Abstract
Background
Microparticles (MPs) are small cell membrane-derived vesicles regarded as both biomarkers and mediators of biological effects. Elevated levels of MPs have previously been associated with endothelial dysfunction and predict cardiovascular death in patients with end-stage renal disease. The objective of this study was to measure change in MP concentrations in contemporary haemodialysis (HD).
Methods
Blood was sampled from 20 consecutive HD patients before and 1 h into the HD session. MPs were measured by flow cytometry and phenotyped based on surface markers.
Results
Concentrations of platelet (CD41+) (P = 0.039), endothelial (CD62E+) (P = 0.004) and monocyte-derived MPs (CD14+) (P < 0.001) significantly increased during HD. Similarly, endothelial- (P = 0.007) and monocyte-derived MPs (P = 0.001) expressing tissue factor (TF) significantly increased as well as MPs expressing Klotho (P = 0.003) and receptor for advanced glycation end products (RAGE) (P = 0.009). Furthermore, MPs expressing platelet activation markers P-selectin (P = 0.009) and CD40L (P = 0.045) also significantly increased. The increase of endothelial (P = 0.034), monocyte (P = 0.014) and RAGE+ MPs (P = 0.032) as well as TF+ platelet-derived MPs (P = 0.043) was significantly higher in patients treated with low-flux compared with high-flux dialysers.
Conclusion
Dialysis triggers release of MPs of various origins with marked differences between high-flux and low-flux dialysers. The MPs carry surface molecules that could possibly influence coagulation, inflammation, oxidative stress and endothelial dysfunction. The clinical impact of these findings remains to be established in future studies.
Keywords: chronic kidney disease, haemodialysis, Klotho, microparticles, RAGE
INTRODUCTION
Patients with chronic kidney disease (CKD) have high incidence of cardiovascular disease (CVD), and CVD remains as the major cause of mortality in patients treated with haemodialysis (HD). In addition to traditional risk factors, the increased risk is attributed to non-traditional risk factors, including inflammation, endothelial dysfunction and oxidative stress [1]. Plasma concentration of membrane microparticles (MPs) has been shown to be associated with cardiovascular risk [2] and patient outcome following myocardial infarction [3].
MPs are small vesicles, 0.1–1.0 µm in size, formed by the outward blebbing of the cell membrane, released from cells upon activation or due to apoptosis and necrosis [4]. Although platelet-derived MPs (PMPs) are most abundant in plasma, MPs from various cell types have been observed, including MPs of endothelial (EMPs) and monocyte (MMPs) origin [5]. MPs retain characteristics of their cell of origin such as cytosolic content and membrane antigens that determine their function and can be used for identification purposes [4]. Today, MPs are accepted as both biomarkers and bioactive particles involved in various disease states [5]. Most MPs express phosphatidylserine (PS) on their outer membrane and some express tissue factor (TF), which in effect makes MPs procoagulant [6]. PS facilitates binding of coagulation cascade proteins Factor (F)VII, IX, X and prothrombin and the assembly of coagulation complexes, and TF is considered the prime activator of the coagulation cascade [7]. In addition to PS and TF, PMPs may also express markers of platelet activation including P-selectin (CD62P) [6] and CD40L [8].
Klotho is a transmembrane protein found predominantly in the distal convoluted tubule of the kidney. Klotho is a co-factor that makes the fibroblast growth factor (FGF) receptor specific for FGF-23, which lowers renal phosphate resorption and suppresses synthesis of calcitriol [1, 25(OH)2D3] in the kidney [9]. Klotho expression declines with reducing renal function and Klotho deficiency has been speculated to contribute to vascular calcification in CKD [10].
Advanced glycation end products (AGEs) are elevated in CKD not only due to hyperglycaemia but also due to oxidative stress. AGEs can reciprocally induce oxidative stress and inflammation and have been linked to CVD [11]. HD decreases the level of AGEs [12]. The receptor for AGEs (RAGE) is expressed by various cells including endothelial cells and macrophages and is upregulated in diabetes and inflammatory diseases [11].
Previous studies on the acute effects of HD on levels of circulating MPs are scarce and to our knowledge, no previous studies have examined Klotho or RAGE expression in MPs. In this study, we investigated MP formation in contemporary HD by measuring plasma concentrations of a wide range of MP subtypes before and during HD.
