Abstract
Background
The course of cryoglobulinaemia varies widely, from asymptomatic patients to severe vasculitis syndrome. Renal involvement (RI) is the major prognostic factor, and frequently occurs several years after diagnosis. However, predictive factors for RI are not well known. The aim of our study was to identify RI predictive factors during cryoglobulinaemia.
Methods
We retrospectively reviewed the clinical charts of a consecutive series of 153 patients positive for cryoglobulinaemia in the University Hospital of Lyon (France). RI was defined either histologically or biologically if cryoglobulinaemia was the only possible cause of nephropathy.
Results
Among the 153 positive patients (mean age 55 years, 37% male), cryoglobulinaemia was associated with RI in 45 (29%) patients. Sixty-five percent of patients had Type II cryoglobulinaemia, 28% had Type III and 7% had Type I. Autoimmune diseases were the most common aetiology (48%), followed by infectious diseases (18%) and lymphoproliferative disorders (13%). Membranoproliferative glomerulonephritis was the main histological pattern (93% of the 14 histological analyses). A multivariable logistic regression showed that Type II cryoglobulinaemia, a high serum cryoglobulin concentration, the presence of an IgG kappa monoclonal component and diabetes were independently associated with the risk for developing RI.
Conclusion
We identified several factors predictive of RI in patients with cryoglobulinaemia, which were different from the diagnostic criteria for cryoglobulinaemic vasculitis. This could suggest a specific pathophysiology for RI. We suggest performing an extensive renal monitoring and ensure nephroprotection when a diagnosis of cryoglobulinaemia is made in patients with these predictive factors.
Keywords: cryoglobulinaemia, kidney failure, predictive factors, proteinuria, renal involvement
INTRODUCTION
Cryoglobulins are immunoglobulins (Ig) that precipitate at temperature below 37°C and dissolve after rewarming. The term cryoglobulinaemic vasculitis is used to describe patients with symptoms related to the presence of cryoglobulins. Many patients with cryoglobulinaemia remain asymptomatic [1]. However, precipitation of cryoglobulins in small vessels can be responsible for vasculitis, with clinical symptoms ranging from mild palpable purpura, arthralgias and fatigue, to severe vasculitis with skin necrosis and glomerulonephritis (GN), as well as involvement of peripheral nerves, central nervous system, gastrointestinal tract, lungs and myocardium [2]. After immunochemical typing, cryoglobulins are sorted according to the classification of Brouet et al. [3]: Type I cryoglobulinaemia comprises single monoclonal Ig; Types II and III are mixed cryoglobulinaemia associating a monoclonal component with polyclonal Ig in Type II and only polyclonal Ig in Type III. These cryoproteins may be associated with distinct underlying diseases encompassing lymphoproliferative disorders in Type I, infectious and autoimmune diseases in Types II and III or, more rarely, may be primary [4, 5].
Renal involvement (RI) during cryoglobulinaemia is mainly characterized by urinary abnormalities [6, 7], proteinuria being reported in 88–100% of cases [8–11], and haematuria in almost all patients [8, 10]. Elevation of plasma creatinine is described in 47–63% of patients with RI [7, 11]. Pathological features observed on kidney biopsies of patients with RI are often characterized by an extensive glomerular infiltration by monocytes with double contours of the basement membrane, and hyaline intraluminal thrombi, evocative of membranoproliferative glomerulonephritis (MPGN) [8]. Immunofluorescence analysis defines the RI as an immune complex-mediated MPGN in the new classification of MPGN [12], showing intra-glomerular sub-endothelial deposits of Ig identical to those of the cryoprecipitates, and complement components. Renal necrotizing vasculitis and extra-capillary crescents are rarely observed.
RI has been reported in 18–40% of patients [13–15], and in patients with cryoglobulinaemia vasculitis, death appears more frequently in those with RI [9, 14–19]. The occurrence of RI follows the diagnosis of cryoglobulinaemia after a mean follow-up of 2.6–4 years [7, 11], and occurs until 41 years after diagnosis [11]. In case of RI, extrarenal manifestations are rarely associated [19], and if they are, their onset is not concomitant to the onset of RI [11]. Long-term predictors of survival in patients with RI are well known [7, 11], but predictive factors of RI have been described in only one study, with no data about cryoglobulin concentration [20]. The aim of our study was to identify predictive factors of RI in cryoglobulinaemia in a large monocentre cohort of patients with cryoglobulinaemia.
MATERIALS AND METHODS
Patients
Clinical charts of a consecutive series of 153 patients with cryoglobulinaemia (from January 2012 to December 2014) from different medical departments of University Hospitals were retrospectively reviewed. Inclusion criteria were: (i) cryoglobulinaemia with cryoglobulin total Ig concentration >20 mg/L and (ii) age >18 years. Exclusion criteria were: (i) RI with a possible cause other than cryoglobulinaemia and (ii) missing data [absence of urinalysis: proteinuria, haematuria; absence of complement exploration or rheumatoid factor (RF) assay; incomplete clinical data].
