Abstract
Post-activation potentiation (PAP) conditioning has been reported to increase performance. Most research has examined PAP effects on strength/power activities, whereas the effects on endurance sports are understudied. The aim of this study was to characterize PAP conditioning stimulus effects on a subsequent 5x1 km running trial. A randomized, within subjects, repeated measures study utilized 12 male, endurance-trained athletes, who performed a full warm-up, conditioning exercise intervention (4x5 repetition maximum band-resisted squat jumps) or a control condition prior to a 5x1 km time trial run. Tests were conducted immediately prior to the intervention, after each kilometer, immediately following the 5x1 km run, and at seven and ten minutes post 5 km run. Measures included the interpolated twitch technique (ITT), evoked contractile properties, maximum voluntary isometric contractions (MVIC) plantar flexor force, drop jump, rating of perceived exertion, and heart rate. The PAP stimulus reduced the time to complete the run (3.6%; p = 0.07, d = 0.51), and decreased the time to complete kilometer one (8%; d = 1.08, p = 0.014). Jump height (p = 0.02; 9.2%) and reactive strength index (p = 0.035; 16%) increased with PAP. F100 (force produced in the first 100ms of the MVIC) and MVIC force with PAP increased at kilometers 3 (p = 0.04, d=0.84), 4 (p = 0.034, d = 0.29), and 7min post-run (p = 0.03, d = 0.60). Voluntary activation (ITT) increased at 7min post-run (p = 0.04, d = 0.59) with PAP, yet decreased at 7min post-run in the control condition (p = 0.03, d = 0.36). A prior band-resisted squat protocol decreased running time and improved neuromuscular properties in endurance athletes running 5x1 km.
Key points.
First study to show that a 5RM jump squat conditioning stimulus positively affected neuromuscular and performance changes resulting in decreased time to complete the running task, increased muscle force generation, voluntary activation, and evoked contractile temporal properties.
A 5RM weighted jump squat protocol as part of a standardized running-specific warm-up will induce significant measurable post-activation potentiation (PAP) effects during the course of a subsequent 5 km time trial run and up to 7-minutes after the running protocol.
The results from this experiment are of particular importance to coaches, athletes and practitioners, who must consider the most effective method of warming up to increase and maintain performance.
Key words: Post-activation potentiation, neuromuscular adaptations, running, endurance, power
Introduction
Endurance running physiology studies have focused on aerobic capacity, metabolic systems and running economy as the limiting factors for performance (Costill et al., 1976). However, these properties cannot solely explain differences observed in endurance performance (Noakes, 2011; Nummela et al., 2006). Neuromuscular based theories propose that, during endurance exercise, the central nervous system integrates all feedback and consequently regulates the skeletal muscle recruitment, such that the levels of intensity and duration do not exceed the threshold where potential damage could occur to the brain, heart, and other vital organs, thus affecting the muscle’s ability to produce power and speed. (Gibson, 2004; Noakes, 2000).
In the case of an endurance athlete, a high rate of fatigue development coupled with a heightened inability to tolerate fatigue, would decrease speed and negatively affect performance. Muscle fatigue can be characterized into central and peripheral components. Peripheral fatigue is due to changes occurring distal to the neuromuscular junction, while central fatigue has its origin proximal to the neuromuscular junction representing activities within the central nervous system (Gandevia, 1998). Research suggests that there are peripheral adjustments that take place at the skeletal muscle to counteract fatigue (Behm 2004) and enhance performance (Boullosa and Tuimil, 2009; Behm et al.., 2018; Hamada et al., 2000a). In addition to peripheral fatigue; reduced neural drive (central fatigue) to the contracting muscles is likely to compromise motor performance during running (Girard et al., 2008, Millet et al., 2003). Thus, the management of fatigue (central and peripheral), commonly known as pacing, becomes an important factor to consider for successful endurance performance.
PAP is a phenomenon that can enhance muscle force output and rate of force development as a result of muscle contractile history (Houston et al., 1987; Grange et al., 1993; Sale, 2004). This phenomenon is highly applicable to sports, and research has increasingly focused on methods for prompting PAP as a form of performance enhancement (Boullosa et al., 2018; Hamada et al., 2000a; Mitchell and Sale 2011). Due to the lack of applicable literature, scientists and performance professionals are often unaware of the potential PAP has to aid in both endurance training and competition. The aim of incorporating a PAP stimulus into an athlete’s training or pre-competition program is to elicit an acute enhancement in muscle performance and consequently a higher jump, faster time, or further throw (Boullosa et al., 2018; Gullich and Schmidtbleicher, 1996; Hamada et al., 2000a; Mitchell and Sale, 2011).
Examining PAP in the endurance phenotype, a widely cited article by Sale (2004) suggests that PAP should have its greatest effect during submaximal contractions in which the motor units are firing at relatively lower frequencies, as is the case with endurance exercise. It follows then that if the levels of fatigue in a sport or activity are relatively lower than the levels of PAP, not only athletes performing maximal activities will benefit, but athletes performing submaximal activities could benefit from this mechanism. These lower firing rates occur during endurance activities and consequently help achieve an improved final force output (Behm, 2004; Sale, 2004).
With the evidence that PAP enhances subsequent endurance performance, the most plausible application of PAP to sport is its use as a warm-up technique prior to training or competition in order to acutely enhance contractile potential (Boullosa et al., 2018). Interestingly, few studies have examined the feasibility of this application in the endurance running population (Palmer et al., 2009; Silva et al., 2014; Skof and Strojnik et al., 2007) though many studies have investigated the enhancement of contractile activity during and after endurance performance activities (Boullosa et al., 2018; Hamada et al., 2000a). As PAP has been shown to persist for approximately 10 minutes (Houston et al., 1987; Grange et al., 1993), and other sports that involve endurance may have prolonged rest periods (e.g. half-time in soccer), it would be of interest to monitor potentiation following an exercise protocol. To our knowledge, there have been very few to no studies that have combined a PAP conditioning stimulus into a warm up while subsequently monitoring the central and peripheral alterations during and after a training-styled run session. This proposed study design offers insights into the confirmation of PAP in endurance athletes and attempts to investigate at what point during an endurance run PAP effects can be measured.
Therefore, the aim of the present study was to evaluate the neuromuscular alterations of a PAP conditioning stimulus on subsequent performance during and after a running time trial. Specifically, we compared the neuromuscular effects (i.e. electromyography, maximal voluntary contractions, evoked contractile properties, and performance measures) of an applicable (Gooyers et al., 2012), five repetition maximum (5RM) band-resisted squat jump on a subsequent 5 x 1 kilometer time trial to highlight possible central or peripheral changes. We hypothesized that performing a 5RM band-resisted jump squat protocol as part of a standardized running-specific warm-up would induce significant measurable PAP effects during the course of a subsequent 5 x 1 kilometer time trial run and up to 10 minutes post-run protocol.
Methods
Experimental design
A within subject (repeated measures) experimental study design was employed in this study. Twelve healthy male recreationally endurance-trained athletes completed two familiarization, and two intervention sessions in a randomized order separated by a minimum of 72 hours. The familiarization sessions included: 1) an incremental running test to volitional exhaustion (V́O2max) and 2) a familiarization of testing equipment, estimation of the individual’s 5RM, and testing of evoked contractile properties. The intervention sessions included a running-specific warm up, with either the intervention (4 x 5RM band resisted squat jumps) or control (no squats) condition, and a 5 x 1 kilometer time trial run. Tests were conducted immediately prior to the intervention, during a 3 minute recovery period between each kilometer, immediately following the 5 x 1 km run, and at minutes seven and ten, post-5 km run. These measures included the interpolated twitch technique (ITT) as a measure of muscle activation, contractile properties (peak twitch torque, rate of force development, percent voluntary activation, potentiated twitch force), maximum voluntary isometric contractions (MVIC) to determine peak ankle plantar flexor force, force produced in the first 100 ms (F100), 30-cm drop jump height (flight time, contact time and reactive strength index), rate of perceived exertion (RPE), and heart rate.
Participants
Based on a statistical power of 0.8, an effect size of 0.5 (Cohen, 1969, p.348), and an alpha level of p < 0.05, an “F test, ‘A’ priori ANOVA: repeated measures” analysis was executed using G*Power (software package 3.1.9.2) with a resultant minimum sample size calculation of 9. Consequently, twelve healthy male endurance-trained runners (28.33 ± 1.37 years, 1.77 ± 0.02 m, 75.07 ± 2.74 kg, 57.98 ± 6.37 mL·kg-1·min-1 [V́02 max]) were recruited for this study. The participants were actively engaged in training (running an average 62 ± 30.9 km/week, running 5 days/week, weight training 1 day/week), had average 5 km times ranging from 16:15 to 22:12, and had a minimum of two years’ experience competing in cross-country/road race events. Participants who had experienced any musculoskeletal injuries in the past six months or who had diagnosed neurological conditions were not eligible to participate in the study. Participants were required to refrain from consuming caffeine or alcohol 24 hours prior to testing, to abstain from food four hours prior to each testing session and to avoid running and high intensity training of any kind 72 hours prior to testing sessions. To ensure healthy, ethically-informed participants, each participant read and signed a Physical Activity Participation Questionnaire (PAR-Q: Canadian Society for Exercise Physiology) and an approved informed consent form. The study adhered to the approval process of the institution’s Interdisciplinary Committee on Ethics in Human Research (20182129-HK) and was conducted according to the Declaration of Helsinki.
