Abstract
This study determined if a single bout of repeated-sprint running under hypoxic (RSH) conditions was associated with impaired cognitive function when compared with repeated-sprint running under normoxic (RSN) conditions. Eleven amateur team-sport athletes performed a repeated-sprint running protocol (4 sets of 4, 4-s all-out sprints; i.e., RSR444) under both conditions (14.5% and 20.9% O2) on a non-motorized treadmill. Changes in SpO2, pre-frontal cortex total haemoglobin (Δ[THb]), oxyhaemoglobin (Δ[O2Hb]), deoxyhaemoglobin (Δ[HHb]) and cognitive function (detection task: DET; identification task: IDN; one card learning task: OCL; performed pre and 20 min post RSR444) were examined. During RSH, SpO2 was lower following each set (p ≤ 0.05), while [HHb] was higher after each set (p ≤ 0.05) compared with RSN. In addition, while there was no effect of condition on DET (p = 0.20) or IDN (p = 0.14), OCL accuracy was lower after, compared with before, RSH (p=0.04), but not RSN (p = 0.52). A significant relationship was observed between Δ[HHB] and ΔOCL accuracy (r = -0.68, p = 0.01). Performance of a single bout of RSH with 14.5% O2 resulted in impaired cognitive function in amateur team-sport athletes. Coaches should be mindful of timing of RSH prescription with regard to other training sessions that challenge speed and movement accuracy.
Key points.
Repeated-sprint training in hypoxia (RSH) can temporarily impair cognitive function in team-sport athletes.
Given that decrements in cognitive function may predispose athletes to knee injuries, coaches should be mindful of the timing of prescription of RSH relative to other training sessions during which knee injuries might occur.
Repeated-sprint training in normoxia (RSN) did not affect cognitive function in the present study.
Key words: Altitude, RSH, sports, cerebral oxygenation, exercise, cognition
Introduction
Repeated-sprint training in hypoxia (RSH) is a promising intervention aimed at evoking greater metabolic (Puype et al., 2013) and performance (Brocherie et al., 2015; Faiss et al., 2013; Galvin et al., 2013; Kasai et al., 2015) adaptations compared with performing repeated-sprint training in normoxia (RSN). Greater increases in muscle enzyme activity (Puype et al., 2013), repeated-sprint ability (RSA; Faiss et al., 2013), Yo-Yo intermittent recovery test level 1 performance (Galvin et al., 2013) and peak and mean power output (Kasai et al., 2015) after 4-6 weeks of RSH compared with RSN have been demonstrated. However, these findings have not been universal (Goods et al., 2015).
In comparison to a single bout of RSN, a single bout of RSH results in higher heart rate, minute ventilation, blood lactate concentration, and muscle deoxygenation; as well as lower blood oxygen saturation, pulmonary oxygen uptake, integrated EMG (Bowtell et al., 2013) and cerebral oxygenation (Smith and Billaut, 2010). While altering the physiological responses is an essential outcome of RSH compared with RSN, it is not known if there are acute adverse effects of performing repeated-sprints in a hypoxic environment that should be considered in the broader context of exercise programming.
Exposure to normobaric hypoxia (FiO2: 0.125-0.150) can elicit reductions in cerebral oxygenation (Komiyama et al., 2015; Seo et al., 2015) and may be related to impaired performance of modified Stroop, i.e., colour-word interference (Leon-Carrion et al., 2008) and Running Memory Continuous Performance (percentage correct/throughput score; Seo et al, 2015) tasks at rest. Nonetheless, prolonged submaximal exercise performed below ~75% peak oxygen uptake has been demonstrated to not only restore (throughput score; Seo et al, 2015), but also improve (reaction time during Go/No-Go task) aspects of cognitive function during hypoxic (FiO2: 0.18, 0.15) exposure despite further reductions in peripheral oxygen saturation and cerebral oxygenation during exercise (Ando et al., 2013). Therefore, submaximal exercise acts as a countermeasure to restore cognitive function during exposure to hypoxic training environments. This may provide a safeguard from accidents or injuries related to acute cognitive impairment, given that individuals exposed to a hypoxic environment that are not aware of their acute cognitive dysfunction, may be at a higher risk of deleterious outcomes such as accidents (Seo et al., 2015). This is particularly important for team-sport athletes who participate in successive training sessions, including exercise in hypoxic environments, who may therefore be at a greater risk of injury.
