Abstract
The objective of the study was to evaluate the alteration in biochemical composition and gender difference within exhaustive exercise in male and female rats using a metabolomics strategy. Sixty male and female rats were randomly assigned to control, exhaustive exercise and one-week recovery groups, respectively. The metabolic profiles of plasma were investigated by gas chromatograph-mass spectrometry (GC-MS) and data further underwent orthogonal partial least-squares (OPLS) analysis. The current study found that gender was a significant determinant of the effects of exhaustive exercise on the cortisol, blood urea nitrogen, creatine kinase, and the ratio of reduced glutathione to oxidized glutathione, whereas, no significant interaction effects between gender and exhaustive exercise were found on the levels of testosterone, malonaldehyde, reduced glutathione, oxidized glutathione and lactic dehydrogenase. In male rats, the altered metabolites within exhaustive exercise included increased tricarboxylic acid cycle intermediates (citric acid, fumaric acid, butanedioic acid), branch-chain amino acids (valine, leucine), fatty acids and metabolite (oleic acid, linoleic acid, 3-hydroxybutyric acid), phosphate and decreased glucose, lactic acid, serine, and glutamic acid. In female rats, the levels of fatty acids and metabolite (linoleic acid, oleic acid, arachidonic acid, 3-hydroxybutyric acid), amino acids (valine, leucine, glutamic acid, 5-oxo-proline, methionine, ornithine), other metabolites urea, myo-inositol and phosphate were increased. The results indicated that exhaustive exercise increased the rates of energy metabolism, glucose metabolism, amino acid catabolism and fatty acid metabolism in male rats, whereas, female rats showed an increased propensity to oxidize lipid and conserve carbohydrate and protein metabolism against physical stress. Disordered urea cycle and inositol metabolism also occurred in female rats with exhaustive exercise. Exhaustive exercise affected the balance of hormone adjustment and caused oxidative stress, subsequent cell membrane damage both in male and female rats. A significant gender-related difference in the metabolic profiles was also found between male and female rats within exhaustive exercise.
Key points.
Exhaustive exercise affected hormonal balance and caused oxidative stress and subsequent cell membrane damage both in male and female rats.
Female rats exhibited higher antioxidant capacity at rest, but showed similar changes to the oxidative stress markers as male rats with exhaustive exercise.
A significant gender-based difference in the metabolic profiles was found in male and female rats within exhaustive exercise.
Exhaustive exercise increased the rates of the TCA cycling, glucose metabolism, amino acid catabolism and fatty acid metabolism in male rats, whereas, female rats demonstrated an increase propensity to lipid utilization and conserve carbohydrate and proteolytic metabolism.
Key words: Exhaustive exercise, physiology, metabolomics, plasma, gender difference
Introduction
Physical exercise is one of the major modulators of systemic metabolism. It increases the rate of metabolic processes and modulates the levels of different metabolites. A persistent, high exercise volume and intensity with limited recovery periods could lead to declined performance, and subsequent accelerated fatigability (Margonis et al., 2007). Various biological markers, hormonal markers and immunological markers have been used to evaluate the physical status within exhaustive exercise (Aguiló et al., 2005; Ferreira et al., 2018; Sarıkaya et al., 2017; Zheng et al., 2018). However, these conventional methods, which involved measuring the concentrations of only a few biochemistry parameters or a few target metabolites in test samples, could not sensitively reflect all physiological differences and would be unable to explain the interaction between varieties of the metabolites (Taysi et al., 2008).
Metabolites are the final biological products of the system, which means that investigating the changes of these endogenous compounds can verify the response endpoint of biological substances (Gao et al., 2014). Metabolomics strategy is a useful analytic platform to determine endogenous metabolites and assess the global and dynamic metabolic responses of living system (Nicholson and Lindon, 2008). Recently, metabolomics has attracted increasing interest in the field of sports medicine and shown great potential for monitoring the changes of physiological state. Huang et al. (2010) used metabolomics to determine the biochemical variations in male rats’ liver with exhaustive and endurance exercises. Kume et al. (2015), using plasma metabolome analysis, identified potential biomarkers of fatigue in male rats. Miao et al. (2018) investigated the mechanism of the anti-fatigue effect of Danggui Buxue Tang on fatigue of male mice induced by forced swimming, and identified 14 metabolites providing evidence for the anti-fatigue effect. Jang et al. (2018) determined changes in urinary metabolic profiles and metabolomics markers in muscle damage following eccentric exercise in men and women, which clarify the metabolic response within eccentric exercise-induced muscle damage and gender-dependent patterns. By measurement of the endogenous metabolites, metabolomics investigations have the potential to distinguish the change of metabolic profiling and to identify the biomarkers associated with exercise performance, fatigue or exercise-induced disorders (Ra et al., 2014; Yan et al., 2009).
Although there have been several studies that examined the metabolic response to exercise, there is a lack of information on the gender-related differences in the metabolic response to exhaustive exercise. Therefore, in this study, the metabolomics study was used to distinguish the variations in metabolite profiles and determine biomarkers changed during exhaustive exercise and recovery in male and female rats.
Methods
Rat blood sampling
Sixty eight-week-old adult male and female Sprague-Dawley rats were randomly subdivided into control, exhaustive exercise and recovery groups consisting of 10 rats each. Before the commencement of the experiment, rats from exhaustive exercise and recovery group were familiarized to adaptive swimming training for 10 min/day for 3 days. Weight-loaded, forced swimming was performed according to the methodology described previously (Xu et al., 2013) with some modifications. The rats swam with a load of aluminum sheets that weighed 5% of their body weight and were attached to their tails. Rats swam individually until exhaustion once a day for 10 consecutive days; exhaustion was reached when the rat was unable to constantly keep its nose out of water and its nose remained below the water surface for 10 seconds (Ma et al., 2017). The recovery group was given a 7-day period to refresh after the exhaustive exercise period, while the rats in the control group were left in cages without swimming. The water temperature was controlled around 32-36 °C. The animals were housed at a controlled ambient temperature of 22-25 °C with 55-65% relative humidity and a 12 h light/12 h dark cycle (lights on at 7:00 AM) and were given food and water ad libitum at the Laboratory Animal Center in Shanghai University of Traditional Chinese Medicine, Shanghai, China. All animal procedures were performed in accordance with Institutional Animal Care and Use Committee guidelines of Shanghai University of Traditional Chinese Medicine. To investigate the metabolites generated in the system, several blood collections from each animal were collected immediately at day 5 and day 10 of swimming, as well as day 3 and day 7 post recovery.
Biochemical assay
Serum testosterone (T) and cortisol (CORT) were analyzed using specific assays strictly according to the manufacturer’s instructions (Nanjing Jiancheng CO., China) and measured by the microplate reader (Biotek, USA). The malondialdehyde (MDA) assay kit (TBA method) was used to detect the MDA level as a marker of lipid peroxidation and the kit was bought from Nanjing Jiancheng CO., China. Blood urea nitrogen (BUN), creatine kinase (CK) and lactic dehydrogenase (LDH) were measured using an automated biochemistry analyzer (Hitachi, Japan). Plasma reduced glutathione (GSH) and oxidized glutathione (GSSG) were evaluated as markers of oxidative stress using specific assay kits from Nanjing Jiancheng CO., China and measured by the microplate reader (Biotek, USA).
