Abstract
Background
Intensive glucose control reduces the risk for microvascular complications in type 2 diabetes (T2DM). Recently, sodium-glucose cotransporter 2 (SGLT2) inhibitors have been shown to exert renoprotection beyond glycemic control, although their effects on the organs are not well known. There are limited data on SGLT2 inhibitors for the biomarkers of kidney injury in type 2 diabetes mellitus (T2DM) patients.
Objective
Our objective was to demonstrate the effect of SGLT2 inhibitors on proximal tubular injury and function in patients with T2DM.
Methods
T2DM patients with persistent glycated hemoglobin (HbA1c) levels >7% were randomly assigned to either dapagliflozin 10 mg/day (n = 28) or standard treatment (n = 29) for 12 weeks. Proximal tubular injury biomarkers, including urine kidney injury molecule-1:creatinine ratio (UKIM1CR), urine cystatin C:creatinine ratio (UCCR), urine albumin:creatinine ratio (UACR), fractional excretion of phosphate (FEPO4) and fractional excretion of uric acid (FEUA) were measured at baseline and study end.
Results
Baseline characteristics were comparable between treatment groups. After 12 weeks, dapagliflozin-treated versus standard-treated patients showed reductions in HbA1c (–0.75 ± 0.21 versus –0.70 ± 0.25%; P = 0.882). There were significant between-group differences in the reduction in UACR {–23.3 [95% confidence interval (CI) –44.4 to –2.2] versus +19.9 (–4.0–43.8) mg/g Cr; P = 0.010} and UKIM1CR [–26.7 (95% CI –232.9–179.5) versus +422.2 (46.7–797.7) ng/g Cr; P = 0.047], but no significant difference in changes in UCCR between the two groups. There was no significant change in glomerular filtration rate, serum phosphate level, FEUA and FEPO4 in the dapagliflozin group. No serious renal-related adverse events were observed in either group.
Conclusions
This study indicates that dapagliflozin in T2DM patients can decrease the levels of urinary proximal tubular injury biomarkers, thus highlighting its renoprotective effect. SGLT2 inhibitors could prove useful in treating T2DM by protecting against renal tubular injury and may lead to reduced long-term renal outcomes.
Keywords: cystatin C, dapagliflozin, kidney injury molecule-1, sodium-glucose cotransporter 2 inhibitor, type 2 diabetes
BACKGROUND
Type 2 diabetes mellitus (T2DM) is one of the most important problems in the world and patients with T2DM are at an increased risk for a number of serious health problems, including cardiovascular disease, premature death, blindness, kidney failure, amputations and cognitive decline [1]. Chronic kidney disease (CKD) with a glomerular filtration rate (GFR) of <60 mL/min/1.73 m2 is a common condition estimated to affect 8.6–14.5% of the Thai population, and 28.5% have both T2DM and CKD [2, 3]. Diabetes is often associated with CKD, and for 30–40% of patients receiving dialysis therapy, diabetes is the primary cause of end-stage renal disease. Optimized glucose control reduces the risk of diabetic micro- and macrovascular complications [4, 5].
One recently approved class of glucose-lowering drugs inhibits sodium-glucose cotransporter type 2 (SGLT2) in the renal proximal tubule and hence reduces renal reabsorption of filtered glucose, resulting in substantial glycosuria [6]. Clinical studies on SGLT2 inhibitors have reported reductions in glycated hemoglobin A1c (HbA1c) levels compared with placebo and other glucose-lowering strategies [7, 8] and a reduction in cardiovascular mortality in individuals with T2DM, as well as a significantly lower risk of worsening nephropathy, progression to macroalbuminuria, doubling of serum creatinine levels and initiation of renal replacement therapy in high cardiovascular-risk patients [9].
SGLT2 inhibitors represent a promising therapeutic approach to preventing and improving nephropathy in patients with T2DM. Current data strongly support that SGLT2 inhibitors have renoprotective properties not only by improving glycemic control, but also through hemodynamic and nonhemodynamic renal effects [10]. A study using diabetic endothelial nitric oxide synthase (eNOS) knockout mice demonstrated the effect of SGLT2 inhibition on diabetic nephropathy, independent of blood glucose lowering [11]. Moreover, initial studies reported that SGLT2 inhibition decreased the production of inflammatory and fibrotic markers induced by high glucose levels in human proximal tubular cells [12]. These in vitro findings suggest that SGLT2 inhibitors may provide renoprotection in diabetes by preventing glucose from entering proximal tubule cells [13, 14]. Thus, in this study, we aimed to determine whether administration of dapagliflozin, an SGLT2 inhibitor, influences proximal tubular cell injuries and function in T2DM patients.