Procedure: Familiarization sessions
Participants attended the laboratory on two separate occasions to 1) perform the Université de Montréal Track Test (V́O2 consumption, ventilatory threshold, maximum aerobic speed, and maximum heart rate) and 2) to estimate 5RM squat, and establish evoked contractile properties measures. Due to the potentially exhausting effects of the familiarization sessions, they were separated by a 48-hour period.
Université de Montréal Track Test (UMTT) & anthropometric measures: Upon arrival to the lab, the participant’s height (cm) and weight (kg) was measured and age was recorded. The participants began the session with a 5-minute warm-up/familiarization period on a treadmill (Cybex 751T, Medway, Massachusetts) at a self-selected pace (recorded). Following the warm-up, the participants were connected to an indirect calorimetric system (MOXUS CD 3-A/S-3A, AEI Technologies, Pittsburgh, Pennsylvania) via a mask secured to the oral/nasal regions of the face. The participants were outfitted with a heart rate monitor (chest strap; Polar H10, Polar, Kempele, Finland), the test protocol was discussed and any questions answered. The test was conducted according to the protocol set out by Léger & Boucher (1980) in which the participant runs on a motor-driven treadmill at a constant slope of 1%. The test began at an initial speed of 7 km·hr-1 with a 1 km·hr-1 speed increase every two minutes until volitional exhaustion. After exhaustion was reached, a five-minute rest/recovery period was allowed before commencing a verification phase (to ensure a true V́O2 maximum [V́O2max]). During the verification phase (Rossiter et al., 2006), the treadmill was set at 105% of the recently determined maximal aerobic speed (MAS) and the participant ran until their limit of tolerance was reached. A self-selected pace cool down period followed and continued until the recovery heart rate reached 120 b·min-1. To further determine if the participants attained V́O2max or true exhaustion, the respiratory exchange ratio (RER: >1.0), a plateau in V́O2 values at maximum intensity, Borg perceived exertion scales (>17/20) and maximum heart rate (approximately 220 – participants’ age) attained were also monitored. Results from this session were used to provide a descriptive statistic into the training status of the participants. MAS and maximum heart rate were used to compare and contrast the measured aerobic speed and heart rate reached during the running trials, enabling a better analysis of the metabolic properties during the trials.
Squat jumps: Based on the evidence demonstrating the potentiating effects of the squat, Rixon et al., (2007) have suggested that potentiation effects can depend on an individual’s training background. Squats and other biomechanically similar exercises such as squat jumps are commonly performed in endurance athlete-focused training sessions (Storen et al., 2008; Willy and Davis, 2011). It was determined prudent to include squat type exercises as the conditioning stimulus of interest as they are ubiquitous in studies examining PAP effects (Bauer et al., 2019; Tillin and Bishop, 2009). As the in-field applicability (Behm and Sale, 1993; Newton et al., 2002) of a variation on the squat (band-resisted jump squat) is extremely high in endurance athletes, this exercise was implemented as the protocol. The equipment was set up such that for every jump squat set, two looped resistance bands (Theraband CLX bands, Akron, Ohio) were attached with carabineers to heavy weights on either side of the participant. The resistance bands were then stretched over the head and placed cross-body on the contralateral clavicle/upper trapezius complex, such that the band secured to the weights on the left side of the participant was placed on the right shoulder of the participant and vice versa. The jump squat began at the downward movement phase (thighs parallel with the floor) and proceeded with a maximally explosive jump (flight phase) against the resistance of the bands (Masamoto et al., 2003.) Every squat maneuver took place on a rubber mat where an exercise physiologist was present to monitor the participant. The same jump squat positioning was ensured by the same test administrators in each instance.
5 Repetition Maximum (RM) Squat Jump Estimation: After a general low-intensity aerobic warm up (5 minutes) on a cycle ergometer, three sets of submaximal squats (five repetitions) with loads of 50-70% body mass were conducted to get an initial idea of the participant’s 5RM (warm up phase). Following this, a series of trials with incrementally larger resistance were conducted and the 5RM was recorded as the maximum amount of resistance that could be lifted for five repetitions through the entire range of motion while maintaining proper form (Mitchell and Sale, 2011). Band resistance (weight) was manipulated with four various coloured bands with standardized resistance (2.6 kg, 3.3 kg, 4.6 kg, and 6.4 kg as prescribed by the manufacturer: Theraband CLX bands, Akron, Ohio). Participants rested for 3 minutes between each 5RM trial. In each trial, if the squat jump was performed with the proper form (thighs parallel to the ground, feet left floor) for all 5 repetitions, the band resistance was incrementally increased by colour and number. Trials continued until the jump fell short of the full range of motion (thigh angle compromised, or evidence of improper gluteal and back form) on two separate trials (spaced by four minutes of rest between sets and 1-3 seconds rest inter-repetitions)(Mitchell and Sale). Following the 5RM estimation trials, the participant was given a brief preview to the interpolated twitch procedure, the treadmill protocol and proper form for completing a 30-cm drop jump.
Evoked contractile properties: Evoked contractile properties were recorded prior to MVIC assessment in the interventions in order to quantify the membrane excitability and excitation-contraction coupling functions. Prior to the application of the stimulating and electromyography (EMG) electrodes, the underlying skin was prepared by shaving, lightly abrading, and cleansing (using isopropyl alcohol.) Peak twitch force of the triceps surae muscle group was evoked using bipolar surface electrodes placed on the popliteal fossa overlying the medial popliteal nerve (cathode) and ~ 2 cm below the distal border of gastrocnemius (Hamada et al., 2000b). The positioning of each electrode was recorded to allow between-session replication, though slight variation in positioning was expected as the electrode location at the beginning of each session was altered to ensure an optimal stimulus response. Both stimulating electrodes were connected to a high-voltage, constant current simulator (Digitimer Stimulator Model DS7AH, Hertfordshire, UK). The 200-μs, 400-V square wave pulse with a current intensity (10 mA – 1 A) was progressively increased (by 10mA increments) until a maximum twitch force was achieved (no further increase in peak twitch torque.) Force-time data was recorded at 2000 Hz using a BioPac/AcqKnowledge data acquisition system (DA 150: analog-digital converter MP100WSW, Holliston, MA). The average of three trials was used to measure peak twitch force, time to peak twitch force, and rate of force development (Behm et al., 2001). This laboratory has previously reported excellent intersession reliability (ICCs of 0.98-0.99 for evoked twitch properties) using the above mentioned technique (Behm et al., 1996; 2002).
Testing Measures
Ankle plantar flexors maximum isometric voluntary contraction (MVIC) force: The participant was seated in a standardized position with ankle, knee, and pelvis angles of 90°, as measured by a goniometer. The isometric ankle plantar flexion of the dominant foot was measured using a custom built boot apparatus (Memorial University Technical Services) outfitted with a strain gauge. Participants were instructed to contract as quickly and powerfully as possible for the entire duration of the MVIC. The MVIC’s were sustained for 4 seconds. All voluntary and evoked forces were detected by the built-in strain gauge (boot apparatus), amplified (x1000) (Biopac Systems Inc., DA 150; analog-digital converter MP100WSW, Holliston, MA) and interfaced with a computer. The data was sampled at 2000 Hz and analyzed with a commercially designed software program (AcqKnowledge III, Biopac Systems Inc., Holliston, MA). Data analysis included triceps surae peak MVIC forces as well as the instantaneous strength defined as the force produced in the first 100 ms (F100).
Interpolated twitch technique (ITT): The ITT has been reported to be a valid and reliable measure of muscle voluntary activation (Behm et al., 1996) and was also used to achieve post-MVIC potentiated twitch measurements. The stimulation intensity was re-adjusted before each testing session with a simplified procedure based on the intensity used during the familiarization session (Rupp et al., 2010). The same electrode configuration and current intensity used for the maximal evoked twitches were used for the ITT. The ITT involved superimposing an electrically stimulated doublet with an inter-pulse interval of 10 ms (100 Hz) in the relaxed dominant triceps surae muscle, at 2.5 s after the onset of a 4-s MVIC of triceps surae, and 5 s after the MVIC (potentiated twitch) (Girard et al., 2012). An interpolation ratio was calculated by comparing the amplitude of the superimposed doublet with the post-contraction potentiated doublet (5 s following the MVIC) to estimate the extent of voluntary activation during the voluntary contraction, where % of muscle voluntary activation = (1- (interpolated doublet force / potentiated doublet force) x 100) (Behm et al., 1996).
Electromyography (EMG): Muscle EMG was recorded from triceps surae (soleus and medial gastrocnemius) during MVIC, evoked and ITT contractions. As mentioned previously, prior to application of the electrodes (1-cm 162 Ag/AgCl; MediTrace 133, Kendall, Technical products Toronto, Ontario, Canada), the designated area was prepared by shaving, lightly abrading, and cleansing (using isopropyl alcohol.) The active recording electrodes were placed on the dominant medial gastrocnemius and dominant soleus (15-cm distal to the stimulating electrode), the reference electrodes were placed distally such that an inter-electrode distance of two centimeters was observed, and the ground electrode was secured on the lateral epicondyle of the femur (Behm et al., 2002). EMG activity was amplified (x1000), band-pass filtered (10-1000 Hz), rectified and directed to a computer. The integrated EMG (iEMG) activity was determined over a 1-s period following the peak force during the MVIC. The reliability of this EMG measurement technique has been demonstrated by this laboratory in previously published literature with reported ICC values ranging from 0.91 to 0.99 (Behm et al., 2001; 2002).