Reagents
4-Chlorophenyl-alanine, heptadecanoic acid, methoxyamine and N,O-bis(trimethylsilyl)-trifluoroacetamide (BSTFA) were purchased from Sigma-Aldrich (Sigma-Aldrich, USA). Methanol (HPLC Grade) and chloroform were obtained from Thermo Fisher (Thermo Fisher, USA). Pyridine was purchased from China National Pharmaceutical Group Corporation (China National Pharmaceutical Group Corporation, China). Pure water was produced by a Milli-Q purification system (Millipore, USA).
Sample preparation for GC-MS analysis
Plasma samples were treated with chemical derivatization following our previously published procedure (Liao et al., 2012). Each plasma sample (100 μL) was added with two internal standard solutions (10 μL of 4-chlorophenyl-alanine in water, 0.3 mg/mL; 10 μL of heptadecanoic acid in methanol, 1 mg/mL) and with 300 μL methanol/chloroform (3:1) to extract the metabolites. After vortex mixing for 30 s, the mixtures were incubated at -20 °C for 10 min and centrifuged at 12,000 rpm for 10 min. A 300 μL supernatant aliquot was transferred into a GC vial and evaporated to dryness under N2 at 30 °C. Methoxyamine (80 μL) in pyridine (15 mg/mL) was added to the dried residue and vortex mixed for 1 min. The methoximation reaction was carried out for 90 min while rocking in an air-shaker at 30 °C, followed by trimethylsilyl for 60 min by adding 80 μL BSTFA at 70 °C. At last, the solution was vortex mixed 30 s and cooled into room temperature for GC-MS analysis.
Metabolite analysis by GC-MS
Each 1 μL derivatized sample was injected onto a HP-5MS capillary column (30 m × 250 μm inner diameter, 0.25 μm film thickness, Agilent J&W Scientific, USA) on an Agilent 7890A GC/5975C MSD (Agilent J&W Scientific, USA). Helium was used as the carrier gas through the column with a constant flow rate of 1.0 mL/min. The sample was injected at 270 °C in splitless mode. The optimized GC-MS gradient temperature programming was selected following our previous experiment (Liao et al., 2012): the GC oven was started at 80 °C for 2 min, then the temperature was increased step-wise, starting at 10 °C /min to 140 °C, 4 °C/min to 210 °C, 10 °C /min to 240 °C, 25 °C/min to 290 °C and then maintained at 290 °C for 3 min. The ion source temperature and the quadrupole temperature were set at 230 °C and 150 °C, respectively. The mass data were acquired in scan (m/z 30-600) mode at a rate of 20 spectra/s with electron impact ionization (70 eV). The solvent delay time was set to 5.0 min.
Data analysis
The data from the GC-MS were converted into CDF formats, and the data were processed by the XCMS toolbox (http://metlin.scripps.edu/download/) to carry out baseline correction, peak deconvolution and alignment using XCMS’s default settings. The result (CSV file) was exported into Microsoft Excel (Microsoft Inc., USA) where normalization was performed. The resulting data were analyzed in the SIMCA-P 11.0 Software (Umetrics, Umea, Sweden) for multivariate statistical analysis. The supervised orthogonal partial least-squares (OPLS) were employed to process the acquired data and to identify the general separation and cluster. Then the differential variables were selected based on a threshold of variable importance in the projection (VIP) value (VIP>1.0) from OPLS model. Subsequently, those variables were validated at a univariate level using the nonparametric Wilcoxon-Mann-Whitney test by SPSS 18.0 (SPSS, Chicago, IL, USA) with the p-value set at 0.05 (Liao et al., 2012). The corresponding fold change showed how these selected metabolites varied between groups in male and female rats. Additionally, compounds were identified by searching in NIST 2011 database. The Kyoto Encyclopedia of Genes and Genomes (KEGG) and Human Metabolome Database (HMDB) were used to give the biochemical interpretation of changed metabolites.
The biochemical parameters data were reported as mean ± Standard Deviations (SD). Statistical analysis was performed using SPSS 18.0 (SPSS Inc. Chicago, IL, USA). A two-way ANOVA was performed to examine the main effects of gender and exhaustive exercise and interaction on the measured variables. When a significant main effect or interaction was detected, data were subsequently analyzed by use of a post-hoc Bonferroni test. The mean differences in biochemical parameters levels between genders from control, exhaustive exercise and recovery group were also examined by running tests for simple main effects. Statistical significance was set at a p < 0.05.
Results
Effects of gender and exhaustive exercise on serum biochemical parameters
A two-way ANOVA was conducted to examine the effect of the gender and exhaustive exercise on T, CORT, BUN, MDA, GSH, GSSG, CK, and LDH; and the results are shown in Figure 1. The T levels were significantly affected by gender (F = 269.28, p = 0.00) and exhaustive exercise (F = 19.61, p = 0.00). However, no significant gender × exhaustive exercise interaction (F = 2.28, p = 0.12) was found for T levels, which indicated the male and female rats exhibited similar patterns of response for T levels. Subsequent post hoc analyses revealed significantly lower values for T in exhaustive exercise group compared to control group. In addition, a significant increase of T levels was observed from exhaustive exercise to recovery in male and female rats. There was a significant interaction effect between gender and exhaustive exercise on CORT level (F=5.89, p=0.008). Analyses for simple main effects indicated that the CORT levels were greater for the male rats than the female rats in the control, exhaustive exercise and recovery groups. The post hoc analyses revealed the CORT increased from control to exhaustive exercise, and returned to basal levels at recovery for the male and female rats. There was a significant interaction effect between gender and exhaustive exercise for the BUN levels (F = 5.90, p = 0.008). Analyses for simple main effects, female rats had higher BUN levels than the male in recovery group, however, no significant difference was found between male and female rats either in control or exhaustive exercise group. The post hoc analyses showed a significant increase of BUN levels from control to exhaustive exercise in male and female rats. From exhaustive exercise to recovery, a significant decrease of BUN values was observed in male rats whereas no changes were found in female rats.
Figure 1.
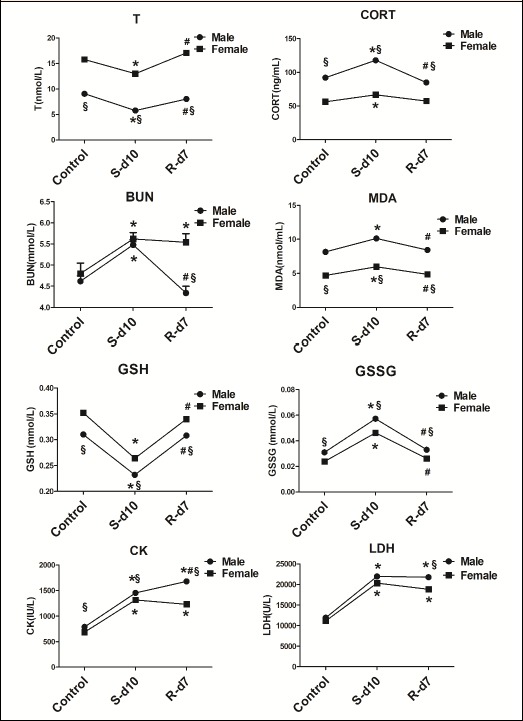
Effects of gender and exhaustive exercise on the levels of T, CORT, BUN, MDA, GSH, GSSG, CK and LDH in male and female rats. The data were analyzed by a two-way ANOVA with post-hoc Bonferroni test and statistical significance was set at a p<0.05. Values are shown as mean ± SD (n=10). * p<0.05: significant change compared with the control group in male or female rats. # p<0.05: significant change compared with the exhaustive exercise group in male or female rats. § p<0.05: significant change between male and female rats from control, exhaustive exercise and recovery group. S-d10: 10-day swimming (exhaustive exercise group); R-d7: 7 days recovery.
