Abstract
Diabetes increases the risk of adverse cardiovascular and renal events. Recently, sodium–glucose co-transporter 2 (SGLT2) inhibitors have been demonstrated to reduce cardiovascular complications and slow diabetic kidney disease progression in patients with type 2 diabetes. The glycaemic control exerted by these drugs is not greater than the one achieved with other classical glucose-lowering medications such as sulphonylureas. For that reason, plausible renoprotective mechanisms independent from glycaemic control have been proposed such as blood pressure control, body weight loss, intraglomerular pressure reduction and a decrease in urinary proximal tubular injury biomarkers. Interestingly, the hypothesis that SGLT2 inhibitors have a direct renoprotective effect has been addressed in diabetic and non-diabetic models. In this editorial, we update the different postulated mechanisms involved in the cardiorenal protection afforded by SGLT2 inhibition in chronic kidney disease.
Keywords: chronic kidney disease, diabetic nephropathy, SGLT2, type 2 diabetes
Patients with type 2 diabetes mellitus (T2D) have a well-known increased risk for premature death and cardiorenal complications. Approximately 35% of patients with T2D will develop diabetic kidney disease. Furthermore, recently published epidemiologic studies demonstrate that diabetic nephropathy contributed 51% of the increased burden of chronic kidney disease (CKD) since 1990 [1]. Before 2015, glucose-lowering medications were shown to only slightly reduce diabetic nephropathy progression during intensive therapy, and were associated with increased adverse effects, mainly in frail patients. Interestingly, no benefit was found in macrovascular complications and death from cardiovascular causes [2–5]. Until 2016, cardiorenal treatment in T2D has been limited to renin–angiotensin system blockade, either by angiotensin-converting enzyme inhibitors (ACEi) or by angiotensin II receptor blockers (ARBs) administration [6, 7]. Of note, the combination of ACEi and ARBs has not demonstrated beneficial effects on cardiorenal protection in T2D over each agent alone [8]. Recently, a new class of anti-diabetic drugs—the sodium–glucose co-transporter 2 (SGLT2) inhibitors—has been demonstrated to slow the progression of diabetic kidney disease and to improve cardiovascular outcomes [9–11].
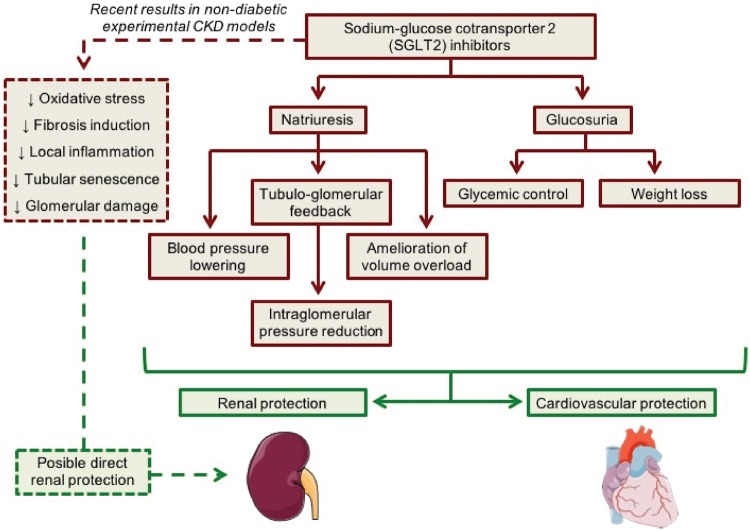
SGLT2 and, to a lesser extent, SGLT1 are responsible for tubular glucose reabsorption in proximal tubules and, thus, they contribute importantly to glucose homoeostasis. The use of SGLT2 inhibitors has proven to be effective in glycaemia control in diabetic patients. SGLT2 inhibition increases urinary glucose excretion, decreasing blood glucose levels [12]. Canagliflozin, dapagliflozin and empagliflozin are the approved SGLT2 inhibitors in the USA and Europe for treatment of T2D. These drugs were introduced into clinical practice between 2013 and 2015, after several clinical trials that demonstrated their efficacy in glucose lowering, and also in weight loss and reduction of blood pressure [13]. Recent studies demonstrated that SGLT2 inhibitors in combination with renin-angiotensin system (RAS) blockade may decrease major cardiovascular events, usually measured as a composite outcome of