MATERIALS AND METHODS
Subjects
This was a randomized, open-label, controlled study conducted over 12 weeks in T2DM patients at Phramongkutklao Hospital, Bangkok, Thailand between 15 January 2015 and 16 December 2016. The study was approved by the institutional review boards of Phramongkutklao Hospital and the College of Medicine. Using blocks of four randomizations, patients were assigned to one of two double-blinded treatment groups. A computer-generated randomization procedure in blocks of four was used. Inclusion criteria comprised age ≥18 years, a diagnosis of T2DM, an estimated GFR (eGFR) >60 mL/min/1.73 m2, stable glycemic treatment with HbA1c levels between 7% and 10% at least 3 months before and no treatment adjusted with antihypertensive drugs within 3 months of starting the study. Exclusion criteria included a history of urinary and genital tract infection, dehydration, a history of diabetic ketoacidosis, use of loop diuretics, pregnancy and any uncontrolled endocrine disorders within 1 year of starting the study. All subjects gave their informed consent before they were enrolled.
Eligible patients were randomly assigned to two groups. One group consisted of 29 patients treated with standard glycemic treatment for T2DM, while the other group consisted of 28 patients treated with dapagliflozin 10 mg/day orally. During the 12 weeks in the control group, patients had their hypoglycemic medications adjusted to maintain HbA1c levels at <7–8%. All patients typically continued with their normal daily activities during both treatments and were instructed to adhere to a disease- and weight-oriented diet and exercise regimen throughout the study.
All subjects underwent laboratory blood tests at baseline and at 12 weeks at the end of the study. All patients were monitored for proximal tubular injury biomarkers, including urine kidney injury molecule-1:creatinine ratio (UKIM1CR), urine cystatin C:creatinine ratio (UCCR), urine albumin:creatinine ratio (UACR), fractional excretion of sodium (FENa), fractional excretion of phosphate (FEPO4) and fractional excretion of uric acid (FEUA). Serum and urine samples were also obtained after overnight fasting and were immediately processed for analysis, including fasting plasma glucose (FPG), HbA1c, blood urea nitrogen, creatinine, complement blood count and urinary analysis. Other conventional parameters, including body weight, systolic and diastolic blood pressure, symptoms and signs related to drug reactions and compliance with medications were closely monitored every 4 weeks.
Safety monitoring
Adverse events that were considered related to dapagliflozin treatment were monitored for at 4 and 12 weeks. The patients were questioned each time in a systematic way about their experiences concerning adverse events, especially ketoacidosis and genital and urinary tract infections, over the 12 weeks. We evaluated the incidence of renal progression using assessments of renal function over time with serum creatinine levels and eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation.
Statistical analyses
Statistical analyses were performed using Stata version 13.0 (StataCorp, College Station, TX, USA). Descriptive statistics were used to summarize the demographics and baseline characteristics. Comparability of treatment groups was assessed using a two-way analysis of variance (ANOVA). Data were presented as mean ± standard error (SE) and mean change. Differences between groups were established using the independent t-test. Repeated measures ANOVA was used to compare the results to baseline at each time point for each of the efficacy parameters. All results were considered significant if P < 0.05.
RESULTS
A total of 68 patients from outpatient clinics were screened for possible study enrollment. Fifty-seven patients were eligible according to the study entry criteria shown in Figure 1. Overall, 28 patients were assigned to the dapagliflozin group (10 mg/day) and 29 patients to the standard treatment group. Characteristics of the study population are shown in Table 1. The baseline characteristics of the treatment and placebo groups did not differ in terms of age, gender, body weight, body mass index, blood pressure, current medications, comorbid diseases and medications. In total, 76% of the patients were taking statins or fibrates at baseline.
FIGURE 1.
Flowchart of the study.
Table 1.
