Abstract
The purpose of the present study is two-fold. First, we evaluated whether 8-weeks of combined training (high-intensity intermittent plus strength training) may change brain derived neurotropic factor (BDNF) and lipid parameters (triacylglycerol, HDL-c and non-HDL) in a fasted state. Second, we investigated the effect of an acute session of high-intensity intermittent exercise followed by strength training on systemic BDNF and lipid parameters pre- and post 8-weeks of training. Twenty-one healthy and physically active men were divided into two groups: high-intensity intermittent exercise combined with strength training (HSG; n = 11) and control (CG; n = 10). The HSG exercised for one minute at 100% of speedVO2max (sVO2max) interspersed with one minute of passive recovery followed by strength training (8 exercises with 8-12 repetition maximum loads) for 8-weeks. Heart rate variability, blood pressure, lipid profile, and BDNF concentrations were measured in the fasted state pre- and post-exercise and before and after the 8-week training period. After 8-weeks of exercise training, there was an increase in spectral high frequency component (ms2) and RR interval (p < 0.05), a decreased spectral low frequency component (nu) and heart rate values (p < 0.05), an increase in HDL-c (p < 0.001), and lower BDNF concentrations (p < 0.001). These results suggest that 8-weeks of high-intensity intermittent exercise combined with strength exercise is an effective protective cardio-metabolic strategy capable of increasing HDL-c and BDNF concentrations after an acute exercise session. In the long-term, the modulation on BDNF and HDL-c concentrations may be a determining factor for protection against neurological and cardiovascular diseases.
Key points.
The combination of these two modalities in the same exercise session is often used by athletes, recreationally active individuals, and coaches.
Present knowledge about the combination of aerobic and strength exercise and the influence of intensity on the physiological parameters is insufficient, especially in relation to the practice of high intensity exercise and the higher organic stress imposed.
The results of this investigation will allow health professionals to use this protocol in an objective and safe way for the purpose of exercise prescription.
Key words: HIIT, combined exercise, cholesterol, autonomic modulation, heart rate variability, health
Introduction
A sedentary lifestyle has been shown to be a precursor for an increase in diseases such as obesity, dyslipidemia, and cardiovascular disease (CVD) (NCD, 2017; Halcox and Misra, 2015). Low levels of physical activity and poor eating habits (e.g. diets rich in simple carbohydrates and saturated lipids) significantly contribute to the development of diseases related to metabolic disorders typically associated with low concentrations of high lipoprotein density cholesterol (HDL-c) and high concentrations of low density lipoprotein cholesterol (LDL-c), triacylglycerol, and fasting blood glucose (Kessler et al., 2012).
Disorders related to lipid metabolism have been shown to modify the functionality of the cardiovascular system and to affect heart rate variability (HRV), heart rate (HR), blood pressure (BP), and endothelial dysfunction (Helkin et al., 2016; Pistorio et al., 2011). In addition, front CVD, endothelial morphology and metabolism are significantly altered in response to elevated lipids concentrations, particularly LDL-c and oxidized LDL, promoting a decrease in nitric oxide bioavailability and an increase in atherogenic markers associated with CVD (Halcox and Misra, 2015; Vanhoutte, 2013).
Furthermore, the cytokine brain derived neurotropic factor (BDNF), which is usually linked to brain health, cognition and mood parameters, has also been shown to have a protective effect against CVD and cardiovascular risk (Zembron-Lacny et al., 2016; Kaess et al., 2015). This protein has also been associated with cardiorespiratorry fitness and maximal oxygen uptake (Santos et al., 2016; Babaei et al., 2014) and recently, our group demonstrated that high-intensity intermittent exercise (HIIE) increased BDNF concentrations (Cabral-Santos et al., 2016). During an acute bout of exercise, BDNF supports metabolic responses mainly related to energy metabolism by increasing insulin sensitivity and lipid oxidation in skeletal muscle. Chronically, BDNF has been shown to be neuroprotective and promote neurogenesis and to be directly associated with development, regeneration, survival, and maintenance of neurons (Marosi and Mattson, 2014).
Regular exercise practice has also been shown to decrease LDL-c and triacylglycerol concentrations and increase HDL-c reducing the probability of atheroma plaque development and adhesion (Marques et al., 2018; Lira et al., 2010; Lira et al., 2009). Several training protocols have been suggested as a strategy to treat and prevent cardio-metabolic risks with respect to anti-inflammatory and anti-atherogenic effects, including high intensity training (Swift et al., 2018; Delgado-Floody et al., 2018; Ramírez-Vélez et al., 2017; Antunes et al., 2016). Additionally, HIIE in combination with strength training has been shown to be effective in increasing HDL-c (Rossi et al., 2016).