death from cardiovascular causes, non-fatal myocardial infarction or non-fatal stroke [14, 15]. The first trial focusing on the evaluation of renal outcomes in diabetic kidney disease was published in 2016. Wanner et al. [9] demonstrated that the administration of empagliflozin in T2D patients at high cardiovascular risk was associated with slower progression of kidney disease and lower rates of clinically relevant renal events as compared with placebo over a median follow-up period of 3.1 years [16]. Subsequently, Heerspink et al. [11] demonstrated that canagliflozin slowed renal disease progression over >2 years of follow-up in T2D patients independently of its glycaemic effects. Recently, dapagliflozin administration was associated with robust reductions in hospitalization for heart failure and progression of renal disease, regardless of baseline atherosclerotic risk category or history of heart failure [10]. These promising outcomes have to be confirmed in future trials with renal progression as the primary endpoint that enrol (or are currently enrolling) patients with more advanced CKD such as Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation, a study to evaluate the effect of dapagliflozin on renal outcomes and cardiovascular mortality in patients with CKD and the study of heart and kidney protection with empagliflozin [17]. In the current study, Satirapoj et al. [18] have shown in a randomized controlled trial that dapagliflozin treatment decreases urinary kidney injury molecule 1 (uKIM-1) levels. In concordance, Dekkers et al. [19] in a post hoc analysis also demonstrated a decrease of uKIM-1 after dapagliflozin treatment. These results suggest that SGLT2 inhibitors exert renoprotection by different mechanisms such as restoring tubuloglomerular feedback, thus decreasing hyperfiltration and albuminuria, and directly decreasing tubular injury, among others in T2D patients [9–11, 16, 18, 19] (Figure 1). For these reason, currently some clinical trials are ongoing to assess the effect of SGLT2 inhibition on non-diabetic CKD patients [20].
FIGURE 1.
Suggested mechanisms for cardiorenal protection with SGLT2 inhibition.
The renoprotective effects of SGLT2 have also been explained by natriuresis resulting from inhibition of sodium and glucose reabsorption. An increased sodium delivery to the macula densa activates the tubuloglomerular feedback that leads to afferent arteriole vasoconstriction and a reduction in intraglomerular pressure. In fact, SGLT2 inhibitors demonstrated a similar pattern of change in renal function to that observed with ACEi or ARBs, where a short-term decrease of glomerular filtration rate is followed by stabilization over time [12]. This initial reduction is also reversible when the drug is discontinued. Other plausible mechanisms that have been proposed to contribute to SGLT2 inhibitor renoprotection are lowering of blood pressure, weight loss, amelioration of the volume overload and glycaemic control itself (Figure 1). However, it is still not clear whether these drugs also exert direct protective effects on the kidney. To determine whether SGLT2 inhibitors have a renoprotective effect independent from glycaemia and blood pressure control, some clinical trials are ongoing to assess its effect on non-diabetic CKD patients.