The MDA levels were significantly affected by gender (F = 173.47, p = 0.00) and exhaustive exercise (F = 12.94, p = 0.00), while no significant interaction effect was found between gender and exhaustive exercise for MDA levels (F = 0.58, p = 0.57). The post hoc analyses revealed that the MDA levels were significantly higher in exhaustive exercise group when compared to the control in male and female rats. Further, the MDA levels significantly decreased from exhaustive exercise to recovery group in male and female rats, and back to basal values. The values of GSH, GSSG and GSH/GSSG were significantly affected by gender (GSH: F = 87.78, p = 0.00; GSSG: F = 69.75. p = 0.00; GSH/GSSG: F = 80.44. p = 0.00) and exhaustive exercise (GSH: F = 198.66, p = 0.00; GSSG: F = 241.91, p = 0.00; GSH/GSSG: F = 150.54, p = 0.00). No significant interaction effect between gender and exhaustive exercise in GSH and GSSG were obtained (GSH: F = 0.78, p =0.47; GSSG: F = 1.80, p = 0.19), while a significant interaction (gender × exercise) was found for the GSH/GSSG (F = 5.91, p = 0.008). Subsequent post hoc analyses revealed that exhaustive exercise decreased GSH levels and increased GSSG levels in male and female rats and their ratio decreased after exercise in both groups. At recovery, the GSH and GSSG levels returned to basal values. In addition, male rats had higher levels of GSSG, as well as lower levels of GSH and GSH/GSSG than female rats in all three groups.
There was a significant interaction (gender × exercise) effect for the CK level (F = 19.87, p = 0.00). Analyses for simple main effects, male rats had higher CK levels than female rats in all three groups. The post hoc analyses revealed that the CK levels were significantly higher in exhaustive exercise and recovery group when compared to the control for the male and female rats. In addition, male rats had higher levels of CK at recovery compared to exhaustive exercise. The LDH level was significantly affected by gender (F = 6.408, p = 0.017) and exhaustive exercise (F = 76.97, p = 0.00), but no significant interaction effect between gender and exhaustive exercise (F = 0.79, p = 0.46) was found. The post hoc analyses revealed that the LDH levels increased from control to exhaustive exercise group for male and female rats. At recovery, however, LDH levels were still greater than the control. The LDH values in female rats were lower than male rats in the recovery group, but no significant differences were found in either the control or exhaustive exercise group.
Metabolomics differences between male and female rats
The data matrix was introduced into SIMCA-P 11.0 Software for multivariate statistical analysis. The OPLS model was employed to process the acquired data to distinguish the separation between groups of observation and to understand which variables contribute to the class-separating. Figure 2A illustrated the score plots of the male and female rats from control, exhaustive-exercise (10-day swimming) and recovery groups (7-day recovery). The X-axis (t1) and Y-axis (t2) indicated the first principle component and second principle component. One point represents one observation sample and the closer the points, the greater the similarity of the samples’ metabolites composition; vice versa, the greater the distance between points, the greater difference in composition. The four principal-component model explained 90.2% R2Y and predicted 82.8% Q2Y of the data according to the cross-validation. This model also explained 65.8% of the variables (R2X).
Figure 2.
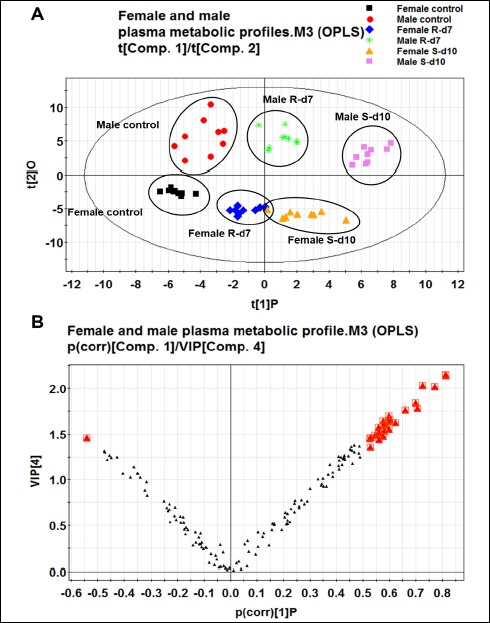
The score plot of OPLS analysis to compare the metabolome of male and female rats from control, exhaustive exercise and recovery groups (n=10 per group).
A: The score plot showed distinct clustering of metabolites with gender and exhaustive exercise periods. Metabolic patterns were grouped by different colored points. One point represents one sample. B: The loading plot was created to display the specific metabolites positively correlated with score plot. Each point presents one variable (metabolite). The bigger red triangle ( ) indicates statistically significant difference in variables that differentiated these groups. S-d10: 10-day swimming (exhaustive exercise group); R-d7: 7-day recovery.
) indicates statistically significant difference in variables that differentiated these groups. S-d10: 10-day swimming (exhaustive exercise group); R-d7: 7-day recovery.
According to the metabolic patterns and the value of R2Y and Q2Y, a distinct cluster of metabolites in male and female rats undergoing control group, exhaustive exercise group and one-week recovery group was found. The observation samples for the control, exhaustive exercise and one-week recovery group from male rats are distributed in the upper part of the Figure 2A and those for female rats are in the lower part, indicating that the model can differentiate between male and female rats at control, exhaustive exercise and one-week recovery stages. Additionally, both in male and female rats, the samples from the exhaustive exercise group are clearly separated from the control group, while those for recovery group are closer to the control group.
To further evaluate the endogenous variations between the male and female rats, the loading plot (Figure 2B) was employed to display the specific metabolites that differentiated the male and female within the control, exhaustive exercise and recovery groups. Each point represents one variable. The differentially expressed metabolites between male and female rats in those three groups were distinguished based on the VIP values (VIP>1.0). Then, univariate statistical analysis, Wilcoxon-Mann-Whitney test (non-normally distributed data), was performed on those variations to evaluate their significance. Male control rats exhibited notably lower levels of citric acid, 3-hydroxybutyric acid and oleic acid as compared with female control rats. Within exhaustive exercise, male rats had higher levels of citric acid, valine, leucine, linoleic acid, oleic acid, 3-hydroxybutyric acid and phosphate than those in female rats. Compared with female recovery group, male rats exhibited significant elevation of citric acid, valine, leucine, oleic acid, 3-hydroxybutyric acid and phosphate. The results are presented in Table 1.
Table 1.