While the aforementioned studies have investigated variables during rest (pre- vs. post-training), it is still not clear how the combination of HIIE and strength exercise training can modulate the autonomic, haemodynamic, and anti-atherogenic variables. Thus, the purpose of the present study was to evaluate the effects of 8-weeks of HIIE combined with strength training on autonomic, haemodynamic and anti-atherogenic responses at rest and in response to an acute exercise session pre- and post-training in healthy men. We hypothesized that BDNF, HDL-c, and HRV values would increase after an acute bout of HIIE plus strength exercise due to the imposed metabolic demand; however, chronically, these responses would be attenuated in response to an adaptation imposed by the training period.
Methods
Participants and procedures
Twenty-one men between the ages of 18 to 35 years, healthy and physically active were included in this study (Table 1). The subjects were divided into two groups: high intensity intermittent exercise combined with strength training (HSG; n =11) and control group (CG; n = 10). Volunteers were informed about the procedures, risks and benefits, and signed a consent form. The University Ethics Committee (22793414.7.0000.5402) approved this study.
Table 1.
Anthropometric markers of volunteers.
| HSG (n=11) | CG (n=10) | |
|---|---|---|
| BMI (kg·m-2) | 23.9 ± 2.5 | 20.7 ± 1.1 |
| Age (years) | 24.5 ± 3.7 | 20.2 ± 2.1 |
| Height (m) | 1.78 ± 6.5 | 1.78 ± 6.5 |
| Body mass (kg) | 74.7 ± 7.6 | 66.8 ± 5.1 |
| 5 km (min) | 42 ± 4 | |
| Mean workload (km/h) | 7.0 ± 0.6 |
Mean ± standard deviation. HSG = high intensity aerobic exercise combined with strength group; CG = control group; ; BMI = body mass index; kg = kilogram; 5 km (min) = time in minutes to run 5km.
Experimental design
Participants visited the laboratory on three separate occasions at the same time of the day for screening as follows: 1) body composition measurements; maximal incremental test on the treadmill, and familiarization with one-repetition maximum strength test (1RM) of the half-squat; 2) 1RM test of the half-squat; 3) experimental session with blood draw, haemodynamic, and autonomic evaluations. The third visit started with participants in a fasted state, followed by HIIE plus strength exercise for the HSG (acute exercise session) while CG performed only fasted measurements. Haemodynamic and autonomic responses to exercise (autonomic assessment, heart rate, and blood pressure) were measured at rest and recovery (30-minutes post-exercise) and anti-atherogenic parameters (BDNF and lipid profile) were evaluated at rest, during exercise (pre-post HIIE, pre-post strength exercises and during recovery at 30- and 60-minutes post-exercise) for the HSG and only at rest for CG. Participants were re-tested at completion of the 8-week exercise training program (Figure 1). The control group was instructed to keep usual activities without performing training protocols.
Figure 1.

Schematic showing the study design.
Maximal incremental test
For determination of maximal aerobic fitness, subjects performed a maximal incremental test to exhaustion on a treadmill with 1% incline (Inbramed-ATL) to determine maximum oxygen uptake (V̇O2max) by gas analyzer (Model Quark PFT Ergo – Cosmed – Rome; V̇O2). Each stage was 2-minutes with 1 km/h increments at the end of each stage; the initial speed was set at 8km/h. The average of the last 30 seconds of the test was defined as the V̇O2max. The maximal velocity reached in the test was defined as the speedVO2max (sVO2max). In case the subject was not able to finish the 2-minute stage, the speed was expressed according to the permanence time in the last stage, determined as the following: sVO2max = velocity of penultimate stage + [(time, in seconds, remaining in the last stage multiplied by 1km/h)/120s] (Kuipers et al., 1985). In addition, a heart rate monitor and subjective effort scale (Borg scale 6-20) were used (Polar Vantage NV, Electro Oy, Finland) and the values expressed integrated to the gas analysis system.
Maximum dynamic strength test
Seventy-two hours after the maximal incremental test, subjects performed the one-repetition maximum (1RM) half-squat on a guided bar. For this, subjects followed recommendations of the American Society of Exercise Physiology (Brown and Weir, 2001). Before performing the 1RM test, subjects were familiarized with the 1RM half-squat, in which they executed two sets of 15-20 repetitions; the first set was kept unloaded while loads for the second set varied between 10 and 15 kilograms.
