Abstract
Exercise program has been associated with improved cardiovascular outcomes in patients sustaining coronary artery disease. However, little is known about the role of exercise after percutaneous coronary intervention (PCI). Published literature was searched from Embase, PubMed, Wanfang Data, Cochrane Database of Systematic Reviews, China National Knowledge Infrastructure (CNKI) and Central Database. Exercise versus no exercise following PCI in the patients with coronary heart disease (CHD) was investigated in randomized trials. Left ventricular end diastolic diameter (LVEDD), left ventricular ejection fraction (LVEF), 6-minute walking distance (6MWD), cardiac death, myocardial infarction, coronary angioplasty, coronary artery bypass surgery (CABG), and angina pectoris or restenosis per randomized patients were analyzed by meta-analytic procedure to compare the curative effect of exercise program with exclusive exercise program after PCI. Ten randomized controlled trials including 1274 subjects (636 in exercise group and 638 in control group) were analyzed. The meta-analysis demonstrated that LVEF was significantly improved in exercise group (MD = 2.82, 95% CI [1.50, 4.14], p < 0.05). In contrast, the incidence rate of cardiac death (RR = 0.24, 95% CI [007, 0.76], p = 0.02), myocardial infarction (RR = 0.23, 95% CI [0.09, 0.57], p = 0.002), coronary angioplasty (RR = 0.47, 95% CI [0.26, 0.84], p = 0.01), angina pectoris (RR = 0.39, 95% CI [0.24, 0.64], p = 0.0002) and restenosis (RR = 0.36, 95% CI [0.16, 0.83], p = 0.02) were significantly lower in exercise group. LVEDD (MD = -2.01, 95% CI [-4.72, 0.70]), 6MWD (MD = 50.85, 95% CI [-13.24, 114.94]), and CABG (RD = -0.01, 95% CI [-0.05, 0.03]) were not significantly different in the patients with or without exercise (p = 0.71). Trial sequential analysis reflected traditional meta-analysis might generate a false positive conclusion for MI and cardiac death. There was no firm evidence to support the beneficial effects of exercise after PCI for the CHD patients to improve heart function or to reduce the incidence of adverse cardiovascular events.
Key points.
The role of exercise after PCI for CAD patients was investigated.
A meta-review was conducted based on 10 RCTs.
There was no firm evidence to support the beneficial effects of exercise after PCI for CHD patients.
Key words: Exercise, percutaneous coronary intervention, coronary heart disease, heart function, cardiovascular events, meta-analysis
Introduction
Cardiovascular disease (CVD) is a common cause of socio-economic and healthcare problems globally. Morbidity, mortality and disability caused by CVD are on the rise annually, accounting for the world 30% of all-cause mortality and 10% of disability (Wong et al., 2012). By far, the coronary heart disease (CHD) is the major cause of death worldwide. Percutaneous coronary intervention (PCI) has been widely used in the treatment of CHD, which greatly improves survival rate and quality of life in patients with CVD, especially angina pectoris or myocardial infarction (Hillis et al., 2012; Yuan and Chen, 2013). However, PCI preoperative coronary stenosis or occlusion following the pathological changes in myocardial ischemia or necrosis may seriously influence cardiac function, and PCI postoperative restenosis and myocardial ischemia may further impact the long-term curative effect of PCI(Buttar et al., 2005). In addition to strengthening the antithrombotic, effective rehabilitation exercise training can improve ischemic myocardial blood supply, improve the patient’s quality of life, reduce mortality, and prevent development of the coronary atherosclerosis.
At present, a large number of randomized controlled trials have shown that exercise can delay the process of atherosclerosis and reduce the incidence of CHD such as arterial hypertension, diabetes mellitus, and hypercholesterolemia. A systematic review of 63 randomized trials of exercise-based cardiac rehabilitation, which included patients suffered a myocardial infarction, or undergone either coronary artery bypass graft (CABG) or PCI procedures, reported an absolute reduction in the risk of cardiovascular mortality from 10.4% to 7.6% (Abell et al., 2017). Most studies also showed improvements in quality of life and a reduction in acute hospital admissions. There were also other evidence-based studies confirmed that exercise-based cardiac rehabilitation reduced cardiovascular mortality and improved quality of life (Anderson et al., 2016; Fletcher et al., 2018; Lavie et al., 2016). Those findings raise a question, that is, whether the combination of exercise and PCI results in better outcome than PCI alone. Therefore, this systematic review was designed to analyze outcomes of combination of PCI and exercise in comparison to PCI alone.
Methods
Search strategy
In this systematic review, randomized controlled trials (RCT) were searched from Embase (1980–July 2018), PubMed (1966–July 2018), Wanfang Data (1998 –July 2018), Cochrane Database of Systematic Reviews (Issue 1, 2018), Chinese National Knowledge Infrastructure (CNKI, 1980–July 2018) and Cochrane Central Register of Controlled Trials (CENTRAL). The following keywords were used for searching databases: coronary disease, CHD, sport, exercise, physical fitness, rehabilitation, physical activity, training, percutaneous coronary intervention, angioplasty, and PCI. The bibliographies of retrieved trials as well as the review articles were retrieved. If there was a lack of information or test report was unknown for the meta-analysis, researchers would write letters to pertinent authors to get the information and report, so that the inclusion of literature data could be maximized.