30-cm drop jump (DJ): Two DJ were performed at 30 second intervals. During the DJ, the participants were instructed to maintain their hands akimbo (hands placed on the iliac crest); this helped remove extraneous variance resulting from arm swing. The DJ maneuver began with the participant standing on a 30-cm platform. When the participant felt inclined, they transferred from the platform, made a 2-footed landing on a jump mat (SmartJump, Fusion Sport, Chicago, Illinois), and jumped vertically for maximum height with a brief contact period. Participants were instructed to maximize jump height by jumping as high as possible with explosive intent to minimize contact time. Flight time, contact time, and jump height were measured by the SmartHub system (Fusion Sport, Chicago, Illinois) and reactive strength index (Jump height / Contact time) was computed accordingly (Young and Behm, 2003).
Rating of perceived exertion (RPE): Participants were asked to measure their level of self-perceived effort in order to determine how hard they felt their body was working. They were told that this rating should reflect how heavy and strenuous the exercise felt to them; combining the feelings of physical stress, effort, and fatigue. They were instructed not to focus on any one factor (e.g. shortness of breath) but to focus on the overall feeling of exertion. As the Borg scale ranges from 6-20, the participants were informed that a 6 represents ‘no exertion at all’ and a 20 means ‘total and maximal exertion.’ At each kilometer interval, the participants were shown the Borg rating scale chart to assist in their response.
Heart Rate (HR): Upon arrival to the laboratory, participants were outfitted with a heart rate monitor (Polar H10, Polar, Kempele, Finland) using a chest strap. Resting heart rate was recorded during a 5 minute period prior to commencing any activity (warm-up or intervention) and the monitor remained on and recorded the heart rate for the complete duration of the testing sessions. Following each testing session, the heart rate monitor was connected to a computer, interfaced with its accompanying software (Polar Weblink and Polar Flow), and the data uploaded for analysis and data extraction.
Testing sessions
Running-specific warm-up: Prior to the first round of tests (pre-intervention), each experimental session began with a standard running warm-up. The warm-up commenced with a five-minute running bout on a treadmill (Cybex 751T, Medway, Massachusetts) at a moderate self-selected pace. Following the treadmill warm-up, the participants performed a 4 x 50m dynamic warm up (Skof and Strojnik, 2007) including ‘A’s, B’s, and C’s (leg whips, knee kicks, heel-to-gluteal kicks). The warm-up component finished with the participants performing 4 x 50m accelerations (start 50 m repeat at a normal pace and accelerating through to a maximum sprint by the 50 m mark). After each of the 50m repeats (dynamic and accelerations) the participants took active rest as they lightly jogged 50 meters back to the starting point (about 30-45 seconds between each 50m repeat.) All of the participants were accustomed to and had previous exposure to this running drill.
Interventions: Using random allocation selection on separate testing days, the participant performed one of two conditions (4 x 5RM band resisted jump squat conditioning stimulus or control). At the beginning of each testing session, proper EMG and stimulating electrode preparation and placement was ensured (as outlined above) and the heart rate monitor was situated on the participant with the resting heart rate observed. All testing measures were performed with uniformity (same tests in the same order) across all conditions.
4 X 5RM squat conditioning stimulus intervention: The PAP stimulus intervention required the participants to perform the standardized running-specific warm-up and subsequently perform the pre-intervention tests (MVIC, ITT, DJ, RPE, HR). At the completion of the pre-intervention tests the participants moved directly to the mat to perform the conditioning stimulus (4 x 5RM band-resisted squat jumps). The pre-determined 5RM resistance bands were anchored on either side of the participant and the resistance bands were placed cross-body on the clavicle/upper trapezius complex and the participant performed 4 sets of 5 repetitions (Ah Sue et al., 2016; Silva et al., 2014). Each of the 4 sets of 5RM were monitored and standardized such that the downward movement phase continued until the thighs were parallel with the floor. Each set was separated by a 2-minute rest. At the completion of the squatting protocol, the participant took an 8-minute rest according to ideal PAP stimulus protocol (Grange et al. 1993; Houston et al. 1987; Silva et al., 2014; Wilson et al., 2013) where sitting and walking were permitted. Subsequent to the 8-minute rest, the participants completed a 5 x 1 km running trial on a motor-driven treadmill (Driller et al., 2016; Paavolainen et al., 1999). Participants were instructed to complete each kilometer repeat as fast as possible and verbal encouragement was given while the participant was running. All of the screens and controls on the treadmill were hidden and the participants were only given access to the control arrows that adjust the treadmill speed. Participants were encouraged to adjust the treadmill speed according to their physiological and pacing needs and were reminded to complete the trial as fast as possible. In an effort to mimic distance markers commonly found in running settings, verbal notice on distance was given when 500 and 250 meters remained in each repeat. The 5 km running trial was separated by test measures at each kilometer (participant dismounted treadmill at each kilometer, performed tests (3 minutes), and remounted the treadmill with 30 seconds allotted to return to speed). Following the completion of the 5 x 1 km running bout, post-intervention tests (MVIC, ITT, DJ, RPE, HR) were conducted immediately and seven and ten minutes (Boullosa and Tuimil, 2009; Hamada et al., 2000b) post-intervention.
Control condition: The control condition required the participants to perform the running-specific warm-up and immediately following, the pre-intervention tests. Following the pre-intervention tests, the participants rested (walked/stood) for a total of 13 minutes (5 minutes for control and 8 additional minutes as part of the PAP rest protocol). After the rest period, the participants ran the 5 x 1 kilometer trial separated by test measures at each kilometer. Post-test measures were taken immediately following, and seven and ten minutes post 5 km run.
Statistical analysis
Statistical analyses were completed using the SPSS software (Version 23.0, SPSS, Inc. Chicago, IL). The assumption of sphericity and normality were tested for all dependent variables and if a violation was noted, the corrected values for non-sphericity with Greenhouse-Geisser were reported. A two-way repeated-measures ANOVA was used to compare triceps surae function (neuromuscular and metabolic) (2 conditions x 8 testing times) in each dependent variable. In the event of significant main effects or interactions, planned pairwise comparisons were made using the Bonferroni method to test for differences among mean value time points. Pearson correlation coefficients were calculated to determine relationships between selected parameters. The level of significance was set at P < 0.05 and all results are expressed as mean ± SD. Effect size (d) magnitude of change were calculated for interactions and reported as trivial (<0.2), small (0.2-0.49), medium (0.5-0.79) or large (≥0.8) effect sizes (d) (Cohen 1988). Inter-session reliability responses were assessed with Cronbach’s alpha intraclass correlation coefficient (ICC).
Results
Other than the ITT measure (0.76), ICC reliability scores for each test measure exceeded 0.90 (Table 1). Tables 2 and 3 illustrate within and between condition significant changes.
Table 1.
Intra-session descriptive and reliability measures.
| Measures | Mean (SD) | ICC |
|---|---|---|
| Gastrocnemius EMG (mV) | 0.284 (0.066) | 0.957 |
| Soleus EMG (mV) | 0.18 (0.04) | 0.963 |
| Triceps Surae MVC (N) | 665.3 (77.5) | 0.984 |
| Triceps Surae F100 (N) | 164.2 (61.9) | 0.976 |
| Evoked Twitch Force (N) | 82.1 (23.9) | 0.962 |
| Potentiated Twitch Force (N) | 98.4 (30.5) | 0.954 |
| ITT %Voluntary Activation | 66.6 (52.9) | 0.763 |
| Contact Time (ms) | 300.1 (34.5) | 0.994 |
| Drop Jump Height (cm) | 30.2 (4.4) | 0.983 |
| Reactive Strength Index | 1.095 (0.180) | 0.989 |
| Time to Complete Kilometer (s) | 257.15 (22.75) | 0.969 |
| Heart Rate (beats per minute) | 151(91) | 0.943 |
| Rate of Perceived Exertion | 13.3 (10.1) | 0.905 |
ICC = intraclass correlation coefficient.
Table 2.
Effect sizes are illustrated for significant within squat condition interactions.
| 2 KM | 3 KM | 4 KM | 5 KM | 7 MIN POST | 10 MIN POST | Overall | |
|---|---|---|---|---|---|---|---|
| MVC | ↑ (0.29) | ↑ (0.36) | |||||
| F100 | ↑ (0.67) | ↑ (0.60) | |||||
| Potentiated TPT | ↓ (0.54) | ↓ (0.51) | |||||
| %VA | ↓ (0.36)* | ||||||
| Reactive Strength Index | ↑ (0.35) | ↑ (0.35) | ↑ (0.51) | ↑ (0.38) | ↑ (0.43) |
Evidence for Post-activation Potentiation (PAP) Arising from Within Condition Effects (as compared to baseline pre-test).
* control condition.
Table 3.