Before the test, subjects warmed-up for five minutes at 50% sVO2max. Subsequently, subjects performed a series of eight repetitions of the estimated intensity at 50%1RM, followed by another set of three repetitions of the estimated 80% 1RM trial to establish the 1RM were executed with progressively heavier weights to volitional fatigue. The rest interval was kept between three to five minutes with no more than five attempts. The highest load lifted during the test was regarded as the 1RM value.
For better control of the 1RM test procedures, each subject had their body position and foot placement in the half-squat exercise recorded and reproduced throughout the study. In addition, a wooden seat with adjustable heights was placed behind the subject in order to keep the bar displacement and knee angle (~90°) constant on each half-squat repetition.
Autonomic evaluation
For autonomic evaluation, the heart rate beat-to-beat intervals that were obtained were used to calculate the HRV indexes. Before data collection, subjects were instructed to avoid physical exercise, and not to consume alcohol and/or stimulants (e.g. coffee, tea, chocolates, soft drinks) 24-hours prior to the test.
A heart rate monitor was used to record heart rate (Polar® S810i, Finland), which contained a recording strap positioned on the distal third of the sternum of the individual, and a receiver placed on the handle of the treadmill (Vanderlei et al., 2008). After placement of the strap and receiver, heart rate was recorded for 30 minutes while participants were in a supine position. This measure was repeated for the HSG after the exercise protocol (high intensity and strength). The RR interval series were analyzed in the following periods: after 5 minutes of rest for both groups, and immediately after the exercise protocol which was divided into 6 periods of 5 minutes each (recovery).
Heart rate variability was analyzed at 1,000 Hz and further filtered to eliminate premature ectopic beats and artifacts, and only the series with more than 95% of sinus beats were included. Heart rate variability time and frequency domains were analyzed using Kubios HRV software (Kubios HRV Analysis Software 2.0, Kuopio, Finland (Tarvainen, 2014).
For time domain, SDNN, RMSSD, RR, and HR mean indices were calculated. The SDNN index represents the standard deviation of all RR intervals and RMSSD is the square root of the square mean of the differences between adjacent normal RR intervals (Vanderlei et al., 2009). The RR and HR refer to mean RR intervals and heart rate respectively. For the analysis of HRV in the frequency domain, spectral high frequency components were analyzed (HF, 0.15 to 0.4 Hz) and low frequency (LF, 0.04 to 0.15 Hz) in ms² and normalized units (nu), and the ratio of these components (LF/HF ratio). Spectral analysis was calculated using the Fourier Transform algorithm (Vanderlei et al., 2009).
Blood pressure measurement
Participants’ blood pressure was measured in the supine position in the morning, following a minimum twelve-hour period of alcohol and caffeine abstention. After resting for 5- to 10-minutes in a quiet environment, the measurement was performed on the right arm with the cuff placed above the antecubital fossa on top of the brachial artery. An OMRON HEM-7200 automatic blood pressure monitor was used to evaluate the measurement before and after training.
Training Protocol – HIIE plus strength exercise
Subjects performed a warm-up consisting of running at 50% sVO2max for five minutes on a treadmill (with inclination fixed at 1%). The HIIE was performed intermittently with subjects running on a treadmill for one minute at 100% sVO2max, interspersed by one minute of passive recovery (without exercise) until the completion of 5 km. Upon completion, subjects rested for 10-minutes before moving to the strength exercise component of training. Strength exercise consisted of the half-squat, bench press, knee extension, back handle, knee flexion, elbow flexion (curl), ankle flexion and elbow extension on the pulley. Subjects performed three sets, with 8-12 repetition maximum (RM), and 90 seconds rest interval between sets. Loads were re-adjusted as necessary to maintain the intensity zone at 8-12 RM as suggested by Campos et al. (2002).
Breakfast
Subsequently, after a fasting blood draw, a standard breakfast consisting of toast, yogurt, and cottage cheese was offered with energy intake amount determined by body composition (approximately 25% of the individual daily energy intake), energy values were distributed between carbohydrates, proteins, lipids, and fiber (Mifflin et al., 1990).
Blood analyses
Approximately 20 mL of blood sample was collected in the morning (between 7:00 and 8:00 am) into tubes containing EDTA and anti-coagulant gel to separate serum and plasma. Blood samples for HSG were collected in the following periods: 1) overnight fast (12-hours); 2) pre-HIIE; 3) immediately after HIIE; 4) pre-strength; 5) immediately after strength; 6) 30-minutes after completion of training; 7) 60-minutes after completion of training. For CG blood samples were collected in the same time condition at rest pre and post 8-weeks. Blood samples were centrifuged at 3.000 rpm for 15-minutes at 4°C. Posteriorly, the supernatant rates of serum and plasma were stored in plastic Eppendorfs tubes, and stored at -80°C for further analysis. Lipid analysis (triacylglycerol (TAG), total cholesterol (TC) and HDL-c) was performed using the colorimetric method, obtained from Labtest® (São Paulo, Brazil) and non-HDL indirectly estimated (TC – HDL-c). The BDNF concentration was analysed according to the manufacturer’s guidelines by enzyme-linked immunosorbent assay (ELISA) using kits from R&D System (Minneapolis, USA) (Cabral-Santos et al., 2016).