Renal involvement
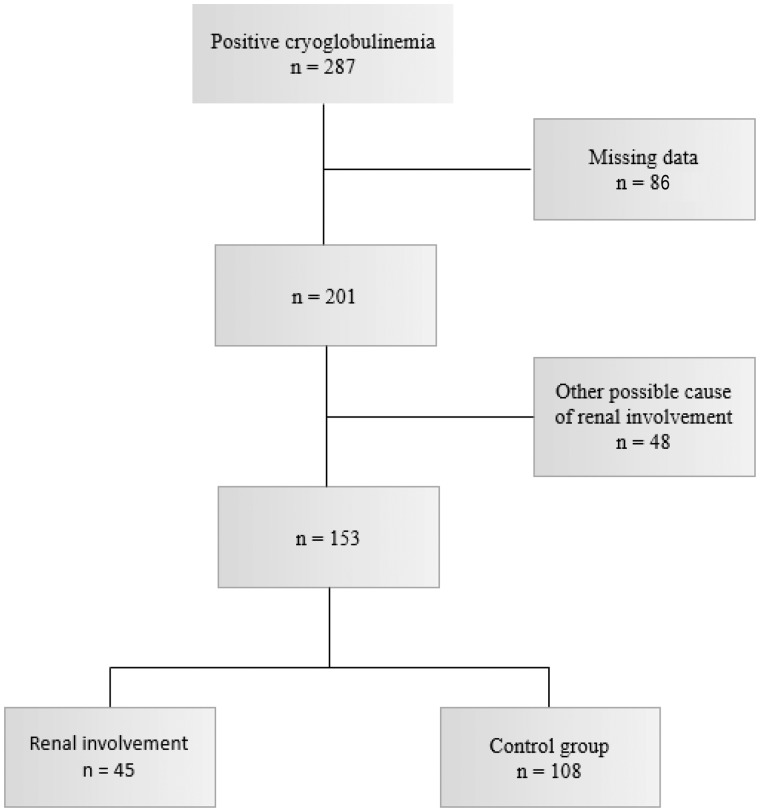
RI was defined either histologically or biologically if no other cause of RI than cryogloclobulinaemia was present. Pathological criterion was a biopsy-proven cryoglobulinaemic GN. Biological criteria were proteinuria (protein to creatinine ratio >0.5 g/g or proteinuria >0.5 g/24 h) and/or haematuria (>10 red blood cells/mm3) and/or estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 as calculated by Chronic Kidney Disease Epidemiology Collaboration equation. In case of RI without renal biopsy, patients were excluded if there was a possible cause of RI other than cryoglobulinaemia (connective tissue diseases, mostly systemic lupus erythematosus and Sjögren syndrome; diabetes if uncontrolled or with other microangiopathic complications). Patients with cryoglobulinaemia and total serum cryoglobulins concentration >20 mg/L without RI were considered as controls. The flow chart of the study is shown in Figure 1. Hypertension was defined by a blood pressure >140/90 mmHg.
FIGURE 1.
Flow chart.
Laboratory analyses
Cryoglobulin detection, purification and characterization were performed in the immunology laboratory of the Hospices Civils de Lyon according to the protocol previously described by Kolopp-Sarda and Miossec [21]. Blood samples were collected by venipuncture for complement exploration and for cryoglobulin detection, conserved for 2 h for complete coagulation at 37°C and rapidly transported to the laboratory at 37°C. Samples were centrifuged (2200g, 15 min) and serum was decanted and stored at 4°C for 7 days. Visual observation at day 7 allowed the detection of any cryoprecipitate. In that case, cryoprecipitate was isolated by +4°C centrifugation (3500 r.p.m. 2200g, 15 min) and purified by three washes with cold phosphate-buffered saline (PBS, pH 7.4, +4°C) to remove serum and proteins that had not precipitated. Pellets were then dissolved at 37°C in 500 µL PBS and stored at 37°C for further analyses.
Characterization of cryoprecipitate was performed by electrophoresis–immunofixation to type cryoglobulins with anti-γ, anti-α, anti-μ, anti-κ and anti-λ antisera (SAS-3®, Helena Bioscience, Gateshead, UK). In dissolved cryoprecipitate stored at 37°C, IgG, IgM and IgA concentrations as well as RF activity (IgM anti-IgG) were assayed by immunonephelometry (BNprospec®, Siemens, Marburg, Germany, reagents for low concentrations). Total Ig concentration in cryoprecipitate was the sum of IgG, IgM and IgA concentrations. A positive threshold of cryoglobulin total Ig concentration >20 mg/L is defined in our laboratory, in line with our technical practice and values described by Vermeersch et al. [22] Serum RF (normal <20 UI/mL), complement C3 (normal range 0.82–1.60 g/L) and C4 (normal range 0.14–0.32 g/L) were quantified by immunonephelometry (BNprospec®) and complement functional activity (CH50) was quantified on SPAplus® (Binding Site, Birmingham, UK; normal range 41–95 U/mL).
Histological analysis
Biopsy specimens were available for 14 patients. Light microscopy was available for all patients, and immunofluorescence was missing for one patient. Renal biopsies were not processed by electron microscopy.
Light microscopy was processed by standard techniques with a minimum of 12 serial sections (2 μm thick), stained with hematoxylin–eosin–safran, periodic acid–Schiff, Masson’s Trichrome and Jones methenamine-silver stains. Routine immunofluorescence was performed on 3 μm-thick cryostat sections using polyclonal FITC-conjugated antibodies to IgA, IgG, IgM, C3, C1q, κ, λ and fibrinogen (Dako).