MATERIALS AND METHODS
Subjects
Twenty patients on thrice weekly maintenance HD at the Department of Nephrology at Uppsala University Hospital who consented to participate in the study were included. There were no other exclusion criteria. At study start, all patients at the dialysis units were asked to participate. Inclusion was stopped as soon as 20 patients meeting the inclusion criteria had given their consent. The study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent, and the Regional Ethics Review Board in Uppsala approved the trial.
Sampling
The patients received their prescribed dialysis with no modifications made for study purpose. For each patient, 2 mL of blood was drawn from the venous line and collected in citrate vacutainer tubes. Samples were taken before the start of the dialysis session and 1 h into dialysis. The blood was centrifuged within 30 min of collection at 1500g for 10 min at room temperature (RT) in order to obtain platelet poor plasma (PPP). The plasma was frozen and stored at –70°C directly.
Measurement of MPs
PPP was thawed in a water bath for 5 min at 37°C and subsequently centrifuged at RT for 20 min at 2000g, in order to further remove any debris or cells that may interfere with the analysis. The supernatant was then re-centrifuged at 13 000g for 2 min at RT. Subsequently, 20 µL of the supernatant were incubated for 20 min in the dark with phalloidin-Alexa 660 (cell-fragment marker, Invitrogen, Paisley, UK) [13], lactadherin-Fluorescein isothiocyanate (FITC) (Haematologic Technologies, VT, USA). For detection of MP origin either CD41-PE (Beckman Coulter, Brea, CA, USA) for PMPs, CD62E-APC (AH diagnostics, Stockholm, Sweden) for EMPs or CD14-FITC (Beckman Coulter, Brea, CA, USA) for MMPs was added. In addition, exposure of TF (CD142-PE, BD, NJ, USA) was measured on PMPs, MMPs and EMPs; CD40L (CD154-APC, AH diagnostics) and P-selectin (CD62P-APC, AH diagnostics) on PMPs and Klotho (Klotho-FITC, Bioss Antibodies Inc, Woburn, MA, USA) and RAGE (Anti-RAGE-FITC, Abcam, Cambridge, UK). MPs were measured using flow cytometry on a Beckman Gallios instrument (Beckman Coulter). The MP gate was determined using Megamix beads (0.5–3.0 µm, BioCytex, Marseille, France). MPs were defined as particles < 1.0 µm in size and positive or negative to the markers described above. Conjugate isotype-matched immunoglobulins with no reactivity against human antigens were used as negative controls. In this study, results are shown as numbers of MPs (MP counted × standard beads added/L)/standard beads counted (FlowCount, Beckman Coulter). The intra- and interassay coefficients of variation for MP measurement were <9%.
Statistical analysis
All statistical analysis was performed using Rstudio (Version 1.1.383). Prior to analysis, data were log-transformed, if necessary, to obtain normal distribution. Histograms and QQ-plots were used to assess normality in combination with Shapiro–Wilk test. Samples taken before and 1 h into dialysis were compared using paired t-test for paired samples with normally or log-normally distributed variables and Wilcoxon signed-rank test for variables with non-normal distribution. Change in MP concentration between groups was compared with Student’s t-test for normally distributed data and Mann–Whitney U test for non-normal data. Correlations between clinical parameters and changes in MP concentrations were assessed using Spearman’s rank correlation. P < 0.05 were considered significant.
RESULTS
Patient characteristics
Twenty patients were included in the study, but samples from one patient were excluded due to failure to comply with the study protocol during sampling. Patient characteristics are presented in Table 1. HD was performed using synthetic dialysers. In eight subjects, high flux polysulphone dialysers were utilized. The rest of the patients were dialysed using polyamide/polyarylether-sulfone/polyvinylpyrrolidone blend dialysers, with nine being low-flux and two high-flux.
Table 1.
