 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lemaire M, Frémeaux-Bacchi V, Schaefer F. et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 2013; 45: 531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Noris M, Mele C, Remuzzi G.. Podocyte dysfunction in atypical haemolytic uraemic syndrome. Nat Rev Nephrol 2015; 11: 245–252 [DOI] [PubMed] [Google Scholar]
- 55. Sanchez Chinchilla D, Pinto S, Hoppe B. et al. Complement mutations in diacylglycerol kinase-ε-associated atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 2014; 9: 1611–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bruneau S, Néel M, Roumenina LT. et al. Loss of DGKε induces endothelial cell activation and death independently of complement activation. Blood 2015; 125: 1038–1046 [DOI] [PubMed] [Google Scholar]
- 57. Derebail V, Parikh P, Jennette JC. et al. A rare cause of the pulmonary-renal syndrome: a case of atypical haemolytic-uraemic syndrome complicated by pulmonary hemorrhage. NDT Plus 2008; 6: 417–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Noris M, Remuzzi G.. Cardiovascular complications in atypical haemolytic uraemic syndrome. Nat Rev Nephrol 2014; 10: 174–180 [DOI] [PubMed] [Google Scholar]
- 59. Sallée M, Daniel L, Piercecchi M-D. et al. Myocardial infarction is a complication of factor H-associated atypical HUS. Nephrol Dial Transplant 2010; 25: 2028–2032 [DOI] [PubMed] [Google Scholar]
- 60. Totina A, Iorember F, El-Dahr SS. et al. Atypical hemolytic-uremic syndrome in a child presenting with malignant hypertension. Clin Pediatr 2013; 52: 183–186 [DOI] [PubMed] [Google Scholar]
- 61. Ardissino G, Tel F, Testa S. et al. Skin involvement in atypical hemolytic uremic syndrome. Am J Kidney Dis 2014; 63: 652–655 [DOI] [PubMed] [Google Scholar]
- 62. Zheng X, Gorovoy IR, Mao J. et al. Recurrent ocular involvement in pediatric atypical hemolytic uremic syndrome. J Pediatr Ophthalmol Strabismus 2014; 51: e62–e65 [DOI] [PubMed] [Google Scholar]
- 63. Larakeb A, Leroy S, Frémeaux-Bacchi V. et al. Ocular involvement in hemolytic uremic syndrome due to factor H deficiency—are there therapeutic consequences? Pediatr Nephrol 2007; 22: 1967–1970 [DOI] [PubMed] [Google Scholar]
- 64. David R, Hochberg-Klein S, Amer R.. Resolution of ocular involvement with systemic eculizimab in atypical hemolytic-uremic syndrome. Eye 2013; 27: 997–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Feng S, Eyler SJ, Zhang Y. et al. Partial ADAMTS13 deficiency in atypical hemolytic uremic syndrome. Blood 2013; 122: 1487–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chapin J, Weksler B, Magro C. et al. Eculizimab in the treatment of refractory idiopathic thrombotic thromobocytopenic purpura. Br J Haemotol 2012; 157; 772–774 [DOI] [PubMed] [Google Scholar]
- 67. Sciascia S, Cuadrado MJ, Khamashta M. et al. Renal involvement in antiphospholipid syndrome. Nat Rev Nephrol 2014; 10: 279–289 [DOI] [PubMed] [Google Scholar]
- 68. Russo P, Doyon J, Sonsino E. et al. A congenital anomaly of vitamin B12 metabolism: a study of three cases. Hum Pathol 1992; 23: 504–522 [DOI] [PubMed] [Google Scholar]
- 69. Lonze BE, Singer AL, Montgomery RA.. Eculizimab and renal transplantation in a patient with CAPS. N Engl J Med 2010; 362: 1744–1745 [DOI] [PubMed] [Google Scholar]
- 70. Bruel A, Kavanagh D, Noris M. et al. Hemolytic uremic syndrome in pregnancy and postpartum. Clin J Am Soc Nephrol 2017; 12: 1237–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cavero T, Rabasco C, López A. et al. Eculizimab in secondary atypical hemolytic uraemic syndrome. Nephrol Doal Transplant 2017; 32: 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. El-Husseini A, Hannan S, Awad A. et al. Thrombotic microangiopathy in systemic lupus erythematosus: efficacy of eculizimab. Am J Kidney Dis 2015; 65: 127–130 [DOI] [PubMed] [Google Scholar]
- 73. Berger B. The alternative pathway of complement and the evolving clinical-pathophysiological spectrum of atypical hemolytic uremic syndrome. Am J Med Sci 2016; 352: 177–190 [DOI] [PubMed] [Google Scholar]
- 74. Fakhouri F, Fila M, Provôt F. et al. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizimab discontinuation. Clin J Am Soc Nephrol 2017; 12: 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mele C, Lemaire M, Iatropoulos P. et al. Characterization of a new DGKE intronic mutation in genetically unsolved cases of familial atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 2015; 10: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rodriguez E, Rallapalli PM, Osborne AJ. et al. New functional and structural insights from updated mutational databases for complement factor H, factor I, membrane cofactor protein and C3. Biosci Rep 2014; 34: 635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mii A, Shimizu A, Kaneko T. et al. Renal thrombotic microangiopathy after hematopoietic stem cell transplantation: involvement of chronic graft-versus-host disease. Kidney Int Rep 2018; 3: 743–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Harboe M, Ulvund G, Vien L. et al. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol 2004; 138: 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ward PA. The dark side of C5a in sepsis. Nature Rev Immunol 2004; 4: 133–142 [DOI] [PubMed] [Google Scholar]