Altered plasma metabolites between male and female rats in the control, exhaustive exercise and recovery groups (n=10).
| Compounds | RT(min) | VIP | MC/FC | MS-d10/FS-d10 | MR-d7/FR-d7 | ||||
|---|---|---|---|---|---|---|---|---|---|
| FC | p | FC | p | FC | p | ||||
| Amino acid | Valine | 7.68 | 1.57 | 2.80 | <0.001 | 2.80 | <0.001 | ||
| Leucine | 8.48 | 1.63 | 2.80 | <0.001 | 2.80 | <0.001 | |||
| Fatty acid | Linoleic acid | 27.65 | 1.62 | 2.35 | 0.002 | ||||
| Oleic acid | 27.73 | 1.84 | 0.46 | 0.005 | 2.29 | 0.003 | 2.11 | 0.007 | |
| Organic aid | 3-Hydroxybutyric acid | 6.88 | 2.14 | 0.45 | 0.004 | 2.80 | <0.001 | 2.80 | <0.001 |
| Citric acid | 19.91 | 1.78 | 0.37 | <0.001 | 2.80 | <0.001 | 2.64 | <0.001 | |
| Other metabolites | Phosphate | 8.65 | 1.61 | 2.80 | <0.001 | 2.80 | <0.001 | ||
RT: The retention time of each compound from GC-MS; VIP: Variable importance in the project; FC: Fold change. Mean ranks were calculated using the nonparametric Wilcoxon-Mann-Whitey test by SPSS 18.0, and FC was obtained by comparing those metabolites’ mean ranks between male and female rats in control, exhaustive exercise and recovery group. FC with a value >1 indicates a relatively higher level present in male rats as compared to the female rats, while a value <1 means a relatively lower level in male rats as compared to the female rats. S-d10: 10 days swimming group (exhaustive exercise group); R-d7: 7 days recovery group; p: Correlation coefficient from Mann-Whitney test. p<0.05 means the change is significant.
Metabolic profiles of male and females induced by exhaustive exercise
The OPLS model was established to analyze metabolic profiles of male and female rats induced by exhaustive exercise. Figure 3 (A, B) illustrates metabolomics movements of the control, 5-day swimming, 10-day swimming, 3-day recovery and 7-day recovery samples from male and female rats, respectively (Male: R2X = 0.472, R2Y = 0.932, Q2Y = 0.811; female: R2X = 0.324, R2Y = 0.820, Q2Y = 0.727). The observation samples for the control and 7-day recovery are located on the left of the Figure 3, whereas those for the 5-day swimming and 10-day swimming are on the right; this plot shows that the metabolic profile of the 10-day swimming (exhaustive exercise group) is the furthest one from the control. As the number of post exercise resting days increased, the metabolic profiles moved closer to the control group. Since clear movement of data points along with exhaustive exercise and recovery periods has been shown, it indicated that exhaustive exercise had significant impact on plasma metabolome both in male and female rats.
Figure 3.
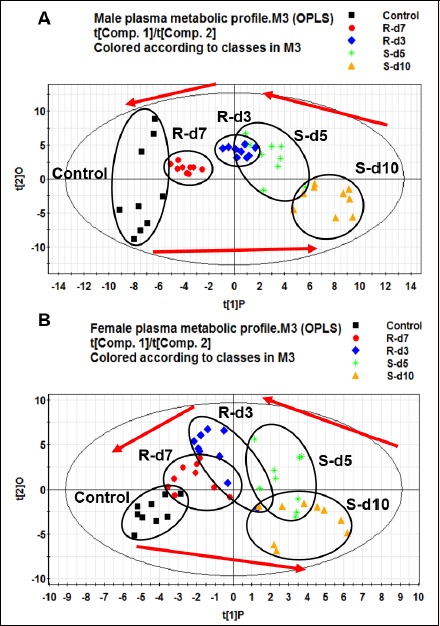
OPLS score plots of male rats (A) and female rats (B) over the whole exercise program (n=10). Both for the male and female rats, clear movement of data points along with exhaustive exercise period and recovery period was shown, indicating that exhaustive exercise had significant impact on plasma metabolome, and that one-week recovery could attenuate the exhaustive exercise induced metabolic perturbation in rats. Metabolic patterns were grouped by different colored points, one point represents one sample. ■/Control, control group;  /S-d5, 5 days swimming;
/S-d5, 5 days swimming;  /S-d10, 10-day swimming;
/S-d10, 10-day swimming;  /R-d3, 3 days recovery;
/R-d3, 3 days recovery;  /R-d7, 7-day recovery.
/R-d7, 7-day recovery.
Endogenous variations contributing to the classification were determined in loading plots. In male rats, a total of 13 variations were selected over the exercise periods (Table 2). Exhaustive exercise decreased the levels of lactic acid, glucose, serine, glutamic acid, and increased the levels of critic acid, fumaric acid, butanedioic acid, valine, leucine, oleic acid, linoleic acid, 3-hydroxybutyric acid, and phosphate in male rats. Among the variations, 7 metabolites in the recovery group still showed significant differences compared with the control, indicating that not all the metabolites were normalized within the one-week recovery period.
Table 2.
Altered plasma metabolites in the control, exhaustive exercise and recovery groups for male and female rats (n=10).
| Compounds | RT (min) |
VIP | S-d10/Control | R-d7/Control | R-d7/S-d10 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FC (Male) |
p | FC (Female) |
p | FC (Male) |
p | FC (Female) |
p | FC (Male) |
p | FC (Female) |
p | |||
| Amino acid | ||||||||||||||
| Valine | 7.68 | 1.43 | 1.88 | 0.016 | 2.62 | <0.001 | 2.00 | 0.008 | 0.35 | <0.001 | 0.52 | 0.013 | ||
| Serine | 8.25 | 1.29 | 0.51 | 0.010 | 0.58 | 0.034 | 1.73 | 0.035 | ||||||
| Leucine | 8.48 | 1.67 | 2.09 | 0.005 | 2.82 | <0.001 | 2.75 | <0.001 | 0.35 | <0.001 | 0.57 | 0.028 | ||
| Methionine | 12.88 | 1.45 | 2.75 | <0.001 | 1.69 | 0.041 | 0.58 | 0.034 | ||||||
| 5-oxo-proline | 12.93 | 1.71 | 2.82 | <0.001 | 2.82 | <0.001 | 0.58 | 0.034 | ||||||
| Glutamic acid | 15.05 | 1.25 | 0.41 | <0.001 | 2.56 | <0.001 | 0.48 | 0.005 | 2.62 | <0.001 | ||||
| Ornithine | 19.65 | 1.34 | 2.82 | <0.001 | 2.44 | <0.001 | ||||||||
| Organic acid | ||||||||||||||
| Lactic acid | 5.56 | 1.68 | 0.35 | <0.001 | 0.35 | <0.001 | ||||||||
| 3-Hydroxybutyric acid | 6.88 | 1.96 | 2.82 | <0.001 | 2.62 | <0.001 | 2.82 | <0.001 | 0.45 | 0.002 | 0.41 | <0.001 | 0.36 | <0.001 |
| Fumaric acid | 9.55 | 1.16 | 2.18 | 0.003 | 2.56 | <0.001 | ||||||||
| Butanedioic acid | 12.37 | 1.26 | 2.50 | <0.001 | 0.47 | 0.003 | ||||||||
| Citric acid | 19.91 | 1.81 | 2.82 | <0.001 | 2.56 | <0.001 | 0.52 | 0.011 | ||||||
| Fatty acid | ||||||||||||||
| Linoleic acid | 27.65 | 1.39 | 2.23 | 0.002 | 2.23 | 0.002 | 0.56 | 0.023 | 0.40 | <0.001 | ||||
| Oleic acid | 27.73 | 1.47 | 2.62 | <0.001 | 2.23 | 0.002 | 2.23 | 0.002 | 0.44 | 0.002 | ||||
| Arachidonic acid | 29.54 | 1.08 | 1.84 | 0.019 | 0.50 | 0.008 | ||||||||
| Carbohydrate | ||||||||||||||
| D-(+)-Glucose | 22.12 | 1.11 | 0.52 | 0.013 | ||||||||||
| Other metabolites | ||||||||||||||
| Urea | 8.16 | 1.40 | 2.39 | 0.001 | ||||||||||
| Phosphate | 8.65 | 1.18 | 2.28 | 0.002 | 2.00 | 0.008 | 2.04 | <0.001 | 0.35 | |||||
| Myo-inostiol | 26.17 | 1.31 | 1.80 | 0.023 | 2.18 | 0.003 | ||||||||
RT: The retention time of each compound from GC-MS; VIP: Variable importance in the project; FC: Fold Change. Mean ranks were calculated using the nonparametric Wilcoxon-Mann-Whitey test by SPSS 18.0, and FC was obtained by comparing those metabolites’ mean ranks among the three groups (exhaustive exercise group vs control group, recovery group vs exhaustive exercise group, and recovery group vs control group) in male and female rats. FC with a value >1 indicates a relatively higher level present in exhaustive exercise or recovery group as compared to the control group, while a value <1 means a relatively lower level in exhaustive exercise or recovery group as compared to the control group. S-d10: 10 days swimming group (exhaustive exercise group); R-d7: 7 days recovery group; p: Correlation coefficient from Mann-Whitney test. p<0.05 means the change is significant.