Statistical analysis
All analyses were performed using SAS software. Data were reported as means and standard deviation (SD). A two-way analysis of variance (group and training) was conducted to compare variables related to heart rate variability, lipid profile and BDNF in the fasted state. A two-way analysis of variance [training period (pre- versus post-) and time of measurement (pre-HIIE, immediately post-HIIE, pre-strength exercise, immediately post-strength exercise, 30-minutes post-exercise session and 60-minutes post-exercise session, before and after 8-weeks training)] was conducted to compare lipid profile and BDNF in HSG. A two-way analysis of variance [training period (pre- and post-) and time of measurement (0-5, 5-10, 10-15, 15-20, 20-25 and 25-30 minutes)] was conducted to compare variables related to heart rate variability. When a significant difference was observed, a Tukey post-hoc test was applied. Statistical significance was set at p < 0.05.
Results
An interaction between group and training period was shown for HRV time domain mean RR intervals (p = 0.01, Table 2). Post-hoc test showed a tendency for higher values post- compared to pre-training period in HSG (p = 0.07). Correspondingly, HR (p = 0.01) also showed lower values for post- compared to pre-training in HSG (p = 0.05). There was also a group main effect for frequency domain measurements of HRV for LF (p = 0.01) and HF (p = 0.01), with lower values in CG compared with HSG for LF (p = 0.01) and higher values for CG compared with HSG for HF (p = 0.01). No significant differences were found for other variables related to HRV and blood pressure at rest.
Table 2.
Mean and standard deviation of HRV indexes and blood pressure (BP) at baseline before and after 8-weeks of HIIE plus strength exercise.
| Variables | HSG (n = 11) | CG (n = 10) | |
|---|---|---|---|
| Mean RR | Pre | 907.9 ± 106.4 | 969.3 ± 159.4 |
| Post | 978.8 ± 115.7 * | 937.7 ± 129.2 | |
| Mean HR (beat/min) | Pre | 67.3 ± 7.5 | 63.8 ± 9.6 |
| Post | 62.5 ± 7.5 * | 65.5 ± 8.4 | |
| SDNN (ms) | Pre | 71.3 ± 26.9 | 82.0 ± 33.6 |
| Post | 79.6 ± 21.5 | 80.8 ± 29.0 | |
| RMSSD (ms) | Pre | 41.4 ± 20.4 | 62.4 ± 26.7 |
| Post | 47.4 ± 17.6 | 57.6 ± 25.0 | |
| LF (nu) | Pre | 62.5 ± 15.6 † | 46.9 ± 11.4 |
| Post | 64.5 ± 10.1 † | 53.4 ± 15.5 | |
| HF (nu) | Pre | 37.4 ± 15.6 † | 52.9 ± 11.4 |
| Post | 35.4 ± 10.1 † | 46.5 ± 15.5 | |
| LF (ms²) | Pre | 1023.1 ± 821.7 | 1261.8 ± 881.1 |
| Post | 1396.6 ± 942.8 | 1046.4 ± 899.6 | |
| HF (ms²) | Pre | 743.9 ± 937.2 | 1063.6 ± 744.2 |
| Post | 788.1 ± 615.9 | 1182.2 ± 728.1 | |
| LF/HF | Pre | 1.3 ± 0.8 | 1.1 ± 1.1 |
| Post | 1.7 ± 1.5 | 0.8 ± 1.2 | |
| SBP (mmHg) | Pre | 122.8 ± 11.3 | 124.9 ± 6.8 |
| Post | 118.7 ± 9.4 | 125.2 ± 5.9 | |
| DBP (mmHg) | Pre | 62.3 ± 5.6 | 65.0 ± 6.6 |
| Post | 62.9 ± 5.4 | 67.1 ± 5.8 | |
Mean ± standard deviation. Mean HR = mean hear rate; Mean RR = mean RR intervals; SDNN = standard deviation of all normal RR intervals written in milliseconds; RMSSD = square root of the square mean of the differences of the adjacent normal RR intervals, in milliseconds; LF ms² = low-frequency component in milliseconds squared; LF nu = low frequency component in unit of measurement; HF ms² = high-frequency component in milliseconds squared; HF nu = high-frequency component in unit of measurement; LF/HF = balance between the low and high frequency component; beat/min = beat per minute; SBP = systolic blood pressure; DBP = diastolic blood pressure; mmHg = millimetres of mercury; HSG = high intensity aerobic exercise combined strength group; CG = control group; Pre = pre-training; Post = post-training;
* = significant difference compared with the pre-training moment (p < 0.05);
† = significant difference compared with the control group (p < 0.05).