Statistical analysis
Patient characteristics are reported as number (percentage) for categorical variables and mean ± SD for continuous variables. Comparison of two groups involved Student’s t-test for quantitative variables and χ2 or Fisher’s exact test as appropriate for categorical variables. We reported group comparisons with odds ratio [OR, 95% confidence intervals (95% CI)]. To identify independent predictors of RI, we used a stepwise procedure to select, among parameters with P < 0.20 in univariable analysis, those to retain in the final multivariable logistic regression model. Results are expressed as OR (95% CI). Statistical analysis was done on SPSS v21.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
RESULTS
Demographic data
The main features of the study groups are listed in Tables 1 and 2. Mean age of patients was 54.6 ± 18.2 years with no difference between the two groups (P = 0.57). Male patients were more prevalent in the RI group (60% versus 27% in the control group, P < 0.0001). Mean follow-up from cryoglobulinaemia diagnosis was similar in both groups, and was 2.0 ± 4.8 years (range 0–26.4 years) in the RI group and 1.2 ± 2.5 years (range 0–18.2 years) in the control group. Diabetes was present in 11% of the population, with a higher proportion in case of RI (29% versus 4% in the control group, P < 0.0001).
Table 1.
Clinical characteristics of patients with and without RI
| Characteristics | Total population | RI | Control group | P |
|---|---|---|---|---|
| n = 153 | n = 45 | n = 108 | ||
| Clinical data | ||||
| Age, years, mean (SD) | 54.6 (18.2) | 56.2 (19.7) | 54 (17.4) | 0.57 |
| Male gender | 56 (37) | 27 (60) | 29 (27) | <0.0001 |
| Years since diagnosis, mean (SD) | 1.46 (3.4) | 2.0 (4.8) | 1.2 (2.5) | 0.22 |
| Diabetes | 17 (11) | 13 (29) | 4 (4) | <0.0001 |
| Purpura | 41 (27) | 17 (38) | 24 (22) | 0.048 |
| Acrosyndrome | 18 (12) | 3 (7) | 15 (14) | 0.21 |
| Arthralgia | 30 (20) | 5 (11) | 25 (23) | 0.09 |
| Neurological involvement | 21 (14) | 7 (16) | 14 (13) | 0.67 |
| Hypertension | 28 (18) | 19 (42) | 9 (9) | <0.0001 |
| Aetiologies | ||||
| Secondary cryoglobulinaemia | 114 (75) | 29 (64) | 85 (79) | 0.07 |
| Lymphoproliferative disorders | 20 (13) | 10 (22) | 10 (9) | 0.03 |
| Autoimmune disease | 74 (48) | 12 (27) | 62 (58) | 0.001 |
| Infectious disease | 28 (18) | 10 (22) | 18 (17) | 0.42 |
| Hepatitis C virus | 21 (14) | 7 (16) | 14 (13) | 0.67 |
Data are presented as n (%) unless otherwise indicated.
Table 2.
Biological characteristics of patients with and without RI
| Total population | RI | Control group | P | |
|---|---|---|---|---|
| n = 153 | n = 45 | n = 108 | ||
| Cryoglobulinaemia composition, n (%) | ||||
| Type I cryoglobulinaemia | 11 (7) | 2 (4) | 9 (8) | 0.51 |
| Type II cryoglobulinaemia | 99 (65) | 38 (84) | 61 (57) | 0.12 |
| Type III cryoglobulinaemia | 43 (28) | 5 (11) | 38 (35) | 0.006 |
| Presence of IgG cryoglobulin | 141 (92) | 44 (98) | 97 (90) | 0.11 |
| Presence of IgA cryoglobulin | 13 (8) | 10 (22) | 3 (3) | 0.0003 |
| Presence of IgM cryoglobulin | 139 (91) | 40 (89) | 99 (92) | 0.55 |
| Cryoglobulin concentration (mg/L) | ||||
| Total: median (IQR, min–max) | 45 (140, 20–4006) | 137 (875, 20–3792) | 39 (75, 20–4006) | 0.001 |
| IgG: median (IQR, min–max) | 17 (62, 0–1833) | 44 (296, 0–1833) | 14 (182, 0–1218) | 0.001 |
| IgA: median (IQR, min–max) | 0 (0, 0–312) | 0 (0, 0–130) | 0 (0, 0–312) | <0.0001 |
| IgM: median (IQR, min–max) | 25 (79, 0–2523) | 30 (360, 0–2408) | 24 (33, 0–2523) | 0.07 |
| Monoclonal cryoglobulin, n (%) | ||||
| Monoclonal IgGκ | 29 (19) | 10 (22) | 7 (7) | 0.005 |
| Monoclonal IgGλ | 16 (10) | 4 (9) | 7 (7) | 0.73 |
| Monoclonal IgA | 12 (8) | 5 (11) | 1 (1) | 0.009 |
| Monoclonal IgMκ | 103 (67) | 27 (60) | 46 (43) | 0.050 |
| Monoclonal IgMλ | 22 (14) | 4 (9) | 14 (13) | 0.48 |
| Polyclonal cryoglobulin, n (%) | ||||
| Polyclonal IgG | 129 (84) | 39 (87) | 90 (83) | 0.61 |
| Polyclonal IgA | 7 (5) | 5 (11) | 2 (2) | 0.024 |
| Polyclonal IgM | 65 (42) | 13 (29) | 52 (48) | 0.03 |
| RF activity | ||||
| Positive RF in cryoprecipitate, n (%) | 45 (29) | 19 (42) | 26 (24) | 0.03 |
| Serum complement | ||||
| Decreased C3 (<0.82 g/L), n (%) | 27 (18) | 8 (18) | 19 (18) | 0.98 |
| Decreased C4 (<0.14 g/L), n (%) | 82 (54) | 23 (51) | 59 (55) | 0.69 |
| Low CH50 (<41 UI/mL), n (%) | 79 (52) | 29 (64) | 50 (46) | 0.041 |
| C3: median (g/L) (IQR, min–max) | 1.04 (0.39, 0.13–1.74) | 1.05 (0.40, 0.13–1.62) | 1.03 (0.35, 0.35–1.74) | 0.78 |
| C4: median (g/L) (IQR, min–max) | 0.15 (0.16, 0.01–0.50) | 0.16 (0.27, 0.01–0.50) | 0.15 (0.13, 0.02–0.46) | 0.85 |
IQR (min–max), interquartile range (minimum–maximum).