Baseline characteristics of patients
| Parameter | Dapagliflozin | Control | P-value |
|---|---|---|---|
| (n = 28) | (n = 29) | ||
| Male, n (%) | 17 (60.7) | 8 (27) | 0.012 |
| Age (years) | 55.9 ± 1.4 | 59.9 ± 1.6 | 0.059 |
| Duration of T2DM (years) | 8.5 ± 0.9 | 9.0 ± 1.0 | 0.685 |
| Systolic blood pressure (mmHg) | 134.8 ± 2.4 | 131 ± 2.7 | 0.302 |
| Diastolic blood pressure (mmHg) | 79.8 ± 2.5 | 75.7 ± 2.2 | 0.221 |
| Body weight (kg) | 75.3 ± 2.4 | 68.7 ± 2.5 | 0.061 |
| Body mass index (kg/m2) | 28.4 ± 0.8 | 27.9 ± 0.9 | 0.692 |
| UACR ≥30 mg/g Cr, n (%) | 11 (39.3) | 8 (27.6) | 0.349 |
| Comorbid diseases, n (%) | |||
| Hypertension | 23 (82.1) | 26 (89.7) | |
| Cerebrovascular disease | 2 (7.1) | 1 (3.5) | 0.532 |
| Dyslipidemia | 26 (92.9) | 28 (96.6) | 0.532 |
| Cardiovascular disease | 1 (3.6) | 5 (17.2) | 0.093 |
| Diabetic retinopathy | 4 (14.3) | 5 (17.2) | 0.760 |
| Medications, n (%) | |||
| RAAS inhibitors | 19 (67.9) | 15 (51.7) | 0.215 |
| Beta-blockers | 6 (21.4) | 9 (31.0) | 0.410 |
| Calcium channel blockers | 10 (35.7) | 15 (51.7) | 0.223 |
| Metformin | 25 (89.3) | 26 (89.7) | 0.964 |
| Sulfonylurea | 16 (57.1) | 14 (48.3) | 0.503 |
| Insulin | 5 (17.9) | 6 (20.7) | 0.786 |
Data are presented as mean ± SE unless stated otherwise. Cardiovascular disease was defined as coronary heart disease, peripheral artery disease and heart failure. Cerebrovascular disease was defined as ischemic and nonischemic stroke.
RAAS, renin–angiotensin–aldosterone system.
Changes in metabolic, glycemic and laboratory parameters
At the end of 12 weeks, body weight was significantly decreased at 1.0 ± 0.4 kg from baseline in the dapagliflozin group (P < 0.05), but there was no difference in mean changes in body weight between the two groups. The mean FPG level significantly decreased from 179.9 ± 9.7 to 157.8 ± 9.5 mg/dL in the dapagliflozin group (P < 0.05) and from 168.3 ± 10.8 to 155.1 ± 9.2 mg/dL in the control group (P < 0.05). Similarly, the mean HbA1c level significantly decreased from 8.7 ± 0.2 to 7.9 ± 0.2% in the dapagliflozin group (P < 0.05) and from 8.6 ± 0.2 to 7.9 ± 0.2% in the control group (P < 0.05). No difference was observed between the two groups regarding FPG and HbA1c levels at baseline or at the study end or in the change in these values during the study (Table 2). The mean hemoglobin level increased after treatment with dapagliflozin and there was a significant difference in increased mean changes in hemoglobin level between the two groups. In contrast, blood pressure and the levels of serum uric acid, phosphate, low-density lipoprotein (LDL) cholesterol and triglycerides did not significantly change in either group throughout the study (Table 2).
Table 2.