Despite the restorative ability of prolonged submaximal cycling on cognitive function in hypoxia, it is unknown if short-duration, repeated treadmill sprinting can attenuate the risk of impaired cognitive function that occurs in hypoxic conditions. Lambourne and Tomporowski (2010) highlighted that cognitive function is impaired in the first 20 min of exercise before an improvement is observed. Therefore, repeated-sprinting protocols used by team-sport athletes may not be long enough to evoke improvements in cognitive function. Furthermore, as exercise intensity increases, arousal levels are thought to increase to the point that cognitive function is compromised (Lambourne and Tomporowski, 2010). This suggests that maximal-intensity, short-duration exercise (i.e., sprinting) may not have the same restorative ability as prolonged submaximal exercise on cognitive function in hypoxia, although the research remains unclear. Also, while it is apparent that cycling is associated with improved cognitive function, treadmill running has been demonstrated to impair cognitive function - possibly due to the greater attentional demands required to maintain a fixed running speed - compared with seated cycling (Lambourne and Tomporowski, 2010). Nevertheless, deleterious effects of treadmill exercise on cognitive function are reversed to values not different from baseline or slightly improved when measured 20 min after exercise (Lambourne and Tomporowski, 2010).
Acute exposure to a hypoxic environment will result in impaired cognitive function in young, healthy men (Seo et al., 2015). Several considerations are important to determine if exercise will restore this deleterious effect, including: i. The exercise mode, i.e., cycle ergometry versus treadmill running (Lambourne and Tomporowski, 2010); ii. The intensity of the exercise, i.e., below or above anaerobic threshold (Brisswalter et al., 1997; Chmura et al., 1994; Kamijo et al., 2004); iii. The type of cognitive tasks selected, i.e., simple versus complex (Lambourne and Tomporowski, 2010); and iv. The timing of the administration of cognitive tasks (Endo et al., 2013). The present study sought to determine if the ability to perform simple and complex cognitive tasks is compromized in individuals 20 min after performance of a single bout of RSN or RSH on a non-motorized treadmill. We predicted that blood oxygen saturation and prefrontal cortex oxygenation would be lower and RPE would be higher during a bout of RSH compared with RSN. We hypothesized that a bout of RSN would not result in any change to baseline cognitive function measured 20 min after exercise due to the inability of the short-duration, repeated treadmill sprinting protocol to enhance mental processes. However, we hypothesized that the performance of simple and complex cognitive tasks would decline from baseline 20 min after a bout of RSH.
Methods
Eleven amateur team-sport athletes (Australian football = 7, soccer = 3, touch-football = 1; age 22.8 ± 3.6 y, body mass 78.3 ± 5.9 kg, and height 1.81 ± 0.03 m) provided their written informed consent before participating in the present study. All procedures were approved by Griffith University Human Research Ethics Committee (AHS/72/14/HREC). All participants had competed for a minimum of two consecutive years in their sport at the local club level immediately prior to participating in the study, trained for a mean (± SD) of 147 ± 31 min·week-1, and competed in their sport for a mean (± SD) of 106 ± 16 min·week-1. Participants performed the RSR test comprising sixteen (four sets of four) 4-s sprints separated by 26 s (and 2 min 26 s between sets) of passive recovery in a standing position (i.e., RSR444) on two occasions in a normobaric hypoxic chamber (Synergy Physical Conditioning Systems, Yatala, Australia). The creation of the hypoxic environment was achieved via the extraction of oxygen from air which was then pumped throughout the chamber. The test was performed once under normoxic (FiO2 0.209) and once under hypoxic (FiO2 0.145) conditions. The study followed a single-blind, crossover design with 5 participants performing the RSR444 in normoxia first and then in hypoxia 7 to 10 d later at the same time of day. The remaining 6 participants performed the RSR tests in the reverse order. Additional information regarding the testing and participant requirements can be viewed elsewhere (Morrison et al., 2015).
Participants completed four standardized familiarization sessions on the treadmill (Curve 3.0, Woodway, Waukesha, Wisconsin, USA) within a 3-wk period, which were designed to: i. Allow participants to acquire the technique required to accelerate/sprint maximally; and ii. Reduce any acute lower-limb soreness during the experimental-trial period due to unaccustomed activity. Specifically, participants completed a standardized warm-up, identical to that performed during testing; i.e., 5 min jogging on the treadmill (~ 10 km/h) interspersed with three 4-s sprints (at 90-s intervals) at self-determined 80%, 90% and 95 % effort, followed by ~ 10 min of dynamic stretching of lower limb musculature. Subsequent to the warm-up participants were required to complete two, three and four sets of four 4-s sprints (with 26 s rest between sprints and 2 min 26 s rest between sets) during familiarization sessions one, two and three/four respectively. Participants completed the cognitive test battery twice on separate days to familiarize them with the procedures and minimize any learning effect. The test battery, CogState (CogState Ltd., Melbourne, VIC, Australia), was presented on a Dell Latitude E6420 14” laptop (Core i5 processor, 2.5 gHz, Dell Inc., Round Rock, Texas, USA), which was set up on a desk with a plain white wall behind it to eliminate the possibility of visual distractions. Noise-cancelling headphones were utilized to amplify audible cues and to eliminate audible distractions. The cognitive-test battery took 10 min to complete and consisted of three tests. Written instructions were presented on the screen prior to the beginning of each test, followed by an interactive demonstration. The three individual tests, in their order of presentation, were: i. The Detection Task (DET), which is a simple reaction-time test that tests psychomotor function; ii. The Identification Task (IDN), which is a choice reaction time test that tests vigilance; and iii. The One Card Learning Task (OCL) which is a test of visual learning and memory. The rationale, method of administration, performance measures and reliability of each task has been described in detail elsewhere (MacDonald and Minahan, 2016).