Patient characteristics
| Age, mean ± SD, years | 74.1 ± 10.1 |
| Male, N (%) | 14 (73.7) |
| BMI, median (range), kg/m2 | 26.6 (21.0–40.7) |
| Arteriovenous fistula, N (%) | 6 (31.6) |
| High-flux membrane, N (%) | 10 (52.6) |
| Haemodiafiltration, N (%) | 9 (47.4) |
| Dialysis duration, median (range), h | 4 (4–5) |
| Ultrafiltration, mean ± SD, L | 1.82 ± 1.21 |
| Diabetes mellitus, N (%) | 7 (36.8) |
| Previous CVD, N (%) | 13 (68.4) |
Microparticles
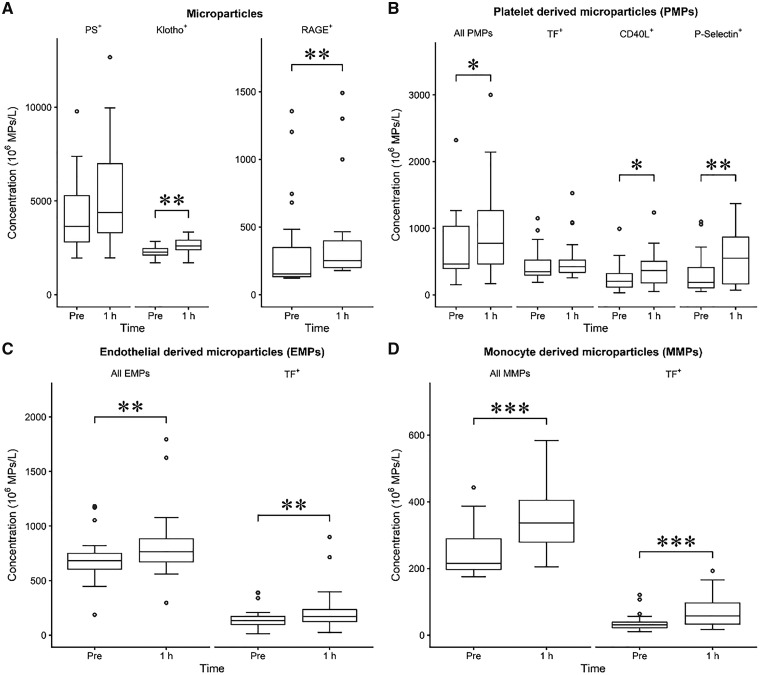
Plasma concentrations of the measured MPs are presented in detail in Table 2 and Figure 1. Plasma concentration of total PS+ MPs did not significantly change during the first hour of HD (P = 0.129) but PMPs (P = 0.039), EMPs (P = 0.004) and MMPs (P < 0.001) increased significantly. Similarly, Klotho+ (P = 0.003) and RAGE+ (0.009) MPs as well as PMPs with platelet activation markers CD40L (P = 0.045) and CD62P (P = 0.009) increased significantly. A significant increase in TF+ EMPs (P = 0.004) and MMPs (P = 0.001) was also observed but not in TF+ PMPs.
Table 2.
Plasma concentration of MPs during HD (106 MPs/L)
| Factor | Pre-dialysis | 1 h into dialysis | P-value |
|---|---|---|---|
| All MPs | |||
| PS+ | 3645 (1960–9784) | 4388 (1966–12672) | 0.129 |
| Klotho+ | 2260 (±276) | 2612 (±414) | 0.003 |
| RAGE+ | 154 (122–1356) | 252 (178–1491) | 0.009 |
| PMPs (CD41+) | |||
| All PMPs | 464 (153–2321) | 774 (169–3000) | 0.039 |
| TF+ | 349 (187–1149) | 424 (258–1526) | 0.242 |
| CD40L+ | 205 (33–992) | 365 (52–1236) | 0.045 |
| P-Selectin+ | 186 (50–1098) | 550 (70–1369) | 0.009 |
| EMPs (CD62E+) | |||
| All EMPs | 683 (188–1183) | 764 (297–1795) | 0.004 |
| TF+ | 135 (15–392) | 171 (26–900) | 0.007 |
| MMPs (CD14+) | |||
| All MMPs | 216 (175–443) | 337 (205–584) | <0.001 |
| TF+ | 31 (11–121) | 58 (17–193) | 0.001 |
Data presented as median (range) and mean (±SD).
FIGURE 1.
Boxplot of plasma concentrations of MPs (not classified by cell type) (A) and PMPs (B), EMPs (C) and MMPs (D) in HD with whiskers representing 1.5 interquartile range and circles representing outliers. *P < 0.05; **P < 0.01; ***P < 0.001.
A/V fistula versus dialysis catheter
Subgroup analysis revealed a significantly higher increase in Klotho+ MPs in patients with arteriovenous fistula (A/V) fistula as vascular access [605.5 (range 272–846) × 106 MPs/L with A/V fistula versus 247 (−762–779) × 106 MPs/L with dialysis catheter, P = 0.036].