Changes in metabolic, glycemic and laboratory parameters after 12 weeks of treatment
| Parameter | Dapagliflozin (n = 28) | Control (n = 29) | P-value |
|---|---|---|---|
| Body weight (kg) | |||
| Baseline | 75.3 ± 2.4 | 68.7 ± 2.5 | 0.061 |
| Week 12 | 74.2 ± 2.5 | 68.2 ± 2.7 | 0.110 |
| Change from baseline | –1.0 ± 0.4* | –0.1 ± 0.4 | 0.081 |
| Fasting plasma glucose (mg/dL) | |||
| Baseline | 179.9 ± 9.7 | 168.3 ± 10.8 | 0.429 |
| Week 12 | 157.8 ± 2.5 | 155.2 ± 9.2 | 0.844 |
| Change from baseline | –19.6 ± 8.2* | –11.1 ± 8.7 | 0.481 |
| HbA1c (%) | |||
| Baseline | 8.7 ± 0.2 | 8.6 ± 0.2 | 0.533 |
| Week 12 | 7.9 ± 0.2 | 7.9 ± 0.3 | 0.776 |
| Change from baseline | –0.7 ± 0.2* | –0.7 ± 0.3 | 0.882 |
| Hemoglobin (g/dL) | |||
| Baseline | 13.5 ± 0.3 | 12.5 ± 0.2 | 0.020 |
| Week 12 | 14.1 ± 0.3 | 12.6 ± 0.3 | 0.000 |
| Change from baseline | 0.5 ± 0.1 | –0.1 ± 0.1 | 0.002 |
| Systolic blood pressure (mmHg) | |||
| Baseline | 134.8 ± 2.4 | 131.0 ± 2.7 | 0.302 |
| Week 12 | 132.8 ± 2.3 | 133.4 ± 2.8 | 0.870 |
| Change from baseline | –2.0 ± 2.8 | 3.3 ± 2.1 | 0.146 |
| Diastolic blood pressure (mmHg) | |||
| Baseline | 79.8 ± 2.5 | 75.7 ± 2.2 | 0.221 |
| Week 12 | 79.9 ± 1.5 | 76.0 ± 2.1 | 0.140 |
| Change from baseline | 0.2 ± 2.3 | 0.6 ± 2.0 | 0.889 |
| Serum phosphate (mg/dL) | |||
| Baseline | 3.2 ± 0.1 | 3.3 ± 0.1 | 0.259 |
| Week 12 | 3.5 ± 0.1 | 3.4 ± 0.2 | 0.861 |
| Change from baseline | 0.3 ± 0.1 | 0.1 ± 0.2 | 0.370 |
| Serum uric acid (mg/dL) | |||
| Baseline | 5.2 ± 0.2 | 4.9 ± 0.3 | 0.605 |
| Week 12 | 5.2 ± 0.3 | 5.3 ± 0.3 | 0.650 |
| Change from baseline | –0.1 ± 0.2 | 0.2 ± 0.1 | 0.265 |
| Triglycerides (mg/dL) | |||
| Baseline | 145.0 ± 14.0 | 161.1 ± 16.7 | 0.463 |
| Week 12 | 142.1 ± 13.4 | 154.7 ± 17.6 | 0.570 |
| Change from baseline | –6.1 ± 12.9 | –7.1 ± 10.0 | 0.950 |
| LDL cholesterol (mg/dL) | |||
| Baseline | 102.5 ± 6.6 | 87.9 ± 4.9 | 0.080 |
| Week 12 | 104.0 ± 5.3 | 89.3 ± 6.5 | 0.085 |
| Change from baseline | 1.3 ± 6.4 | –1.8 ± 4.5 | 0.949 |
Data are mean ± SE. Week 12 values compared with baseline. *P < 0.05.