In female rats, 13 variations were identified (Table 2). Exhaustive exercise significantly increased the levels of valine, leucine, methionine, 5-oxo-proline, glutamic acid, ornithine, linoleic acid, oleic acid, arachidonic acid, 3-hydroxybutyric acid, as well as urea, phosphate, and myo-inositol. Within the one-week recovery, the levels of the amino acids valine, leucine, methionine, 5-oxo-proline, glutamic acid and ornithine were still higher in comparison to the control group. The fatty acids linoleic acid, oleic acid and arachidonic acid diminished within the one-week recovery period and showed no significant differences compared with the control.
Discussion
The current study demonstrated significant gender-related differences in biochemical parameters and metabolic responses to exhaustive exercise. It was found that the gender was a significant determinant of the effects of exhaustive exercise on the CORT, BUN, CK, and the ratio of GSH to GSSG. The levels of T, LDH, MDA, GSH and GSSG were affected by gender and exhaustive exercise, however, no significant interaction effects were found. Exhaustive exercise increased the rates of energy metabolism, glucose metabolism, amino acid catabolism and fatty acid metabolism in male rats, whereas, female rats showed an increased propensity to oxidize lipid and conserve carbohydrate and protein against physical exercise.
Biochemical parameters
It has been proven that endogenous hormones are essential for physiological adaptions during exercise and influence the recovery phase by modulating anabolic and catabolic processes (Urhausen et al., 1995). In the current study, serum CORT, which was considered as a catabolic hormone (Elloumi et al., 2003), was significantly increased both in male and female rats within exhaustive exercise period, whereas the levels of T (which was considered as anabolic hormone) (Elloumi et al., 2003) decreased in male and female rats, indicating that exhaustive exercise affected the balance of hormone adjustment (Lac and Maso, 2004). Within the recovery period, the CORT and T levels returned to base values in both male and female rats. The basal levels of urea derived from nitrogen metabolism are reported to be amplified by intensive exercise (Haralambie and Berg, 1976). In the current study, the BUN levels in male and female rats were significantly increased by exhaustive exercise, suggesting an increased breakdown of nitrogen-containing compounds, in line with a reported rise in blood serum urea after heavy exercise (Haralambie and Berg, 1976). Within the recovery period, the BUN levels returned to base values in male rats, whereas no significant change was found in female rats compared to exhaustive exercise group, which was considered as to be an indicator of insufficient regeneration with recovery period.
Regarding the fact that GSH/GSSG in blood plasma stand for clinical measure of oxidative stress (Johns, 2006), reduction in plasma GSH and GSH/GSSG ration causes a shift in redox balance towards a more oxidizing environment. In other words, significant increase in GSSG and further decrease in the GSH/GSSG immediately after exhaustive exercise probably indicate elevation in free-radical production (Aguiló et al., 2005). In the current study, as compared with the control group, significant increases in GSSG level, as well as significant decreases in GSH level and GSH/GSSG were found within exhaustive exercise in plasma from male and female rats, reflecting exercise-induced oxidative stress. MDA is a byproduct of oxidation of polyunsaturated fatty acid which has been used as a marker of lipid peroxidation (Powers et al., 2016; Vollaard et al., 2005). In the present study, MDA level was increased within exhaustive exercise and gender showed similar alteration, which also imply that exercise-induced oxidative stress occurred both in male and female rats. Our results are consistent with the research by Goldfarb et al. (2007). In addition, female rats exhibited higher levels of GSH and GSH/GSSG than male rats in all three groups. Similarly, some studies have found higher levels of antioxidant molecules or lower levels of oxidative molecules in the blood in females than in males (Balog et al. 2006, Goldfarb et al. 2007). Another experimental study in rats suggested that the mitochondria from females exhibited higher antioxidant capacity than in males at rest (Borrás et al., 2003). Our data also revealed that female rats had lower levels of MDA than male rats in all three groups. Several studies have reported that the MDA levels in women are lower than in men at rest (Bloomer et al., 2008). These differences between males and females have been attributed to estrogen action (Borrás et al., 2003). Taken together, the present study does support the findings of Goldfarb et al. (2007) that females are slightly better protected from oxidative stress than males. However, male and female rats showed similar changes to the oxidative stress markers undergoing the exhaustive exercise.
For the most part, increased levels of tissue enzymes in serum have been treated as indirect indicators of increased cell permeability caused by tissue membrane damage (Kanter et al., 1988). In the current study, post-exercise CK and LDH values were significantly greater than the control both in male and female rats, suggesting that exhaustive exercise might cause cell membrane damage. Additionally, persistently elevated CK and LDH levels within recovery period in male and female rats were suggestive of over-exercise. Besides these effects, male rats had higher CK and LDH levels at recovery than female rats, which is consistent with the intriguing work of Sewright et al. (2008), who have reported that male rats showed a large CK response 7 d after exercise than female rats.
Plasma metabolomics
Exhaustive exercise significantly increased the tricarboxylic acid cycle (TCA) intermediates levels including citric acid, fumaric acid and butanedioic acid in male rats. However, no changes in TCA intermediates levels were found in female rats. It is generally accepted that energy expenditure will be elevated with increasing exercise volume and intensity, and the TCA cycle will be activated accordingly to oxidize and produce more ATP for the body (Peake et al., 2014), with a concomitant, marked increase in plasma TCA intermediates (Gibala et al., 1988). Additionally, it has been known that carbohydrate oxidation is the primary ATP-producing system during exercise and subsequent to maintain high levels of TCA intermediates (Bowtell et al., 2007). However, since there were no significant changes of TCA intermediates in female rats, females might rely less on carbohydrate utilization and TCA cycling during exercise. In addition, although male rats had lower levels of citric acid than females in the control group, male levels became higher than in females within exhaustive exercise, which also suggested that males might rely more on the TCA cycle to provide energy for the body. During exhaustive exercise, glucose is an important energy source supplying most part of the oxidative energy production (Hargreaves, 2015; Jensen and Richter, 2012). In the current study, the level of glucose was decreased in male rats undergoing exhaustive exercise, which suggested greatly increased energy expenditure. Moreover, the decrease in lactate content indicated a reduction in the whole body rate of glycosis, which was in agreement with previous report in which lowered lactate levels were found in mice doing the wheel running exercise (Monleon et al., 2014).