Low-flux versus high-flux dialysers
Increase in EMPs (P = 0.034), MMPs (P = 0.014), RAGE+ MPs (P = 0.032), and TF+ PMPs (P = 0.043), TF+ EMPs (P = 0.027) and TF+ MMPs (P = 0.048) were significantly higher in patients treated with low-flux compared with high-flux dialysers (Table 3). In the low-flux group, EMPs (P = 0.004), MMPs (P < 0.001), Klotho+ (P = 0.039) and RAGE+ MPs (P = 0.003), and TF+ EMPs (P = 0.004) and TF+ MMPs (P = 0.009) increased significantly during dialysis and in the high-flux group MMPs (P = 0.014), Klotho+ MPs (P = 0.019) and P-selectin+ PMPs (P = 0.049) increased significantly. Patients in the low-flux group were significantly older (P = 0.011), had lower body mass index (BMI) (P = 0.006), and were dialysed for shorter duration (P = 0.013) and with less ultrafiltration volume (P = 0.028) than those in the high-flux group.
Table 3.
Comparison of changes in MP concentrations (Δ106 MPs/L) between high- and low-flux dialysers
| Factor | High flux | Low flux | P-value |
|---|---|---|---|
| All MPs | |||
| PS+ | 366 (±1851) | 1722 (±2815) | 0.241 |
| Klotho+ | 250 (−289 to 758) | 617 (−762 to 846) | 0.053 |
| RAGE+ | 24 (±103) | 128 (±91) | 0.032 |
| PMPs | |||
| All PMPs | 241 (±352) | 340 (±755) | 0.725 |
| TF+ | −42 (−647 to 694) | 74 (4 to 758) | 0.043 |
| CD40L+ | 74 (±105) | 143 (±301) | 0.527 |
| P-Selectin+ | 142 (−58 to 1040) | 89 (−82 to 1124) | 0.720 |
| EMPs | |||
| All EMPs | 38 (−319 to 626) | 127 (95 to 804) | 0.034 |
| TF+ | 18 (−217 to 560) | 61 (8 to 552) | 0.027 |
| MMPs | |||
| All MMPs | 75 (±78) | 136 (±61) | 0.014 |
| TF+ | 24 (±40) | 44 (±38) | 0.048 |
Data presented as median (range) and mean (±SD).
Other subpopulations
No significant difference in the change of plasma concentration was found for any of the MP subpopulations depending on gender, smoking status, previous CVD events, presence of diabetes, current statin treatment or HD modality (haemodiafiltration (HDF) versus standard HD).
Correlations
Correlations between MP levels and dialysis and patient-specific factors are presented in detail in the supplementary material (Supplementary data, Table S1). Changes in Klotho+ and RAGE+ MP levels correlated negatively with ultrafiltration volume and BMI. Change in RAGE+ MP concentrations correlated positively with age. Changes in levels of PS+ MPs and TF+ PMPs and MMPs correlated positively with pre-dialysis systolic blood pressure (BP) and changes in levels of PS+ MPs, MMPs and TF+ PMPs, EMPs and MMPs correlated positively with post-dialysis systolic BP.
DISCUSSION
Summary of findings
In this study, we set out to obtain a comprehensive overview of the acute effect of contemporary HD on MP levels by measuring MPs in plasma before and 1 h into dialysis. The major findings of this study are (i) plasma concentration of PMPs, EMPs and MMPs increased during HD; (ii) PMPs expressing platelet activation markers P-selectin and CD40L, and TF+ PMPs, TF+ EMPs and TF+ MMPs increased during HD; (iii) Klotho+ and RAGE+ MPs, which have, to our knowledge, never been demonstrated before, increased significantly during HD; and (iv) the increase of MPs was generally greater in patients treated with low-flux compared with high-flux dialysers.
Previous data on MPs in relation to HD are conflicting, which could possibly be explained by difference in inclusion criteria, dialyser type and choice of antibodies used for analysis. In particular, antibodies for detection of EMPs vary greatly between studies with most utilizing CD31 [14–18], and others CD66E [19], CD144 [15, 19, 20] and CD146 [20, 21]. In this study, E-selectin was used, which is upregulated on endothelial cells as an inflammatory response. Lactadherin was used to label PS+ MPs, which binds more selectively than the more commonly used Annexin V [13].