Clinical symptoms
Purpura was the most frequent symptom (27% of the patients). Its prevalence was greater in RI than in the control group (38% versus 22%, P = 0.048). The most common symptoms were respectively arthralgias (20%), neurological involvement (14%) and acrosyndrome (12%). Regarding aetiologies, the majority (75%) of cryoglobulinaemias were secondary, with only 14% of cases related to hepatitis C virus. Autoimmune diseases were the most frequent aetiology (48%), with a higher frequency in the control group than in the RI group (58% versus 27%, P = 0.001). Lymphoproliferative disorders were more common in case of RI (22% versus 9% in the absence of RI, P = 0.03).
Cryoglobulin characteristics
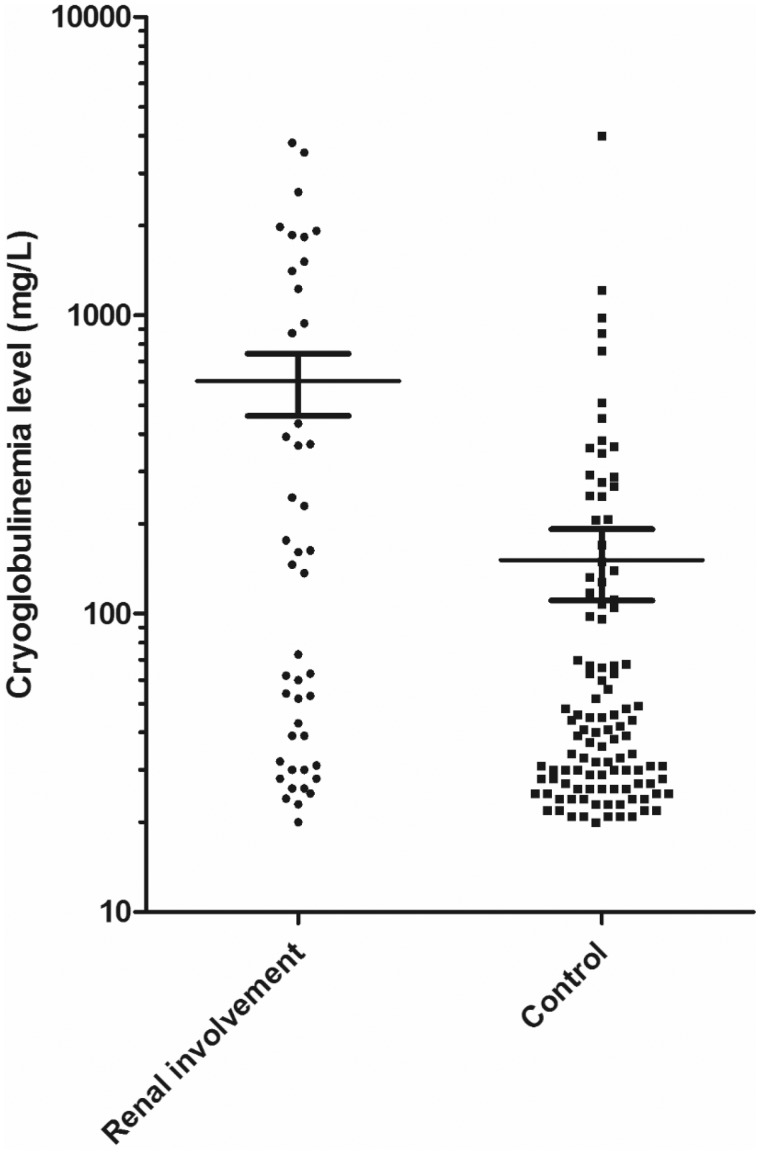
Type II cryoglobulinaemia was the main type (65%), followed by Type III (28%) and Type I (7%). IgG and IgM cryoglobulins were found in 92% and 91% of patients, respectively, whereas IgA cryoglobulin was found in only 8% of patients. IgA cryoglobulin was more common in cases of RI (22% versus 3% in the control group, P = 0.0003). Monoclonal IgM kappa was the most common monoclonal cryoglobulin (67%), whereas regarding the polyclonal cryoglobulin, IgG was the most common (84%). IgM kappa and IgG kappa were more frequent in cases of RI than in the absence of RI (60% and 22%, versus 43% and 7%, P = 0.05 and P = 0.005, respectively). Median of the total cryoglobulin concentration was 45 mg/L, and was higher in cases of RI than in the absence of RI (137 mg/L versus 39 mg/L, P = 0.001). Figure 2 shows the distribution of total cryoglobulin concentration (defined as cryoglobulinaemia level in the figure) in patients with or without RI.
FIGURE 2.
Total cryoglobulin levels (mean ± SD) in patients with or without RI; P<0.001, Student’s t-test.
Complement and RF
The majority of patients had low serum C4 and CH50, but a normal C3, with no difference between both groups except for CH50 (64% of low CH50 in cases of RI versus 46% in the control group, P = 0.041). IgM anti-IgG RF was present in cryoprecipitate in 29% of patients, with a higher proportion in the RI group (42%) than in the control group (24%, P = 0.03).