Generally, the increased levels of free fatty acids and ketone bodies suggest that lipolysis is involved (Romijn et al., 1993; Turcotte et al., 1992). Free fatty acids represent a major energy source supporting whole body energy flux in men and women (Friedlander et al., 1998). 3-Hydroxybutyrate is an important byproduct produced by oxidation of fatty acides in the liver and kidney (Liao et al., 2012). Current results reveal that the levels of fatty acids including oleic acid, linoleic acid and metabolite 3-hydroxybutyric acid were increased in male and female rats within exhaustive exercise, indicating enhanced lipolysis. In addition, these data show that male rats had lower levels of 3-hydroxybutyric acid and oleic acid than females in control group, but those two variations together with linoleic acid were significantly elevated and became higher than in female rats within exhaustive exercise, which suggest that female rats might oxidize more fatty acids for energy consumption during exhaustive exercise. Results of another study (Henderson et al., 2007) also suggest that fatty acids comprise a greater proportion of energy source in women than in men for a given relative work intensity. In our study, no significant changes of carbohydrate and TCA intermediates were found in female rats within exhaustive exercise, indicating that females might have an increased propensity to oxidize lipid and conserve carbohydrate against physical stress. It has been reported that the relative abundance of ovarian hormones in women might alter the regualtion of metabolic pathways to favor fat oxidation and conserve carbohydrate (D’Eon and Braun, 2002; Ruby and Robergs, 1994). Other studies indicated that elevated estrogen and progesterone in women altered sensitivity to catecholamines and enhanced lipolysis and lipid utilization, consequently, carbohydrate utilization decreased (Braun and Horton, 2001; Horton et al., 1998). During the recovery period, the same trends were found in declining levels of those fatty acids for male and female rats.
Valine and leucine, the branched-chain amino acids (BCAAS) participating in blood glucose regulation, were found to be signicantly increased in male and female rats within exhaustive exercise. It has been recognized that skeletal muscle is a major site of BCAAs utilization (She et al., 2010). As a result of the reduced availability of the muscular glycogen during exercise, there were higher blood levels of BCAAs on account of increased proteolysis in skeletal muscle (Kume et al., 2015). The other amino acids serine and glutamic acid were significantly decreased in male rats, which are in agreement with the results of Dohm et al. (1981). They found significantly decreased levels of alaline, glutamate, and glutamine in plasma following exercise. In constrast, plasma concentrations of the BCAAs were generally elevated by exercise. These data were, thus, quite similar to those of Kasperek (1989). The results suggest an increased metabolic use of these amino aicds with exercise. Our data also show that male rats have higher valine and leucine levels than female rats in the exhaustive exercise group, whereas no significant differences are found between male control rats and female control rats for the levels of valine and leucine, which suggests that male rats might use more proteolysis to supply energy. Tarnopolsky et al. (1990) found that the lower insulin and higher epinephrine levels in males might partially explain the greater glycogenolysis and protein catabolism observed in the exercise group.
As mentioned before, plasma GSH couple GSH/GSSG is a critically important redox biomarkers (Seifi-Skishahr et al. 2016). Our data suggest that exhaustive exercise-induced oxidative stress were observed in both male and female rats. GSH, a tripeptide composed of glutamate, cysteine and glycine, is a major non-enzymatic endogenous antioxidant (Ji, 2002). In the present study, elevated level of glutamate was detected in female rats within exhaustive exercise. It has been known that the cystine/glutamate antiporter is a plasma membrane-bound protein critically involved in glutamatergic transmission. Upon oxidative stress, it starts to pump out glutamate in exchange for cysteine, which is essential for glutathione synthesis (Lu, 2013). The increase in the influx of cysteine during increased glutathione synthesis results in increased export of glutamate to extracellular compartment and to the circulation, which ultimately results in the increase of extracellular glutamate levels (Tapiero et al., 2002; Anderson, 1998). Additionally, the levels of 5-oxoproline, methionine and arachidonic acid were also significantly elevated in female rats within exhaustive exercise, suggesting that exhaustive exercise induced diverse responses to oxidative stress in female rats. In our study, exhaustive exercise also increased the levels of urea and ornithine in female rats, which suggested that the urea cycle might be elevated in activity within exercise. Myo-inositol is the primary biologically active form of inostiol which could improve glucose tolerance by insulin sensitivity (Croze et al., 2013). The increased levels of myo-inositol in female rats indicated the disorder of inostiol metabolim caused by exhaustive exercise.
Actually, selected body fluid is important in detection of oxidative stress. GSH is found in all extracellular biological fluids and here we chose plasma as an extracellular fluid circulating between body cells and exchanging oxidative biomarkers. However, according to the study from Seifi-Skishahr et al. (2016), they found that the changes in erythrocyte GSH/GSSG are not parallel with plasma GSH/GSSG, which suggest that plasma redox biomarker might not accurately reflect tissue redox status compared with erythrocyte biomarkers. One of limitations of the current study is lack of data on erythrocyte oxidative stress biomarkers after exhaustive exercise which is suggested for future studies.
Conclusion
It is concluded that exhaustive exercise affects hormone balance and causes oxidative stress, and subsequent cell membrane damage both in male and female rats. Females are slightly better protected from oxidative stress than males. However, undergoing exhaustive exercise, male and female rats showed similar changes to the oxidative stress markers. Significant gender-related differences in metabolic profiles in male and female rats within exhaustive exercise occur, with increased rates of TCA cycling, glucose metabolism, amino acid catabolism and fatty acid metabolism in male rats, whereas, female rats might have an increased propensity to oxidize lipid and conserve carbohydrate and protein metabolism against physical stress. Disordered urea cycle and inositol metabolism also occur in females with exhaustive exercise. The one-week recovery period attenuated many, but not all, of the metabolic perturbation by exhaustive exercise in rats.
Acknowledgements
Wenbin Zhou and Guigang Zeng equally contributed to this paper. Hai Wei and Shen Zhang are co-corresponding authors. This study would not have been possible without our participants. This work was supported by the Shanghai Administration of Sports under Grant 16Z006. The authors declare that they have no conflict of interest. All experiments comply with the current laws of the country.
Biographies
Wenbin ZHOU
Employment
Institute of Interdisciplinary Integrative Biomedical Research, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Degree
Ph.D
Research interest
Enduring and exhaustive exercise, metabolomics, pharmacokinetics
E-mail: zhouwenbin41@163.com
Guigang ZENG
Employment
Department of Rehabilitation, Changzheng Hospital, The Second Military Medical University, Shanghai 200003, China.
Degree
Ph.D
Research interest
Enduring and exhaustive exercise, volleyball exercise, rehabilitation medicine
E-mail: newbees@smmu.edu.cn
Chunming LYU
Employment
Shanghai Zhulian Intelligent Technology CO., LTD, Shanghai 201323, China.