In agreement with our findings, some previous studies have reported an increase in PMP levels during dialysis [21, 22], whereas others have not [14, 19, 23]. EMP levels were reported to increase in one previous study [14], similar to our results, whereas two other studies did not observe any significant alterations [19, 21]. Only one previous study investigated MMPs, with no significant changes between before and after HD [19].
We demonstrated a significant increase in TF+ EMPs and TF+ MMPs but not TF+ PMPs. The only previous study on the effect of HD on TF+ MPs did not demonstrate a significant change in TF+ MP plasma concentrations [19]. One study compared TF+ MPs between HD, peritoneal dialysis, non-dialysed uraemic patients and healthy controls and found no significant difference between groups [17]. However, neither of these studies divided TF+ MPs by subpopulations which makes comparison difficult.
RAGE+ MPs increased significantly during HD and to our knowledge, this is the first time RAGE+ MPs have been observed. The cellular origin of these MPs is not known, and we cannot conclude if the increase is specific to RAGE or just reflects a general increase in MPs. In the absence of disease, RAGE is expressed predominantly in lung tissue but under pathological conditions, including inflammatory diseases, RAGE is upregulated and found on various cells including monocytes and endothelial cells [24, 25]. This suggests that our findings possibly reflect an inflammatory response induced by HD. Studies on HD and RAGE are scarce, but soluble RAGE has been shown to be elevated in HD patients compared with healthy controls [26]. However, HD has been shown to decrease the level of AGEs [12].
We observed a slight increase in Klotho+ MPs during HD. Klotho expression in MPs has to our knowledge never been demonstrated before and interestingly, levels of Klotho+ MPs were substantially higher than the other MP subtypes, except for PS+ MPs. The origin of these MPs remains unknown. Klotho is predominantly expressed in the kidney, most abundantly, but not exclusively, in the distal convoluted tubule [27]. The kidney has also been shown to be the prime source of circulating Klotho in mice [27], which suggests that Klotho+ MPs could also be of renal origin. Increase in Klotho+ MPs was higher in subjects where an A/V-fistula was used as access compared with dialysis catheter. Previous studies have to our knowledge only included patients dialysed through A/V fistula [19, 21, 23, 28].
Interestingly, the increase in EMPs, MMPs, Klotho+ MPs and TF+ PMPs, EMPs and MMPs was higher in patients treated with low-flux dialysers compared with high-flux dialysers. However, it should be noted that this study was not specifically designed for the purpose of detecting differences between different dialysers. The intended advantage of high-flux membranes is the greater clearance of middle molecules, which might influence MP formation indirectly. Proteins involved in bioincompatibility reactions against biomaterials, including complement factors C3a and C5a, are significantly removed by filtration in high-flux HD [29]. In vitro data suggest that complement activation triggers MP release [30]. It is debated whether high-flux membranes are more bio-compatible than their low-flux counterparts [31, 32] although two multicentre studies have reported survival benefits [33, 34]. Pore size would not influence MP levels directly since the average pore sizes of the two high-flux dialysers used were 3.3 nm and 40.1 nm, far smaller than the lower size limit of MPs. It is possible that the lower MP formation observed in the high-flux group could in part be dependent on the use of HDF since it was predominant (90%) in this group. This is supported by previous studies which have shown that patients treated with HDF have lower EMP levels compared with patients treated with standard HD [16], with no differences between HDF modalities [18, 22]. Other significant differences between the high- and low-flux groups included age, BMI, dialysis duration and ultrafiltration, further stressing the need for additional adequately powered studies specifically designed for this purpose to draw any major conclusions. Previous studies have not compared low- and high-flux membranes although dialysers of different materials have been compared. No differences between polysulphone and cellulose triacetate dialysers have been shown in previous studies, nor between synthetic and celluloid dialysers [23].
Possible mechanism
The changes to MP levels observed during HD could be attributed to many factors. The adverse effects of HD in triggering coagulation, inflammation and oxidative stress are well established [35]. The inflammatory response has been attributed to blood–biomaterial interactions resulting in protein adsorption on the dialysis membrane with subsequent activation of the complement system [36]. Dialysers made from synthetic polymers such as polysulphone, most used in contemporary HD, are considered more biocompatible but their hydrophobic nature leads to greater protein adsorption, resulting in a more indirect interaction with the blood [37]. Studies have shown that the protein complex, C5b-9, formed in the last step of the complement cascade induces release of MPs in vitro [30, 38].