Upon arrival to the laboratory for the first RSR444 trial, participants were required to sit in an upright posture, using the same chair and desk and complete the cognitive test battery. After the cognitive test, participants completed a standardized warm-up on the treadmill (identical to that performed during familiarization), as well as two all-out 4-s sprints (separated by 26 s passive rest). At the completion of the warm-up, participants stood quietly on the treadmill for 7 min and were instrumented with the near-infrared spectroscopy (NIRS) probe (Oxymon MKIII, Artinis, Netherlands) to assess changes in the concentrations of total haemoglobin (Δ[THb]), oxyhaemoglobin (Δ[O2Hb]) and deoxyhaemoglobin (Δ[HHb]) in cerebral tissue. A NIRS optode pair (two transmitters and one receiver) was placed over the left prefrontal cortex region of the forehead between Fp1 and F3, international EEG 10-20 system and, if needed, further adjusted marginally (less than 5 mm) to ensure a strong signal. Optodes were fixed at a distance of 5 cm apart with a plastic spacer that was held in place with double-sided tape and a black elastic headband. A detailed explanation of NIRS during exercise as well as photographs of optode placement can be viewed elsewhere (Perrey, 2008). Data were sampled at 10 Hz and transferred from the Oxymon MKIII to a laptop computer for subsequent analysis. Photos of the position of the optode spacer allowed for accurate positioning during the subsequent testing day. Collection of prefrontal cortex oxygenation data commenced during the final 2 min of this quiet stance period (baseline) and continued throughout the RSR444. Participants were instructed to assume the starting stance and the RSR444 began on the command of the chief investigator. Participants stopped each 4-s sprint by straddling the treadmill belt. Upon completion of the test, participants rested in a seated position for 3 min in the environmental condition for that testing day, before exiting the environmental chamber and remaining under normoxic conditions thereafter. The cognitive test battery was performed again exactly 20 min after the completion of the RSR444 in normoxic conditions. The timing of performance of the post-test cognitive test battery was chosen because team-sport athletes performing successive training sessions involving RSH followed by a skills session, for example, would commonly have at least a short break in normoxia to relocate to the field/venue for the skills session, potentially change footwear and have drills explained.
NIRS data were analysed to examine changes in [THb], [O2Hb] and [HHb] relative to the average of values recorded during the final 30 s of the 2-min quiet stance collection period (baseline). A single value was obtained for each set by finding the maximum/minimum value for the set, which in every case was at the very end or immediately after the final sprint of the set, and averaging the value with ten data points either side of it. Four participant’s NIRS data were omitted due to unclear readings resulting from either i) dark skin, ii) profuse sweating, or possibly, iii) frontal bone thickness (Okada and Delpy, 2003).
Peak speed (m.s-1) and distance (m) data were obtained via Pacer Performance System software (Fitness Technology, Adelaide, Australia) as described previously (Morrison et al., 2015). Estimations of arterial oxygen saturation (SpO2) were also made in normoxia before the warm-up, immediately prior to the start of the RSR444, and immediately after sprints 4, 8, 12 and 16 in the environmental condition for that testing day using a portable pulse oximeter (Octive Tech, 300CSE, Beijing Choice Electronic Technology Co., Ltd. Beijing, China). Participants’ rating of perceived exertion (RPE, modified Borg’s 0-10 scale) scores were also obtained immediately following each set (Borg, 1982).
Statistical analyses
Statistical analyses were performed using the IBM SPSS Statistics 19 software package. A fully-factorial ANOVA with repeated measures was used to determine the interaction (INT) between, or main effect (ME) of, condition and time for performance, physiological and cognitive function variables. Least squares difference pairwise comparisons were used to detect the specific site of any significant effect identified. Where significant changes were detected from pre to post in NIRS and cognitive function variables, a Pearson’s correlation coefficient was calculated to detect any relationships. The Pearson’s correlation coefficient was calculated using data from both the normoxic and hypoxic trials. Data are expressed as means±standard deviation (SD), and significance was set at p ≤ 0.05.