Selection of studies
The inclusion criteria were the articles that were associated with: 1) Patients with established CHD who underwent coronary stenosis < 50% after PCI; 2) Comparisons of exercise versus usual care without exercise; 3) More than one outcome measurements as following: Left ventricular end diastolic diameter (LVEDD), Left ventricular ejection fraction (LVEF), 6-minute walking distance (6MWD), cardiac death, myocardial infarction, coronary angioplasty, angina pectoris, coronary artery bypass surgery (CABG), and restenosis; 4) Publication in English or Chinese; 5) RCTs. The articles were excluded based on the four exclusion criteria: 1) Studies related to neither exercise nor PCI; 2) Inaccurate or incomplete report, or providing no outcome study; 3) Duplicated publications; 4) Observational studies or case report.
Each aforementioned trials identified by searching activities were distributed to one review topic or topics. The data that were extracted from review were input in Thomson Research Software (EndNote X4) to examine their accuracy. If there were any unclear information, further details would be offered by the original reports. “Pending”, “included” and “excluded (reason)” were listed in the column of “notes”, and from references, the “pending” reports would be retraced.
Quality assessment
Based on the Cochrane handbook for Systematic Reviews of Interventions 5.1.0 recommended by Cochrane Collaboration, an assessment was made on the quality of the inclusive studies. There are 7 aspects included in the evaluation items: allocation concealment, double blind of both provider and participants, blind of the outcome assessment, generation of random sequence, selective reporting, incomplete outcome data and other biases. All documents conforming to these 7 items were judged as “low risk”, “unclear risk” as well as “high risk”.
Data extraction
One form was designed for the extraction of data, which included first author, publication year, objects` features, follow-up, treatment types and outcome measurements.
The selection of literature, extraction of data and quality assessment were implemented by the two authors (HZ and RC). In case of disagreement, it was discussed and consulted with a third person.
Statistical analysis
The statistical analysis was conducted in Review Manager (RevMan5.3) produced by Cochrane Collaboration. The Risk Ratio (RR) and 95% CI showed the meta-analysis result for binary data classification. Mean difference (MD) and 95% CI showed the meta-analysis result for continuous outcomes.
Chi-square test was used to measure the heterogeneity among all studies, and the estimation of I2 was also made. A fixed effect model would be adopted if heterogeneity was not significant in all the inclusive studies (P > 0.1, I2 ≤ 50%); while random model was used if the heterogeneity was statistically significant in all-inclusive studies (p ≤ 0.1, I2 >50%). The subgroup analyses on the basis of interventions were supposed to be proposed, so that heterogeneity sources would be detected, if sources could not be identified, the combined random effects model was used. Small-study publication bias was assessed by examining Begg’s and Egger’s test.
Trial sequential analysis
Trial sequential analysis (TSA) is a method for estimating sample size, which can adjust random errors and calculate the sample size, by using the TSA 0.9 Beta (available at http://www.ctu.dk/tsa). We estimated a diversity-adjusted and required information size (DARIS), consisted of 2-sided testing, type I error α = 5% and power = 80%. We tested the hypothesis that IVUS guidance could yield a 50% relative reduction in the risk of myocardial infarction and cardiac death, respectively, with an anticipated event rate of 5% for myocardial infarction and 4% for cardiac death in the control group. The main results were displayed in a graph of the cumulative Z curve, and the O’Brien-Fleming α-spending function was used to determine the boundaries in this graph for concluding superiority, inferiority, or non-inferiority.
Results
Study selection
Total 502 articles were found with the search strategy. After excluding the duplicates, 467 studies were screened, and 447 impertinent citations were excluded. After the full texts of 20 inclusive articles and those in pending lists were reviewed, other 10 articles were excluded. Finally, only 10 published RCTs (Belardinelli et al., 2001; Hofman-Bang et al., 1999; Kubo et al., 1992; Lee et al., 2013; Mei et al., 2009; Munk et al., 2009; Niu et al., 2017; Vasiliauskas et al., 2007; Wang, 2016; Xu et al., 2017) from 1992 to 2017 were included in this meta-analysis, with 6 English articles and 4 Chinese articles (Figure 1).
Figure 1.
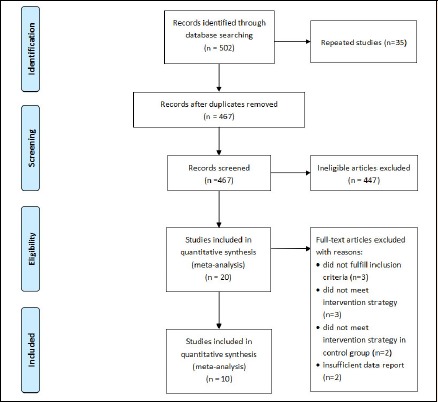
Flow diagram of study searching strategy.