Proximal tubular injury biomarkers and renal function
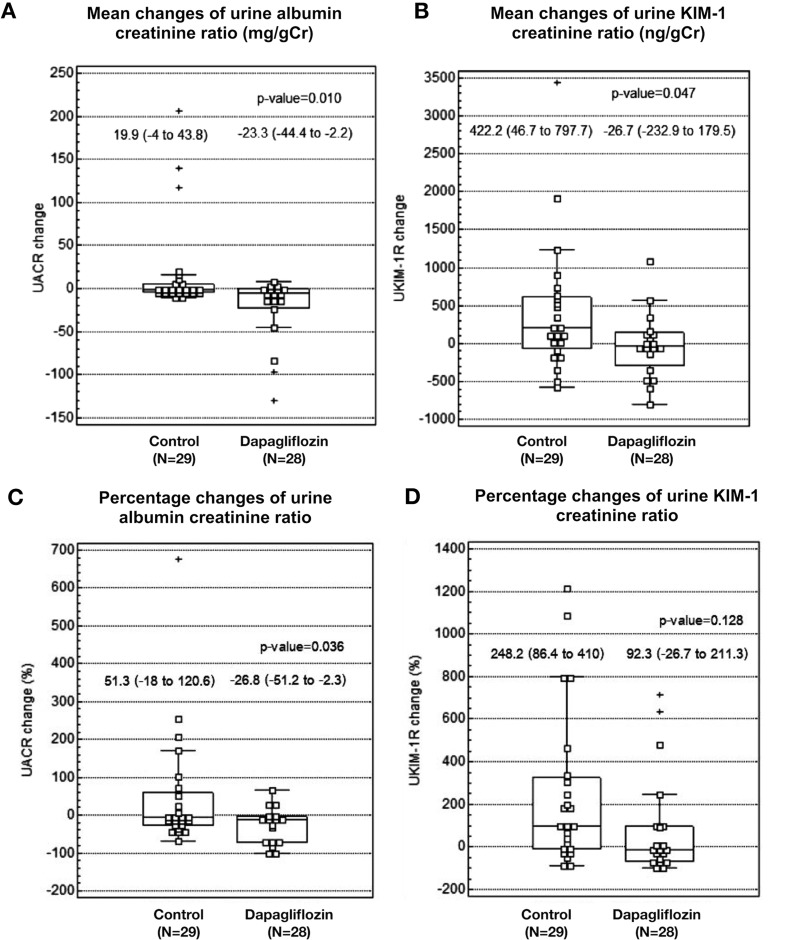
After 12 weeks of treatment, the mean UACR significantly decreased from 51.1 ± 14.2 to 37.3±9.4 mg/g Cr (P = 0.032) in the dapagliflozin group and there was a significant between-group difference in the reduction of UACR {−23.3 [95% confidence interval (CI) −44.4 to −2.2] versus +19.9 (−4.0–43.8) mg/g Cr; P = 0.010} and in percentage change in UACR (−26.8 ± 11.5 versus 51.3 ± 33.4%; P = 0.036) (Figure 2). Similarly, the mean UKIM1CR decreased from 502.7 ± 106.8 to 338.4 ± 85.7 mg/g Cr (P = 0.789) in the dapagliflozin group and significantly increased from 563.3 ± 117.2 to 910.5 ± 230.6 mg/g Cr in the control group (P = 0.029). There were significant between-group differences in the reduction of UKIM1CR [−26.7 (95% CI −232.9–179.5) versus + 422.2 (46.7–797.7) ng/g Cr; P = 0.047] and in the percentage change in UKIM1CR (92.3 ± 56.7 versus 248.2 ± 78.0%; P = 0.128) (Figure 2). UCCR significantly increased in both groups during the study, but there was no significant difference in the mean change in UCCR between the two groups. There was no significant change in serum creatinine levels, GFR, FENa, FEUA and FEPO4 in either treatment group (Table 3).
FIGURE 2.
Mean and percentage changes in UACR and UKIM1CR after 12 weeks of treatment. There were no significant changes in mean UACR and UKIM1CR from baseline in both control and dapagliflozin treatment groups, but significantly increased UKIM1CR in the control treatment group. There were significant between-group differences in the mean changes in (A) UACR and (B) KIM1CR (P < 0.05). There was (C) a significant between-group difference in the percentage changes of UACR (P = 0.036) but (D) no significant between-group difference in the percentage changes of UKIM1CR (P = 0.128).
Table 3.