Lipid profile showed an interaction for HDL-c during the fasted state between groups and training period (p = 0.001) with lower values post- compared to pre-training in CG (p = 0.001) and pre-training values in CG higher than pre-training values in HSG (p = 0.02). There was also group main effect for TAG (p < 0.001) with higher values in CG compared to HSG (p <0.001) (Table 3). A trend was observed for group main effect (p = 0.06) with lower values in CG compared to HSG (p = 0.06).
Table 3.
Mean and standard deviation of anti-atherogenic variables at baseline before and after 8-weeks, with (HSG) and without (CG) training.
| HSG (n = 11) | CG (n = 10) | ||
|---|---|---|---|
| HDL-c (mmol/L) | Pre | 0.8 ± 0.1 † | 1.7 ± 0.1 |
| Post | 0.9 ± 0.1 | 0.9 ± 0.1 * | |
| TAG (mmol/L) | Pre | 1.1 ± 0.2 † | 1.4 ± 0.2 |
| Post | 1.1 ± 0.1 | 1.3 ± 0.2 | |
| Non HDL (mmol/L) | Pre | 3.5 ± 0.7 | 2.8 ± 0.6 |
| Post | 3.7 ± 1.3 | 2.9 ± 0.7 | |
| BDNF (ng·mL-1) | Pre | 108.8 ± 56.7 | 66.1 ± 40.2 |
| Post | 92.6 ± 15.9 | 67.1 ± 43.9 |
Mean ± standard deviation. HDL-c = high density lipoprotein; TAG= triacylglycerol; Non HDL= non high density lipoprotein; BDNF= brain derived neurotropic factor; mmol/L = millimole per liter; ng·mL-1 = nanograms per milliliter; HSG = high intensity aerobic exercise combined strength group; CG = control group;
* = significant difference compared with the pre-training moment (p < 0.05);
† = significant difference compared with the control group (p < 0.05).
HSG also showed a main effect for HDL-c (p < 0.001) with higher values post- compared with pre-training (p < 0.001), and a main effect for time of measurement of HDL (p < 0.001) with post greater than pre-HIIE (p = 0.001); post-HIIE higher than post- 30- and 60-minutes of acute exercise session (p < 0.001 and p = 0.04 respectively) and higher values immediately post-strength exercise compared with 30-minutes post-acute exercise session (Figure 2).
Figure 2.

A and B Mean and standard deviation of TAG and BDNF before (●) and after (■) 8-weeks of HIIE plus strength exercise. a = lower than post-HIE, pre-SE post-SE; and post-30 exercise session; b = lower than pre-training values. C and D Mean and standard deviation of non-HDL and HDL-c before (■) and after (●) 8-weeks of HIIE plus strength exercise. a = higher than pre training values; b = higher than pre-HIE, 30- and 60-min post exercise session; c = higher than 30-min post exercise session.
TAG showed a main effect for time of measurement (p <0.001) with values pre-session lower than post-HIIE (p =0.001), pre-strength exercise (p = 0.001) and immediately post-strength exercise (p = 0.005), and post-HIIE higher than 30-minutes post-acute exercise session (p = 0.005). BDNF showed a main effect for training period (p < 0.001) with lower values post- compared to pre-training (p < 0.001) (Figure 2).
For RR there was a time of measurement main effect (p < 0.001), with values at rest being higher than all other time of measurement (p < 0 .001 for all comparisons); 5-minutes post-exercise session lower than 20-, 25-, and 30-minutes post-exercise session (p < 0.001 for all comparisons); 10-minutes post-exercise session lower than 20 and 30-minutes post-exercise session (p < 0.001 for both comparisons), and 20-minutes post-exercise session lower than 25- and 30-minutes post-exercise session (p = 0.01 and p =0.001 respectively). There was also a training period and time of measurement interaction (p <0.001) with all values higher post- versus pre-training (p <0.001 for all comparisons) except in the first 5-minutes of recovery where the values did not differ with 8-weeks of training (Figure 3).
Figure 3.