Quality assessment and publication bias
Figure 2 and Figure 3 showed the summary of results of the quality assessment for inclusive studies. One trial was double-blinded (Belardinelli et al., 2001). There were 5 trials (Belardinelli et al., 2001; Mei et al., 2009; Munk et al., 2009; Wang, 2016; Xu et al., 2017) reference to randomization used computer-generated random sequence as well as random number tables, while other studies no description about how to carry out randomization. None of the included trials described how to allocate patients concealment and there were comparable baselines in 9 trials (Belardinelli et al., 2001; Hofman-Bang et al., 1999; Kubo et al., 1992; Lee et al., 2013; Mei et al., 2009; Munk et al., 2009; Vasiliauskas et al., 2007; Wang, 2016; Xu et al., 2017). The blinded data analysis was independent of trial of each study. The incomplete or selective report did not exist. Generally, the quality of the studies of this meta-analysis was moderate.
Figure 2.
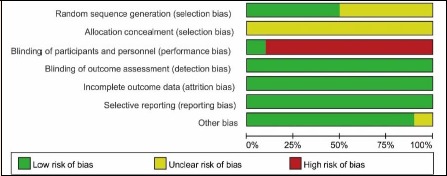
Quality assessment summary for included studies.
Figure 3.
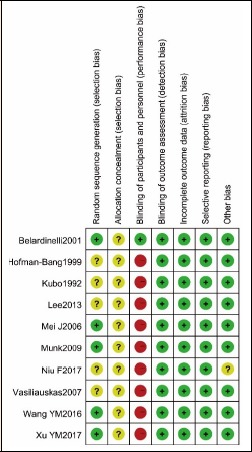
Methodological quality assessment for each included study
The P value of Begg’s test for LVEF was 1.000, Egger’s test was 0.996. The P value of Begg’s test for MI was 0.707, Egger’s test was 0.76. Neither Begg’s test nor Egger’s test showed the potential for publication bias.
Characteristics of study selection
The characteristics of the inclusive studies were shown in Table 1. This meta-analysis involved a total of 1274 CHD patients (randomized population, 636 in exercise group and 638 in control group) who received PCI. The mean age of participants ranged between 53 and 61, the sample size was between 38 and 300 people, and the follow-up time was between 6 and 38 months. The characteristics of the experimental interventions and control interventions were variable.
Table 1.
Characteristics of included studies.
| Author, year | Sample size, gender M/F | Mean age, years (SD) | Description of PCI | Exercise interventions | Control interventions | Outcome measures | Follow-up |
|---|---|---|---|---|---|---|---|
| Belardinelli, 2001 | Exercise: 59,49/10 Control: 59,50/9 |
53(11) 59(10) |
PCI with or without stent | Begin exercise 25d after PCI; 30 min stretching, calisthenics and pedaling; program were supervised by cardiologist 3 times per week lasted 6 months. | Basic daily mild physical activities and pharmacological therapy | LVEF; LVEDD; cardiac death; myocardial infarction; coronary angioplasty; CABG; restenosis | 6 months |
| Hofman-Bang, 1999 | Exercise: 46,37/9 Control: 41,36/5 |
53(7) 53(7) |
PCI with stent | Begin physical exercise after PCI; 4 times per week lasted 12 months. | Usual care | Myocardial infarction; CABG | 24 months |
| Kubo, 1992 | Exercise: 18,14/4 Control: 20,16/4 |
59(12) 58(10) |
PCI | Begin exercise 7d after PCI; 30-40 min treadmill or out-door walk-run program; 6 times per week lasted 12 weeks | No exercise | Restenosis | 12 weeks |
| Lee, 2013 | Exercise: 37,30/7 Control: 37,31/6 |
58.8 (10.8) 60.3(8.7) |
PCI with drug-eluting stent | Begin exercise after PCI; 10 minute warm-up, 40 minute aerobic exercise on a treadmill or bicycle ergometer, 10 minute cool-down phase; supervised exercise under prescription for 6 weeks and community-based / self-managed exercise 6 times per week over 9 months | No exercise | Cardiac death; myocardial infarction; CABG | 9 months |
| Mei J, 2006 | Exercise: 150 Control: 150 |
67.0 (9.1) | PCI with stent | Begin exercise after PCI; 10-20 minutes walking every time; 2 times per day and 14 times per week lasted 6 months | Usual care | Cardiac death; myocardial infarction; coronary angioplasty; CABG; restenosis; angina pectoris | 6, 12, 38 months |
| Munk, 2009 | Exercise: 20,17/3 Control: 20,16/4 |
57(14) 61(10) |
