Abstract
In April 2019, two major Phase 3 randomized clinical trials were published that assessed primary renal outcomes in diabetic kidney disease (DKD) in type 2 diabetes mellitus (T2DM). The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) tested an already available antidiabetic drug, canagliflozin, and the Study of Diabetic Nephropathy with Atrasentan (SONAR) tested a novel molecule, the endothelin-1 receptor blocker atrasentan, both on top of renin–angiotensin system blockade. Both trials demonstrated significant nephroprotection in patients with overt DKD (albuminuria >300 mg/g urinary creatinine) for combined primary endpoints of end-stage kidney disease (ESKD), doubling of serum creatinine or death from renal or cardiovascular causes in CREDENCE {hazard ratio [HR] 0.70 [95% confidence interval (CI) 0.59–0.82]} and ESKD and doubling of serum creatinine in SONAR [HR 0.65 (95% CI 0.49–0.88)]. Canagliflozin also decreased the secondary renal endpoint ESKD, doubling of serum creatinine or renal death [HR 0.66 (95% CI 0.53–0.81)], which was similar in nature and impact to the primary endpoint in SONAR. In addition, canagliflozin decreased a secondary endpoint of cardiovascular death or hospitalization for heart failure [HR 0.69 (95% CI 0.57–0.83)], whereas atrasentan had no significant impact on a secondary cardiovascular composite endpoint or on hospital admissions for heart failure and, despite restrictive exclusion criteria, there was a non-significant trend towards more frequent episodes of heart failure. Based on these results, canagliflozin will likely be approved for the indication of treating DKD in T2DM and the estimated glomerular filtration rate threshold for prescribing it will be lifted, whereas the future and place of atrasentan in the treatment of DKD remain unclear.
Keywords: albuminuria, atrasentan, canagliflozin, chronic kidney disease, diabetic kidney disease, endothelin, sodium-glucose cotransporter-2 (SGLT2) inhibitor
April 2019 saw the publication of positive results from two major Phase 3 randomized clinical trials (RCTs) assessing primary renal outcomes in diabetic kidney disease (DKD) of type 2 diabetes mellitus (T2DM) patients. The approach was very different: The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) tested an already available antidiabetic drug, canagliflozin [1] and the Study of Diabetic Nephropathy with Atrasentan (SONAR) tested a novel molecule, the endothelin-1 receptor blocker atrasentan [2]. We now discuss what these results will mean in the near future for the practicing nephrologist, as they will likely result in a dramatic paradigm change in the management of DKD. Thus, in the past 20 years, only renin–angiotensin system (RAS) blockers have shown a nephroprotective effect on DKD as a primary endpoint in a placebo-controlled RCT, whereas numerous other trials have failed to demonstrate a benefit [3, 4].
WHAT IS DKD?
A recent consensus conference considered DKD as chronic kidney disease (CKD) attributable to diabetes, where CKD is defined by an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or a urinary albumin:creatinine ratio (UACR) ≥30 mg/g creatinine [5, 6]. Thus DKD is usually a clinical diagnosis not confirmed by kidney biopsy. Pathological features of DKD may be present in the absence of and may precede pathological albuminuria [7]. The terms DKD and diabetic nephropathy are frequently used interchangeably, although it has been argued that diabetic nephropathy should be reserved for those patients with histopathological confirmation of the diagnosis [7]. However, diabetic patients may also have non-diabetic causes of CKD, especially if reduced eGFR coexists with normoalbuminuria and additional red flags. A diagnosis of DKD implies both an increased risk of end-stage renal disease (ESRD) ultimately requiring renal replacement therapy (RRT) and an increased risk of premature death, mainly due to cardiovascular disease [8]. The classical clinical spectrum of DKD consists of the progression from microalbuminuria (UACR 30–300 mg/g, currently termed A2 albuminuria) to macroalbuminuria (UACR >300 mg/g, currently termed A3 albuminuria). While ∼25–35% of DKD patients have decreased renal function despite normoalbuminuria (UACR <30 mg/g, currently termed as A1 albuminuria) or microalbuminuria [9], those with A3 albuminuria (overt DKD) are at the highest risk of progression and premature death and should be targeted by trials of novel therapeutic approaches on top of the current state of the art [10, 11].
WHAT IS THE BURDEN OF DKD?
CKD is one of the fastest growing causes of death worldwide. It is estimated that, at the current pace of growth, it will become the second most frequent cause of death before the end of the century in some developed countries [12]. DKD is a key contributor to mortality from CKD and the most frequent cause of need for RRT worldwide [13]. According to Global Burden of Disease data, DKD was the cause of >425 000 deaths in 2017, an increase of 37% in the last decade, and accounted for 35% of deaths from CKD worldwide [14]. T2DM was responsible for >80% of these DKD deaths [14]. This illustrates the insufficient implementation or the lack of effective therapeutic approaches [15]. A critical analysis of currently available therapies and recent results from RCTs suggests that suboptimal efficacy of current approaches does play a key role in the dismal outcome of DKD patients.
WHAT IS THE CURRENT TREATMENT FOR DKD?