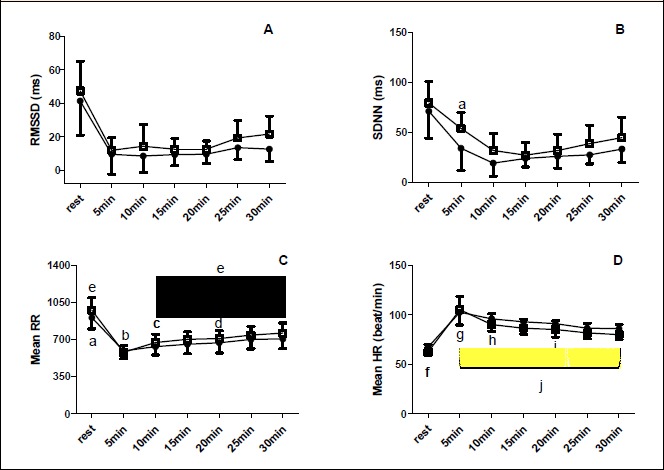
A and B: RMSSD and SDNN recovery before (●) and after (■) 8-weeks of HIIE plus strength exercise. a = higher than post 5-10- and 20-min post exercise session. C and D: Mean RR and Mean HR recovery before (■) and after (●) 8-weeks of HIIE plus strength exercise. a = higher than all other time of measurement; b = lower than 20-, 25- and 30-min post exercise session; c = lower than 20- and 30- min post exercise session; d = lower than 25- and 30- min post acute exercise session; e = pre-training lower than post-training; f = lower than all other time of measurement g= higher than 25- and 30-min post exercise session; h = higher than 25- and 30-min post exercise session; I = higher than 30-min post-exercise session; j = post lower than pre-training.
For HR there was time of measurement main effect (p <0 .001) with values at rest being lower than all other times of measurement (p < 0.001 for all comparisons), post 5-minutes exercise session greater than post 25- and 30-minutes post-exercise session (p < 0.001 for both comparisons), post 10-minutes exercise session greater than 25- and 30-minutes post-exercise session (p < 0.001 for both comparisons), and finally 20-minutes post-exercise session greater than post 30-minutes post-exercise session (p = 0.02). There was also training period and time of measurement interaction (p < 0.001), with values at 5-, 10-, 20- and 30- minutes post-exercise session lower in post than pre-training (p = 0.01; p = 0.003; p =0.011; and p = 0.01 respectively) (Figure 3). For SDNN there was time of measurement main effect (p < 0.001) with values at rest higher than 5- (p = 0.004), 10- (p = 0.004) and 20-minutes post-exercise session (p = 0.02) (Figure 3). No significant difference were found for RMSSD variable (Figure 3).
For LF (nu) there was a time of measurement main effect (p = 0.03) with values post 30-minutes of acute exercise session lower than post 10- and 20-minutes of exercise session (p = 0.04 and p = 0.03 respectively) (Figure 4). For HF (ms2) there was time of measurement main effect (p = 0.02) with post 20-minutes exercise session lower than post 30-minutes exercise session (p = 0.03). For HF (nu) there was also time of measurement main effect (p = 0.01) with values at post 30-minutes exercise session greater than post 10-, 20-, and 25-minutes post-exercise session (p = 0.03, p = 0.02 and p = 0.03, respectively) (Figure 4).
Figure 4.
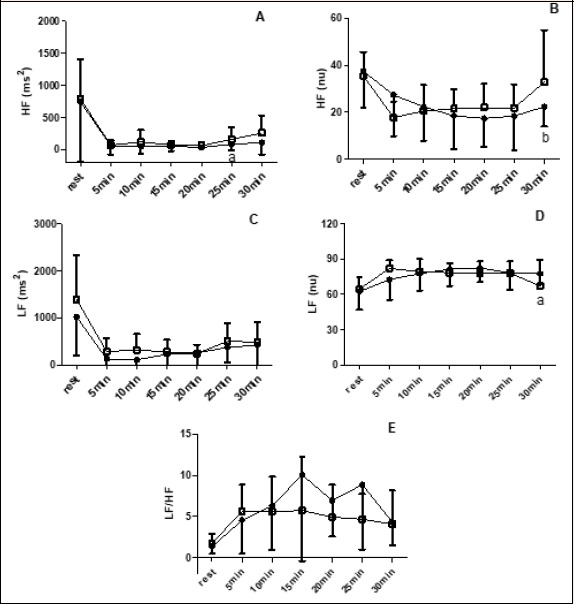
A and B: HF before (●) and after (■) 8-weeks of HIIE plus strength exercise. a = lower than 30-min post exercise session; b = higher than 10-, 20- and 30-min post exercise session. C and D: LF before (■) and after (●) 8-weeks of HIIE plus strength exercise. a = lower than 10 and 20-min post exercise session. E: Behavior of LF/HF ratio before (■) and after (●) 8-weeks of HIIE plus strength exercise.