Effect sizes are illustrated for significant between jump squat condition interactions.
| 1 KM | 2 KM | 3 KM | 4 KM | 7 MIN POST | 10 MIN POST | Overall | |
|---|---|---|---|---|---|---|---|
| MVC | ↑ (0.55) | ||||||
| Resting TPT | ↑ (0.50) | ↓ (0.60) | ↓ (0.70) | ||||
| Potentiated TPT | ↓ (0.51) | ||||||
| %VA | ↑ (0.54) | ↑ (0.53) | |||||
| Jump Height | ↑ (0.47) | ↑ (0.42) | ↑ (0.51) | ↑ (0.62) | |||
| Reactive Strength Index | ↑ (0.60) | ↑ (0.74) | ↑ (0.76) | ↑ (0.58) | |||
| Time to complete km | ↓ (1.08) | ↑ (0.36) * | ↓ (0.51) |
Evidence for Post-activation Potentiation (PAP) Arising from Between Condition Effects.
* control condition.
Triceps Surae MVC: There were no significant main effects of the conditioning squat jump stimulus or time for triceps surae MVC force. There was a significant (F(7, 77) = 2.3, p = 0.039) interaction effect between the conditioning stimulus and the data time points for force produced between running trials. Between group post-hoc contrasts revealed significantly (F(1, 11) = 4.679, p = 0.05, d = 0.55, 3.0% ↑) greater MVC force when comparing squat stimulus to control at 10 minutes post-run as compared to pre-test. Within group post-hoc analyses revealed significantly higher MVC force for the squat condition at kilometer 4 (d = 0.29, p = 0.034, 8% ↑) and 10 minutes post-run (d = 0.36, p = 0.036, 9.5% ↑) compared to pre-test (Figure 1).
Figure 1.
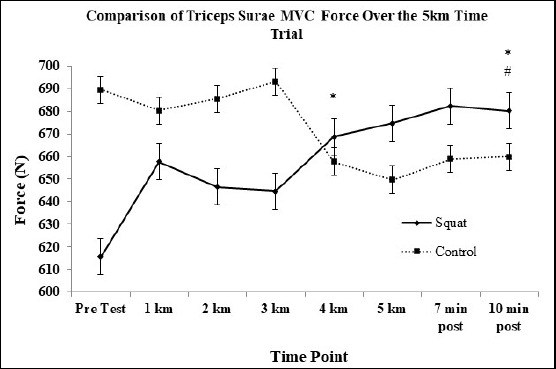
Estimated changes in triceps surae maximum voluntary contraction (MVC) force means between the control and squat condition. Overall increase noted in the squat condition. * indicates the squat condition value was significantly greater than pre-test. # indicates the squat condition value was significantly greater than the control condition.
Gastrocnemius and Soleus EMG: No significant overall main effects or interactions were observed.
Triceps Surae F100: There was a tendency for a main effect of time for F100 in the 5 km time trial (F(7, 77) = 2.034, p = 0.061). Contrasts revealed that F100 at kilometer 3 (F(1, 11) = 9.605, p = 0.01, d = 0.7, 24% ↑) and 7 minutes post run (F(1, 11) = 6.042, p = 0.03, d = 0.6, 20% ↑) were significantly higher than pre-test (Figure 2).
Figure 2.
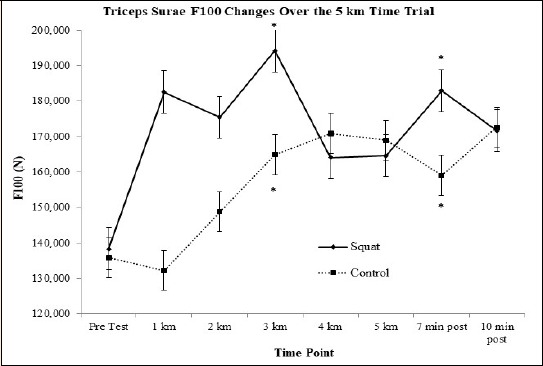
Representative graph of force developed in the first 100 milliseconds (F100) at each time point. Evident interaction of changes over time. * indicates the condition value was significantly greater than pre-test.
Evoked Contractile Properties
Resting Twitch Force Properties: There were no significant trends, effects, or interactions observed for resting twitch force. There was a main effect of condition for the resting time to peak twitch force (TPT) with the 5km time trial (F(1, 11) = 3.684, p = 0.05). Between group post-hoc contrasts revealed that resting TPT in the squat condition (F(1, 11) = 3.684, p = 0.05, d = 0.7) was lower than control. The contrasts further revealed that resting TPT in the squat condition at kilometer 3 (F(1, 11) = 4.365, p = 0.061, d = 0.5, 5% ↓) and 10 minute post run (F(1, 11) = 6.057, p = 0.032, d = 0.6, 5% ↓) was lower than control (Figure 3).
Figure 3.
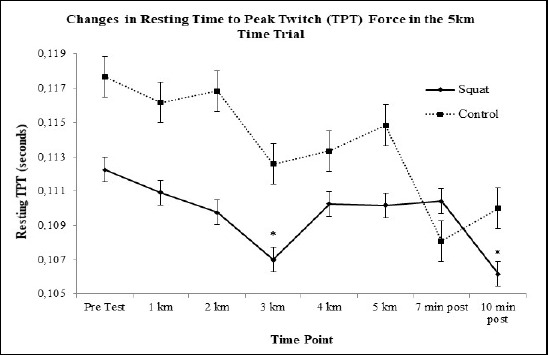
Estimated changes (as computed by means) noted between the two conditions. Overall decrease can be noted in the control condition. * indicates the squat condition value was significantly greater than pre-test.
Potentiated Twitch Force Properties: There were no significant trends, effects, or interactions observed for potentiated twitch force. There was a significant main effect for potentiated TPT observed over time in the time trial (F(7, 77) = 2.073, p = 0.05). Contrasts revealed that potentiated TPT at kilometer 4 (F(1, 11) = 4.447, p = 0.05, d = 0.54, 6% ↓) was significantly lower than pre-test. A near-significant main effect for condition (F(1, 11) = 3.908, p = 0.07, d = 0.51) exhibited that potentiated TPT was lower in the jump squat condition.
Potentiated Twitch Force Properties: There were no significant trends, effects, or interactions observed for potentiated twitch force. There was a significant main effect for potentiated TPT observed over time in the time trial (F(7, 77) = 2.073, p = 0.05). Contrasts revealed that potentiated TPT at kilometer 4 (F(1, 11) = 4.447, p = 0.05, d = 0.54, 6% ↓) was significantly lower than pre-test. A near-significant main effect for condition (F(1, 11) = 3.908, p = 0.07, d = 0.51) exhibited that potentiated TPT was lower in the jump squat condition.
ITT Voluntary Muscle Activation (%VA): There was a significant (F(7, 77) = 2.699, p = 0.015) interaction effect between the conditioning stimulus and the data time points for %VA between running trials (Figure 4). Between group post-hoc contrasts revealed significantly greater %VA when comparing jump squat stimulus to control at 7 minutes post-run (F(1, 11) = 4.573, p = 0.05, d = 0.54, 10% ↑) and 10 minutes post-run (F(1, 11) = 4.370, p = 0.05, d = 0.53, 11.5% ↑) as compared to pre-test. In the control condition, there was a significant decrease noted in %VA from pretest to 7 minutes post run (d = 0.36, p = 0.030, 19% ↓).
Figure 4.
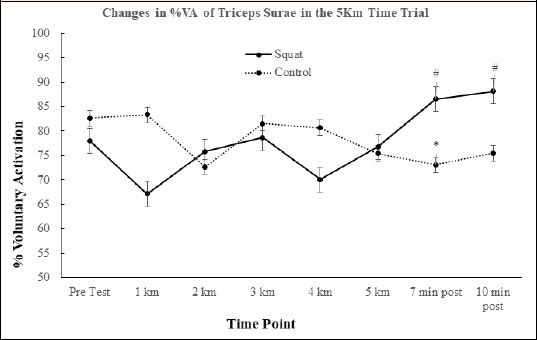
Representative graph demonstrating the change in %VA (voluntary activation) over the course of the interventions. Potentiated can be seen at seven and 10 minutes post-run (squats). * indicates the control condition value was significantly lower than pre-test. # indicates the squat condition value was significantly greater than the control condition.
Drop Jump (DJ) Performance Measures: There were no significant trends, effects, or interactions for contact time. There was a main effect of DJ height observed for condition in the time trial (F(1, 11) = 6.815, p = 0.02). Between group post-hoc contrasts revealed that DJ height in the jump squat condition was significantly elevated (F(1, 11) = 6.815, p = 0.02, d = 0.62) as compared to the control condition (Figure 5). When comparing the between group differences in the jump squat and control conditions, on average, participants showed significantly increased DJ height in the jump squat condition during kilometers 2 (d = 0.47, p = 0.015, 9% ↑), 3 (d = 0.42, p = 0.05, 8% ↑), and 4 (d = 0.51, p = 0.011, 8.5% ↑) as compared to the control.
Figure 5.
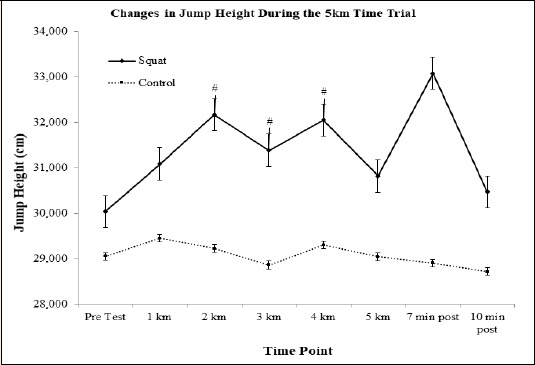
Representative graph demonstrating the changes seen in jump height during the squat and control conditions. Significant changes observed in the squat condition. # indicates the squat condition value was significantly greater than the control condition.