Renal involvement
Biological results
Mean eGFR was 54 mL/min/1.73 m2 in the RI group and 95 mL/min/1.73 m2 in the control group. In case of RI, haematuria was present in 71% of patients, with a median of 40/mm3, and proteinuria was present in 62% of patients, with a median of 0.79 g/24 h (or g/g). In the RI group, patients with kidney biopsy had a more severe RI than patients without kidney biopsy: mean proteinuria was 4.65 ± 7.90 g/24 h versus 0.58 ± 0.71 g/24 h (P = 0.007), and mean eGFR was 36 ± 20 versus 70 ± 28 mL/min/1.73 m2 (P < 0.001).
Histological results
Table 3 describes the results of kidney biopsies in 14 patients. MPGN was the main histopathological pattern, in 93% of patients. The important monocyte infiltrate with double contours of the basement membrane was the most characteristic histological feature on 10 biopsies. Intraluminal thrombi and endocapillary proliferation were respectively present in 77% and 63% of cases. By immunofluorescence, Ig deposits observed on the biopsy were similar to those characterizing cryoglobulinaemia in 75% of cases.
Table 3.
Pathological features of 14 patients with RI
| Histopathological pattern | n (%) |
|---|---|
| MPGN | 13 (93) |
| Focal segmental glomerulosclerosis | 1 (7) |
| Lesion | |
| Double contours of the basement membrane | 10 (70) |
| Intraluminal thrombi | 11 (77) |
| Endocapillary proliferation | 9 (63) |
| Extracapillary crescents | 5 (35) |
| Necrotizing vasculitis | 1 (7) |
| Immunofluorescence | |
| IgG | 10 (70) |
| IgA | 7 (49) |
| IgM | 11 (77) |
| IgE | 1 (7) |
| C3 | 10 (70) |
| C1q | 6 (42) |
| Kappa light chain | 11 (77) |
| Lambda light chain | 11 (77) |
Predictive factors of RI
Univariable analyses
Male gender, diabetes, hypertension and purpura were significantly associated with RI (Table 4). Lymphoproliferative disorders were correlated with RI, whereas no difference was found for the other aetiologies. Regarding biological data, several features were significantly associated with RI (total cryoglobulin concentration, Type II cryoglobulinaemia, presence of a monoclonal component, IgG kappa monoclonal component, presence of RF in cryoprecipitate, decreased CH50). Type III cryoglobulinaemia and the presence of polyclonal IgM were significantly associated with the absence of RI.
Table 4.
Univariable and multivariable analyses of the clinical and biological factors associated with RI
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Clinical factors | ||||||
| Male gender | 4.07 | 1.96–8.50 | <0.0001 | 8.15 | 2.84–23.39 | <0.0001 |
| Hypertension | 7.80 | 3.16–19.24 | <0.0001 | 11.51 | 3.40–38.90 | <0.0001 |
| Diabetes | 10.25 | 3.12–33.67 | <0.0001 | 6.31 | 1.49–26.74 | 0.01 |
| Arthralgia | 0.42 | 0.15–1.16 | 0.10 | – | – | – |
| Purpura | 2.12 | 0.99–4.51 | 0.05 | – | – | – |
| Secondary cryoglobulinaemia | 0.49 | 0.23–1.05 | 0.07 | – | – | – |
| Lymphoproliferative disorders | 2.80 | 1.08–7.30 | 0.04 | – | – | – |
| Biological factors | ||||||
| Total cryoglobulin concentration (per 100 mg/L increase) | 1.12 | 1.04–1.21 | 0.002 | 1.10 | 1.02–1.18 | 0.02 |
| Type II cryoglobulinaemia | 4.18 | 1.72–10.20 | 0.002 | 4.53 | 1.36–15.09 | 0.03 |
| Type III cryoglobulinaemia | 0.23 | 0.08–0.63 | 0.004 | – | – | – |
| Monoclonal component | 4.34 | 1.58–11.92 | 0.004 | – | – | – |
| IgG kappa monoclonal component | 4.12 | 1.46–11.65 | 0.008 | 6.18 | 1.50–25.42 | 0.01 |
| IgM polyclonal component | 0.44 | 0.21–0.92 | 0.03 | – | – | – |
| IgA polyclonal component | 3.79 | 0.61–23.47 | 0.15 | – | – | – |
| RF presence in cryoprecipitate | 2.31 | 1.10–4.82 | 0.03 | – | – | – |
| Decreased CH50 | 2.10 | 1.03–4.31 | 0.04 | – | – | – |
Dashes represent P > 0.05.
Multivariable analyses
Several factors were identified as independent predictive factors for developing RI in case of cryoglobulinaemia: male gender (OR = 8.15, 95% CI 2.84–23.39, P < 0.0001) Type 2 diabetes (OR = 6.31, 95% CI 1.49–26.74, P = 0.01), hypertension (OR = 11.51, 95% CI 3.40–38.90, P < 0.0001), Type II cryoglobulinaemia (OR = 4.53, 95% CI 1.36–15.09, P = 0.03), IgG kappa monoclonal component (OR = 6.18, 95% CI 1.50–25.42, P = 0.01) and high cryoglobulin total concentration (OR 1.10, 95% CI 1.02–1.18) for each increase of 100 mg/L of the total cryoglobulin concentration, P = 0.02) (Table 4).
DISCUSSION
This study aimed to describe the clinical, biological and histological characteristics of patients with cryoglobulinaemia with or without RI in a large monocentre cohort between 2012 and 2014, and to identify predictive factors of RI. This is the first study focusing on the predictive factors for developing RI in the presence of cryoglobulinaemia.