Results
There was no difference in peak sprint speed (INT: F = 1.37, p = 0.27, ƞ2 = 0.12; ME of condition: F = 4.37, p = 0.06, ƞ2 = 0.30) or distance (INT: F = 0.82, p = 0.49, ƞ2 = 0.08; ME of condition: F = 2.18, p = 0.17, ƞ2 = 0.18) between RSN and RSH across the entire RSR444; however, peak sprint speed (F = 8.87, p < 0.01, ƞ2 = 0.47, Figure 1) and distance (F = 6.62, p < 0.01, ƞ2 = 0.40, Figure 2) deteriorated over the four sets (from 7.82 to 7.68 m·s-1 and 24.59 to 24.07 m;, respectively).
Figure 1.
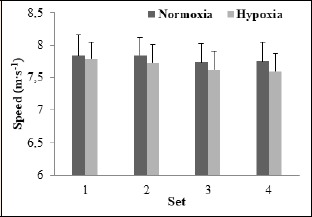
Peak speed values averaged across four, 4-s sprints for each set in normoxia and hypoxia achieved in male amateur team-sport athletes.
Figure 2.
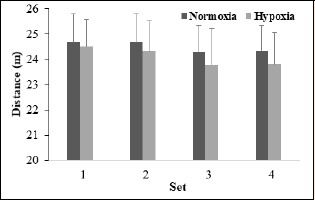
Distance values averaged across four, 4-s sprints for each set in normoxia and hypoxia achieved in male amateur team-sport athletes.
There was a significant INT between condition and set for SpO2 (F = 12.04, p < 0.01, ƞ2 = 0.41). Measures of SpO2 were not different (p = 0.44) on arrival to the laboratory between RSN and RSH but were significantly lower in RSH during quiet stance (p<0.01) and following each set (Table 1; p < 0.01) of the RSR444.
Table 1.
Arterial oxygen saturation (SpO2) and rating of perceived exertion (RPE) measured in team-sport athletes before (SpO2), during, and immediately after performing the RSR444 (4 sets of 4, 4-s sprints) in normoxic (20.9%) and hypoxic (14.5%) conditions. Data are means (±SD).
| Variable | Condition | Baseline | Pre-test | Post-set 1 | Post-set 2 | Post-set 3 | Post-set 4 |
|---|---|---|---|---|---|---|---|
| SpO2 (%) | Normoxia | 97.3 (0.9) | 97.7 (1.0) | 95.0 (3.1) | 95.0 (2.3) | 94.1 (2.4) | 94.2 (1.8) |
| Hypoxia | 98.0 (0.8) | 91.2 (1.8)* | 83.2 (2.9)* | 80.6 (3.2)* | 79.9 (3.7)* | 79.4 (4.0)* | |
| RPE | Normoxia | - | - | 4.4 (1.1) | 5.6 (1.2) | 6.9 (0.9) | 7.9 (1.1) |
| Hypoxia | - | - | 5.0 (1.2) | 6.4 (1.1) | 7.6 (1.2) | 8.4 (0.9) |
*Different from normoxia (p < 0.05). Baseline measurements were obtained in normoxic conditions before the warm up. Pre-test measurements were performed in the environmental condition, after the warm up and immediately before the commencement of the RSR444
Figure 3 displays average concentration changes for THb, O2Hb and HHb across sets 1-4. There was no ME of condition on [THb] at any time throughout the RSR444 (F = 0.01, p = 0.91, ƞ2 < 0.001); but [THb] was higher than baseline after set 3 in both conditions (p < 0.05) and continued to increase after set 4 (p < 0.05). [O2Hb] decreased to a value different from baseline after set 1 in both conditions and the value was lower in hypoxia (p = 0.01). There were no differences in [O2Hb] between conditions following sets 2 (p = 0.09), 3 (p = 0.27), or 4 (p = 0.28). [O2Hb] returned to a value no different from baseline after set 4 (p = 0.73) in normoxia, while [O2Hb] remained lower than baseline in hypoxia (p = 0.02). [HHb] displayed an INT between condition and set (F = 5.16, p = 0.04 ƞ2 = 0.67). [HHb] was higher after set 1 (p=0.03), set 2 (p=0.01), set 3 (p=0.02) and set 4 (p = 0.04) in hypoxia than normoxia. In both conditions, [HHb] was higher than baseline after set 1 (p < 0.01) and continued to increase after set 2 (p < 0.01). While [HHb] did not change after set 2 in normoxia (p > 0.05), [HHb] continued to increase after set 3 (p = 0.02) in hypoxia, but not after set 4 (p = 0.22).
Figure 3.
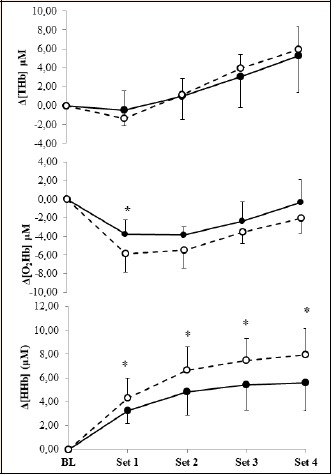
Near-infrared spectroscopy concentration changes from baseline (BL) and following each set of repeated sprints in normoxia (filled circle) and hypoxia (open circle). THb total haemoglobin, O2Hb oxyhaemoglobin, HHb deoxyhaemoglobin. Brackets indicate concentration. * = significant difference between conditions (p < 0.05).