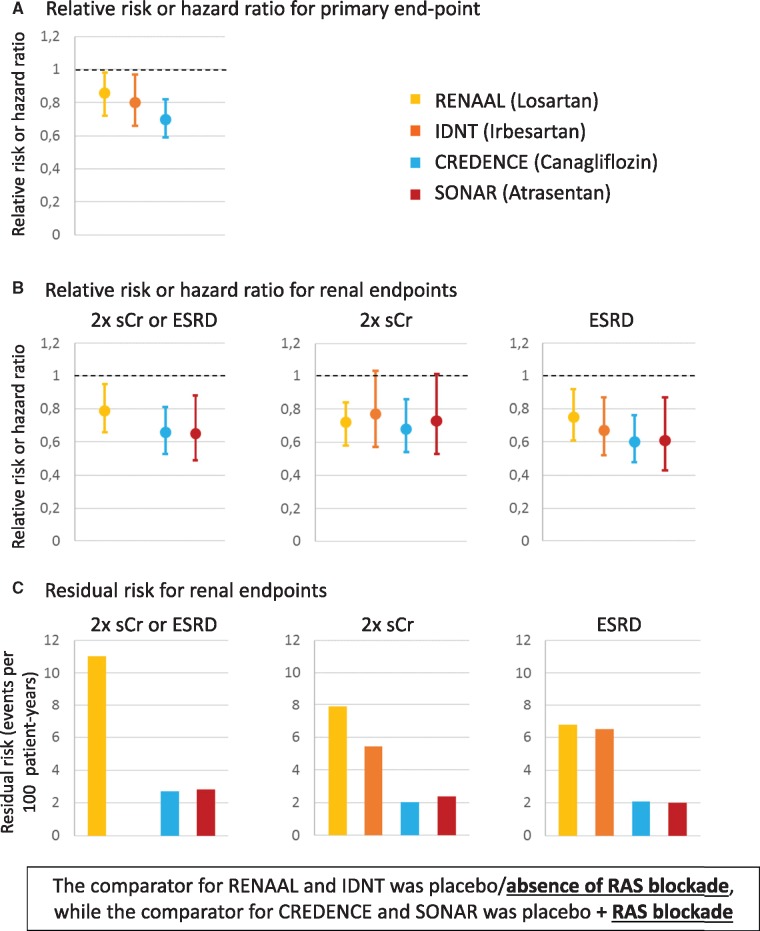
Hyperglycaemia is the driving force for the development of diabetic complications, and tight glycaemic control prevents the development of micro- and macrovascular complications [16]. However, once kidney injury has occurred, additional injury pathways are recruited and optimal kidney care requires the combination of antidiabetic with nephroprotective medication. Nephroprotection based on antiproteinuric therapy with RAS blockers is the current standard of therapy for non-pregnant diabetic patients with either hypertension or pathological albuminuria [17]. Angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) are indicated for nephroprotection. However, their beneficial nephroprotective effect has only been demonstrated as secondary prevention, and American Diabetes Association (ADA) guidelines still do not recommend ACEis or ARBs for primary prevention of CKD in diabetic patients with normal blood pressure or UACR <30 mg/g and normal eGFR [17]. Dual RAS blockade with ACEis, ARBs or aliskiren is formally contraindicated following RCT-derived concerns about the efficacy and safety of these combinations [18, 19]. Key RAS blockade trials for DKD in T2DM, such as Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL; losartan versus placebo) or the Irbesartan Diabetic Nephropathy Trial (IDNT; irbesartan versus placebo or amlodipine) enrolled patients with overt DKD (UACR >300 mg/g) (Figure 1) and observed a risk reduction for doubling of serum creatinine or ESRD versus placebo (Figure 2A and B), but the residual risk was still in the range of 6.0–8.0/100 patient-years for individual endpoints and 11/100 patient-years for the combined endpoint of doubling of serum creatinine or ESRD (Figure 2C) [3, 4]. Thus, despite the current standard of care, the residual risk for CKD progression is still substantial, in line with the global statistics for mortality from CKD and DKD. In recent years, several clinical trials assessing new molecules, such as paricalcitol, aliskiren, sulodexide and bardoxolone, have failed to demonstrate a renoprotective effect on DKD [24–27].
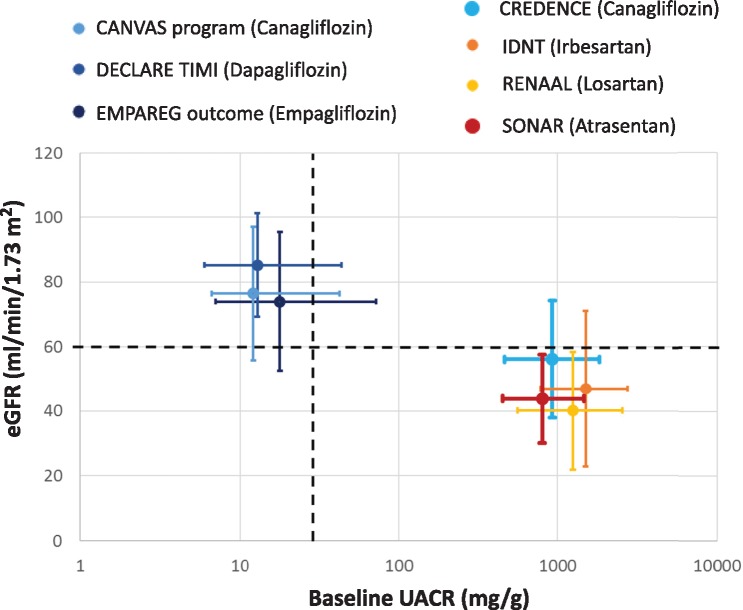
FIGURE 1.