In 2011, De Vita et al. [23] proposed a preliminary classification criteria for the cryoglobulinaemic vasculitis: the diagnosis was made if two or three items (questionnaire, clinical, laboratory) were positive. These items were different from our results. The proposed classification included several clinical symptoms, reduced serum C4, positive serum RF and positive serum monoclonal component as criteria for diagnosis of cryoglobulinaemic vasculitis, whereas none of these features was independently associated with RI in our study. Moreover, predictive factors of RI defined in our study were not present in this classification. This could suggest a specific pathophysiology for RI, different from that which targeted the onset of cryoglobulinaemic vasculitis.
First, our results show that a higher cryoglobulin level is associated with a higher risk of RI: for each increase of 100 mg/L of the total cryoglobulin level, the risk of RI increased by 10%. The importance of the cryoglobulin level was not recognized, either in the classification for the cryoglobulinaemic vasculitis [23] or in the study of Hurwitz et al. [24] in 1975, who did not find any difference for this criterion between patients with or without RI. The only previous study about predictive factors of RI did not analyse the cryoglobulin level [20]. However, a similar figure was found by Trejo et al. [13] in a cohort of 443 patients in Spain: patients with a cryocrit >5% had a higher frequency of GN. Our study strongly suggests a dose-dependent relationship to this association.
In rheumatological surveys, patients with Type III cryoglobulinaemia outnumbered those with Type II cryoglobulinaemia [17, 25]. Conversely, surveys based on RI showed a greater prevalence of Type II mixed cryoglobulins [8, 11, 20]. We therefore confirm these analyses, showing a statistically significant association between Type II cryoglobulinaemia and RI in the multivariable analysis (Table 4).
In our study, the presence of an IgG kappa cryoglobulin was independently associated with risk for developing RI. A link between IgG monoclonal cryoglobulins and severe manifestations of cryoglobulinaemia, including RI, has been reported by Néel et al. [19] in 2014 in Type I cryoglobulinaemia. However, our results indicate the special role of the monoclonal IgG kappa component in all types of cryoglobulinaemia, while polyclonal IgG or the presence of an IgG cryoglobulin was not associated with RI.
The role of IgA cryoglobulins in RI of cryoglobulinaemia requires investigation in further studies. Indeed, presence of IgA cryoglobulins was strongly associated with RI in univariate analyse, but this result was not found in multivariate analyse because of associations between IgA cryoglobulin and three of the predictive factors of RI (presence of IgG kappa cryoglobulin, Type II cryoglobulinaemia and male gender). These associations are not described in the literature, although it is known that IgA have an important role in some glomerulopathies. So perhaps, the lack of statistical power explains the absence of statistically significant difference.
The high frequency of Type II diabetes in cryoglobulinaemic patients has been shown by Antonelli et al. [26], and is confirmed in our study. However, diabetes was independently associated with risk for developing RI in our study. To prevent potential patients with diabetic nephropathy in the RI group, we have excluded patients with uncontrolled diabetes or with other microangiopathic complications. The analysis of diabetic patients with RI shows that a high proportion had haematuria (62%, similar to the 71% of the total RI group), whereas haematuria is infrequent in diabetic nephropathy [27]. Furthermore, in patients with a biopsy-proven cryoglobulinaemic RI, 21% had diabetes, which is not different from the 29% in the total RI group (Table 1), and widely superior to the 4% of diabetic patients in the control group (Table 1). These results are in favour of absence or a very low proportion of patients with diabetic nephropathy in the RI group, although we cannot exclude RI due to diabetes versus cryoglobulinaemia. Patients with diabetes are therefore possibly more at risk of developing RI in case of cryoglobulinaemia, but this notion requires further studies.
No particular aetiology independently predicted RI, although lymphoproliferative disorders were more common in cases of RI (22% versus 9%), but this was not statistically significant. The existing literature about cryoglobulinaemia has extensively studied the hepatitis C virus (HCV)-related cryoglobulinaemias [7, 18, 28–30], but our study showed different results concerning cryoglobulinaemia-related diseases, with a low proportion of HCV (14% of the total population, without any difference between both groups). Beddhu et al. [8] studied 17 patients with cryoglobulinaemia and RI; 11 patients (65%) had an HCV infection. In a Barcelona cohort of 441 patients with cryoglobulinaemia, Trejo et al. [13] found a proportion of 71% patients with an HCV infection, while there only 14% of patients had HCV in our study. This low proportion of HCV can be explained by the geographical characteristic of our monocentre cohort, where the prevalence of HCV infection is much lower than in some Mediterranean populations [31]. It also confirms the current trend towards a decreasing prevalence of HCV infection in France since the introduction of effective antiviral treatments [32]. In our study, because of the exclusion of 48 patients with a possible cause of RI other than cryoglobulinaemia, some results about aetiologies should be considered with caution, because most (31 of these 48 patients) had a connective tissue disease, possibly leading to underestimation of the proportion of patients with connective tissue disease in the RI group. For the same reason, the correlation we found between male gender and RI should be interpreted with caution, because 28 of these 48 excluded patients were women. Therefore, male gender is potentially overestimated in the RI group.
Clinically, no single symptom was an independent predictive factor of RI. Purpura was more frequent in the RI group, but this difference was not statistically significant in multivariate analysis. Hypertension was much more present in cases of RI, but seems to be a consequence of RI. Indeed, several studies have described the high prevalence of hypertension in cryoglobulinaemic patients with RI: 75% and 64% of patients in two US studies [8, 9], 80% in a French study [33] and 82% in an Italian study [11]. Furthermore, in cases of RI, blockers of the renin–angiotensin system are sometimes stopped, which may partly explain the elevation of blood pressure. However, treatments were not recorded; therefore, it is impossible to analyse the influence of drug withdrawal on blood pressure in our study. Hypertension could be the cause of the RI in some patients. However, only 2 of the 45 patients with RI had neither haematuria nor proteinuria, which is hardly compatible with a high proportion of hypertensive nephrosclerosis, and the mean eGFR in the RI group was quite high (54 mL/min/1.73 m2), which is not in support of a high proportion of secondary focal glomerulosclerosis.