RPE increased (p < 0.01) after each set under both normoxia and hypoxia (Table 1). While RPE was not statistically higher after set 4 in normoxia, compared with hypoxia, the ANOVA revealed a significant ME of condition (F = 4.01, p = 0.04, ƞ2 = 0.32), suggesting that perceived exertion was higher in hypoxia, compared with normoxia, for the entire RSR444 protocol.
There were no INTs (F = 0.29, p = 0.60, ƞ 2= 0.03) between condition (RSN vs. RSH) and time (pre v post), nor any ME of condition (F = 0.30, p = 0.56, ƞ2 = 0.03) observed for OCL reaction time. There was a ME of time indicating a speeding of the response time to the OCL task under both conditions (F = 10.62, p = 0.01, ƞ2 = 0.52). There was no difference in OCL accuracy between conditions (p = 0.20) measured before the RSR444 (pre), and OCL accuracy remained unchanged after the RSR444 in normoxia (p = 0.52). However, there was a decay in OCL accuracy from pre to post the RSR444 in hypoxia (p = 0.04), and the mean OCL accuracy recorded after the RSR444 was significantly lower than that achieved in normoxia (p = 0.02). We observed a significant correlation between the change in [HHb] and the change in OCL accuracy measured in seven participants during both their normoxic and hypoxic RSR444 trials (r = -0.68, p = 0.01, Figure 4). There was no difference between conditions on the DET task either pre (p = 0.12) or post (p = 0.20) performance of the RSR444. The IDN task was not different either pre (p = 0.31) or post (p = 0.14) performance of the RSR444 in hypoxia or normoxia.
Figure 4.
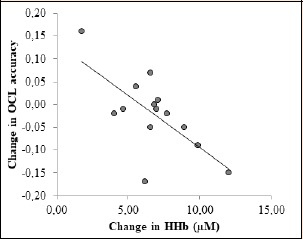
Change in [HHb] and OCL accuracy measured in seven participants during normoxic and hypoxic RSR444 trials showing a significant correlation (r = -0.68, p = 0.01). Only participants with both OCL accuracy and NIRS data were included (n = 7).
Discussion
The present study examined the effect of performing a single bout of repeated-sprint training in both normoxia (RSN) and hypoxia (RSH) on cognitive function in amateur team-sport athletes. While physical performance was maintained under hypoxic, compared with normoxic conditions, SpO2 and prefrontal cortex oxygenation were lower, and a single marker of cognitive function (visual learning and memory) was impaired following RSH only.
Performance of RSH elicits altered physiological responses (Bowtell et al., 2013; Smith and Billaut, 2010) and has been demonstrated to evoke greater training outcomes than RSN (Brocherie et al., 2015; Faiss et al., 2013; Galvin et al., 2013; Kasai et al., 2015; Puype et al., 2013) despite many previous studies reporting impaired acute performance during RSH, compared with RSN (Bowtell et al., 2013; Morrison et al., 2015; Smith and Billaut, 2010). Results from the present study are in agreement with previous research demonstrating reduced arterial oxygen saturation (Bowtell et al., 2013; Smith and Billaut, 2010) and prefrontal cortex oxygenation (Smith and Billaut, 2010) during RSH, compared with RSN. Interestingly, sprint speed and distance were maintained during RSH compared with RSN in the present study, despite lower prefrontal cortex oxygenation and arterial oxygen saturation, which is in contrast to previous findings (Smith and Billaut, 2010). This may have been due to differences in repeated-sprint protocols, mode of exercise and/or the FiO2 utilized. Regardless of the effect on performance, our results suggest that RSH may be associated with impaired cognitive function.