- 80. Legendre CM, Licht C, Muus P. et al. Terminal complement inhibitor eculizimab in atypical hemolytic-uremic syndrome. N Engl J Med 2013; 368: 2169–2181 [DOI] [PubMed] [Google Scholar]
- 81. Fakhouri F, Delmas Y, Provot F. et al. Insights from the use in clinical practice of eculizimab in adult patients with atypical hemolytic uremic syndrome affecting the native kidneys: an analysis of 19 cases. Am J Kidney Dis 2013; 63: 40–48 [DOI] [PubMed] [Google Scholar]
- 82. Li A, Makar RS, Hurwitz S. Therapeutic plasma exchange for the treatment of thrombotic microangiopathy without severe ADAMTS13 deficiency: a propensity score-matched study. Blood 2015; 126: 3471. [DOI] [PubMed] [Google Scholar]
- 83. Licht C, Greenbaum LA, Muus P. et al. Efficacy and safety of eculizimab in atypical hemolytic uremic syndrome from 2-year extension of phase 2 studies. Kidney Int 2015; 87: 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Greenbaum LA, Fila M, Ardissino G. et al. Eculizimab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 2016; 89: 701–711 [DOI] [PubMed] [Google Scholar]
- 85. Ardissino G, Testa S, Possenti I. et al. Discontinuation of eculizimab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis 2014; 64: 633–637 [DOI] [PubMed] [Google Scholar]
- 86. Macia M, de Alvaro Moreno F, Dutt T. et al. Current evidence on the discontinuation of eculizimab in patients with atypical hemolytic uremic syndrome. Clin Kidney J 2017; 10: 310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Puissant-Lubrano B, Puissochet S, Congy-Jolivet N. et al. Alternative complement pathway hemolytic assays reveal incomplete blockade in patients treated with eculizimab. Clin Immunol 2017; 183: 1–7 [DOI] [PubMed] [Google Scholar]
- 88.Centers for Disease Control and Prevention (CDC). Meningococcal Vaccination 2017. https://www.cdc.gov/vaccines/vpd/mening/index.html
- 89. Sinha A, Gulati A, Saini S.. Prompt plasma exchanges and immunosuppressive treatment improves the outcomes of anti-factor H autoantibody-associated hemolytic uremic syndrome in children. Kidney Int 2013; 85: 1151–1160 [DOI] [PubMed] [Google Scholar]
- 90. Girardi G, Berman J, Redecha P. et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 2003; 112: 1644–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bakhtar O, Thajudeen B, Braunhut BL. et al. A case of thrombotic microangiopathy associated with antiphospholipid antibody syndrome successfully treated with eculizimab. Transplantation 2014; 98: e17–e18 [DOI] [PubMed] [Google Scholar]
- 92. Canaud G, Kamar N, Anglicheau D. et al. Eculizimab improves posttransplant thrombotic microangiopathy due to antiphospholipid syndrome recurrence but fails to prevent chronic vascular changes. Am J Transplant 2013; 13: 2179–2185 [DOI] [PubMed] [Google Scholar]
- 93. Strakhan M, Hurtado-Sbordoni M, Galeas N. et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizimab: a case report and review of literature. Case Rep Hematol 2014; 2014: 704371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hadaya K, Ferrari-Lacraz S, Fumeaux D. et al. Eculizimab in acute recurrence of thrombotic microangiopathy after renal transplantation. Am J Transplant 2011; 11: 2523–2527 [DOI] [PubMed] [Google Scholar]
- 95. Kronbichler A, Frank R, Kirschfink M. et al. Efficacy of eculizimab in a patient with immunoabsorption-dependent catastrophic antiphospholipid syndrome: a case report. Medicine 2014; 93: e143–e147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shapira I, Andrade D, Allen SL. et al. Induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizimab. Arthritis Rheum 2012; 64: 2719–2723 [DOI] [PubMed] [Google Scholar]
- 97. Zapantis E, Furle R, Horowitz D.. Response to eculizimab in the antiphospholipid antibody syndrome. Ann Rheum Dis 2015; 74: 34124285491 [Google Scholar]
- 98. Noris M, Galbusera M, Gastoldi S. et al. Dynamics of complement activation in aHUS and how to monitor eculizimab therapy. Blood 2014; 124: 1715–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gavriilaki E, Yuan X, Ye Z. et al. Modified Ham test for atypical hemolytic uremic syndrome. Blood 2015; 125: 3637–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]