CKD G (GFR) and A (albuminuria) categories of patients enrolled in RCTs for current DKD therapies, cardiovascular SGLT2i trials, CANVAS and SONAR. Data from references [1, 2, 4, 20–23]. Data presented as mean ± SD and albuminuria data as median (IQR). The percentage of patients with eGFR <60 mL/min/1.73 m2 was 25.9, 22.7 and 9.11% in EMPA-REG, CANVAS and DECLARE, respectively. For albuminuria >300 mg/g, the percentages were 11, 7.1 and 6.8%, respectively.
FIGURE 2.
Efficacy of nephroprotective therapies. (A) Relative risk or HR for primary endpoint (IDNT and RENAAL: doubling of the serum creatinine concentration, ESRD or death; CANVAS: doubling of the serum creatinine concentration, ESRD or cardiovascular or renal death). SONAR was omitted because the primary endpoint did not include death. (B) Relative risk or HR for key renal endpoints explored in all the trials. The original IDNT manuscript did not provide the combined doubling of the serum creatinine concentration and ESRD endpoint. (C) Residual risk for key renal endpoints. In IDNT, the residual risk per 100 person-years was estimated from mean follow-up and percentage of patients with events. Data from references [2–4]. The comparator for RENAAL and IDNT was placebo/absence of RAS blockade, whereas the comparator for CREDENCE and SONAR was placebo + RAS blockade.
WHAT IS THE CURRENT ANTIDIABETIC DRUG OF CHOICE FOR PATIENTS WITH DKD?
Regarding the optimal antidiabetic drugs to prevent DKD in diabetic patients or to prevent DKD progression once it has developed, the recommendations are evolving rapidly. As recently as 2015, the European Renal Best Practice recommended metformin in a dose adapted to renal function as a first-line agent in DKD patients with eGFR <45/mL/min/1.73 m2 [28, 29], despite a formal European Medicines Agency (EMA) contraindication for eGFR <30 mL/min/1.73 m2 that extends to <45 mL/min/1.73 m2 for fixed-dose combinations of metformin with sodium–glucose cotransporter (SGLT) inhibitors (SGLT2is) [30]. The recommendation to use metformin as a first-line agent whenever not contraindicated is in line with the Standards of Medical Care in Diabetes of the ADA 2018, which additionally recommended not to initiate metformin if eGFR <45 mL/min/1.73 m2 and to stop it if eGFR <30 mL/min/1.73 m2 and added no further advice for patients with DKD regarding the choice of antidiabetic agent [31], as well as with the 2018 Consensus Report by the ADA and the European Association for the Study of Diabetes (EASD) for haemoglobin A1c (HbA1c) goals, treatments and lifestyle management for T2DM patients with and without complications [32]. However, this report, published at the end of 2018, clearly differentiated patients with CKD from non-complicated T2DM patients and recommended metformin as a first-line agent to lower glucose in patients with T2DM and CKD without contraindications and to consider adding an SGLT2i if HbA1c targets were not achieved or replacing a second antidiabetic drug for an SGLT2i if HbA1c was on target. If the SGLT2 is contraindicated, then a glucagon-like peptide-1 (GLP-1) receptor agonist shown to reduce CKD progression may be considered. The recommendation is based on secondary outcome results of the cardiovascular outcomes trials EMPA-REG OUTCOME (empagliflozin; Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients), CANVAS Program (canagliflozin; CANagliflozin cardioVascular Assessment Study), LEADER (liraglutide; Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) and SUSTAIN 6 (semaglutide; Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes) [17, 20, 32–35]. Thus only SGLT2i and GLP-1 receptor agonist have shown any nephroprotective effect beyond their glucose-lowering effect, albeit up to CREDENCE, as secondary endpoints in populations not necessarily having DKD [36]. The ADA-EASD document does not provide a GFR threshold for the indication of SGLT2i, given that it differs for different regions, but acknowledged the existence of such thresholds. Thus they recommended considering SGLT2i to lower glucose when metformin failed or could not be used or on top of metformin in patients with a DKD diagnosis based on albuminuria but, essentially, with preserved renal function, given the explicit caveats on the use of SGLT2i in patients with low eGFR. Thus, based on the evidence available in 2018, they recommend considering SGLT2i to lower serum glucose for albuminuric DKD patients with relatively preserved renal function based on the potential nephroprotective effects, but stopped short of recommending SGLT2i to treat DKD. A European Renal and Cardiovascular medicine and DIABESITY consensus statement are aligned with the ADA-EASD consensus report [37]. Despite the recommendations on oral antidiabetics, insulin is still commonly used in T2DM, especially as eGFR decreases [38, 39].
WHAT ARE SGLT2is?
SGLT2is are the newest class of oral agents to treat T2DM. These drugs block the SGLT2 cotransporter involved in 90% of glucose reabsorption in the proximal renal tubule, resulting in increased urinary glucose excretion and lower blood glucose levels [37]. In cardiovascular safety trials enrolling patients at high cardiovascular risk (Figure 1), the SGLT2is canagliflozin, dapagliflozin and empagliflozin reduced cardiovascular risk (Figure 3A) and also incident or worsening nephropathy as secondary or post hoc kidney outcomes [20, 33, 37, 40, 41].