A recent meta-regression analysis concluded that exposure (for ≤ 6 days) to acute hypoxia has a negative effect on central executive and non-executive, i.e., perception, attention and short-term memory, tasks (McMorris et al., 2017). However, analyses of a considerable body of research examining the effects of acute bouts of exercise on cognitive function have indicated that overall there is a small positive effect (Chang et al., 2012). Importantly, the effect that exercise has on cognitive function may be dependent on the exercise intensity (Dupuy et al., 2018; Okada and Delpy, 2003) and induced changes in cerebral oxygenation (Ando et al., 2011). Previous studies have reported that augmented prefrontal cortex oxygenation during constant (Endo et al., 2013) and intermittent (Kujach et al., 2018) submaximal cycling in normoxia accompanied improved performance during a colour-word matching Stroop task. Meanwhile, the RSH employed in the present study resulted in significantly lower prefrontal cortex oxygenation and impaired performance during OCL task. It is not implausible that this observed impairment during the OCL task was due in part to reduced prefrontal cortex oxygenation. This notion is supported by the moderate correlation found between Δ[HHb] and the change in OCL accuracy; however, we cannot ascertain if the impairment in cognitive function was directly attributable to the reduced prefrontal cortex oxygenation, since [HHb] was not measured during performance of the cognitive task. Furthermore, we only observed a decrease in OCL accuracy, but no change in performance of the DET or IDN following RSH. The OCL task is a test of executive function (visual learning and memory) while the DET and IDN tasks are tests of non-executive or ‘simpler’ cognitive processes (psychomotor function and vigilance, respectively) and, therefore, activate different, and require varied levels of integration between, brain regions (Corbetta and Shulman, 2002; Driver and Frackowiak, 2001; Posner and Fan, 2008). Previous studies have highlighted that ‘higher-order’ or more difficult cognitive function tests are more easily affected by hypoxic exposure (Bartholomew et al., 1999; Bonnon et al., 1999). However, a recent meta-analysis by McMorris et al. (2017) reported no differences in the effects of hypoxia on central executive compared with non-executive cognitive tasks. Nevertheless, to our knowledge, the present study is the first to examine the combined effect of repeated-sprint exercise and hypoxia on cognitive function.
If the observed reduction in prefrontal cortex oxygenation during RSH did indeed contribute to impaired cognitive function, it could be suggested that performing maximal intensity exercise in a hypoxic environment may have been associated with a reduction in cerebral oxygenation in brain regions associated with executive function, but not in brain regions associated primarily with psychomotor function and vigilance. This is supported by research demonstrating a disparity in levels of deoxygenation in different cortices during maximal exercise in hypoxia (Subudhi et al., 2009), as well as by stress research in animals which highlights that during highly stressful situations the brain undergoes neurophysiological processes that allow it to detect high priority, dangerous stimuli whilst allowing it to ignore non-threatening stimuli (Arnsten, 2009). This may explain why psychomotor function (DET) and vigilance (IDN) were unaffected by RSH. However, these possible explanations can only be suggested as the present study measured cerebral oxygenation within the prefrontal cortex and [HHb] was not measured during performance of the cognitive tests.
It is also possible that higher perceived exertion during RSH (i.e., RPE =8.4) compared with RSN (i.e., RPE = 7.9) in the present study contributed to the impairment in OCL performance since the higher mental load associated with perception of effort may negatively affect motivation and attention (Brisswalter et al., 2002). Improvements in reaction time during performance of modified Eriksen flanker task elicited by submaximal cycling have been demonstrated to result from moderate (60% VO2 peak), but not higher intensity (80% VO2 peak) exercise, during which RPE scores were significantly increased (Ando et al., 2011). Meanwhile, impairments in cognitive function have been demonstrated during exhaustive exercise, but only during more complex cognitive tasks (Fery et al., 1997).
Regardless of the mechanisms that resulted in a decline in performance of the OCL task following RSH, it would appear to be of substantial importance for team-sport coaches to consider the timing of RSH prescription with respect to other sessions given the association between lower cognitive function and non-contact injuries in team-sport athletes (Swanik et al., 2007). In light of our results and the findings of Swanik and colleagues (2007), we recommend that amateur team-sport athletes are not prescribed RSH immediately prior to any type of training session during which they could be at risk of sustaining a non-contact injury (e.g. skills session, agility session). Limitations of the study include that cognitive function was not tested immediately after exercise and that prefrontal cortex oxygenation was not measured during cognitive function testing. Further research is required to: 1) examine prefrontal cortex oxygenation during cognitive function testing before and after the performance of RSN and RSH; 2) elucidate the time period for which cognitive function is adversely affected following the performance of RSH, beginning immediately after exercise; 3) determine if performance of a single bout of RSH by elite athletes results in the same impairment in cognitive function; and 4) determine if similar (chronic) RSH results in adaptations that alter either the cerebral oxygenation response to repeated-sprint exercise, as previously demonstrated utilising single-set RSH (Galvin et al., 2013), or the cognitive function response following RSH.
Conclusion
This study is the first to examine the effect of RSH on cognitive function. The main finding was that performance during a visual learning and memory task was impaired following RSH but not RSN. This may be partially explained by reduced prefrontal cortex oxygenation as well as greater perception of effort, and may have implications regarding the prescription of training in team sports.
Acknowledgements
This work was supported by Griffith Sports Physiology and Performance. The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The study complied with the laws of the country of the authors’ affiliation.
Biographies
Jaime MORRISON
Employment
PhD candidate at Griffith University, in the Griffith Sports Physiology and Performance research group.
Degree
MSc
Research interests
Repeated-sprinting in simulated altitude in team sport athletes.