Reactive Strength Index: There was a main effect of time for the reactive strength index (F(7, 77) = 2.305, p = 0.035). Between group post-hoc contrasts revealed that reactive strength index was significantly higher at kilometers 2 (F(1, 11) = 6.307, p = 0.03, d = 0.6), 3 (F(1, 11) = 13.132, p = 0.004, d = 0.74), 4 (F (1, 11) = 15.032, p = 0.003, d = 0.76), and 7 minutes post-run (F(1, 11) = 5.497, p = 0.039, d = 0.58). Within group contrasts in the squat condition revealed a significant increase in reactive strength index from pre-test to kilometers 2 (d = 0.35, p = 0.046, 11.1% ↑), 3 (d = 0.35, p = 0.019, 11.4% ↑), 4 (d = 0.51, p = 0.001, 15.7% ↑), 5 (d = 0.38, p = 0.008, 12.0% ↑), and from pre-test to 7 minutes post-run (d = 0.43, p = 0.018, 13.5% ↑)(Figure 6).
Figure 6.
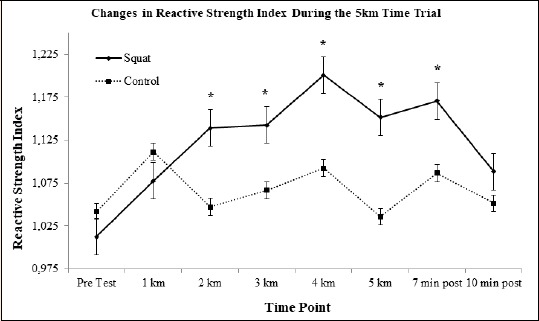
Reactive Strength Index (RSI) means graphed to represent changes over time. * indicates the squat condition value was significantly higher than pre-test.
Time to Complete the Time Trial: Eight of the twelve participants ran the summed 5 kilometer time trial faster in the jump squat condition than in the control condition (Figure 7). There was a significant decrease in the time to complete kilometer 1 in the jump squat condition (d = 1.08, p = 0.014, 8% ↓) compared to the control condition (Figure 8 and Table 3). An interaction effect (F(4, 44) = 2.694, p = 0.04) between condition and time demonstrated an increase in time to complete kilometer 4 (F(1, 11) = 7.974, p = 0.017, d = 0.65, 1.5% ↑) in the control condition. A main condition effect (F(1, 11) = 3.954, p = 0.05) revealed that the average time to complete each kilometer was less in the jump squat (4:20 min ± 23.2 sec) condition than in the control (4:37 min ± 34.5 sec) condition (Figure 8). The jump squat condition yielded a 3.6% reduction in the aggregate time to complete the 5 kilometers (Jump squat 21:07 min ± 1:83 min vs. Control 21:87 min ± 2:74 min).
Figure 7.
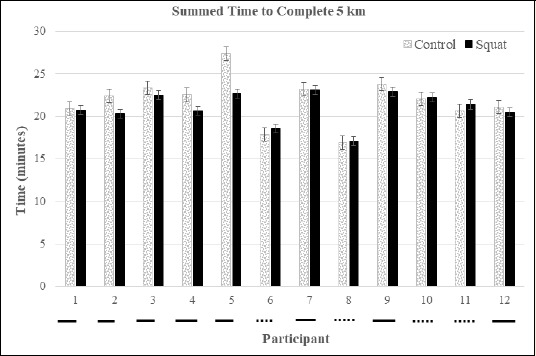
Total summed times for each participant. Solid underline indicates a decrease in time from control to squat and a dashed underline indicates an increase in time.
Figure 8.
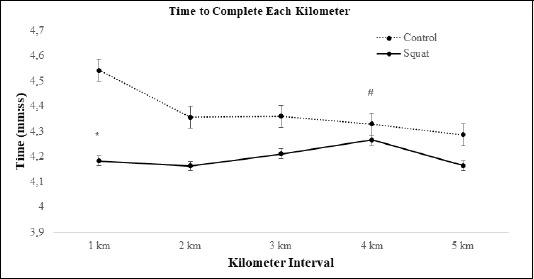
Representative graph demonstrating the changes seen in time to complete each kilometer during the squat and control conditions. * indicates a significant decrease in run time in the squat condition as compared to the control condition. # symbol indicates a significant interaction effect where the control condition run time was higher.
Heart Rate: Figure 9 illustrates the mean average heart rate in each condition throughout the experiment. There was a main effect of condition for heart rate in the time trial (F(1, 11) = 9.742, p = 0.01). There was a significant increase noted in the heart rate at kilometers 1 (d = 0.84, p = 0.001) and 2 (d = 0.48, p = 0.009) in the control condition as compared to the jump squat condition.
Figure 9.
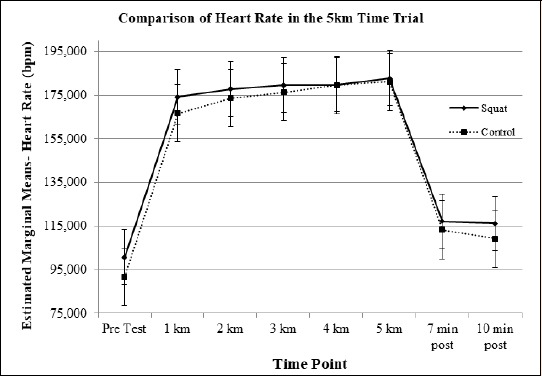
Heart Rate (bpm) means graphed to represent changes over time and highlights differences between the conditions.
Rating of Perceived Exertion (RPE): No significant trends, nor effects, or interactions were observed for RPE. Mean RPE was similar in both the jump squat (13.552 ± 0.323) and control (13.042 ± 0.387) condition respectively.
Discussion
The main finding of this study was the improved aggregate time to complete 5 km run trials in the jump squat conditioning stimulus intervention as compared to the standard running-specific warm-up. Factors contributing to this improvement could be related to jump squat conditioning stimulus-induced increases in voluntary activation at seven and 10 minutes post-run, MVC force at kilometer four and ten minutes post-run, MVC F100 at three and seven minutes post-run, decreased TPT at kilometer 4, DJ height at kilometers 2, 3 and 4, as well as reactive strength index at kilometers 2-5, immediately and 7 min post-run. These performance improvements contrasted with control condition decrements in triceps surae MVIC F100, potentiated time to peak twitch force, and time to complete the kilometer repeats.
The intent of the present study was to examine at what time point during a 5km run, might run performance enhancement and potentiation be observed. The balance of fatigue/force production (pacing) is an important element for any endurance runner, so the intermittent testing during each kilometer of the 5x1km run permitted insight into the effect of PAP on pacing throughout the time trial. Very few studies have examined running repeats (Del Rosso et al., 2016) with more prior studies investigating the neuromuscular effects of a standalone 5 km run (Girard et al., 2012; Nummela et al., 2006; Paavolainen et al., 1999). Perhaps most notable from these findings is the physiological and performance changes that combined to produce a faster time to complete kilometer one and four in the jump squat condition, as well as a 3.6% reduction in the aggregate 5 kilometer time after performing band-resisted squat jumps as compared to the control. The similar changes in the descriptive metabolic measures illustrated the increased intensity during the intervention sessions, (RPE: 17.5 ± 0.31, HRpeak: 181.96 ± 1.85, HRaverage: 151.25 ± 2.07) with no significant differences overall between jump squat and control condition. This fact eliminates the possibility that these changes could be caused be varying levels of involvement between the two sessions.
To the author’s knowledge, this study is the first to demonstrate such a result in an endurance-running population, though similar studies conducted in endurance cyclist populations support this main finding (Boullosa et al., 2018; Hamada et al., 2000a). Comparatively, in a study with trained cyclists, it was found that adding a 5RM leg press strength exercise bout to a 5-minute warm-up improved performance in a subsequent 20-km cycling time trial (Silva et al., 2014). This study found no significant difference in blood lactate measures, but suggested that the improved performance (~6.1% reduction in time to complete the trial) after heavy resistance was explained by improved cycling economy. The authors also suggested the possibility that, in addition to the neuromuscular effects observed, an increased capacity to access aerobic performance reserves also aided in explaining increased performance during the trial. In another related study, Palmer et al. (2009) examined the effects of prior heavy exercise (5 minutes of heavy cycling at 50% difference between lactate threshold and V́O2peak) in well-trained cyclists but measured performance in a subsequent 4-km cycling time trial. Athletes were subjected to either no prior exercise, prior heavy exercise, or self-selected prior exercise, all conducted on a cycle ergometer. Similar to Silva et al. (2014), they found faster times to complete the trial post-heavy exercise, although different from the Silva study was the overall increase in the mean power output (5.4%) post-heavy exercise. Specific to the population of interest, these effects were also observed in a study examining the effects of two different warm-up protocols on the neuromuscular systems of well-trained distance runners (Skof and Strojnik, 2007). This study found that adding sprinting and bounding exercises to an athletes’ warm-up would improve neuromuscular efficiency via PAP, though the authors did not rule out increased efficiency of the central nervous system (improved central input or enhanced excitation of the α-motor neuron) as a possible mechanism.