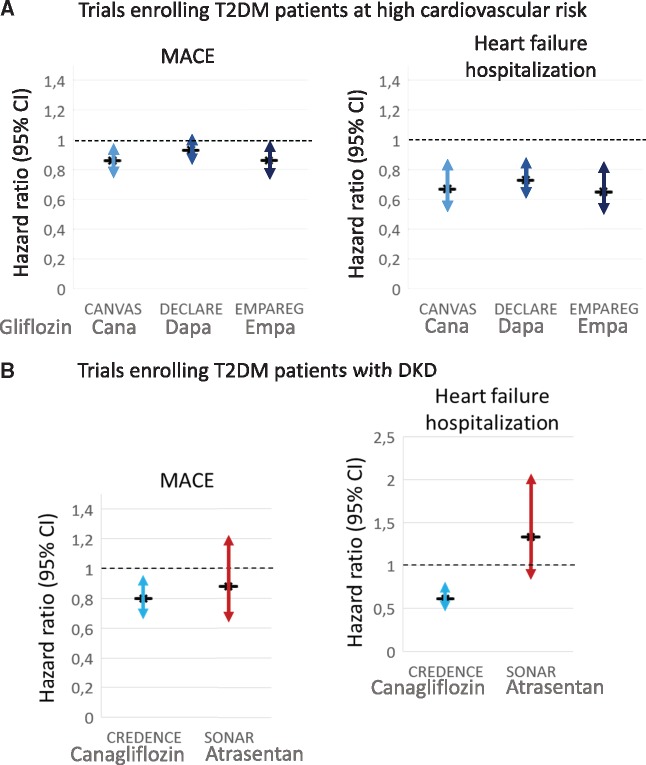
FIGURE 3.
Cardiovascular safety of SGLT2i and atrasentan. Data from (A) cardiovascular outcome trials enrolling T2DM patients at high cardiovascular risk (CANVAS Program, DECLARE-TIMI and EMPA-REG OUTCOME) [20, 33, 40] and (B) trials enrolling patients with DKD (CANVAS and SONAR) [2, 20] are presented. MACE: major cardiovascular events: cardiovascular death, myocardial infarction or stroke.
ARE SGLT2is INDICATED FOR THE TREATMENT OF DKD?
Not as of April 2019. The EMA lists as indications for SGLT2i the treatment of adults with T2DM in monotherapy or combination therapy and (for dapagliflozin) also for type 1 diabetes mellitus (T1DM) in combination with insulin (Table 1) [42–44]. There is no mention of specific indications for DKD patients or to treat DKD. On the contrary, there is a warning for starting these drugs in DKD patients that fulfil the eGFR criterion for CKD (SGLT2i not to be initiated when eGFR <60 mL/min/1.73 m2). This warning is based on the lower antidiabetic effect of the drugs when renal function is decreased as well as on concerns about volume depletion. This warning is expected to change soon once the regulatory authorities examine the results from CREDENCE. In this regard, scientific society guidelines and consensus documents have moved faster than regulatory authorities by recommending considering SGLT2i preferentially to treat diabetes in patients with DKD and will likely continue to advance in this major paradigm change by making recommendations on their role in the treatment of DKD, not just of T2DM.
Table 1.
SGLT2i and atrasentan current (15 April 2019) indications and use in renal disease by the EMA [42–44]
| Indications |
| Canagliflozin, dapagliflozin and empagliflozin: adults with insufficiently controlled T2DM as an adjunct to diet and exercise, either as monotherapy when metformin is considered inappropriate due to intolerance or in addition to other medicinal products for the treatment of diabetes. |
| Dapagliflozin: adults with insufficiently controlled T1DM as an adjunct to insulin in patients with BMI ≥27 kg/m2, when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy. |
| Atrasentan: no indication, experimental drug. |
| Use in renal disease patients |
| Canagliflozin, dapagliflozin or empagliflozin should not be initiated in patients with an eGFR <60 mL/min/1.73 m2 and should be discontinued at a GFR persistently <45 mL/min/1.73 m2. |
WHAT WAS THE EVIDENCE BASE TO INDICATE SGLT2i IN PATIENTS WITH DKD PATIENTS PRIOR TO CREDENCE?
Before CREDENCE, cardiovascular outcomes trials of SGLT2is used to lower glucose had enrolled patients at a high cardiovascular risk [20, 33, 40]. These included a significant number of patients with DKD defined as either eGFR 30–60 mL/min/1.73 m2 or UACR >30 mg/g: as an example, in EMPA-REG OUTCOME, 40% of patients had pathological albuminuria and an additional 20% had eGFR 30–60 mL/min/1.73 m2 with normoalbuminuria [45]. However, only 11% had baseline albuminuria >300 mg/g. As a consequence, the mean levels of eGFR and median levels of UACR for the full SGLT2i cardiovascular cohort were not in the DKD range (Figure 1). In addition to significantly lower cardiovascular events as defined by at least one primary endpoint (Figure 3A), trials with empagliflozin, canagliflozin and dapagliflozin observed a significantly lower incidence of renal outcomes defined by secondary endpoints in these mixed DKD and non-DKD populations [20, 33, 40].