E-mail: j.morrison@griffith.edu.au
Karlee QUINN
Employment
PhD candidate at Griffith University and a scholar at the Queensland Academy of Sport
Degree
MSc
Research interests
Female athletes, specifically the influence of the female sex hormones on physiology and performance.
E-mail: karlee.quinn@griffithuni.edu.au
Luke MACDONALD
Employment
Performance Scientist in the Prospecting for Gold Talent Transfer Program at the Queensland Academy of Sport.
Degree
PhD
Research interests
The effects of mindfulness training on cognition and physiological stress in athletes.
E-mail:luke.macdonald@griffithuni.edu.au
Francois BILLAUT
Employment
Prof., Département de kinésiologie, Université Laval, Québec, QC, Canada
Degree
PhD
Research interests
Understanding and optimising the effects of exercise, training and the hypoxic environment on physiological responses and physical work capacity for performance and health outcomes
E-mail: francois.billaut@kin.ulaval.ca
Clare MINAHAN
Employment
Prof., Griffith Sports Physiology and Performance, School of Allied Health Sciences, Griffith University
Degree
PhD
Research interests
The unique and distinct response of the female athlete to exercise and training.
E-mail: c.minahan@griffithuni.edu.au
References
- Ando S., Hatamoto Y., Sudo M., Kiyonaga A., Tanaka H., Higaki Y. (2013) The Effects of Exercise Under Hypoxia on Cognitive Function. PLoS One 8, May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S., Kokubu M., Yamada Y., Kimura M. (2011) Does cerebral oxygenation affect cognitive function during exercise? European Journal of Applied Physiology 111, 1973-1982. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T. (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience 10, 410-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew C.J., Jensen W., Petros T.V., Ferraro F.R., Fire K.M., Biberdorf D., Fraley E., Schalk J., Blumkin D. (1999) The effect of moderate levels of simulated altitude on sustained cognitive performance. International Journal of Aviation Psychology 9, 351-3599. [DOI] [PubMed] [Google Scholar]
- Bonnon M., Noel-Jorand M.C., Therme P. (1999) Criteria for psychological adaptation to high-altitude hypoxia. Perceptual and Motor Skills 89, 3-18. [DOI] [PubMed] [Google Scholar]
- Borg G.A.V. (1982) Psychophysical Bases of Perceived Exertion. Medicine and Science in Sports and Exercise 14, 377-381. [PubMed] [Google Scholar]
- Bowtell J.L., Cooke K., Turner R., Mileva K.N., Sumners D.P. (2013) Acute physiological and performance responses to repeated sprints in varying degrees of hypoxia. Journal of Science and Medicine in Sport 17, 399-403. [DOI] [PubMed] [Google Scholar]
- Brisswalter J., Arcelin R., Audiffren M., Delignieres D. (1997) Influence of physical exercise on simple reaction time: Effect of physical fitness. Perceptual and Motor Skills 85, 1019-1027. [DOI] [PubMed] [Google Scholar]
- Brisswalter J., Collardeau M., Arcelin R. (2002) Effects of acute physical exercise characteristics on cognitive performance. Sports Medicine 32, 555-566. [DOI] [PubMed] [Google Scholar]
- Brocherie F., Girard O., Faiss R., Millet G.P. (2015) High-intensity intermittent training in hypoxia: a double-blinded, placebo-controlled field study in youth football players. Journal of Strength and Conditioning Research 29, 226-37, Jan. [DOI] [PubMed] [Google Scholar]
- Chang Y.K., Labban J.D., Gapin J.I., Etnier J.L. (2012) The effects of acute exercise on cognitive performance: A meta-analysis. Brain Research 1453, 87-101. [DOI] [PubMed] [Google Scholar]
- Chmura J., Nazar K., Kaciubauscilko H. (1994) Choice-Reaction Time during Graded-Exercise in Relation to Blood Lactate and Plasma-Catecholamine Thresholds. International Journal of Sports Medicine 15, 172-176. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002) Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience 3, 201-215. [DOI] [PubMed] [Google Scholar]
- Driver J., Frackowiak R.S. (2001) Neurobiological measures of human selective attention. Neuropsychologia 39, 1257-1262. [DOI] [PubMed] [Google Scholar]
- Dupuy O., Billaut F., Raymond F., Benraiss A., Theurot D., Bosquet L., Fraser S., Tremblay J. (2018) Effect of Acute Intermittent Exercise on Cognitive Flexibility: the Role of Exercise Intensity. Journal of Cognitive Enhancement 2, 146-156. [Google Scholar]