Complement analyses were similar in both groups, with frequently normal C3, but decreased C4 and CH50. These are classic data for complement system in cryoglobulinaemia, as described in the 1970s [34–36]. However, the fact that low C4 was not an independent predictive factor of RI supports the hypothesis that in cryoglobulinaemia, low C4 is not the consequence but the cause of the disease. Indeed, Menegatti et al. [37] showed in 2016 that polymorphisms of the C4 gene were less frequent in patients with mixed cryoglobulinaemia than in healthy patients. RF presence was not an independent predictive factor of RI, whereas it is a predictive factor of cryoglobulinaemic vasculitis [23].
Morphological analyses of kidney biopsies showed that MPGN was the most frequent histopathological pattern, as described in earlier studies [7, 8, 10, 11, 29, 33], but extra-capillary crescents were present in 35% of biopsies, which is quite high [8] and partly responsible for the RI. Deposits of Ig were frequent, and identical to those of the cryoprecipitates in most cases. Histological analysis was only available in 14 patients. However, in case of biological RI, we excluded patients with possible causes of RI other than cryoglobulinaemia, such as connective tissue disease and diabetic nephropathy. Moreover, the population of the RI group had similar biological renal characteristics to those of patients from previous studies of RI in cryoglobulinaemia [6, 8–11], with a very high proportion of haematuria and/or proteinuria (only two patients had RI because of an isolated low eGFR without haematuria or proteinuria). The proportion of patients with RI and without kidney biopsy could be explained by the fact that RI frequently appears several years after the diagnosis of cryoglobulinaemia [7, 11], and in high-risk patients (e.g. anticoagulant drugs, elderly patients). In cases of typical and non-severe RI, renal biopsy is deemed unnecessary in patients with already known cryoglobulinaemia. We compared patients with and without biopsy in case of RI, and indeed, patients with kidney biopsy had a more severe RI.
There are caveats to our study: the small sample size yielded large confidence intervals, which reduces the robustness of the analysis. Furthermore, the monocentre source of data may limit the validity of our results, which should be confirmed in further studies.
In summary, we identified several predictive factors of developing RI in case of cryoglobulinaemia: Type II cryoglobulinaemia, a high cryoglobulin concentration, the presence of an IgG kappa monoclonal component and diabetes. These factors are different from the diagnostic criteria for cryoglobulinaemic vasculitis. This could suggest a specific RI pathophysiology. In patients with predictive factors for RI at diagnosis, kidney function monitoring and nephroprotection should be intensified.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
V.C., M.N.-S., M.L., S.R., C.T.-C., J.-C.L. and D.F. declared no competing interests. R.C. reports grants and personal fees from Merck, Amgen and Chugai-Roche, and personal fees from UCB, Pfizer, Abbvie, BMS, Lilly, Sandoz and Radius outside the submitted work. P.S. reports personal fees from Pfizer, Abbvie and Novartis outside the submitted work.
REFERENCES
- 1. Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis 2008; 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramos-Casals M, Stone JH, Cid MC. et al. The cryoglobulinaemias. Lancet 2012; 379: 348–360 [DOI] [PubMed] [Google Scholar]
- 3. Brouet J-C, Clauvel J-P, Danon F. et al. Biologic and clinical significance of cryoglobulins. Am J Med 1974; 57: 775–788 [DOI] [PubMed] [Google Scholar]
- 4. Ferri C, Zignego AL, Pileri SA.. Cryoglobulins. J Clin Pathol 2002; 55: 4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Retamozo S, Brito-Zerón P, Bosch X. et al. Cryoglobulinemic disease. Oncol (Williston Park) 2013; 27: 1098–1105, 1110–1116 [PubMed] [Google Scholar]
- 6. Tarantino A, De Vecchi A, Montagnino G. et al. Renal disease in essential mixed cryoglobulinaemia. Long-term follow-up of 44 patients. Q J Med 1981; 50: 1–30 [PubMed] [Google Scholar]
- 7. Roccatello D, Fornasieri A, Giachino O. et al. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis 2007; 49: 69–82 [DOI] [PubMed] [Google Scholar]
- 8. Beddhu S, Bastacky S, Johnson JP.. The clinical and morphologic spectrum of renal cryoglobulinemia. Medicine 2002; 81: 398–409 [DOI] [PubMed] [Google Scholar]
- 9. Gorevic PD, Kassab HJ, Levo Y. et al. Mixed cryoglobulinemia: clinical aspects and long-term follow-up of 40 patients. Am J Med 1980; 69: 287–308 [DOI] [PubMed] [Google Scholar]
- 10. Li SJ, Xu ST, Chen HP. et al. Clinical and morphologic spectrum of renal involvement in patients with HBV-associated cryoglobulinaemia. Nephrol 2017; 22: 449–455 [DOI] [PubMed] [Google Scholar]