The repetitive mechanical stress from the HD treatment itself could contribute to MP elevation during HD, as high shear stress has previously been demonstrated to promote MP release from platelets [39]. Contrary to what we observed in this study, the removal of uraemic toxins during HD could theoretically suppress EMP formation. These toxins have been linked to endothelial dysfunction, and in vitro studies have demonstrated that the uraemic toxins p-cresol and indoxyl sulphate induce MP release from endothelial cells [21]. Considering the conflicting data on EMP levels in HD in previous studies, other mechanisms seem to be involved as well. In addition, these toxins are highly protein bound that makes removal during HD less effective with high variation between different dialyser types [21].
Clinical significance
The pro-coagulatory potential of MPs is widely recognized [7]. In vitro studies have shown that MPs from uraemic patients have greater procoagulant activity than MPs from healthy control [17]. PMP count has also been shown to be significantly higher in CKD patients with a recent history of thrombotic events [23]. It is conceivable that the observed increase in TF+ MPs could act as a source for the TF-dependent clotting during extracorporeal treatments [31]. The activation of platelets is also considered an important step in these clotting reactions, which could be reflected in the observed increases in MPs positive for platelet activation markers [40]. MPs have been linked to CVD and endothelial dysfunction [5]. In a prospective study, EMP concentration at baseline was found to be an independent predictor of cardiovascular and all-cause mortality in patients’ dialysed with polysulphone and AN69 dialysers [41]. EMP count has also been found to correlate with pulse wave velocity (PWV) and carotid augmentation index, markers of arterial stiffness, in HD patients, independently of age and BP [15]. Correlations were not found for total MPs or PMPs. Another study similarly demonstrated an independent association between EMP count and PWV in children with CKD [20]. Arterial stiffness is an independent predictor of cardiovascular and all-cause mortality in end-stage renal disease [42]. In vitro studies using rat aortas incubated in MPs from HD patients show that MPs impair endothelial function by reducing vasorelaxation in response to acetylcholine and cyclic guanosine monophosphate generation [15].
Limitations
To appreciate these findings, a number of limitations need to be addressed. First, the sample size was limited and the studied patients were heterogeneous with regard to prescribed dialysis and clinical characteristics. However, these consecutive patients reflected the real-life setting. Patient and dialysis-specific characteristics with the potential to influence the studied biomarkers need to be addressed in future trials. Secondly, the measured MP concentration was not corrected for alterations in haematocrit resulting from haemoconcentration induced by HD. However, the observed concentration changes occurred already at 1 h into dialysis. Furthermore, total MP concentration was not altered during HD and none of the measured MPs correlated positively with ultrafiltration volume. Thirdly, the limited number of sample instances limits our understanding of the full dynamics of MP levels during HD. The typical HD sessions lasts for up to 5 h and it could be that these observed alterations are of a transient nature. Fourthly, the observed increase in Klotho+ and RAGE+ MPs has never been demonstrated before, and it is necessary to reproduce these results before any major conclusions are reached. Finally, the suggested differences in MP formation between HD using low-flux and high-flux dialysers may also be explained by other patient or dialysis-related factors as subjects were not randomly assigned treatments and groups were not matched.
CONCLUSION
HD triggers an acute increase in MPs of various origins and our data suggest a marked difference between high- and low-flux dialysers. Interestingly, MPs expressing Klotho and RAGE, which also increase during HD, have been demonstrated for the first time. The surface molecules found on MPs are not only biological markers but also carry out biological effects that are known to influence coagulation, inflammation, levels of oxidative stress and endothelial dysfunction. The clinical impact of MPs in HD remains to be established in future studies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Yvonne Lundholm, R.N., for sampling and skilful sample handling.