In EMPA-REG OUTCOME, empagliflozin significantly reduced incident or worsening nephropathy (defined as decreasing renal function, developing UACR > 300 mg/g, doubling of serum creatinine or developing ESRD or death from ESRD) by almost 40% {hazard ratio [HR] 0.61 [95% confidence interval (CI) 0.53–0.70]} [33, 41]. In the CANVAS Program, canagliflozin patients developed less macroalbuminuria than placebo patients and the composite outcome (40% reduction in eGFR, RRT or renal death) was less frequent in canagliflozin than in placebo patients [HR 0.60 (95% CI 0.47–0.77)] [20]. The Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI) was published later in 2019. Patients randomized to dapagliflozin had a 47% lower incidence of the composite renal outcome [decrease of at least 40% in eGFR to <60 mL/min/1.73 m2, ESRD or death from renal causes: HR 0.53 (95% CI: 0.43−0.66)] [40]. However, the incidence of ESRD in the control population was low (e.g. 0.2/100 patient-years) in EMPA-REG OUTCOME [41].
In EMPA-REG OUTCOME, 7.7% of the patients had an eGFR <45 mL/min/1.73 m2 and in the CANVAS Program, 5.5%. In both studies, the cardiovascular and renal benefits were observed in both patients with normal and reduced eGFR.
Thus hypothesis-generating evidence had been gathered prior to CREDENCE from secondary endpoints of cardiovascular outcomes trials that were very suggestive of a drug class nephroprotective effect for SGLT2is. Furthermore, the cardiovascular and renal benefits of SGLT2is appear to extend to DKD patients with eGFR in the 30–60 mL/min/1.73 m2 range. But this hypothesis had not been formally tested in an RCT with primary renal outcomes nor in a full cohort of DKD patients, which is the novelty of CREDENCE.
WHAT WAS LEARNED FROM THE CREDENCE TRIAL TESTING CANAGLIFLOZIN TO TREAT DKD?
The results of the CREDENCE trial were published in April 2019. This is the first RCT of any SGLT2i in which the whole study population had DKD and the primary endpoint was renal, thus providing solid evidence for a novel therapeutic indication for canagliflozin to treat DKD. CREDENCE randomized 4401 T2DM patients with DKD to canagliflozin or placebo and followed them for a median of 2.62 years in an event-driven trial (Figure 1) [2]. It was terminated early because of the evidence of benefit in a pre-specified interim analysis. The key result is a risk reduction of 30% in the canagliflozin group for the composite primary renal outcome, and what is more relevant, these results were obtained in subjects with eGFR 30–90 mL/min/1.73 m2 and UACR 300–5000 mg/g [2]. It should be noted that patients were enrolled with an eGFR up to 30 mL/min/1.73 m2, thus potentially expanding a putative canagliflozin indication ‘treatment of DKD’ outside the eGFR limits of the current EMA statement that canagliflozin should not be initiated when eGFR is <60 mL/min/1.73 m2 for the indication ‘treatment of T2DM’. In fact, at baseline, eGFR was 30–44 mL/min/1.73 m2 in 31% of the patients and 45–60 mL/min/1.73 m2 in 29%. Furthermore, canagliflozin was maintained until patients started RRT. Thus this trial established the efficacy for kidney protection and the safety of the use of canagliflozin at any eGFR as long as the patient is not on RRT.
Canagliflozin decreased the combined primary endpoint [ESRD, doubling of serum creatinine or death from renal or cardiovascular cause; HR 0.70 (95% CI 0.59–0.82)] and the secondary renal endpoint ESRD, doubling of serum creatinine or renal death [HR 0.66 (95% CI 0.53–0.81)] (Figure 2B). In addition, canagliflozin decreased the secondary endpoint of cardiovascular death or hospitalization for heart failure [HR 0.69 (95% CI 0.57–0.83)] (Figure 3B).
Overall, canagliflozin was safe, with both any and serious adverse events significantly less frequent than for placebo [HR 0.87 (95% CI 0.82–0.93) and HR 0.87 (95% CI 0.79–0.97), respectively]. Some adverse effects that raised concerns in prior canagliflozin trials were not increased in frequency [HR 1.11 (95% CI 0.79–1.56) for amputation and HR 0.98 (95% CI 0.70–1.37) for fracture], whereas the HR for diabetic ketoacidosis was 10, although the absolute risk was low (11/2200 patients).
A perspective on the combined cardiovascular and renal effects of canagliflozin in DKD patients is provided by the meagre advances in cardiovascular outcomes provided by the introduction of RAS blockade. In IDNT (irbesartan), a secondary cardiovascular outcome of cardiovascular death, myocardial infarction, heart failure resulting in hospitalization, stroke or lower limb amputation was not significantly different from placebo [HR 0.91 (95% CI 0.72–1.14)] [4]. In RENAAL (losartan), there were no significant differences for a similar secondary cardiovascular outcome (cardiovascular death, myocardial infarction or unstable angina, stroke, first hospitalization for heart failure or coronary or peripheral revascularization, risk reduction 10%; P = 0.26) [3]. However, irbesartan reduced the incidence of congestive heart failure necessitating hospitalization by 23% and there was a non-significant trend (P = 0.08) for a reduction of this outcome for losartan.
IS THERE BIOLOGICAL PLAUSIBILITY FOR CANAGLIFLOZIN BEING NEPHROPROTECTIVE ONCE EGFR IS <60 mL/MIN/1.73 m2?