Discussion
The aim of the present study was to investigate the effects of an acute bout of combined aerobic and strength training and the influence of 8-weeks of the same exercise protocol on the cardio-metabolic indexes (autonomic, haemodynamic and anti-atherogenic parameters) in healthy physically active men. The present study showed that 8-weeks of HIIE combined with strength exercise increased mean RR intervals as well as decreased HR during the fasted state while no alterations in CG were observed. In agreement with the outcomes from the acute exercise session, 8-weeks of HIIE combined with strength exercise induced a faster parasympathetic reactivation post- compared with pre-training due to higher mean RR intervals and lower HR post-acute exercise session and post-training intervention. Moreover, HDL-c increased and BDNF decreased post- compared with pre-training values, also in agreement with the acute exercise session outcomes. There was also an increase in HDL-c and TAG after an acute exercise bout.
Regarding the modifications to HRV post-training, the mean RR intervals increased, and HR values decreased at baseline in the fasted condition. These changes suggest that the training protocol was effective in increasing cardiac parasympathetic modulation at rest, suggesting lower cardiac stress evidenced by the reduction of HR. In addition, although not statistically significant, the RMSSD parasympathetic index showed an increase after 8-weeks of training in contrast to the control group that decreased. The increase in the parasympathetic modulation reflects a better autonomic adaptation in response to training. The HRV is an effective and practical method to evaluate the neurocardiac function, the increase of its values has been shown to relate to lower cardiovascular risks and greater autonomic function (Vanderlei et al., 2009).
Changes in catecholamine plasmatic concentrations (Vlcek et al., 2008), excitatory and inhibitory pathway regulation of the paraventricular nucleus by nitric oxide (Zheng et al., 2005), and the action of BDNF on the activity of cholinergic neurons (Marosi et al., 2014) are some of the proposed physiological mechanisms that contribute to changes in autonomic modulation. Studies in populations with autonomic dysfunction and healthy individuals have shown that aerobic exercise performed at moderate and high intensities are sound strategies to increase autonomic modulation, by regulation of sympatho-vagal balance (de Sousa et al., 2018; Besnier et al., 2017). On the other hand, strength training has been linked to minimal improvements in cardiac autonomic control in healthy individuals, presenting greater expression in diseased individuals (Bhati et al., 2018). Thus, a combination of these two modalities with distinct characteristics and action under autonomic modulation was effective to increase cardiac protection of healthy individuals after 8-weeks.
As for the lipid profile and BDNF, the training period did not provide changes in serum resting concentrations of these variables in the HSG. However, CG showed a reduction in HDL-c concentrations after 8-weeks, thus, we suggest that HIIE plus strength exercise can prevent any reduction in HDL-c concentrations and consequently promote the maintenance of cardio-metabolic health. Regarding modifications to the lipid profile, HDL-c concentrations increased in response to an acute HIIE plus strength exercise session after the training period, with higher values in HSG. These data suggest that this training model provides influence over the lipid profile, and consequently, on the cardio-metabolic parameters through improvements in lipid metabolism. Physical exercise, mainly aerobic protocols, are an efficient tool for anti-inflammatory and anti-atherogenic responses since it has been shown to activate the Lecithin-cholesterol acyltransferase (L-CAT) and Cholesteryl ester transfer protein (CETP) modulation increaseing reverse cholesterol transport by transferring cholesterol ester of other lipoproteins to HDL-c (Marques et al., 2018).
Another possible mechanism related to the increase in HDL-c is the hydrolysis of the TAG from VLDL by Lipoprotein Lipase (LPL), activated during exercise, which may result in parts on the HDL-c particles (Magkos et al., 2008). This modulation generated in response to exercise may explain the lower TAG concentration 30-minutes post- HIIE plus strength exercise. In addition, Magkos et al. (2008) investigated the effects of a single resistance (3 sets of 10 repetitions for 12 exercises at 80% of peak torque production) or endurance (90-minutes at ≥ 30% of peak oxygen consumption) exercise session on the kinetics of VLDL/TAG of seven healthy subjects in the post-absorptive period (morning after) and found that only resistance exercise lowered fasting plasma VLDL-TG concentration (p = 0.034), besides increasing the plasma clearance rate (p = 0.003) and shortening the mean residence time (p = 0.016). On the other hand, Tsekouras et al. (2008) demonstrated that a short period of high-intensity interval training (3 sessions/week; running at 60 and 90% of peak oxygen consumption in 4-minutes intervals for a total of 32-minutes) lowered the rate of VLDL-TG secretion by the liver in previously sedentary men. These findings may explain the results observed in this study that found changes in the lipid profile, especially in the HDL-c and TAG, favoring a protective condition with respect to the cardiovascular system and associated risk factors.