- Endo K., Matsukawa K., Liang N., Nakatsuka C., Tsuchimochi H., Okamura H., Hamaoka T. (2013) Dynamic exercise improves cognitive function in association with increased prefrontal oxygenation. Journal of Physiological Sciences 63, 287-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiss R., Leger B., Vesin J.M., Fournier P.E., Eggel Y., Deriaz O., Millet G.P. (2013) Significant molecular and systemic adaptations after repeated sprint training in hypoxia. PLoS One 8, e56522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fery Y.A., Ferry A., VomHofe A., Rieu M. (1997) Effect of physical exhaustion on cognitive functioning. Perceptual and Motor Skills 84, 291-298. [DOI] [PubMed] [Google Scholar]
- Galvin H.M., Cooke K., Sumners D.P., Mileva K.N., Bowtell J.L. (2013) Repeated sprint training in normobaric hypoxia. British Journal of Sports Medicine 47, 74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goods P.S.R., Dawson B., Landers G.J., Gore C.J., Peeling P. (2015) No Additional Benefit of Repeat-Sprint Training in Hypoxia than in Normoxia on Sea-Level Repeat-Sprint Ability. Journal of Sports Science and Medicine 14, 681-688. [PMC free article] [PubMed] [Google Scholar]
- Kamijo K., Nishihira Y., Hatta A., Kaneda T., Wasaka T., Kida T., Kuroiwa K. (2004) Differential influences of exercise intensity on information processing in the central nervous system. European Journal of Applied Physiology 92, 305-311. [DOI] [PubMed] [Google Scholar]
- Kasai N., Mizuno S., Ishimoto S., Sakamoto E., Maruta M., Goto K. (2015) Effect of training in hypoxia on repeated sprint performance in female athletes. Springerplus 4, Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T., Sudo M., Higaki Y., Kiyonaga A., Tanaka H., Ando S. (2015) Does moderate hypoxia alter working memory and executive function during prolonged exercise? Physiology & Behavior 139, 290-296. [DOI] [PubMed] [Google Scholar]
- Kujach S., Byun K., Hyodo K., Suwabe K., Fukuie T., Laskowski R., Dan I., Soya H. (2018) A transferable high-intensity intermittent exercise improves executive performance in association with dorsolateral prefrontal activation in young adults. Neuroimage 169, 117-125. [DOI] [PubMed] [Google Scholar]
- Lambourne K., Tomporowski P. (2010) The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Research 1341, 12-24. [DOI] [PubMed] [Google Scholar]
- Leon-Carrion J., Damas-Lopez J., Martin-Rodriguez J.F., Dominguez-Roldan J.M., Murillo-Cabezas F., Martin J.M.B.Y., Dominguez-Morales M.R. (2008) The hemodynamics of cognitive control: The level of concentration of oxygenated hemoglobin in the superior prefrontal cortex varies as a function of performance in a modified Stroop task. Behavioural Brain Research 193, 248-256. [DOI] [PubMed] [Google Scholar]
- MacDonald L.A., Minahan C.L. (2016) Indices of cognitive function measured in rugby union players using a computer-based test battery. Journal of Sports Sciences 34, 1669-1674. [DOI] [PubMed] [Google Scholar]
- McMorris T., Hale B.J., Barwood M., Costello J., Corbett J. (2017) Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci Biobehav Rev 74, 225-232. [DOI] [PubMed] [Google Scholar]
- Morrison J., McLellan C., Minahan C. (2015) A Clustered Repeated-Sprint Running Protocol for Team-Sport Athletes Performed in Normobaric Hypoxia. Journal of Sports Science and Medicine 14, 857-863. [PMC free article] [PubMed] [Google Scholar]
- Okada E., Delpy D.T. (2003) Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Applied Optics 42, 2915-2922. [DOI] [PubMed] [Google Scholar]
- Perrey S. (2008) Non-invasive NIR spectroscopy of human brain function during exercise. Methods 45, 289-299. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Fan J. (2008) Attention as an organ system. Topics in Integrative Neuroscience 31-61. [Google Scholar]
- Puype J., Van Proeyen K., Raymackers J.M., Deldicque L., Hespel P. (2013) Sprint Interval Training in Hypoxia Stimulates Glycolytic Enzyme Activity. Medicine & Science in Sports & Exercise 45, 2166-214 [DOI] [PubMed] [Google Scholar]
- Seo Y., Burns K., Fennell C., Kim J.H., Gunstad J., Glickman E., McDaniel J. (2015) The Influence of Exercise on Cognitive Performance in Normobaric Hypoxia. High Altitude Medicine and Biology 16, 298-305. [DOI] [PubMed] [Google Scholar]
- Smith K.J., Billaut F. (2010) Influence of cerebral and muscle oxygenation on repeated-sprint ability. European Journal of Applied Physiology 109, 989-99. [DOI] [PubMed] [Google Scholar]
- Subudhi A.W., Miramon B.R., Granger M.E., Roach R.C. (2009) Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia. Journal of Applied Physiology 106, 1153-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanik C.B., Covassin T., Stearne D.J., Schatz P. (2007) The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. American Journal of Sports Medicine 35, 943-948. [DOI] [PubMed] [Google Scholar]