PCI with drug-eluting stent or bare metal stent | Begin 11 ± 4 d after PCI; 1 hour of interval training on treadmill or treadmill + strength training; 3 times a week lasted 6 months | No exercise and pharmacological therapy | Myocardial infarction; coronary angioplasty; CABG;angina pectoris | 6 months |
| Niu F, 2017 | Exercise: 124,63/61 Control: 132,73/59 |
62.63 (2.23) 62.32 (2.53) |
PCI | Begin 30 days after PCI; 20-30 minutes walking every time; 2 times per week lasted 8-12 months. | Usual care | LVEF; cardiac death; myocardial infarction; angina pectoris | 6 months |
| Vasiliauskas, 2007 | Exercise: 95 Control: 90 |
58.4 (4.3) 59.5 (5.2) |
PCI with balloon dilatation | Begin shortly after PCI; 1month stationary, 3 months outpatient, 2 months at home; 4 months 20 to 40 min treadmill, 2 months 30–60 min treadmill; 7 times per week lasted 6 months | Pharmacological therapy | LVEF; LVEDD; CABG; angina pectoris; restenosis; 6MWD | 6 months |
| Wang, 2016 | Exercise: 29,20/9 Control 29,19/10 |
55.28(3.10) 54.14(4.87) |
PCI with stent | Begin exercise after PCI; 30-60 minutes treadmill every time; 3-4 times per week lasted 6 weeks | Pharmacological therapy | LVEF | 6 weeks |
| Xu, 2017 | Exercise: 58,46/132 Control: 60,47/13 |
56.40(8.12) 58.62(7.96) |
PCI | Begin exercise after PCI; 1-2 months 30 to 40 minutes walking, 2-3 months 30–40 minutes rehabilitation gymnastics exercise; 3 times per week lasted 3 months. | Usual care | LVEF; LVEDD; 6MWD | 6 months |
Cardiac function: LVEF
Five RCTs (Belardinelli et al., 2001; Niu et al., 2017; Vasiliauskas et al., 2007; Wang, 2016; Xu et al., 2017) reported 373 and 362 patients who received PCI in exercise group and control group, respectively. This meta-analysis demonstrated that the statistical heterogeneity existed between the two groups (p = 0.69, I2 = 0.01%), and thus, the random effect model was utilized to merge. A statistically significant difference of LVEF between the two groups was found (MD = 2.82, 95% CI [1.50, 4.14], p < 0.0001, Figure 4).
Figure 4.
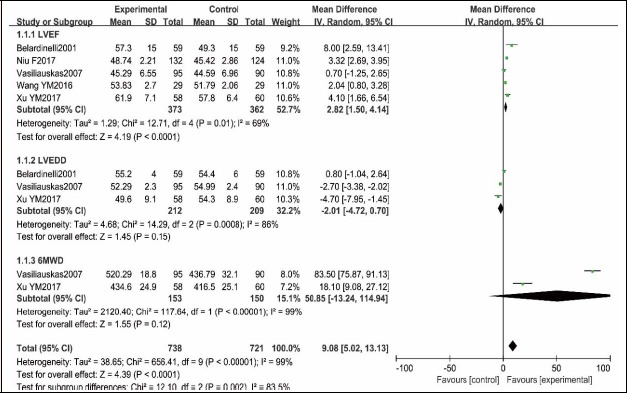
Cardiac function comparison between exercise group and no exercise group.
LVEDD
Three RCTs (Belardinelli et al., 2001; Vasiliauskas et al., 2007; Xu et al., 2017) reported that 212 and 209 patients received PCI in the exercise group and control group, respectively. Meta-analysis of these studies demonstrated that statistically significant heterogeneity existed between the two groups (p = 0.0008, I2 = 86%), and thus, random effect model was used to merge. No statistically significant difference of LVEDD was found between the two groups (MD = -2.01, 95% CI [-4.72, 0.70], p = 0.15, Figure 4).
6MWD
Two RCTs (Vasiliauskas et al., 2007; Xu et al., 2017) reported that 153 and 150 patients received PCI in the exercise group and control group, respectively. Meta-analysis of the studies demonstrated that statistically significant heterogeneity existed between the two groups (p < 0.00001, I2 = 99%), and thus, random effect model was used to merge. No significant difference of 6MWD between the two groups was found (MD = 50.85, 95% CI [-13.24, 114.94], p = 0.12, Figure 4).
Major adverse cardiovascular events Cardiac death
Four RCTs (Belardinelli et al., 2001; Lee et al., 2013; Mei et al., 2009; Niu et al., 2017) reported that, of the 378 and 372 patients who received PCI with or without exercise, respectively, 3 and 14 patients had cardiac death, respectively. There was no statistically significant heterogeneity in the two groups (p = 0.39, I2 = 0%), and thus, fixed effect model was used to merge. A significant difference in the cardiac death rate between both groups was found (RR = 0.24, 95% CI [007, 0.76], p = 0.02, Figure 5).
Figure 5.
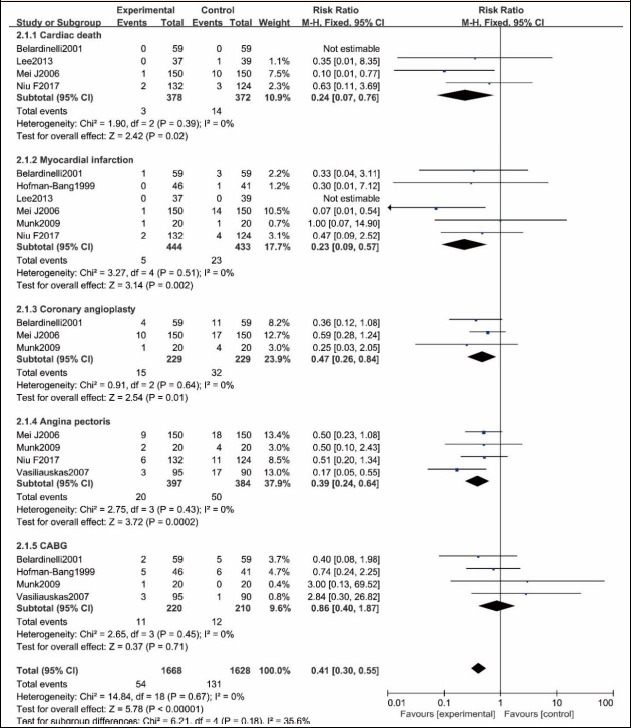
Adverse cardiovascular events comparison between exercise group and no exercise group.