FUNDING
This study was supported by grants from CUWX Counties Renal Patients Foundation, ALF Grant Region Uppsala and Uppsala-Örebro Regional Research Council.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Sarnak MJ, Levey AS, Schoolwerth AC. et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003; 108: 2154–2169 [DOI] [PubMed] [Google Scholar]
- 2. Baron M, Boulanger CM, Staels B. et al. Cell-derived microparticles in atherosclerosis: biomarkers and targets for pharmacological modulation? J Cell Mol Med 2012; 16: 1365–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morel O, Hugel B, Jesel L. et al. Sustained elevated amounts of circulating procoagulant membrane microparticles and soluble GPV after acute myocardial infarction in diabetes mellitus. Thromb Haemost 2004; 91: 345–353 [DOI] [PubMed] [Google Scholar]
- 4. Hugel B, Martínez MC, Kunzelmann C.. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005; 20: 22–27 [DOI] [PubMed] [Google Scholar]
- 5. Burger D, Schock S, Thompson CS. et al. Microparticles: biomarkers and beyond. Clin Sci 2013; 124: 423–441 [DOI] [PubMed] [Google Scholar]
- 6. Mobarrez F, He S, Bröijersen A. et al. Atorvastatin reduces thrombin generation and expression of tissue factor, P-selectin and GPIIIa on platelet-derived microparticles in patients with peripheral arterial occlusive disease. Thromb Haemost 2011; 106: 344–352 [DOI] [PubMed] [Google Scholar]
- 7. Owens AP, Mackman N.. Microparticles in hemostasis and thrombosis. Circ Res 2011; 108: 1284–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mobarrez F, Sjövik C, Soop A. et al. CD40L expression in plasma of volunteers following LPS administration: a comparison between assay of CD40L on platelet microvesicles and soluble CD40L. Platelets 2015; 26: 486–490 [DOI] [PubMed] [Google Scholar]
- 9. Kuro-O M. Phosphate and Klotho. Kidney Int 2011; 79121: S20–S23 [DOI] [PubMed] [Google Scholar]
- 10. Hu MC, Kuro-o M, Moe OW.. The emerging role of Klotho in clinical nephrology. Nephrol Dial Transplant 2012; 27: 2650–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stinghen AEM, Massy ZA, Vlassara H. et al. Uremic toxicity of advanced glycation end products in CKD. J Am Soc Nephrol 2016; 27: 354–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Floridi A, Antolini F, Galli F. et al. Daily haemodialysis improves indices of protein glycation. Nephrol Dial Transplant 2002; 17: 871–878 [DOI] [PubMed] [Google Scholar]
- 13. Mobarrez F, Antovic J, Egberg N. et al. A multicolor flow cytometric assay for measurement of platelet-derived microparticles. Thromb Res 2010; 125: e110–e116 [DOI] [PubMed] [Google Scholar]
- 14. Boulanger CM, Amabile N, Guérin AP. et al. In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal sisease. Hypertension 2007; 49: 902–908 [DOI] [PubMed] [Google Scholar]
- 15. Amabile N, Guérin AP, Leroyer A. et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 2005; 16: 3381–3388 [DOI] [PubMed] [Google Scholar]
- 16. Ariza F, Merino A, Carracedo J. et al. Post-dilution high convective transport improves microinflammation and endothelial dysfunction independently of the technique. Blood Purif 2013; 35: 270–278 [DOI] [PubMed] [Google Scholar]
- 17. Gao C, Xie R, Yu C. et al. Thrombotic role of blood and endothelial cells in uremia through phosphatidylserine exposure and microparticle release. PLoS One 2015; 10: e0142835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esquivias-Motta E, Martín-Malo A, Buendia P. et al. Hemodiafiltration with endogenous reinfusion improved microinflammation and endothelial damage compared with online-hemodiafiltration: a hypothesis generating study. Artif Organs 2017; 41: 88–98 [DOI] [PubMed] [Google Scholar]
- 19. Trappenburg MC, van Schilfgaarde M, Frerichs FCP. et al. Chronic renal failure is accompanied by endothelial activation and a large increase in microparticle numbers with reduced procoagulant capacity. Nephrol Dial Transplant 2012; 27: 1446–1453 [DOI] [PubMed] [Google Scholar]
- 20. Dursun I, Poyrazoglu HM, Gunduz Z. et al. The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrol Dial Transplant 2009; 24: 2511–2518 [DOI] [PubMed] [Google Scholar]