Changes in renal function and levels of proximal tubular biomarkers after 12 weeks of treatment
| Renal parameters | Dapagliflozin (n = 28) | Control (n = 29) | P-value |
|---|---|---|---|
| Serum creatinine (mg/dL) | |||
| Baseline | 0.9 ± 0.0 | 0.8 ± 0.0 | 0.309 |
| Week 12 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.381 |
| Change from baseline | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.982 |
| eGFR (mL/min/1.73 m2) | |||
| Baseline | 88.7 ± 3.1 | 87.3 ± 2.9 | 0.747 |
| Week 12 | 87.4 ± 3.3 | 85.3 ± 3.2 | 0.653 |
| Change from baseline | –1.0 ± 1.4* | –1.9 ± 1.9 | 0.695 |
| UACR (mg/g Cr) | |||
| Baseline | 51.1 (22.0–80.1) | 127.4 (–75.4–330.1) | 0.456 |
| Week 12 | 36.7 (12.1–61.4) | 46.5 (10.7–82.4) | 0.677 |
| Change from baseline | –23.3 (–44.4 to –2.2) | 19.9 (–4.0–43.8) | 0.010 |
| UKIM1CR (ng/g Cr) | |||
| Baseline | 502.7 ± 106.8 | 563.3 ± 117.2 | 0.705 |
| Week 12 | 338.4 ± 85.7 | 910.5 ± 230.6 | 0.028 |
| Change from baseline | –26.7 (–232.9–179.5) | 422.2 (46.7–797.7) | 0.047 |
| UCCR (mcg/gCr) | |||
| Baseline | 12.5 ± 2.6 | 15.8 ± 3.4 | 0.455 |
| Week 12 | 27.8 ± 6.1 | 29.4 ± 4.9 | 0.841 |
| Change from baseline | 15.3 ± 7.2* | 14.1 ± 5.7* | 0.898 |
| FEPO4 (%) | |||
| Baseline | 11.6 ± 0.8 | 11.3 ± 0.9 | 0.826 |
| Week 12 | 10.8 ± 1.1 | 10.8 ± 2.9 | 0.986 |
| Change from baseline | –0.7 ± 0.9 | –0.6 ± 3.1 | 0.959 |
| FEUA (%) | |||
| Baseline | 9.1 ± 0.7 | 9.8 ± 0.8 | 0.525 |
| Week 12 | 8.8 ± 0.7 | 7.0 ± 0.5 | 0.046 |
| Change from baseline | –0.1 ± 0.8 | –1.3 ± 0.6 | 0.130 |
| FENa (%) | |||
| Baseline | 1.1 ± 0.2 | 1.3 ± 0.2 | 0.473 |
| Week 12 | 1.1 ± 0.2 | 1.2 ± 0.2 | 0.528 |
| Change from baseline | –0.1 ± 0.2 | 0.1 ± 0.2 | 0.619 |
Data are mean ± SE or mean (95% CI). Week 12 values compared with baseline. *P < 0.05.
Adverse events
No significant acute kidney injury, volume depletion and hypotension in the dapagliflozin group was detected. Finally, there were no reports of urinary tract infections, genital tract infections, ketoacidosis, deaths or drug-related serious adverse events in either group.
DISCUSSION
The present study is a randomized controlled trial of the effect of SGLT2 inhibitors on proximal tubular injuries and function in T2DM patients. Dapagliflozin 10 mg/day as add-on therapy appears as efficacious as standard treatment in terms of improving glycemic control (HbA1c and FPG). Interestingly, dapagliflozin also has inhibitory effects on urine kidney injury molecule-1 (KIM-1) and albuminuria in proximal tubular cells in response to hyperglycemia.
In a recent larger randomized controlled trial, empagliflozin was associated with slower progression of kidney disease and lower rates of new or worsening nephropathy, including macroalbuminuria, doubling of serum creatinine levels with a GFR of <45 mL/min/1.73 m2 and initiation of renal replacement therapy in patients with T2DM at high cardiovascular risk [15]. In addition, several clinical studies showed a significant reduction in albuminuria and a slow decline in GFR after the initiation of SGLT2 inhibitors [16–18]. Our study also demonstrated improved albuminuria, the earliest clinical manifestation of diabetic renal injury and which serves as a biomarker of renal and vascular injury in diabetes [19]. SGLT2 inhibitors might be able to limit high glucose–induced inflammatory and fibrotic markers, most likely due to blocking glucose entry into proximal tubular cells.