Diabetic mice and rat models seem to respond to SGLT2 inhibitors similarly to humans in terms of glycaemia and body weight control [21]. In addition, the experimental models of diabetic nephropathy also showed the cardiorenal protection phenotype [22–25]. In contrast, in non-diabetic CKD experimental models, the results are unclear. Some studies were not able to demonstrate that SGLT2 inhibitors prevented kidney damage [26, 27], whereas others demonstrated clear renoprotective effects [28–32]. In mice with tubular damage induced by chronic oxalosis, empagliflozin did not improve renal function or fibrosis [26]. In concordance, dapagliflozin did not improve the glomerular filtration rate in the subtotal nephrectomy model of glomerulosclerosis in the rat [27]. However, in a rat model of kidney damage induced by unilateral ureteral obstruction, SGLT2 inhibition decreased kidney fibrosis and inflammation biomarkers, such as transforming growth factor-beta 1 (TGF-β1), alpha smooth muscle actin (α-SMA) or fibronectin. Moreover, they exhibited a downregulation of the inflammatory Nuclear factor kappa B/Toll-like receptor 4 (NF-κB/TLR4) signalling pathway, as well as a partial recovery of tubular klotho levels suggesting that empagliflozin may have a protective effect against inflammation and fibrosis [30]. Panchapakesan et al. showed similar results in cultured proximal tubular cells where empagliflozin attenuated the NF-κB/TLR4 pathway. They also demonstrated that SLGT2 expression was not only induced by high glucose levels, but also by the profibrotic factor TGF-β1 [28]. In a mouse model, luseogliflozin prevented fibrosis after kidney injury induced by ischaemia–reperfusion. Increased expression of vascular endothelial growth factor A (VEGF-A) in the kidneys of these animals was also observed. Both the decrease of fibrosis and the VEGF-A overexpression were suppressed when luseogliflozin was associated with sunitinib—a VEGF receptor inhibitor. These results suggest that the protective effects of luseogliflozin were in part mediated by the VEGF-A pathway [29]. In a murine protein-overload proteinuria model, dapagliflozin reduced proteinuria and glomerular damage in a similar way to lisinopril—an ACEi. In the in vivo model and in cultured cells, bovine serum albumin upregulated SGLT2 expression in podocytes in an NF-κB-dependent manner. This induced cytoskeleton changes that reverted with the administration of dapagliflozin. Interestingly, SGLT2 inhibition may directly target the podocytes and contribute to maintain the actin cytoskeleton architecture [31]. Hyperglycaemia-induced senescence and oxidative stress on the tubular cells have also been related to glucose overload. In a type 1 diabetic rat model, senescence was mediated by SGLT2 and p-21 [32]. Moreover, in cultured tubular cells, high glucose concentrations induce an inflammatory and proapoptotic state mainly caused by oxidative stress that was prevented by tofogliflozin [33].
The results obtained in non-diabetic CKD models suggest that SGLT2 inhibitors could also have a direct beneficial effect on the kidney, which would be independent of the glycaemic and blood pressure control (Figure 1). Not all the biological pathways involved in the cardiorenal protection exerted by SGLT2 inhibitors have been characterized. In addition to high glucose levels, several studies have observed SGLT2 upregulation by profibrotic factors like TGF-β1 and protein overload. These findings may explain the implication of this co-transporter in non-diabetic kidney disease. Furthermore, SGLT2 blockade interacts with several pathways and signalling molecules such as NF-κB/TLR4, VEGF-A or klotho, suggesting that these drugs modulate inflammatory and fibrotic responses. As not all of the non-diabetic CKD animal models responded to SGLT2 inhibitors [26, 27], it is possible that the direct effects on the kidney are dependent on the specific CKD experimental model studied.
In conclusion, SGLT2 inhibitors have been shown to reduce cardiovascular complications and to slow diabetic kidney disease progression in patients with T2D. Interestingly, this effect was also associated with decreased urinary proximal tubular injury biomarkers. Beside glycaemic and blood pressure control, these drugs seem to exert direct renoprotective effects that may explain the results obtained in non-diabetic models of CKD. However, more evidence is needed to confirm the results, as well as to explain how and in which specific non-diabetic kidney diseases they could be effective.
ACKNOWLEDGEMENTS
The authors are current recipients of research grants from the FONDO DE INVESTIGACIÓN SANITARIA-FEDER, ISCIII, PI17/00257 and REDINREN, RD16/0009/0030. A.V. performed this work within the basis of the Doctorate of Medicine of the Autonomous University of Barcelona (UAB).
CONFLICT OF INTEREST STATEMENT
M.J.S. reports conflict of interest with NovoNordisk, Janssen, Boehringer, Eli Lilly, AstraZeneca, Abbvie and Esteve, outside of the submitted work.