The EMA states that SGLT2i should not be initiated at low eGFR as the degree of glycosuria and antidiabetic efficacy is lower when less glucose is filtered by glomeruli. However, there are several solid hypotheses that may explain nephroprotection even when the antidiabetic effect is mild [37]. Foremost among these is a single-nephron impact on glomerular hyperfiltration related to tubuloglomerular feedback inducing afferent arteriole vasoconstriction, thus complementing efferent arteriole vasodilation by RAS blockade and further lowering intraglomerular pressure, glomerular hyperfiltration and albuminuria [37]. The tubuloglomerular feedback would be triggered by increased sodium availability in the distal nephron, as SGLT2 inhibition prevents both sodium and glucose reabsorption in proximal tubules.
Additional mechanisms of action have been proposed for the cardiovascular and nephroprotective effects that exceed those of other antidiabetic drugs with a greater impact on glucose control as summarized recently [37]. These may include but are not limited to lowering body weight, blood pressure, uric acid, plasma volume and proximal tubular cell glucotoxicity, oxidative stress, inflammation and oxygen consumption and increasing natriuresis, haemoglobin levels and insulin sensitivity [37].
WHAT IS ATRASENTAN?
Endothelin-1 is a vasoactive peptide with cytokine-like properties shown to contribute to the pathogenesis of DKD in preclinical studies [46]. Atrasentan is a selective endothelin receptor type A (ETA) antagonist initially studied for the potential treatment of cancer [47]. However, clinical development for cancer was discontinued. Early success with ETA antagonists in decreasing albuminuria in DKD patients was marred by volume overload, which led to the termination of RCTs with avosentan for nephroprotection due to an excess of heart failure episodes [48]. The SONAR Phase 3 RCT explored nephroprotection by atrasentan in DKD and incorporated an innovation to prior trials: the exclusion of patients at risk of heart failure. This was done in two sequential steps: exclusion of patients with baseline brain natriuretic peptide (BNP) >200 pg/mL or a history of heart failure or of severe peripheral oedema, followed by an enrichment period that selected patients for albuminuria response plus lack of substantial fluid retention (an increase in bodyweight ≥3 kg and a BNP increase to ≥300 pg/mL) once on atrasentan [2]. In our opinion, these sequential steps to improve safety may severely compromise the external validity of the results and their use in routine clinical practice.
WHAT WAS LEARNED FROM THE SONAR TRIAL TESTING ATRASENTAN TO TREAT DKD?
SONAR randomized 2648 adults with T2DM, an eGFR 25–75 mL/min/1.73 m2 and a UACR 300–5000 mg/g on RAS blockade to atrasentan or placebo and followed them for a median of 2.2 years [2] (Figure 1). The trial was event-driven and was terminated prematurely when it was predicted that the pre-specified number of events would not be reached. The primary composite renal outcome of doubling of serum creatinine or ESRD was reached in 6.0% of atrasentan and 7.9% of placebo patients with an HR of 0.65 (95% CI 0.49–0.88; P = 0.0047) (Figure 2B). The difference was also significant for doubling of serum creatinine [HR 0.61 (95% CI 0.43–0.87)] but not for ESRD [HR 0.73 (95% CI 0.53–1.01)], despite a greater number of ESRD than doubling of serum creatinine events.
Atrasentan, used under the conditions of the trial, was found to be safe but, at the same time, it did not provide a cardiovascular advantage. There were no significant differences in a secondary cardiovascular composite endpoint (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) or in hospital admissions for heart failure [HR 1.33 (95% CI 0.85–2.07)] or deaths (Figure 3B). Still, there were significantly more episodes of fluid retention (36.6% versus 32.3%, P = 0.022), but not of cardiac failure (5.5% versus 3.9%, P = 0.064).
HOW WILL THIS NEW KNOWLEDGE IMPACT CLINICAL PRACTICE? THE NEW ERA OF SGLT2i/RAS BLOCKADE FOR DKD
The impact of CREDENCE is expected to be more immediate than that of SONAR. In our view, CREDENCE sets the stage for an indication of canagliflozin for the treatment of DKD as the combination of canagliflozin and RAS blockade achieved a very low residual renal risk (Figure 2C). This would result in a major paradigm change from using SGLT2is to treat T2DM to using them to treat DKD, independent from any antidiabetic effect. Furthermore, it should expand the indication of canagliflozin to patients with eGFR below the current limits of 60 mL/min/1.73 m2 for initiation and 45 mL/min/1.73 m2 for maintenance. Indeed, since the drug was not stopped until patients initiated RRT, there should be no limits for canagliflozin prescription based on eGFR. It is likely that CREDENCE evidenced a nephroprotective class effect, but regulatory indications for other SGLT2is for DKD will likely await the results of RCTs with similar primary endpoints. This new knowledge will likely open the door for testing SGLT2i/RAS blockade for nephroprotection in T1DM as well as in other forms of CKD. The later will be explored by the EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin; NCT03594110) and DAPA-CKD (A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease; NCT03036150) [49]. An improved understanding of nephroprotective effects obtained from clinical studies may also help advance the field of CKD therapy. In this regard, a recent RCT disclosed evidence of proximal tubular cell protection by dapagliflozin [50].