Novel biomarkers of tubulointerstitial changes prove to better predict renal progression and prognosis in T2DM. Tubular biomarkers such as cystatin C, KIM-1, neutrophil gelatinase-associated lipocalin (NGAL) and monocyte chemoattractant protein-1 reflect tubular injury [20, 21]. The effects of SGLT2 inhibitors on markers of tubular injury in patients with T2DM are as yet unknown, with no previous clinical study of SGLT2 inhibitors on biomarkers of proximal tubular injury in patients with T2DM. Proximal tubular basement membrane thickening, hyperplasia and hypertrophy, followed by tubular atrophy, were detected in progressive diabetic kidney injury [22]. Interestingly, experimental knockout of SGLT2 in diabetic mice had no effect on the increase in markers of tubular damage such as NGAL and KIM-1 [23]. An in vitro study using human proximal tubular cells indicated that SGLT2 inhibitors augmented advanced glycation end-product–induced apoptotic death of tubular cells and decreased the level of high glucose–induced inflammatory and fibrotic markers by reducing glucose transport to the proximal tubular cells [12, 24]. Recently, in a post hoc analysis of a crossover trial, dapagliflozin treatment for 6 weeks decreased albuminuria, urinary KIM-1 excretion and urinary interleukin-6 (IL-6) excretion compared with placebo, which could be the result of reduced tubular cell injury [25]. Consistent with our findings, dapagliflozin protects against proximal tubular injury and decreases urinary KIM-1 and albuminuria, independent of glycemic control and HbA1c lowering. However, there was no difference in changes in urine cystatin C levels between the two treatment groups. There was a significant increase in urine cystatin C levels after treatment with both the SGLT2 inhibitor and control in our study, but these changes were very small.
In terms of proximal tubular function, FEUA, FEPO4 and serum uric acid levels did not significantly change from baseline after 12 weeks of treatment with dapagliflozin. In agreement with a previous study in healthy Japanese men, the greatest change in urinary excretion of uric acid from baseline was observed on day 1, with the magnitude of change decreasing on days 3 and 7 after SGLT2 inhibitor administration [26, 27]. However, dapagliflozin treatment was associated with a small decrease in serum uric acid levels, in line with many previous studies, which could explain the uricosuric effect of SGLT2 inhibitors. Glucose transporter 9 (GLUT9) is a countertransporter that exchanges glucose for uric acid, whereby the two isoforms of the counter-transporter act concertedly to reabsorb glucose from the lumen of the tubule in exchange for uric acid. In the presence of high glucose concentrations on the luminal side, coupled with volume depletion (due to SGLT2 inhibitors), one would expect primary proximal uric acid reabsorption and elevated serum uric acid levels. GLUT9 might counterbalance primary uric acid reabsorption, acting as an efflux transporter of urate from the tubular cells [28]. In our study, the uricosuric effect, as demonstrated by FEUA, decreased in both treatment groups and was more marked at week 12 in the control group compared with the dapagliflozin-treated group, but there was no significant difference in the mean change in FEUA between the groups. This probably reflects the low baseline serum uric acid levels and the biochemical interaction between serum glucose and purine metabolism, with decreased excretion of uric acid in the presence of lower plasma glucose levels and glycosuria [29].
Several limitations are associated with the present study. First, the long-term outcomes and serious adverse effects in patients with T2DM could not be demonstrated in this study. The study also did not show that the increased levels of urine KIM-1 would be associated with long-term effects on clinical endpoints. Additional research is needed to confirm the results and determine the long-term clinical outcomes. Second, the study included the relative difference in baseline urine albumin between the two groups of patients, and only 50–70% of the patients received renin–angiotensin–aldosterone system inhibition. Third, we did not perform renal histopathology to confirm evidence of proximal tubular injuries. Finally, the study had an open-label, randomized controlled design. The strength of the study stemmed from measurements of several tubular biomarkers, including KIM-1, cystatin C, FEUA and FEPO4.
In summary, this study showed that dapagliflozin in T2DM patients decreased urinary levels of proximal kidney injury biomarkers, including KIM-1 and albuminuria, which indicates its renoprotective effects, independent of blood glucose levels and HbA1c lowering. These data suggest that SGLT2 inhibitors might ameliorate glucose toxicity on proximal tubular cells, leading to potential long-term renoprotective effects in T2DM patients. Large long-term clinical trials are needed to confirm the main renal benefits of SGLT2 inhibitors in terms of the development of tubular injury in diabetic nephropathy.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the contributions to this study of staff from the Division of Nephrology and Biomedical Clinical Research Center in Phramongkutklao Hospital. This study was supported by a grant from Phramongkutklao Hospital and College of Medicine and the National Science and Technology Development Agency (NSTDA, P-13-00505), Bangkok, Thailand.
CONFLICT OF INTEREST STATEMENT
None declared. Thai Clinical Trials Registry: TCTR20180424002.