Myocardial infarction
Six RCTs (Belardinelli et al., 2001; Lee et al., 2013; Mei et al., 2009; Munk et al., 2009; Niu et al., 2017; Vasiliauskas et al., 2007) reported that, of the 444 and 433 patients who received PCI with or without exercise, respectively, 5 and 23 patients had myocardial infarction, respectively. There was no statistically significant heterogeneity between the two groups (p = 0.51, I2 = 0%), and thus, fixed effect model was used to merge. A statistically significant difference in the myocardial infarction rate was found between the two groups (RR = 0.23, 95% CI [0.09, 0.57], p = 0.002, Figure 5).
Coronary angioplasty
Three RCTs(Belardinelli et al., 2001; Mei et al., 2009; Munk et al., 2009) reported that, of the 229 and 229 patients who received PCI with or without exercise, 15 and 32 patients had coronary angioplasty, respectively. There was no significant heterogeneity between the two groups (P = 0.64, I2 = 0%), and thus, fixed effect model was used to merge. A statistically significant difference in the coronary angioplasty rate was found between the two groups (RR =0.47, 95% CI [0.26, 0.84], p = 0.01, Figure 5).
Angina pectoris
Four RCTs (Mei et al., 2009; Munk et al., 2009; Niu et al., 2017; Vasiliauskas et al., 2007) reported that, of the 397 and 384 patients who received PCI with or without exercise, 20 and 50 patients had angina pectoris, respectively. There was no significant heterogeneity between the two groups (p = 0.43, I2 = 0%), and thus, fixed effect model was used to merge. A statistically significant difference in the angina pectoris rate was found between the two groups (RR = 0.39, 95% CI [0.24, 0.64], p = 0.0002, Figure 5).
CABG
Four RCTs (Belardinelli et al., 2001; Hofman-Bang et al., 1999; Munk et al., 2009; Vasiliauskas et al., 2007) reported that, of the 220 and 210 patients received PCI with or without exercise, 11 and 12 patients had CABG, respectively. There was no significant heterogeneity between the two groups (p = 0.45, I2 = 0%), and thus, fixed effect model was used to merge. No significant difference in CABG rate was noticed between the two groups (RR = 0.86, 95% CI [0.40, 1.87], p = 0.71, Figure 5).
Restenosis
Five RCTs (Belardinelli et al., 2001; Kubo et al., 1992; Lee et al., 2013; Mei et al., 2009; Vasiliauskas et al., 2007) reported that, of the 359 and 358 patients who received PCI with or without exercise, 23 and 53 patients had restenosis, respectively. There was significant heterogeneity between the two groups (p = 0.06, I2 = 56%), and thus, random effect model was used to merge. A statistically significant difference in the restenosis rate was found between the two groups (RR = 0.36, 95% CI [0.16, 0.83], p = 0.02, Figure 6).
Figure 6.
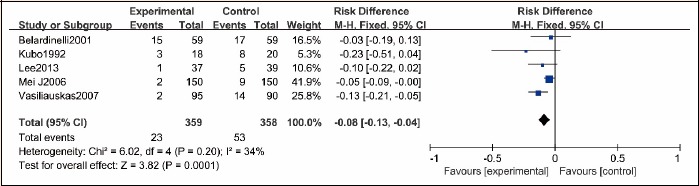
Restenosis comparison between exercise group and no exercise group.
Trial sequential analysis
As shown Figure 7 and 8, trial sequential analysis for the evaluation of myocardial infarction revealed that only 48.35% of the required sample size (1814 patients) was accrued in the current analysis. For the assessment of cardiac death, only 32.82% of the required sample size (2285 patients) was accrued in the current analysis. The cumulative Z curves crossed the traditional boundary for superiority, but did not cross TSA boundary, indicating the traditional meta-analysis might generate a false positive conclusion. Therefore, more trials need to be included in the future analysis in order to confirm the efficacy.
Figure 7.
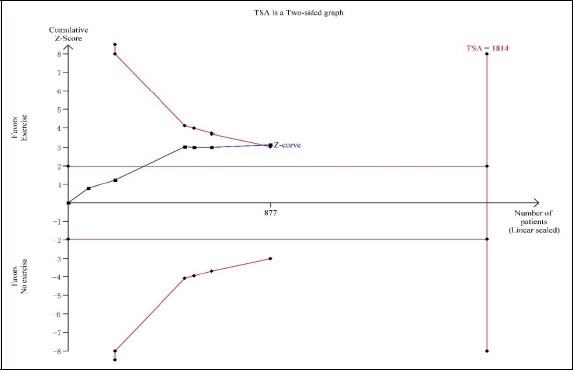
Trial sequential analyses for myocardial infarction.
Figure 8.