- 11. Tarantino A, Campise M, Banfi G. et al. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int 1995; 47: 618–623 [DOI] [PubMed] [Google Scholar]
- 12. Masani N, Jhaveri KD, Fishbane S.. Update on membranoproliferative GN. Clin J Am Soc Nephrol 2014; 9: 600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trejo O, Ramos-Casals M, García-Carrasco M. et al. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine 2001; 80: 252–262 [DOI] [PubMed] [Google Scholar]
- 14. Saadoun D, Sellam J, Ghillani-Dalbin P. et al. Increased risks of lymphoma and death among patients with non–hepatitis C virus-related mixed cryoglobulinemia. Arch Intern Med 2006; 166: 2101–2108 [DOI] [PubMed] [Google Scholar]
- 15. Ferri C, Sebastiani M, Giuggioli D. et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum 2004; 33: 355–374 [DOI] [PubMed] [Google Scholar]
- 16. Terrier B, Carrat F, Krastinova E. et al. Prognostic factors of survival in patients with non-infectious mixed cryoglobulinaemia vasculitis: data from 242 cases included in the CryoVas survey. Ann Rheum Dis 2013; 72: 374–380 [DOI] [PubMed] [Google Scholar]
- 17. Meltzer M, Franklin EC, Elias K. et al. Cryoglobulinemia – a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med 1966; 40 : 837–856 [DOI] [PubMed] [Google Scholar]
- 18. Della Rossa A, Tavoni A, D'Ascanio A. et al. Mortality rate and outcome factors in mixed cryoglobulinaemia: the impact of hepatitis C virus. Scand J Rheumatol 2010; 39: 167–170 [DOI] [PubMed] [Google Scholar]
- 19. Néel A, Perrin F, Decaux O. et al. Long-term outcome of monoclonal (type 1) cryoglobulinemia. Am J Hematol 2014; 89: 156–161 [DOI] [PubMed] [Google Scholar]
- 20. Zaidan M, Terrier B, Pozdzik A. et al. Spectrum and prognosis of noninfectious renal mixed cryoglobulinemic GN. J Am Soc Nephrol 2016; 27: 1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kolopp-Sarda M, Miossec P.. Cryoglobulins: an update. Autoimmun Rev 2018; 17: 457–464 [DOI] [PubMed] [Google Scholar]
- 22. Vermeersch P, Gijbels K, Knockaert D. et al. Establishment of reference values for immunoglobulins in the cryoprecipitate. Clin Immunol 2008; 129: 360–364 [DOI] [PubMed] [Google Scholar]
- 23. De Vita S, Soldano F, Isola M. et al. Preliminary classification criteria for the cryoglobulinaemic vasculitis. Ann Rheum Dis 2011; 70: 1183–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hurwitz D, Quismorio FP, Friou GJ.. Cryoglobulinaemia in patients with infectious endocarditis. Clin Exp Immunol 1975; 19: 131–141 [PMC free article] [PubMed] [Google Scholar]
- 25. Invernizzi F, Pioltelli P, Cattaneo R. et al. A long-term follow-up study in essential cryoglobulinemia. Acta Haematol 1979; 61: 93–99 [DOI] [PubMed] [Google Scholar]
- 26. Antonelli A, Ferri C, Fallahi P. et al. Type 2 diabetes in hepatitis C-related mixed cryoglobulinaemia patients. Rheumatology (Oxford) 2004; 43: 238–240 [DOI] [PubMed] [Google Scholar]
- 27. Chong YB, Keng TC, Tan LP. et al. Clinical predictors of non-diabetic renal disease and role of renal biopsy in diabetic patients with renal involvement: a single centre review. Ren Fail 2012; 34: 323–328 [DOI] [PubMed] [Google Scholar]
- 28. Barsoum RS. Hepatitis C virus: from entry to renal injury—facts and potentials. Nephrol Dial Transplant 2007; 22: 1840–1848 [DOI] [PubMed] [Google Scholar]
- 29. D’Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int 1998; 54: 650–671 [DOI] [PubMed] [Google Scholar]
- 30. Terrier B, Cacoub P.. Renal involvement in HCV-related vasculitis. Clin Res Hepatol Gastroenterol 2013; 37: 334–339 [DOI] [PubMed] [Google Scholar]
- 31. Sansonno D, Carbone A, De Re V. et al. Hepatitis C virus infection, cryoglobulinaemia, and beyond. Rheumatology (Oxford) 2007; 46: 572–578 [DOI] [PubMed] [Google Scholar]
- 32. Cacoub P, Dabis F, Costagliola D. et al. Burden of HIV and hepatitis C co-infection: the changing epidemiology of hepatitis C in HIV-infected patients in France. Liver Int Off J Int Assoc Study Liver 2015; 35: 65–70 [DOI] [PubMed] [Google Scholar]
- 33. Matignon M, Cacoub P, Colombat M. et al. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis C virus infection. Medicine 2009; 88: 341–348 [DOI] [PubMed] [Google Scholar]
- 34. Linscott WD, Kane JP.. The complement system in cryoglobulinaemia. Interaction with immunoglobulins and lipoproteins. Clin Exp Immunol 1975; 21: 510–519 [PMC free article] [PubMed] [Google Scholar]
- 35. Rother U, Rother K, Flad HD. et al. Bithermic complement activation in cryoglobulinaemic serum. Eur J Clin Invest 1972; 2: 59–65 [DOI] [PubMed] [Google Scholar]
- 36. Naish PF, Collins C, Barratt J.. Classical pathway complement activation in association with paraproteinaemia. Immunology 1977; 33: 517–521 [PMC free article] [PubMed] [Google Scholar]
- 37. Menegatti E, Messina M, Oddone V. et al. Immunogenetics of complement in mixed cryoglobulinaemia. Clin Exp Rheumatol 2016; 34 (3 Suppl 97): S12–S15 [PubMed] [Google Scholar]