Degree
Ph.D
Research interest
Pharmacokinetics
E-mail: chunming83g@126.com
Fang KOU
Employment
Institute of Interdisciplinary Integrative Biomedical Research, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Degree
MSc
Research interest
Biopharmaceutics, pharmacokinetics
E-mail: samantha_kou@hotmail.com
Shen ZHANG
Employment
Prof., Department of Rehabilitation, Changzheng Hospital, The Second Military Medical University, Shanghai 200003, China.
Degree
Ph.D
Research interest
Enduring and exhaustive exercise, rehabilitation medicine
E-mail: johnsonzs33@smmu.edu.cn
Hai WEI
Employment
Prof., Institute of Interdisciplinary Integrative Biomedical Research, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Degree
Ph.D
Research interest
Biopharmaceutics, enduring and exhaustive exercise, metabolomics, pharmacokinetics
E-mail: wei_hai@hotmail.com
References
- Aguiló A., Tauler P., Fuentespina E., Tur J.A., Córdova A., Pons A. (2005) Antioxidant response to oxidative stress induced by exhaustive exercise. Physiology & Behavior 84, 1-7. [DOI] [PubMed] [Google Scholar]
- Anderson M.E. (1998) Glutathione: an overview of biosynthesis and modulation. Chemico-Biological Interactions 111-112, 1-14. [DOI] [PubMed] [Google Scholar]
- Balog T., Sobocanec S., Sverko V., Krolo I., Rocić B., Marotti M., Marotti T. (2006) The influence of season on oxidant-antioxidant status in trained and sedentary subjects. Life Sciences 78, 1441-1447. [DOI] [PubMed] [Google Scholar]
- Bloomer R.J., Fisher-Wellman K.H. (2008) Blood oxidative stress biomarkers: influence of sex, exercise training status, and dietary intake. Gender Medicine 5, 218-228. [DOI] [PubMed] [Google Scholar]
- Borrás C., Sastre J., García-Sala D., Lloret A., Pallardó F.V., Viña J. (2003) Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radical Biology and Medicine 34, 546-552. [DOI] [PubMed] [Google Scholar]
- Bowtell J.L., Marwood S., Bruce M., Constantin-Teodosiu D., Greenhaff P.L., (2007) Tricarboxylic acid cycle intermediate pool size: functional importance for oxidative metabolism in exercising human skeletal muscle. Sports Medicine 37, 1071-1088. [DOI] [PubMed] [Google Scholar]
- Braun B., Horton T. (2001) Endocrine regulation of exercise substrate utilization in women compared to men. Exercise and Sport Sciences Reviews 29, 149-154. [DOI] [PubMed] [Google Scholar]
- Croze M.L., Vella R.E., Pillon N.J., Soula H.A., Hadji L., Guichardant M., Soulage C. O. (2013) Chronic treatment with myo-inositol reduces white adipose tissue accretion and improves insulin sensitivity in female mice. Journal of Nutritional Biochemistry 24, 457-466. [DOI] [PubMed] [Google Scholar]
- D’Eon T., Braun B. (2002) The roles of estrogen and progesterone in regulating carbohydrate and fat utilization at rest and during exercise. Journal of Women’s Health & Gender-Based Medicine 11, 225-237. [DOI] [PubMed] [Google Scholar]
- Dohm G.L., Beecher G.R., Warren R.Q., Williams R.T. (1981) In fluence of exercise on free amino acid concentrations in rat tissues. Journal of applied physiology: respiratory, environmental and exercise physiology 50, 41-44. [DOI] [PubMed] [Google Scholar]
- Elloumi M., Maso F., Michaux O., Robert A., Lac G. (2003) Behaviour of saliva cortisol [C], testosterone [T] and the T/C ratio during a rugby match and during the post-competition recovery days. European Journal of Applied Physiology 90, 23-28. [DOI] [PubMed] [Google Scholar]
- Ferreira G.A., Felippe L.C., Silva R.L.S., Bertuzzi R., De Oliveira F.R., Pires F.O., Lima-Silva A.E. (2018) Effect of pre-exercise carbohydrate availability on fat oxidation and energy expenditure after a high-intensity exercise. Brazilian Journal of Medical and Biological Research 51, e6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander A.L., Casazza G.A., Horning M.A., Buddinger T.F., Brooks G.A. (1998) Effects of exercise intensity and training on lipid metabolism in young women. American Journal of Physiology 275, E853-E863. [DOI] [PubMed] [Google Scholar]
- Gao X., Guo B., Yang L., Liu J., Zhang X., Qin X., Du G. (2014) Selection and dynamic metabolic response of rat biomarkers by metabonomics and multivariate statistical analysis combined with GC–MS. Pharmacology Biochemistry and Behavior 117, 85-91. [DOI] [PubMed] [Google Scholar]
- Gibala M.J., MacLean D.A., Graham T.E., Saltin B. (1988) Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. American Journal of Physiology 275, E235-E242. [DOI] [PubMed] [Google Scholar]
- Goldfarb A.H., McKenzie M.J., Bloomer R.J. (2007) Gender comparisons of exercise-induced oxidative stress: influence of antioxidant supplementation. Applied Physiology, Nutrition, and Metabolism 32, 1124-1131. [DOI] [PubMed] [Google Scholar]
- Haralambie G., Berg A. (1976) Serum urea and amino nitrogen changes with exercise duration. European Journal of Applied Physiology and Occupational Physiology 36, 39-48. [DOI] [PubMed] [Google Scholar]
- Hargreaves M. (2015) Exercise, muscle, and CHO metabolism. Scandinavian Journal of Medicine & Science in Sports 25, 29-33. [DOI] [PubMed] [Google Scholar]
- Henderson G.C., Fattor J.A., Horning M.A., Faghihnia N., Johnson M.L., Mau T.L., Luke-Zeitoun M., Brooks G.A. (2007) Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. The Journal of Physiology 584, 963-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton T.J., Pagliassotti M.J., Hobbs K., Hill J.O. (1998) Fuel metabolism in men and women during and after long-duration exercise. Journal of Applied Physiology 85, 1823-1832. [DOI] [PubMed] [Google Scholar]
- Huang C.C., Lin W.T., Hsu F.L., Tsai P.W., Hou C.C. (2010) Metabolomics investigation of exercise-modulated changes in metabolism in rat liver after exhaustive and endurance exercises. European Journal of Applied Physiology 108, 557-566. [DOI] [PubMed] [Google Scholar]
- Jang H.J., Lee J.D., Jeon H.S., Kim A.R., Kim S., Lee H.S., Kim K.B., (2018) Metabolic Profiling of Eccentric Exercise-Induced Muscle Damage in Human Urine. Toxicological Research 34, 199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T.E., Richter E.A. (2012) Regulation of glucose and glycogen metabolism during and after exercise. The Journal of Physiology 590, 1069-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L.L. (2002) Exercise-induced modulation of antioxidant defense. Annals of the New York Academy of Sciences 959, 82-92. [DOI] [PubMed] [Google Scholar]
- Johns D.P. (2006) Redefining oxidative stress. Antioxidants & redox signaling 8, 1865-1879. [DOI] [PubMed] [Google Scholar]
- Kanter M.M., Lesmes G.R., Kaminsky L.A., La Ham-Saeger J., Nequin N.D. (1988) Serum creatine kinase and lactate dehydrogenase changes following an eighty kilometer race. Relationship to lipid peroxidation. European Journal of Applied Physiology and Occupational Physiology 57, 60-63. [DOI] [PubMed] [Google Scholar]