REFERENCES
- 1. Xie Y, Bowe B, Mokdad AH. et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018; 94: 567–581 [DOI] [PubMed] [Google Scholar]
- 2. Patel A, MacMahon S, Chalmers J. et al. ; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 3. Palazzolo G, Albanese NN, DI Cara G. et al. Proteomic analysis of exosome-like vesicles derived from breast cancer cells. Anticancer Res 2012; 32: 847–860 [PubMed] [Google Scholar]
- 4. Gerstein HC, Miller ME, Byington RP. et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UPDS Group (UKPDS). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853 [PubMed] [Google Scholar]
- 6. Lewis EJ, Hunsicker LG, Bain RP. et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993; 329: 1456–1462 [DOI] [PubMed] [Google Scholar]
- 7. Brenner BM, Cooper ME, de Zeeuw D. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 8. Mann JFE, Anderson C, Gao P. et al. Dual inhibition of the renin–angiotensin system in high-risk diabetes and risk for stroke and other outcomes. J Hypertens 2013; 31: 414–421 [DOI] [PubMed] [Google Scholar]
- 9. Wanner C, Inzucchi SE, Lachin JM. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 10. Wiviott SD, Raz I, Bonaca MP. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357 [DOI] [PubMed] [Google Scholar]
- 11. Heerspink HJL, Desai M, Jardine M. et al. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 2017; 28: 368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeFronzo RA, Norton L, Abdul-Ghani M.. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 2017; 13: 11–26 [DOI] [PubMed] [Google Scholar]
- 13. Monami M, Nardini C, Mannucci E.. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16: 457–466 [DOI] [PubMed] [Google Scholar]
- 14. Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 [DOI] [PubMed] [Google Scholar]
- 15. Neal B, Perkovic V, Mahaffey KW. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 16. Perkovic V, de Zeeuw D, Mahaffey KW. et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 2018; 6: 691–704 [DOI] [PubMed] [Google Scholar]
- 17. Jardine MJ, Mahaffey KW, Neal B. et al. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 2018; 46: 462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Satirapoj B, Korkiatpitak P, Supasyndh O.. Effect of SGLT-2 inhibitor to proximal tubular function and injury in 2 patients with type 2 diabetes: a randomized controlled trial. Clin Kidney J 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dekkers CCJ, Petrykiv S, Laverman GD. et al. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab 2018; 20: 1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herrington WG, Preiss D, Haynes R. et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018; 11: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liang Y, Arakawa K, Ueta K. et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One 2012; 7: e30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shin SJ, Chung S, Kim SJ. et al. Effect of sodium-glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS One 2016; 11: e0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ye Y, Bajaj M, Yang H-C. et al. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther 2017; 31: 119–132 [DOI] [PubMed] [Google Scholar]
- 24. Tahara A, Takasu T.. Prevention of progression of diabetic nephropathy by the SGLT2 inhibitor ipragliflozin in uninephrectomized type 2 diabetic mice. Eur J Pharmacol 2018; 830: 68–75 [DOI] [PubMed] [Google Scholar]
- 25. Leng W, Ouyang X, Lei X. et al. The SGLT-2 inhibitor dapagliflozin has a therapeutic effect on atherosclerosis in diabetic ApoE −/− mice. Mediators Inflamm 2016; 2016: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma Q, Steiger S, Anders H-J.. Sodium glucose transporter-2 inhibition has no renoprotective effects on non-diabetic chronic kidney disease. Physiol Rep 2017; 5: e13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Thai K, Kepecs DM. et al. Sodium-glucose linked cotransporter-2 inhibition does not attenuate disease progression in the rat remnant kidney model of chronic kidney disease. PLoS One 2016; 11: e0144640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panchapakesan U, Pegg K, Gross S. et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells—renoprotection in diabetic nephropathy? PLoS One 2013; 8: e54442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y, Nakano D, Guan Y. et al. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor–dependent pathway after renal injury in mice. Kidney Int 2018; 94: 524–535 [DOI] [PubMed] [Google Scholar]
- 30. Abbas NAT, El Salem A, Awad MM.. Empagliflozin, SGL T2 inhibitor, attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction: potential role of klotho expression. Naunyn Schmiedebergs Arch Pharmacol 2018; 39: 1347–1360 [DOI] [PubMed] [Google Scholar]
- 31. Cassis P, Locatelli M, Cerullo D. et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 2018; 3: pii: 98720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kitada K, Nakano D, Ohsaki H. et al. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complications 2014; 28: 604–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ishibashi Y, Matsui T, Yamagishi S.. Tofogliflozin, a highly selective inhibitor of SGLT2 blocks proinflammatory and proapoptotic effects of glucose overload on proximal tubular cells partly by suppressing oxidative stress generation. Horm Metab Res 2015; 48: 191–195 [DOI] [PubMed] [Google Scholar]