The impact of SONAR on the regulatory fate and eventual clinical use of atrasentan is less clear. On the one hand, SONAR has clearly demonstrated that atrasentan preserves renal function in addition to decreasing albuminuria. However, this was shown against the old benchmark of RAS blockade, and the field has shifted towards a new benchmark of RAS blockade plus SGLT2i. Thus, even if atrasentan is approved for a DKD indication, its current role in clinical practice would be unclear. On the other hand, the particular design of the SONAR trial, and especially the need for a first BNP determination at baseline and a second measurement in the enrichment period, can represent a serious hindrance to the widespread use of atrasentan in diabetic patients. In addition, nephroprotection afforded by atrasentan was not associated with cardiovascular benefit, even after multiple baseline and early exclusions based on cardiovascular safety. This again benchmarks negatively with current drugs such as SGLT2is that provide both kidney and cardiovascular benefit. However, there is the distinct possibility that the combined nephroprotection afforded by atrasentan and SGLT2i on top of RAS blockade further reduces the residual risk and that additionally the combination offsets any potential deleterious effect of atrasentan on heart failure. Nevertheless, as attractive as this hypothesis might seem, it requires formal assessment in new, specifically designed RCTs. In addition, although T1DM was not explored in SONAR, T1DM patients may be a niche for atrasentan, given the potential for ketoacidosis with SGLT2is in these patients.
FUNDING
Research by the authors is funded by FIS/Fondos FEDER (PI16/02057, PI16/01814. PI17/00257, ISCIII-RETIC REDinREN RD016/0009), Sociedad Española de Nefrología, FRIAT, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM. E.P. is a researcher in the Ramón y Cajal Program.
CONFLICT OF INTEREST STATEMENT
A.O. is a consultant for Sanofi Genzyme and has received speaker fees or travel support from Amicus, Amgen, Fresenius Medical Care, Menarini, Kyowa Kirin, Alexion, Otsuka and Vifor Fresenius Medical Care Renal Pharma. J.F.N.G. has served as a consultant and has received speaker fees or travel support from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Esteve, Genzyme, Lilly, Novartis, Servier, Shire and Vifor Fresenius Medical Care Renal Pharma. J.L.G. has received fees for giving talks from AstraZeneca, Boehringer Ingelheim, Janssen, Mundipharma, Novartis, Novo Nordisk, Otsuka and Vifor Pharma. M.J.S. has received speaker fees or travel support from Otsuka, Menarini, AstraZeneca, Boehringer Ingelheim, Janssen, Mundipharma, Novartis, Eli Lilly, Esteve and Novo Nordisk. B.F.F. has received speaker fees or travel support from Abbvie, AstraZeneca, Boehringer Ingelheim, Esteve, Menarini, Mundipharma, Novartis and Novo Nordisk.
REFERENCES
- 1. Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 2. Heerspink HJL, Parving H-H, Andress DL. et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 2019; 393: 1937–1947 [DOI] [PubMed] [Google Scholar]
- 3. Brenner BM, Cooper ME, de Zeeuw D. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 4. Lewis EJ, Hunsicker LG, Clarke WR. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [DOI] [PubMed] [Google Scholar]
- 5. Tuttle KR, Bakris GL, Bilous RW. et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014; 37: 2864–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perez-Gomez MV, Bartsch LA, Castillo-Rodriguez E. et al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019; 12: 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamanouchi M, Furuichi K, Hoshino J. et al. Nonproteinuric versus proteinuric phenotypes in diabetic kidney disease: a propensity score-matched analysis of a nationwide, biopsy-based cohort study. Diabetes Care 2019; 42: 891–902 [DOI] [PubMed] [Google Scholar]
- 8. Fernández Fernández B, Elewa U, Sánchez-Niño MD. et al. 2012 update on diabetic kidney disease: the expanding spectrum, novel pathogenic insights and recent clinical trials. Minerva Med 2012; 103: 219–234 [PubMed] [Google Scholar]
- 9. Porrini E, Ruggenenti P, Mogensen CE. et al. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diab Endocrinol 2015; 3: 382–391 [DOI] [PubMed] [Google Scholar]
- 10. Perez-Gomez M, Sanchez-Niño M, Sanz A. et al. Horizon 2020 in diabetic kidney disease: the clinical trial pipeline for add-on therapies on top of renin angiotensin system blockade. J Clin Med 2015; 4: 1325–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandez-Fernandez B, Ortiz A, Gomez-Guerrero C. et al. Therapeutic approaches to diabetic nephropathy–beyond the RAS. Nat Rev Nephrol 2014; 10: 325–346 [DOI] [PubMed] [Google Scholar]
- 12. Ortiz A, Sanchez-Niño MD, Crespo-Barrio M. et al. The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia 2019; 39: 29–34 [DOI] [PubMed] [Google Scholar]
- 13. Kramer A, Pippias M, Noordzij M. et al. The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry annual report 2015: a summary. Clin Kidney J 2018; 11: 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1736–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ortiz A. Translational nephrology: what translational research is and a bird’s-eye view on translational research in nephrology. Clin Kidney J 2015; 8: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2019. Diabetes Care 2019; 42(Suppl 1): S61–S70 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes—2019. Diabetes Care 2019; 42(Suppl 1): S124–S138 [DOI] [PubMed] [Google Scholar]