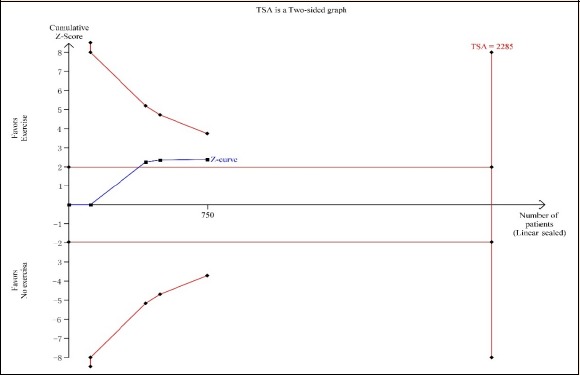
Trial sequential analyses for cardiac death.
Discussion
In the present meta-analysis of 10 RCTs comprising a total of 1274 patients (636 in exercise group and 638 in control group), we found that combination of exercise and PCI was associated with a reduced risk of cardiac death, MI, coronary angioplasty, angina pectoris and restenosis in comparison to PCI alone; and that LVEF was significantly improved in the patients with exercise than that in the patients without exercise. There was no significant difference in LVEDD, 6MWD or CABG between the two groups of PCI with or without exercise. In addition, trial sequential analysis reflected traditional meta-analysis might generate a false positive conclusion in MI and cardiac death. Findings of the current analysis did not support the beneficial effects of exercise after PCI.
CHD is caused by coronary atherosclerotic plaque formation leading to vascular stenosis or obstruction caused by the supply area of myocardial ischemia, hypoxia or necrotic lesions. In recent years, diagnosis and treatment of coronary heart disease has been in the increasing trend and improved by the traditional drug treatment as well as by PCI as the main treatment strategy. After the PCI, exercise rehabilitation has become a secondary postoperative treatment in addition to the conventional drug therapy. The guidelines published by WHO in 2007 on “the cardiovascular risk factor evaluation and treatment” recommended that the incidence of cardiovascular disease and mortality are closely related to whether the individual exercise regardless of gender, age, and that 33.3% of coronary heart disease-associated deaths are due to lack of exercise(Back et al., 2008; Kachur et al., 2017). For patients with CHD who had PCI and conventional pharmacological therapy, individualized exercise rehabilitation training, can improve the patient’s cardiac function and reduce the incidence of adverse cardiovascular events and restenosis (Deniz Acar et al., 2014). While safety should be concerned, Lavie et al. (2016) found that the safety of patients with exercise rehabilitation following PCI was not an issue in these patients, and that beneficiary effect of exercise rehabilitation lasted long-term even after quitting the exercise, which was consistent with the finding of this study.
Cardiac function is important in predicting long-term prognosis in the patients had PCI. In this regard, if a patient’s heart function improved significantly following PCI treatment, he/she will have a favored long-term prognosis. LVEF, LVEDD, 6MWD are commonly used for the evaluation of cardiac function. Cardiac function in the patients who had exercise rehabilitation 6 months after PIC was significantly improved compared with the patients received conventional treatment group. Long-term standardized exercise rehabilitation can prevent or even reverse the occurrence of myocardial remodeling, which may improve patient’s heart functions including myocardial contractility, ejection fraction, and blood supply to the tissues. This was demonstrated by the significant increase of 6MWD in the patients had exercise other than the conventional treatment. Haykowsky et al. (Haykowsky et al., 2011) found that LVEF was significantly improved in 1029 patients with acute myocardial infarction who had exercise rehabilitation for 3 months. Jelinek et al (Jelinek et al., 2013) reported that 6MWD and peak oxygen uptake (VO2max) were significantly improved after a period of 6 weeks of cardiac rehabilitation in 38 patients with coronary heart disease. Poortaghi et al. (Poortaghi et al., 2013) conducted a randomized controlled trial in 40 cases of control or 40 patients with 45 days of rehabilitation training, found that the exercise capacity and cardiac function were improved more significantly in experimental group than control group.
Studies have also shown that exercise rehabilitation can reduce the level of hypersensitive C-reactive protein (C-RP) and inflammatory cytokines (TNF-α, IL-6) in patients with coronary heart disease after PCI (Kim et al., 2008; Munk et al., 2011). Cardiovascular protective factor (growth differentiation factor – 15) and vascular endothelial nitric oxide synthase were up-regulated after exercise training, which further improved the coronary artery endometrial relaxation through increasing coronary endothelial cell nitric oxide levels (Hambrecht et al., 2003; Munk et al., 2009). Moderate exercise is also conducive to the coronary artery by reducing the risk of atherosclerosis formation. Rehabilitation exercise can improve the autonomic nervous function, improve the maximum oxygen uptake capacity of the muscle, reduce the level of myocardial oxidative stress, prevent the extracellular matrix collagen abnormal degradation and fibrosis, and improve ventricular remodeling and pumping function (Shuichi et al., 2004; Zheng et al., 2008). By which mechanisms, cardiac function of the patients with CHD and PCI treatment was significantly improved while the incidence rate of the major adverse cardiac events (MACE) was reduced.