Neuromuscular adjustments were manifest in the present study as the results show that plantar flexor MVIC in the squat condition increased by 8% at kilometer four and 9.5% 10-minutes post-run. The jump squat condition revealed increased F100 at various points throughout the subsequent running trial, EMG remained unchanged while voluntary activation (ITT) increased. As EMG and contractile force are key features of fatigue, this could suggest that a bout of band-resisted squat jumps could increase muscle performance by overcoming possible onset of fatigue. This idea of unchanged (or increased) central properties supporting endurance activities has been demonstrated in other running studies (Skof and Strojnik et al., 2006a; 2006b), cycling studies (Booth et al., 1997; Sahlin, 1986), and intermittent isometric exercises (Vollestad et al., 1997). It has further been suggested that the increase in muscle force potentiation during running could act as a mechanism to prolong endurance activities, as seen in this study (Mettler and Griffin, 2012). This unchanged, or increased neural drive, is in stark contrast to the results of previous studies that found a 10-30% decrease in contractile force (plantar flexors MVIC) after completing running protocols lasting from one to several hours (Avela and Komi, 1998; Racinais et al., 2007). These studies would suggest that central fatigue could be observed in endurance activities lasting longer than one hour, which was not the case in the present study. Perhaps most relevant to this study are the contrasting results presented by Girard et al., (2012) and Nummela et al., (2006) where a decrease in plantar flexors (27%) and knee extensors (15%) MVIC force was revealed after 5 km running time trials. A notable difference between these two studies and the present study is the 5 versus 3 (present study) minutes of neuromuscular testing that were conducted between each of the five-kilometer repeats. Though this testing included maximal contractions and plyometric testing, heart rate profiles notably dropped between each repeat. In a review, Behm (2004) further explains that a preceding contraction could provide improved central adjustments via motoneuron excitation from both supraspinal and afferent input. This review gives evidence that supraspinal motor-evoked potentials have been facilitated in studies of different durations and intensities (Balbi et al., 2002). Further evidence is presented in the review that suggests that, at the spinal level, short term increases in explosive force following a conditioning contraction (MVIC) are attributable to enhanced neuromuscular activation (Gullich and Schmidtbleicher, 1996). These changes are supported by observed increases in reflex amplitude 10 minutes post-conditioning contraction (Trimble and Harp, 1998), and the possible 30% contribution of the motoneuron excitation to the discharge frequency from Ia afferents of the intrafusal stretch receptors (Gandevia, 1998).
Another possible explanation arising from the results of the well-trained runners in the present study is the presence of a centrally mediated pacing strategy that aims to inhibit substantial deterioration of muscle force, augmenting possible PAP effects. Noakes (2000) suggests that at, or shortly after, the onset of exercise of different durations and intensities, well-trained endurance athletes adopt a centrally mediated pacing strategy in order to complete the activity as efficiently as possible, while maintaining physiological capacity. In the case of the current study, the results suggest that the participants effectively avoided significant strength loss despite instructions to complete the running trial as fast as possible. This is not surprising as a review (Fuller et al., 1996) of pacing strategies demonstrates that well trained athletes effectively employ racing strategies that aid their ability to maintain a steady submaximal physiological effort. This idea of improved running economy during paced submaximal activities is in line with suggestions by Behm (2004) and Sale (2004) wherein they indicate that PAP may be most evident with submaximal contractions. As an athlete employs an effective pacing strategy to maintain lower submaximal frequency stimulation, suboptimal Ca2+activation (less than full release of Ca2+ from the sarcoplasmic reticulum) may be present, enabling PAP effects. This is explained by myosin regulatory light chain (RLC) phosphorylation (Grange et al., 1993; Houston et al., 1987) that takes place at the peripheral level under conditions of suboptimal Ca2+ activation and is thought to increase the number of force-producing cross-bridges (Sweeney and Stull, 1986), enabling an enhanced running economy. Thus, with the training background of the participants in this study, it is important to note that the results may be specific to this type of athlete rather than less-trained individuals.
The lack of fatigue also suggests the possibility of PAP performance improvements occurring at the peripheral level. The most notable finding at the peripheral level was a lack of impairment of the evoked contractile temporal properties (with the exception of potentiated and resting TPT both decreased by ~5% at kilometers 3-4 and 10 minutes post run), which is in line with other studies examining the peripheral effects of running (Millet et al., 2003a; Pageaux et al., 2017). This maintenance of contractile capacity has been demonstrated in endurance athletes after performing endurance exercises of different durations (Finni et al., 2003; Nicol et al., 1991; 2007). This was initially evident after a 65 km ultra-marathon running event in which a severely depressed maximal voluntary force capacity was reported concurrently with a surprising potentiated twitch mechanical response and shorter contraction/half-relaxation times (Millet et al., 2003a).
These results are contradicted by a study examining the fatigue effects of endurance running (Nicol et al., 1991). Nine experience male runners were recruited to run a timed marathon with neuromuscular tests pre- and post-marathon. Sprint velocity, MVIC force, ground reaction forces, and stride profiles were all negatively affected by the marathon intervention. This coincides with a study conducted by Finni et al. (2003) where reductions in EMG amplitude, superimposed doublet twitch, and rate of force development were found after participants completed a 10-km running trial. A notable difference between these studies and the present one is the timing of the neuromuscular tests, as the present study required testing at each kilometer interval instead of a basic pre-test and post-test.
Furthermore, another obvious difference between these studies is the selected distance. Exertion during a marathon distance of 42 kilometers or more is more likely to cause low frequency fatigue (Giandolini et al., 2016; Millet, 2011) and is presumably greater than that of a five kilometer time trial. Though these differences could explain the contradicting results, peripheral PAP effects have been demonstrated in other studies examining performance measures at intervals during running trials (Del Rosso et al., 2016). Additionally, in a similar study with endurance runners, participants ran 6km at anaerobic threshold (VOBLA) and the results showed no sign of contractile enhancement, suggesting a balance of fatigue and potentiation via an impairment of the excitation-contraction coupling (Skof et al., 2006a). The mean maximum twitch torque, EMG activation, and maximal voluntary force either dropped or showed no change at both stimulation frequencies though maximum twitch torque was enhanced 10 minutes post run. Skof et al. (2006b) performed another study that demonstrated potentiating effects in twitch torque 10 minutes after a 5 X 300 m interval workout despite evidence of low-frequency fatigue.
With the nature of the shorter distance being run at a higher intensity in the present study, low frequency fatigue would have been the most likely to prevail. The lack of impairments and prevalence of improvements (increase potentiated twitch force) would suggest that low frequency fatigue was not manifested to a great extent in the current study. An explanation for this finding could come from the proposed mechanism of PAP (membrane excitability and excitation-contraction coupling). Peripheral fatigue is often linked to excitation-contraction coupling failure and correlates with the reduced efficiency of the calcium cycle (Fitts, 1994). Sahlin (1986) provided evidence that intracellular pH is directly connected to calcium cycle efficiency. Consequently, a drop in pH resulting from increased amounts of metabolites like H+, ADP, Pi can inhibit the release of Ca2+ from the sarcoplasmic reticulum (Fitts, 1994; Metzger and Moss, 1987) and could reduce Ca2+ affinity for troponin (Hultman et al., 1986), reducing the muscle contractile force. The fact that the contractile properties remained the same, or increased demonstrates that peripheral fatigue was either not present or its effects were masked or balanced due to the effects of PAP. When calcium molecules are released during a muscle contraction, myosin light chain kinase is released, which catalyzes the phosphorylation of the RLC’s at the end of the myosin head (Tillin and Bishop, 2009). The PAP-induced phosphorylation of the RLC is believed to potentiate the following contractions by causing it to move away from its thick filament backbone, more poised to efficiently interact with the actin (Hodgson et al., 2005). It is these proposed molecular-level changes that are thought to explain the noted enhancement in evoked contractile temporal properties and consequent decrease in time to complete the running trials.
Possibly, the most significant effects of PAP leading to faster run times were evident in the increased performance of the DJ. This was manifested in the 15.7% increase in reactive strength index from pre-test to kilometer four in the jump squat condition. A 9% increase in jump height from pre-test to kilometer 1, 8% in kilometer two, and 8.5% in kilometer four in the jump squat condition also provide evidence for PAP. This is supported by two studies by Paavolainen et al., (1999; 2003) where positive correlations between jump performance and velocity in running a five kilometer time trial were observed. These results suggest that rapid force production can be beneficial not only for jumpers and sprinters but also for endurance runners. PAP can induce greater muscle stiffness positively affecting the stretch-shortening cycle (SSC) increasing muscle force and power (Behm, 2004), contributing to more efficient running economy (Nicol et al., 2007). For example, Heise and Martin (1998) observed an inverse relationship between leg stiffness and running economy and concluded that those with a less economical running style were more compliant during ground contact. Likewise, Dalleau et al., (1998) discovered that energy cost during treadmill running was significantly correlated to the stiffness of the propulsive leg. These authors explain that increased leg stiffness would increase the ability to absorb and restitute power during a forceful SSC (as seen in running). Furthermore, an increase in reactive power from increased leg stiffness would render the SSC more efficient and consequently decrease the energy cost of running, enabling a faster pace and time. Moreover, findings of other studies (Cleak and Eston, 1992; Spurrs et al., 2003) examining increased leg stiffness following intense exercise bouts could imply that the band-resisted squat jumps served to increase leg stiffness and consequent running economy. It is on this basis that we propose that the increased jump height, reactive strength index, and subsequent time to complete the kilometer repeats can also be explained by an increased leg stiffness and reactive power, rendering the SSC more effective and consequently more economical, enabling the participant to run at a faster pace.