REFERENCES
- 1. Goff DC Jr, Gerstein HC, Ginsberg HN. et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99: 4i–20i [DOI] [PubMed] [Google Scholar]
- 2. Ingsathit A., Thakkinstian A, Chaiprasert P. et al. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant 2010; 25: 1567–1575 [DOI] [PubMed] [Google Scholar]
- 3. Satirapoj B, Supasyndh O, Mayteedol N. et al. Metabolic syndrome and its relation to chronic kidney disease in a Southeast Asian population. Southeast Asian J Trop Med Public Health 2011; 42: 176–183 [PubMed] [Google Scholar]
- 4. Satirapoj B, Adler SG.. Comprehensive approach to diabetic nephropathy. Kidney Res Clin Pract 2014; 33: 121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Satirapoj B. Review on pathophysiology and treatment of diabetic kidney disease. J Med Assoc Thai 2010; 93(Suppl 6): S228–S241 [PubMed] [Google Scholar]
- 6. Kasichayanula S, Liu X, Lacreta F. et al. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet 2014; 53: 17–27 [DOI] [PubMed] [Google Scholar]
- 7. Bailey CJ, Gross JL, Pieters A. et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 375: 2223–2233 [DOI] [PubMed] [Google Scholar]
- 8. Perkins BA, Cherney DZI, Partridge H. et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care 2014; 37: 1480–1483 [DOI] [PubMed] [Google Scholar]
- 9. Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 [DOI] [PubMed] [Google Scholar]
- 10. Satirapoj B. Sodium-glucose cotransporter 2 inhibitors with renoprotective effects. Kidney Dis 2017; 3: 24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gangadharan Komala M, Gross S, Mudaliar H. et al. Inhibition of kidney proximal tubular glucose reabsorption does not prevent against diabetic nephropathy in type 1 diabetic eNOS knockout mice. PLoS One 2014; 9: e108994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panchapakesan U, Pegg K, Gross S. et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells–renoprotection in diabetic nephropathy? PLoS One 2013; 8: e54442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vallon V, Rose M, Gerasimova M. et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 2013; 304: F156–F167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vallon V, Gerasimova M, Rose MA. et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 2014; 306: F194–F204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wanner C, Inzucchi SE, Lachin JM. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 16. Heerspink HJ, Johnsson E, Gause-Nilsson I. et al. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab 2016; 18: 590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cherney D, Lund SS, Perkins BA. et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 2016; 59: 1860–1870. [DOI] [PubMed] [Google Scholar]
- 18. Heerspink HJ, Desai M, Jardine M. et al. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 2017; 28: 368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viberti GC, Hill RD, Jarrett RJ. et al. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1: 1430–1432 [DOI] [PubMed] [Google Scholar]
- 20. Satirapoj B. Tubulointerstitial biomarkers for diabetic nephropathy. J Diabetes Res 2018; 2018: 2852398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Satirapoj B, Aramsaowapak K, Tangwonglert T. et al. Novel tubular biomarkers predict renal progression in type 2 diabetes mellitus: a prospective cohort study. J Diabetes Res 2016; 2016: 3102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tervaert TWC, Mooyaart AL, Amann K. et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010; 21: 556–563 [DOI] [PubMed] [Google Scholar]
- 23. Maltese G, Abou-Saleh A, Gnudi L. et al. Preventing diabetic renal disease: the potential reno-protective effects of SGLT2 inhibitors. Br J Diabetes 2015; 15: 114–118. [Google Scholar]
- 24. Maeda S, Matsui T, Takeuchi M. et al. Sodium-glucose cotransporter 2-mediated oxidative stress augments advanced glycation end products-induced tubular cell apoptosis. Diabetes Metab Res Rev 2013; 29: 406–412 [DOI] [PubMed] [Google Scholar]
- 25. Dekkers CCJ, Petrykiv S, Laverman GD. et al. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab 2018; 20: 1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chino Y, Samukawa Y, Sakai S. et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 2014; 35: 391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. List JF, Woo V, Morales E. et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009; 32: 650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doblado M, Moley KH.. Facilitative glucose transporter 9, a unique hexose and urate transporter. Am J Physiol Endocrinol Metabol 2009; 297: E831–E835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cook DG, Shaper AG, Thelle DS. et al. Serum uric acid, serum glucose and diabetes: relationships in a population study. Postgrad Med J 1986; 62: 1001–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]