- 21. Faure V, Dou L, Sabatier F. et al. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost 2006; 4: 566–573 [DOI] [PubMed] [Google Scholar]
- 22. Sakurai K, Saito T, Yamauchi F. et al. Comparison of the effects of predilution and postdilution hemodiafiltration on neutrophils, lymphocytes and platelets. J Artif Organs 2013; 16: 316–321 [DOI] [PubMed] [Google Scholar]
- 23. Ando M, Iwata A, Ozeki Y. et al. Circulating platelet-derived microparticles with procoagulant activity may be a potential cause of thrombosis in uremic patients. Kidney Int 2002; 62: 1757–1763 [DOI] [PubMed] [Google Scholar]
- 24. Kierdorf K, Fritz G.. RAGE regulation and signaling in inflammation and beyond. J Leukoc Biol 2013; 94: 55–68 [DOI] [PubMed] [Google Scholar]
- 25. Yan SF, Yan SD, Ramasamy R. et al. Tempering the wrath of RAGE: an emerging therapeutic strategy against diabetic complications, neurodegeneration and inflammation. Ann Med 2009; 41: 408–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakashima A, Carrero JJ, Qureshi AR. et al. Effect of circulating soluble receptor for advanced glycation end products (sRAGE) and the proinflammatory RAGE ligand (EN-RAGE, S100A12) on mortality in hemodialysis patients. Clin J Am Soc Nephrol 2010; 5: 2213–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olauson H, Mencke R, Hillebrands J-L. et al. Tissue expression and source of circulating αKlotho. Bone 2017; 100: 19–35 [DOI] [PubMed] [Google Scholar]
- 28. Daniel L, Fakhouri F, Joly D. et al. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int 2006; 69: 1416–1423 [DOI] [PubMed] [Google Scholar]
- 29. Jørstad S, Smeby LC, Balstad T. et al. Generation and removal of anaphylatoxins during hemofiltration with five different membranes. Blood Purif 1988; 6: 325–335 [DOI] [PubMed] [Google Scholar]
- 30. Sims PJ, Faioni EM, Wiedmer T. et al. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem 1988; 263: 18205–18212 [PubMed] [Google Scholar]
- 31. Davenport A. The role of dialyzer membrane flux in bio-incompatibility. Hemodial Int 2008; 12 (Suppl 2): S29–S33 [DOI] [PubMed] [Google Scholar]
- 32. Kerr PG, Huang L.. Review: membranes for haemodialysis. Nephrology (Carlton) 2010; 15: 381–385 [DOI] [PubMed] [Google Scholar]
- 33. Krane V, Krieter DH, Olschewski M. et al. Dialyzer membrane characteristics and outcome of patients with type 2 diabetes on maintenance hemodialysis. Am J Kidney Dis 2007; 49: 267–275 [DOI] [PubMed] [Google Scholar]
- 34. Delmez JA, Yan G, Bailey J. et al. Cerebrovascular disease in maintenance hemodialysis patients: results of the HEMO Study. Am J Kidney Dis 2006; 47: 131–138 [DOI] [PubMed] [Google Scholar]
- 35. Ekdahl KN, Soveri I, Hilborn J. et al. Cardiovascular disease in haemodialysis: role of the intravascular innate immune system. Nat Rev Nephrol 2017; 13: 285–296 [DOI] [PubMed] [Google Scholar]
- 36. Nilsson B, Ekdahl KN, Mollnes TE. et al. The role of complement in biomaterial-induced inflammation. Mol Immunol 2007; 44: 82–94 [DOI] [PubMed] [Google Scholar]
- 37. Cheung AK. Biocompatibility of hemodialysis membranes. J Am Soc Nephrol 1990; 1: 150–161 [DOI] [PubMed] [Google Scholar]
- 38. Moskovich O, Fishelson Z.. Live cell imaging of outward and inward vesiculation induced by the complement C5b-9 complex. J Biol Chem 2007; 282: 29977–29986 [DOI] [PubMed] [Google Scholar]
- 39. Miyazaki Y, Nomura S, Miyake T. et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 1996; 88: 3456–3464 [PubMed] [Google Scholar]
- 40. Davenport A. What are the anticoagulation options for intermittent hemodialysis? Nat Rev Nephrol 2011; 7: 499–508 [DOI] [PubMed] [Google Scholar]
- 41. Amabile N, Guérin AP, Tedgui A. et al. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: a pilot study. Nephrol Dial Transplant 2012; 27: 1873–1880 [DOI] [PubMed] [Google Scholar]
- 42. London GM. Cardiovascular calcifications in uremic patients: clinical impact on cardiovascular function. J Am Soc Nephrol 2003; 14: S305–S309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.