There are some limitations in the current analysis. Most importantly, the studies enrolled into this review were heterogeneous. The exercise programs varied that limited comparability. The degree of cardiac function in patients with regular exercise programs is related to exercise intensity and energy consumption.
Therefore, it would be more accurate if the impact of different exercise intensity, time and frequency on coronary artery restenosis, cardiac function as well as the incidence rate of MACE were analyzed. Various PIC procedures were used by different clinicians, that is, some were used only with balloon dilatation, while some with stent implantation, partly performing drug-eluting stents, partly bare-metal stents. Finally, only few RCT literatures on rehabilitation exercise therapy has been reported and the total sample size is small. Therefore, it is necessary to incorporate more prospective randomized controlled trials with larger sample size.
Conclusion
This systematic review and meta-analysis showed that exercise after PCI might be beneficial for patients with CHD by improving LVEF, reducing risk of cardiac death, myocardial infarction, coronary angioplasty, angina pectoris and restenosis. Exercise did not significantly affect LVEDD, 6MWD or CABG in the patients with CHD after PCI treatment. Nevertheless, due to limited number of RCTs and lower power of this review, the effect of exercise on cardiac function and risk of adverse cardiovascular events after PCI remains to be further confirmed through more well designed and large sample size of RCTs.
Acknowledgements
The experiments comply with the current laws of the country in which they were performed. The authors have no conflicts of interests to declare.
Biographies
Hu ZHANG
Employment
Attending physician
Degree
MD
Research interests
Coronary heart disease
E-mail: zhang2005hu@sina.com
Rong CHANG
Employment
Attending physician
Degree
PhD
Research interests
Hypoxia and thrombus
E-mail: qhschangrong@126.com
References
- Abell B., Glasziou P., Hoffmann T. (2017) The Contribution of Individual Exercise Training Components to Clinical Outcomes in Randomised Controlled Trials of Cardiac Rehabilitation: A Systematic Review and Meta-regression. Sports Medicine - open 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L., Oldridge N., Thompson D.R., Zwisler A.D., Rees K., Martin N., Taylor R.S. (2016) Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. Journal of the American College of Cardiology 67, 1-12. [DOI] [PubMed] [Google Scholar]
- Back M., Wennerblom B., Wittboldt S., Cider A. (2008) Effects of high frequency exercise in patients before and after elective percutaneous coronary intervention. European Journal of Cardiovascular Nursing 7, 307-313.. [DOI] [PubMed] [Google Scholar]
- Belardinelli R., Paolini I., Cianci G., Piva R., Georgiou D., Purcaro A. (2001) Exercise training intervention after coronary angioplasty: the ETICA trial. Journal of the American College of Cardiology 37, 1891-1900. [DOI] [PubMed] [Google Scholar]
- Buttar H.S., Li T., Ravi N. (2005) Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Experimental and Clinical Cardiology 10, 229-249. [PMC free article] [PubMed] [Google Scholar]
- Deniz Acar R., Bulut M., Ergun S., Yesin M., Alici G., Akcakoyun M. (2014) Effect of cardiac rehabilitation on left atrial functions in patients with acute myocardial infarction. Annals of Pphysical and Rehabilitation Medicine 57, 105-113. [DOI] [PubMed] [Google Scholar]
- Fletcher G.F., Landolfo C., Niebauer J., Ozemek C., Arena R., Lavie C.J. (2018) Promoting Physical Activity and Exercise: JACC Health Promotion Series. Journal of the American College of Cardiology 72, 1622-1639. [DOI] [PubMed] [Google Scholar]
- Hambrecht R., Adams V., Erbs S., Linke A., Krankel N., Shu Y., Baither Y., Gielen S., Thiele H., Gummert J.F., Mohr F.W., Schuler G. (2003) Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107, 3152-3158. [DOI] [PubMed] [Google Scholar]
- Haykowsky M., Scott J., Esch B., Schopflocher D., Myers J., Paterson I., Warburton D., Jones L., Clark A.M. (2011) A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: start early and go longer for greatest exercise benefits on remodeling. Trials 12, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis L.D., Smith P.K., Anderson J.L., Bittl J.A., Bridges C.R., Byrne J.G., Cigarroa J.E., DiSesa V.J., Hiratzka L.F., Hutter A.M., Jr., Jessen M.E., Keeley E.C., Lahey S.J., Lange R.A., London M.J., Mack M.J., Patel M.R., Puskas J.D., Sabik J.F., Selnes O., Shahian D.M., Trost J.C., Winniford M.D., Jacobs A.K., Anderson J.L., Albert N., Creager M.A., Ettinger S.M., Guyton R.A., Halperin J.L., Hochman J.S., Kushner F.G., Ohman E.M., Stevenson W., Yancy C.W., American College of Cardiology Foundation/American Heart Association Task Force on Practice, G. (2012) 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. The Journal of Thoracic and Cardiovascular Surgery 143, 4-34. [DOI] [PubMed] [Google Scholar]