- Kasperek G.J. (1989) Regulation of branched-chain 2-oxo acid dehydrogenase activity during exercise. The American journal of physiology 256, 186-190. [DOI] [PubMed] [Google Scholar]
- Kume S., Yamato M., Tamura Y., Jin G., Nakano M., Miyashige Y., Eguchi A., Ogata Y., Goda N., Iwai K., Yamano E., Watanabe Y., Soga T., Kataoka Y. (2015) Potential biomarkers of fatigue identified by plasma metabolome analysis in rats. PloS One 10, e0120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lac G., Maso F. (2004) Biological markers for the follow-up of athletes throughout the training season. Pathologie-Biologie 52, 43-49. [DOI] [PubMed] [Google Scholar]
- Liao W., Wei H., Wang X., Qiu Y., Gou X., Zhang X., Zhou M., Wu J., Wu T., Kou F., Zhang Y., Bian Z., Xie G., Jia W. (2012) Metabonomic variations associated with AOM-induced precancerous colorectal lesions and resveratrol treatment. Journal of Proteome Research 11, 3436-3448. [DOI] [PubMed] [Google Scholar]
- Lu S.C. (2013) Glutathione synthesis. Biochimica et Biophysica Acta 1830, 3143-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Kong L., Qi S., Wang D. (2017) Exhaustive exercise decreases L-type calcium current by activating endoplasmic reticulum stress. The Journal of Sports Medicine and Physical Fitness 57, 483-489. [DOI] [PubMed] [Google Scholar]
- Margonis K., Fatouros I.G., Jamurtas A.Z., Nikolaidis M.G., Douroudos I., Chatzinikolaou A., Mitrakou A., Mastorakos G., Papassotiriou I., Taxildaris K., Kouretas D. (2007) Oxidative stress biomarkers responses to physical overtraining: implications for diagnosis. Free Radical Biology & Medicine 43, 901-910. [DOI] [PubMed] [Google Scholar]
- Miao X, Xiao B, Shui S, Yang J, Huang R, Dong J. (2018) Metabolomics analysis of serum reveals the effect of Danggui Buxue Tang on fatigued mice induced by exhausting physical exercise. Journal of Pharmaceutical and Biomedical Analysis 151, 301-309. [DOI] [PubMed] [Google Scholar]
- Monleon D., Garcia-Valles R., Morales J.M., Brioche T., Olaso-Gonzalez G., Lopez-Grueso R., Gomez-Cabrera M.C., Viña J. (2014) Metabolomic analysis of long-term spontaneous exercise in mice suggests increased lipolysis and altered glucose metabolism when animals are at rest. Journal of Applied Physiology 117, 1110-1119. [DOI] [PubMed] [Google Scholar]
- Nicholson J.K., Lindon J.C. (2008) Systems biology: metabonomics. Nature 455, 1054-1056. [DOI] [PubMed] [Google Scholar]
- Peake J.M., Tan S.J., Markworth J.F., Broadbent J.A., Skinner T.L., Cameron-Smith D. (2014) Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. American Journal of Physiology. Endocrinology and Metabolism 307, E539-E552. [DOI] [PubMed] [Google Scholar]
- Powers S.K., Radak Z., Ji L.L. (2016) Exercise-induced oxidative stress: past, present and future. The Journal of Physiology 594, 5081-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ra S.G., Maeda S., Higashino R., Imai T., Miyakawa S. (2014) Metabolomics of salivary fatigue markers in soccer players after consecutive games. Applied Physiology, Nutrition, and Metabolism 39, 1120-1126. [DOI] [PubMed] [Google Scholar]
- Romijn J.A., Coyle E.F., Sidossis L.S., Gastaldelli A., Horowitz J.F., Endert E. (1993) Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. The American Journal of Physiology 265, E380-391. [DOI] [PubMed] [Google Scholar]
- Ruby B.C., Robergs R.A. (1994) Gender differences in substrate utilisation during exercise. Sports Medicine 17, 393-410. [DOI] [PubMed] [Google Scholar]
- Sarıkaya B., Dursun A.D., Taylan Deveden E.Y., Pınar L. (2017) Interleukin-6 and hepcidin expression changes in cardiac tissue of long-term trained and untrained rats after exhaustive exercise. Turkish Journal of Medical Sciences 47, 1940-1946. [DOI] [PubMed] [Google Scholar]
- Seifi-Skishahr F., Damirchi A., Farjaminezhad M., Babaei P. (2016) Physical training status determines oxidative stress and redox changes in response to an acute aerobic exercise. Biochemistry Research International 2016: 3757623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewright K.A., Hubal M.J., Kearns A., Holbrook M.T., Clarkson P.M. (2008) Sex differences in response to maximal eccentric exercise. Medicine and Science in Sports and Exercise 40, 242-251. [DOI] [PubMed] [Google Scholar]
- She P., Zhou Y., Zhang Z., Griffin K., Gowda K., Lynch C.J. (2010) Disruption of BCAA metabolism in mice impairs exercise metabolism and endurance. Journal of Applied Physiology 108, 941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H., Mathé G., Couvreur P., Tew K.D. (2002) II. Glutamine and glutamate. Biomedicine & pharmacotherapy 56, 446-457. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky L.J., MacDougall J.D., Atkinson S.A., Tarnopolsky M.A., Sutton J.R. (1990) Gender differences in substrate for endurance exercise. Journal of applied physiology 68, 302-208. [DOI] [PubMed] [Google Scholar]
- Taysi S., Oztasan N., Efe H., Polat M.F., Gumustekin K., Siktar E., Canakci E., Akcay F., Dane S., Gul M. (2008) Endurance training attenuates the oxidative stress due to acute exhaustive exercise in rat liver. Acta Physiologica Hungarica 95, 337-347. [DOI] [PubMed] [Google Scholar]
- Turcotte L.P., Richter E.A., Kiens B. (1992) Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. The American Journal of Physiology 262, E791-799. [DOI] [PubMed] [Google Scholar]
- Urhausen A., Gabriel H., Kindermann W. (1995) Blood hormones as markers of training stress and overtraining. Sports Medicine 20, 251-276. [DOI] [PubMed] [Google Scholar]
- Vollaard N.B., Shearman J.P., Cooper C.E. (2005) Exercise-induced oxidative stress: myths, realities and physiological relevance. Sports Medicine 35, 1045-1062. [DOI] [PubMed] [Google Scholar]
- Yan B., A, J., Wang G., Lu H., Huang X., Liu Y., Zha W., Hao H., Zhang Y., Liu L., Gu S., Huang Q., Zheng Y., Sun J. (2009) Metabolomic investigation into variation of endogenous metabolites in professional athletes subject to strength-endurance training. Journal of Applied Physiology 106, 531-538. [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhang P., Wang C., Shan Y., Wang D., Qian F., Sun M., Zhu C. (2013) Effect of ginsenoside Rg3 on tyrosine hydroxylase and related mechanisms in the forced swimming-induced fatigue rats. Journal of Ethnopharmacology 150, 138-147. [DOI] [PubMed] [Google Scholar]
- Zheng C., Chen X.K., Zhou Y. (2018) Acute glutamine ingestion modulates lymphocytic responses to exhaustive exercise in the heat. Applied Physiology, Nutrition, and Metabolism 43, 213-220. [DOI] [PubMed] [Google Scholar]