A limitation could be considered that, as with any study conducted on a treadmill in a laboratory setting, it is also important to note that different results could possibly be obtained from conducting a similar study in a free-living environment. Participant bias and pacing is always a possible limitation, however, we attempted to control for pre-conceived pacing notions by blinding the participants to speed, time, and pacing. Future research should investigate these effects with females or populations with varying training status.
Conclusion
This is the first study to show that a 5RM jump squat conditioning stimulus positively affected neuromuscular and performance changes resulting in decreased time to complete the running task, increased force generation, voluntary activation, and evoked contractile temporal properties. The transient nature of the improvements observed post-conditioning contraction suggest that there are multiple factors responsible for mediating the changes in neuromuscular and performance measures during running. Based on the evidence presented, the researchers affirm the hypothesis that performing a 5RM weighted jump squat protocol as part of a standardized running-specific warm-up will induce significant measurable PAP effects during the course of a subsequent 5 km time trial run and up to 7-minutes post-run protocol. The results from this experiment are of particular importance to coaches, athletes and practitioners, who must consider the most effective method of warming up to increase and maintain performance.
Acknowledgements
The authors report no conflict of interest. All experimental procedures used, comply with local governmental laws for human subjects.
Biographies

Jonathan L. LOW
Employment
Product Performance Analyst (Garmin)
Degree
MSc (Kinesiology)
Research interests
Exercise Science, Fitness wearables
E-mail: jllow@mun.ca

Hamid AHMADI
Employment
Graduate Student
Degree
MSc (Kinesiology)
Research interests
Exercise Science
E-mail: hahmadi@mun.ca

Liam P. KELLY
Employment
Clinical Exercise Physiologist
Degree
PhD
Research interests
Stroke Rehabilitation and Performance
E-mail: lpkelly@mun.ca

Jeffrey WILLARDSON
Employment
Professor
Degree
PhD
Research interests
Exercise Science, resistance training
E-mail:Jeffrey.willardson@msubillings.edu

Daniel BOULLOSA
Employment
Professor
Degree
PhD
Research interests
Exercise Science
E-mail: d_boullosa@yahoo.es

David G BEHM
Employment
Professor
Degree
PhD
Research interests
Applied neuromuscular physiology
E-mail: dbehm@mun.ca
References
- Ah Sue R., Adams K., Debeliso M. (2016) Optimal timing for post-activation potentiation in women collegiate volleyball players. Sports 4, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avela J., Komi P.V. (1998) Interaction between muscle stiffness and stretch reflex sensitivity after long-term stretch-shortening cycle exercise. Muscle Nerve 21, 1224-1227. [DOI] [PubMed] [Google Scholar]
- Balbi P., Perretti A., Sannino ,, Marcantonio L., Santoro L. (2002) Post-exercise facilitation of motor evoked potentials following transcranial magnetic stimulation: A study in normal subjects. Muscle Nerve 25, 448-452. [DOI] [PubMed] [Google Scholar]
- Bauer P., Sansone P., Mitter B., Makivic B., Seitz L.B., Tschan H. (2019) Acute effects of back squats on countermovement jump performance across multiple sets of a contrast training protocol in resistance-trained males. Journal of Strength and Conditioning Research 33(4), 995-1000. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Sale D.G. (1993) Intended rather than actual movement velocity determines velocity-specific training response. Journal of Applied Physiology 74, 359-368. [DOI] [PubMed] [Google Scholar]
- Behm D.G., St-Pierre D.M., Perez D. (1996) Muscle inactivation: Assessment of interpolated twitch technique. Journal of Applied Physiology 81(5), 2267-2273. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Button D.C., Butt J.C. (2001) Factors affecting force loss with prolonged stretching. Canadian Journal of Applied Physiology 26(3), 262-272. [PubMed] [Google Scholar]
- Behm D.G., Whittle J., Button D., Power K. (2002) Intermuscle differences in activation. Muscle Nerve 25(2), 236-243. [DOI] [PubMed] [Google Scholar]
- Behm D.G. (2004) Force maintenance with submaximal fatiguing contractions. Canadian Journal of Applied Physiology 29(3), 274-290 [DOI] [PubMed] [Google Scholar]
- Booth J., Mckenna M.J., Ruell P.A., Gwinn T.H., Davis G.M., Thompson M.W., Harmer A.R., Hunter S.K., Sutton J.R. (1997) Impaired calcium pump function does not slow relaxation in human skeletal muscle after prolonged exercise. Journal of Applied Physiology 83(2), 511-521. [DOI] [PubMed] [Google Scholar]
- Boullosa D., Tuimil J. (2009) Postactivation potentiation in distance runners after two different field running protocols. Journal of Strength and Conditioning Research 23, 1560-1565. [DOI] [PubMed] [Google Scholar]
- Boullosa D., Rosso S.D., Behm D.G., Foster C. (2018) Post-activation potentiation (PAP) in endurance sports: A review. European Journal of Sport Science 18, 595-610. [DOI] [PubMed] [Google Scholar]
- Cleak M.J., Eston R.G. (1992) Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. British Journal of Sports Medicine 26, 267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioural sciences. Hillside, N.J [Google Scholar]
- Costill D.L., Fink W.J., Pollock M.L. (1976) Muscle fiber composition and enzyme activities of elite distance runners. Medicine Science Sports and Exercise 8(2), 96-100. [PubMed] [Google Scholar]
- Dalleau G., Belli A., Bourdin M., Lacour J.R. (1998) The spring-mass model and the energy cost of treadmill running. European Journal of Applied Physiology and Occupational Physiology 77, 257-263 [DOI] [PubMed] [Google Scholar]
- Del Rosso S., Barros E., Tonello L., Oliveira-Silva I., Behm D., Foster C., Boullosa D. (2016) Can pacing be regulated by post-activation potentiation? Insights from a self-paced 30 km trial in half-marathon runners. PLoS ONE 11(3), e0150679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driller M., Brophy-Williams N., Walker A. (2016) The reliability of a 5km run test on a motorized treadmill. Measurement in Physical Education and Exercise Science 21(3), 121-126. [Google Scholar]
- Finni T., Kyröläinen H., Avela J., Komi P. (2003) Maximal but not submaximal performance is reduced by constant-speed 10-km run. Journal of Sports Medicine and Physical Fitness 43(4), 411-417 [PubMed] [Google Scholar]
- Fitts R.H. (1994) Cellular mechanisms of muscle fatigue. Physiological Reviews 74, 49-94. [DOI] [PubMed] [Google Scholar]
- Fuller D., Sullivan J., Fregosi R.F. (1996) Expiratory muscle endurance performance after exhaustive submaximal exercise. Journal of Applied Physiology 80, 1495-502 [DOI] [PubMed] [Google Scholar]
- Gandevia S.C. (1998) Neural control in human muscle fatigue: Changes in muscle afferents, motoneurones and motocortical drive. Acta Physiologica Scandinavica 162, 275-283 [DOI] [PubMed] [Google Scholar]
- Giandolini M., Gimenez P., Temesi J., Arnal P.J., Martin V., Rupp T., Millet G.Y. (2016) Effect of the fatigue induced by a 110-km ultramarathon on tibial impact acceleration and lower leg kinematics. Plos One 11(3), e0151687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A.S. (2004) Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. British Journal of Sports Medicine 38(6), 797-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard O., Lattier G., Maffiuletti N., Micallef J., Millet G. (2008) Neuromuscular fatigue during a prolonged intermittent exercise: Application to tennis. Journal of Electromyography and Kinesiology 18, 1038-1046. [DOI] [PubMed] [Google Scholar]
- Girard O., Millet G., Micallef J., Racinais S. (2012) Alteration in neuromuscular function after a 5 km running time trial. European Journal of Applied Physiology 112, 2323-2330. [DOI] [PubMed] [Google Scholar]
- Gooyers C.E., Beach T.A., Frost D.M., Callaghan J.P. (2012) The influence of resistance bands on frontal plane knee mechanics during body-weight squat and vertical jump movements. Sports Biomechanics 11(3), 438-439. [DOI] [PubMed] [Google Scholar]
- Grange R., Vandenboom R., Houston M. (1993) Physiological significance of myosin phosphorylation in skeletal muscle. Canadian Journal of Applied Physiology 18, 229-242. [DOI] [PubMed] [Google Scholar]
- Gullich A., Schmidtbleicher D. (1996) MVC-induced short-term potentiation of explosive force. New Studies in Athletics 11(4), 67-81. [Google Scholar]
- Hamada T., Sale D.G., MacDougall J.D. (2000a) Post-activation potentiation in endurance-trained male athletes. Medicine Science Sports and Exercise 32, 403. [DOI] [PubMed] [Google Scholar]
- Hamada T., Sale D.G., MacDougall J.D., Tarnopolsky M.A. (2000b) Post-activation potentiation, fiber type, and twitch contraction time in human knee extensor muscles. Journal of Applied Physiology 88(6), 2131-2137. [DOI] [PubMed] [Google Scholar]