- 18. Esteras R, Perez-Gomez MV, Rodriguez-Osorio L. et al. Combination use of medicines from two classes of renin-angiotensin system blocking agents: risk of hyperkalemia, hypotension, and impaired renal function. Ther Adv Drug Saf 2015; 6: 166–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makani H, Bangalore S, Desouza KA. et al. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346: f360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neal B, Perkovic V, Mahaffey KW. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 21. Zinman B, Inzucchi SE, Lachin JM. et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOMETM). Cardiovasc Diabetol 2014; 13: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raz I, Mosenzon O, Bonaca MP. et al. DECLARE-TIMI 58: participants’ baseline characteristics. Diabetes Obes Metab 2018; 20: 1102–1110 [DOI] [PubMed] [Google Scholar]
- 23. Riphagen IJ, Deetman PE, Bakker SJL. et al. Bilirubin and progression of nephropathy in type 2 diabetes: a post hoc analysis of RENAAL with independent replication in IDNT. Diabetes 2014; 63: 2845–2853 [DOI] [PubMed] [Google Scholar]
- 24. de Zeeuw D, Agarwal R, Amdahl M. et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2010; 376: 1543–1551 [DOI] [PubMed] [Google Scholar]
- 25. Parving HH, Brenner BM, McMurray JJV. et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367: 2204–2213 [DOI] [PubMed] [Google Scholar]
- 26. Packham DK, Wolfe R, Reutens AT. et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol 2012; 23: 123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Zeeuw D, Akizawa T, Agarwal R. et al. Rationale and trial design of Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes: the Occurrence of Renal Events (BEACON). Am J Nephrol 2013; 37: 212–222 [DOI] [PubMed] [Google Scholar]
- 28.Guideline development group. Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol Dial Transplant 2015; 30(Suppl 2): ii1–ii142. [DOI] [PubMed] [Google Scholar]
- 29. Martínez-Castelao A, Górriz JL, Ortiz A. et al. ERBP guideline on management of patients with diabetes and chronic kidney disease stage 3B or higher. Metformin for all? Nefrologia 2017; 37: 567–571 [DOI] [PubMed] [Google Scholar]
- 30.Committee for Medicinal Products for Human Use. Assessment Report: Metformin containing medicinal products2016. https://www.ema.europa.eu/en/documents/variation-report/vipdomet-h-c-2654-a31-1432-epar-assessment-report-article-31_en.pdf (19 April 2019, date last accessed)
- 31.American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care 2018; 41: S73–S85 [DOI] [PubMed] [Google Scholar]
- 32. Davies MJ, D’Alessio DA, Fradkin J. et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 [DOI] [PubMed] [Google Scholar]
- 34. Marso SP, Bain SC, Consoli A. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844 [DOI] [PubMed] [Google Scholar]
- 35. Mann JFE, Ørsted DD, Brown-Frandsen K. et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017; 377: 839–848 [DOI] [PubMed] [Google Scholar]
- 36. Górriz JL, Nieto J, Navarro-González JF. et al. Nephroprotection by hypoglycemic agents: do we have supporting data? J Clin Med 2015; 4: 1866–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sarafidis P, Ferro CJ, Morales E. et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transplant 2019; 34: 208–230 [DOI] [PubMed] [Google Scholar]
- 38. Weinrauch LA, Segal AR, Bayliss GP. et al. Changes in treatment of hyperglycemia in a hypertensive type 2 diabetes population as renal function declines. Clin Kidney J 2017; 10: 661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Imam TH. Changes in metformin use in chronic kidney disease. Clin Kidney J 2017; 10: 301–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wiviott SD, Raz I, Bonaca MP. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357 [DOI] [PubMed] [Google Scholar]
- 41. Wanner C, Inzucchi SE, Lachin JM. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 42.Committee for Medicinal Products for Human Use. Annex I. Summary of Product Characteristics. Invokana https://www.ema.europa.eu/en/documents/product-information/invokana-epar-product-information_en.pdf (19 April 2019, date last accessed)
- 43.Committee for Medicinal Products for Human Use. Annex I. Summary of Product Characteristics Jardiance https://www.ema.europa.eu/en/documents/product-information/jardiance-epar-product-information_en.pdf (19 April 2019, date last accessed)
- 44.Committee for Medicinal Products for Human Use. Annex I. Summary of Product Characteristics Forxiga.https://www.ema.europa.eu/en/documents/product-information/forxiga-epar-product-information_en.pdf (19 April 2019, date last accessed)
- 45. Cherney DZI, Zinman B, Inzucchi SE. et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diab Endocrinol 2017; 5: 610–621 [DOI] [PubMed] [Google Scholar]
- 46. Benigni A, Colosio V, Brena C. et al. Unselective inhibition of endothelin receptors reduces renal dysfunction in experimental diabetes. Diabetes 1998; 47: 450–456 [DOI] [PubMed] [Google Scholar]
- 47. Norman P. Atrasentan Abbott. Current Opin Invest Drugs 2002; 3: 1240–1248 [PubMed] [Google Scholar]
- 48. Mann JFE, Green D, Jamerson K. et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 2010; 21: 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Herrington WG, Preiss D, Haynes R. et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018; 11: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Satirapoj B, Korkiatpitak P, Supasyndh O.. Effect of sodium-glucose cotransporter 2 inhibitor on proximal tubular function and injury in patients with type 2 diabetes: a randomized controlled trial. Clin Kidney J 2019; 12: 326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]