- Hofman-Bang C., Lisspers J., Nordlander R., Nygren A., Sundin O., Ohman A., Ryden L. (1999) Two-year results of a controlled study of residential rehabilitation for patients treated with percutaneous transluminal coronary angioplasty. A randomized study of a multifactorial programme. European Heart Journal 20, 1465-1474. [DOI] [PubMed] [Google Scholar]
- Jelinek H.F., Huang Z.Q., Khandoker A.H., Chang D., Kiat H. (2013) Cardiac rehabilitation outcomes following a 6-week program of PCI and CABG Patients. Frontiers in Physiology 4, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachur S., Chongthammakun V., Lavie C.J., De Schutter A., Arena R., Milani R.V., Franklin B.A. (2017) Impact of cardiac rehabilitation and exercise training programs in coronary heart disease. Progress in Cardiovascular Diseases 60, 103-114. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Shin Y.O., Bae J.S., Lee J.B., Ham J.H., Son Y.J., Kim J.K., Kim C., Lee B.K., Oh J.K., Othman T., Min Y.K., Yang H.M. (2008) Beneficial effects of cardiac rehabilitation and exercise after percutaneous coronary intervention on hsCRP and inflammatory cytokines in CAD patients. Pflugers Archiv : European Journal of Physiology 455, 1081-1088. [DOI] [PubMed] [Google Scholar]
- Kubo H., Yano K., Hirai H., Yabuki S., Machii K. (1992) Preventive effect of exercise training on recurrent stenosis after percutaneous transluminal coronary angioplasty (PTCA). Japanese Circulation Journal 56, 413-421. [DOI] [PubMed] [Google Scholar]
- Lavie C.J., Arena R., Franklin B.A. (2016) Cardiac Rehabilitation and Healthy Life-Style Interventions: Rectifying Program Deficiencies to Improve Patient Outcomes. Journal of the American College of Cardiology 67, 13-15. [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Kim J.H., Kim B.O., Byun Y.S., Cho S., Goh C.W., Ahn H., Rhee K.J., Kim C. (2013) Regular exercise training reduces coronary restenosis after percutaneous coronary intervention in patients with acute myocardial infarction. International Journal of Cardiology 167, 2617-2622. [DOI] [PubMed] [Google Scholar]
- Mei J., Guo X., Sun J., Qi S., Yang L., Lei S. (2009) Effects of Early Exercise Presecription on Acute Myocardial Infarction Patients’ Rehabilitation and Restenosis after Stent Implantation plus Percutaneous Transluminal Coronary Angiogplasty. Nursing Journal of Chinese PLA 26, 11-13. [Google Scholar]
- Munk P.S., Staal E.M., Butt N., Isaksen K., Larsen A.I. (2009) High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation A randomized controlled trial evaluating the relationship to endothelial function and inflammation. American Heart Journal 158, 734-741. [DOI] [PubMed] [Google Scholar]
- Munk P.S., Valborgland T., Butt N., Larsen A.I. (2011) Response of growth differentiation factor-15 to percutaneous coronary intervention and regular exercise training. Scandinavian Cardiovascular Journal 45, 27-32. [DOI] [PubMed] [Google Scholar]
- Niu M., Gan K., Sun S., Li F. (2017) Application of decomposition-ensemble learning paradigm with phase space reconstruction for day-ahead PM2.5 concentration forecasting. Journal of Environmental Management 196, 110-118. [DOI] [PubMed] [Google Scholar]
- Poortaghi S., Baghernia A., Golzari S.E., Safayian A., Atri S.B. (2013) The effect of home-based cardiac rehabilitation program on self efficacy of patients referred to cardiac rehabilitation center. BMC Research Notes 6, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuichi T., Satoru S., Takeshi B., Hiroshi T., Naohiko A., Yoshio Y., Hitoshi S., Hiroshi N., Yoichi G. (2004) Predictors of left ventricular remodeling in patients with acute myocardial infarction participating in cardiac rehabilitation. Circulation Journal 68, 214-219. [DOI] [PubMed] [Google Scholar]
- Vasiliauskas D., Benetis R., Jasiukeviciene L., Grizas V., Marcinkeviciene J., Navickas R., Leimoniene L. (2007) Exercise training after coronary angioplasty improves cardiorespiratory function. Scandinavian Cardiovascular Journal 41, 142-148. [DOI] [PubMed] [Google Scholar]
- Wang Y. (2016) Effects of training on cardiac function and quality of life in CHD patients treated with PCI. Jilin University Journal 1-39. [Google Scholar]
- Wong W.P., Feng J., Pwee K.H., Lim J. (2012) A systematic review of economic evaluations of cardiac rehabilitation. BMC Health Services Research 12, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Feng Y., Su P., Li C., Qiao J. (2017) Impact of Exercise Rehabilitation on Cardiac Function in Coronary Artery Disease Patients After Percutaneous Coronary Intervention. Chinse Circulation Journal 32, 326-330. [Google Scholar]
- Yuan Y., Chen F. (2013) Progress of Rehabilitation Exercise Therapy Mechanism after Percutaneous Coronary Intervention. AJCCPVD 21, 7-9. [Google Scholar]
- Zheng H., Luo M., Shen Y., Ma Y., Kang W. (2008) Effects of 6 months exercise training on ventricular remodelling and autonomic tone in patients with acute myocardial infarction and percutaneous coronary intervention. Journal of Rehabilitation Medicine 40, 776-779. [DOI] [PubMed] [Google Scholar]