- Heise G.D., Martin P.E. (1998) ‘Leg spring’ characteristics and the aerobic demand of running. Medicine Science Sports and Exercise 30, 750-754 [DOI] [PubMed] [Google Scholar]
- Hodgson M., Docherty D., Robbins D. (2005) Post-activation potentiation underlying physiology and implications for motor performance. Sports Medicine 35(7), 585-595. [DOI] [PubMed] [Google Scholar]
- Houston M., Lingley M., Stuart D., Grange R. (1987) Myosin light chain phosphorylation in intact human muscle. FEBS Letters 219(2), 469-471. [DOI] [PubMed] [Google Scholar]
- Hultman E., Spriet L.L., Soderlund K. (1986) Biochemistry of muscle fatigue. Biomedica Biochimica Acta 45(supplement), 97-106. [PubMed] [Google Scholar]
- Léger L., Boucher R. (1980) An indirect continuous running multistage field test: the Université de Montréal track test. Canadian Journal of Sport Sciences 5, 77-84. [PubMed] [Google Scholar]
- Masamoto N., Larson R., Gates T., Faigenbaum A. (2003) Acute effects of plyometric exercise on maximum squat performance in male athletes. Journal of Strength and Conditioning Research 17(1), 68-71. [DOI] [PubMed] [Google Scholar]
- Mettler J., Griffin L. (2012) Post-activation potentiation and muscular endurance training. Muscle Nerve 45, 416-425. [DOI] [PubMed] [Google Scholar]
- Metzger J.M., Moss R.L. (1987) Shortening velocity in skinned single muscle fibres. Biophysical Journal 52, 127-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet G.Y. (2011) Can neuromuscular fatigue explain running strategies and performance in ultra-marathons? Sports Medicine 41(6), 489-506. [DOI] [PubMed] [Google Scholar]
- Millet G.Y., Lepers R., Maffiuletti N., Babault N., Martin V., Lattier G. (2003) Alterations of neuromuscular function after an ultramarathon. Journal of Applied Physiology 92, 486-492. [DOI] [PubMed] [Google Scholar]
- Millet G.Y., Millet G.P., Lattier G., Maffiuletti N., Candau R. (2004) Alteration of neuromuscular function after a prolonged road cycling race. International Journal of Sports Medicine 24, 190-194. [DOI] [PubMed] [Google Scholar]
- Mitchell C., Sale D. (2011) Enhancement of jump performance after a 5-RM squat is associated with post-activation potentiation. European Journal of Applied Physiology 111, 1957-1963. [DOI] [PubMed] [Google Scholar]
- Nicol C., Komi P., Marconnet P. (1991) Changes in muscle force and stiffness characteristics. Scandinavian Journal of Medicine & Science in Sports 1, 10-17. [Google Scholar]
- Nicol C., Komi P.V., Marconnet P. (2007) Fatigue effects of marathon running on neuromuscular performance. Scandinavian Journal of Medicine & Science in Sports 1(1), 18-24. [Google Scholar]
- Newton R.U., Robertson M., Dugan E., Hanson C., Cecil J., Gerber A., Hill J., Schwier L. (2002) Heavy elastic bands alter force, velocity, and power output during back squat lifts. Journal of Strength Conditioning Research 16, 1-18 [Google Scholar]
- Noakes T.D. (2000) Physiological models to understand exercise fatigue and the adaptations that predict or enhance athletic performance. Scandinavian Journal of Medicine & Science in Sports 10, 123-145 [DOI] [PubMed] [Google Scholar]
- Noakes T.D. (2011) Time to move beyond a brainless exercise physiology: the evidence for complex regulation of human exercise performance. Applied Physiology, Nutrition, and Metabolism 36(1), 23-35. [DOI] [PubMed] [Google Scholar]
- Nummela A., Paavolainen L., Sharwood K., Lambert M., Noakes T., Rusko H. (2006) Neuromuscular factors determining 5 km running performance and running economy in well-trained athletes. European Journal of Applied Physiology 97, 1-8. [DOI] [PubMed] [Google Scholar]
- Paavolainen L., Hakkinen K., Hamalainen I., Nummela A., Rusko H. (2003) Explosive-strength training improves 5-km running time by improving running economy and muscle power. Scandinavian Journal of Medicine & Science in Sports 13(4), 272-272. [DOI] [PubMed] [Google Scholar]
- Paavolainen L.M., Nummela A.T., Rusko H.K. (1999) Neuromuscular characteristics and muscle power as determinants of 5-km running performance. Medicine Science Sports and Exercise 31, 124-130 [DOI] [PubMed] [Google Scholar]
- Pageaux B., Theurel J., Lepers R. (2017) Cycling versus uphill walking: Impact on locomotor muscle fatigue and running exercise. International Journal of Sports Physiology and Performance 12, 1310-1318. [DOI] [PubMed] [Google Scholar]
- Palmer C., Jones A., Kennedy G., Cotter J. (2009) Effects of prior heavy exercise on energy supply and 4000-m cycling performance. Medicine Science Sports and Exercise 41, 221-229. [DOI] [PubMed] [Google Scholar]
- Racinais S., Girard O., Micallef J.P., Perrey S. (2007) Failed excitability of spinal motoneurons induced by prolonged running exercise. Journal of Neurophysiology 97(1), 596-603. [DOI] [PubMed] [Google Scholar]
- Rixon K.P., Lamont H.S., Bemben M.G. (2007) Influence of type of muscle contraction, gender, and lifting experience on post-activation potentiation performance. Journal of Strength and Conditioning Research 21(2), 500-505. [DOI] [PubMed] [Google Scholar]
- Rossiter H.B., Kowalchuk J.M., Whipp B.J. (2006) A test to establish maximum O2 uptake despite no plateau in the O2 uptake response to ramp incremental exercise. Journal of Applied Physiology 100(3), 764-770. [DOI] [PubMed] [Google Scholar]
- Rupp T., Girard O., Perrey S. (2009) Redetermination of the optimal stimulation intensity modifies resting H-reflex recovery after a sustained moderate-intensity muscle contraction. Muscle Nerve 41(5), 642-650. [DOI] [PubMed] [Google Scholar]
- Sahlin K. (1986) Muscle fatigue and lactic acid accumulation. Acta Physiologica Scandinavica 128, 83-91 [PubMed] [Google Scholar]
- Sale D.G. (2004) Post-activation potentiation: role in human performance. Exercise and Sport Sciences Reviews 30(3), 138-143. [DOI] [PubMed] [Google Scholar]
- Silva R., Silva-Júnior F., Pinheiro F., Souza P., Boullosa D., Pires F. (2014) Acute prior heavy strength exercise bouts improve the 20-km cycling time trial performance. Journal of Strength and Conditioning Research 28, 2513-2520. [DOI] [PubMed] [Google Scholar]
- Škof B., Strojnik V. (2006a) Neuro-muscular fatigue and recovery dynamics following anaerobic interval workload. International Journal of Sports Medicine 27, 220-225. [DOI] [PubMed] [Google Scholar]
- Škof B., Strojnik V. (2006b) Neuromuscular fatigue and recovery dynamics following prolonged continuous run at anaerobic threshold. British Journal of Sports Medicine 40, 219-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Škof B., Strojnik V. (2007) The effect of two warm-up protocols on some biomechanical parameters of the neuromuscular system of middle distance runners. Journal of Strength and Conditioning Research 21(2), 394-399 [DOI] [PubMed] [Google Scholar]
- Spurrs R.W., Murphy A.J., Watsford M.L. (2003) The effect of plyometric training on distance running performance. European Journal of Applied Physiology 89(1), 1-7. [DOI] [PubMed] [Google Scholar]
- Støren Ø., Helgerud J., Støa E.M., Hoff J. (2008) Maximal strength training improves running economy in distance runners. Medicine Science Sports and Exercise 40(6), 1087-1092. [DOI] [PubMed] [Google Scholar]
- Sweeney H.L., Stull J.T. (1986) Phosphorylation of myosin in permeabilized mammalian cardiac and skeletal muscle cells. American Journal of Physiology-Cell Physiology 250(4), C657-660. [DOI] [PubMed] [Google Scholar]
- Tillin N.A., Bishop D. (2009) Factors modulating post-activation potentiation and its effect on performance of subsequent explosive activities. Sports Medicine 39(2), 147-166. [DOI] [PubMed] [Google Scholar]
- Trimble M.H., Harp S.S. (1998) Post-exercise potentiation of the H-reflex in humans. Medicine Science Sports and Exercise 30, 933-941 [DOI] [PubMed] [Google Scholar]
- Vøllestad N.K., Sejersted I., Saugen E. (1997) Mechanical behavior of skeletal muscle during intermittent voluntary isometric contractions in humans. Journal of Applied Physiology 83(5), 1557-1565. [DOI] [PubMed] [Google Scholar]
- Willy R.W., Davis I.S. (2011) The effect of a hip-strengthening program on mechanics during running and during a single-leg squat. Journal of Orthopaedic & Sports Physical Therapy 41(9), 625-632. [DOI] [PubMed] [Google Scholar]
- Wilson J.M., Duncan N.M., Marin P.J., Brown L.E., Loenneke J.P., Wilson S.M., Jo E., Lowery R.P., Ugrinowitsch C. (2013) Meta-analysis of post-activation potentiation and power. The Journal of Strength & Conditioning Research 27(3), 854-859. [DOI] [PubMed] [Google Scholar]
- Young W.B., Behm D.G. (2003) Effects of running, static stretching and practice jumps on explosive force production and jumping performance. Journal of Sports Medicine and Physical Fitness 43, 21-27. [PubMed] [Google Scholar]