Physical fitness is associated with anti-atherogenic factors like BDNF; however, the response of this variable might change when analyzed either chronically or acutely. According to Babaei et al. (2014), chronic exercise promotes an inverse relationship between serum BDNF and VO2max. Furthermore, improvements in cardiovascular fitness promotes beneficial cardio-metabolic adaptations, and consequently, less concentrations of this biomarker due to greater BDNF sensitivity and efficiency (Babaei et al., 2014; Numakawa et al., 2009). Other studies reinforce these findings showing a negative correlation between serum BDNF and total energy expenditure (r= -0.507; p < 0.05), movement-related energy expenditure (r= -0.503; p < 0.05), and step count (r= -0.480; p < 0.05) (Nofuji et al., 2008). Our findings are in agreement with these studies, since the condition effect for BDNF during the acute HIIE and strength exercise session were also shown. Thus, HIIE and strength exercise were both efficient in increasing serum BDNF synthesis and uptake after the 8-week intervention resulting in lower acute serum concentrations. Clinically, the efficiency of resting BDNF uptake favors neurogenesis and neuroprotection, on the other hand, increased uptake during exercise may potentiate lipid oxidation and insulin sensitivity (Marosi and Mattson, 2014).
In relation to the autonomic modulation before and after 8-weeks of HIIE and strength exercise, frequency domain HRV variables showed a time of measurement main effect. It is important to note that immediately after training, HF (ms2) significantly increased and LF decreased in the 30th minute of recovery. This may represent a more effective parasympathetic recovery after 8-weeks of HIIE plus strength exercises than before training, which could be indicative of an acute cardio-protective situation (Cygankiewicz and Zareba, 2013). To reinforce this finding, we also found that both the HR and mean RR intervals were affected by the training intervention with lower and higher values displayed post-training, respectively. In addition, there was an increase in the values of RMSSD after 8-weeks (although not significant). Due to experimental design, we were not able to determine HRV recovery for a longer time, which could have shown differences in long-term HRV recovery.
In general, HRV decreased during exercise (paraympathetic withdrawal and sympathetic activation) according to exercise intensity, modality, and volume (Cabral-Santos et al., 2016; Seiler et al., 2007). To our knowledge, this is the first study to analyze HRV after HIIE combined with strength exercise, and the effect of 8-weeks of training on HRV recovery after an acute exercise bout. HRV recovery may influence training prescription either for trained (Seiler et al., 2007) or physically active subjects (Cabral-Santos et al., 2016). Our data shows that, the same acute session after 8-weeks of training (i.e. with similar relative intensity), might induce dissimilar HRV recovery in active subjects, indicating a positive autonomic modulation and consequently lower cardiovascular stress after training.
It is important to highlight that the fixed volume of 5 km in the HIIE session provided different running times and consequently different energy expenditure among the volunteers. It is already well established that energy expenditure is an important variable for metabolic adaptations to physical exercise (Pontzer et al., 2016; Lira et al., 2009). Thus, future studies that address isocaloric HIIE sessions are needed to better elucidate the influence of this protocol combined with strength exercise on cardio-metabolic parameters. In addition, a limitation of the present study is the lack of one exercise session pre- and post 8-weeks for the CG, aiming to evaluate possible acute changes in the evaluated parameters.
Conclusion
In summary, we suggest that 8-weeks of HIIE combined with strength exercise is an effective protective cardio-metabolic strategy capable of modifying HDL-c and BDNF concentration after an acute exercise session. In the long-term, the modulation of BDNF and HDL-c concentrations may be a determining factor for protection against neurological and cardiovascular diseases. In addition, 8-weeks of HIIE and strength exercise appear to affect HRV recovery after similar intensity exercise sessions and decrease HR with a concomitant increase in RR at rest.
Acknowledgements
Fabio Santos Lira and Caique Figueiredo thanks the “Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2013/25310-2 and 2015/23127-1)” for the support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The reported experiments comply with the current laws of the country in which they were performed. The authors have no conflicts of interests to declare.
Biographies

Caique FIGUEIREDO
Employment
Exercise and Immunometabolism Research Group, Department of Physical Education, Universidade Estadual Paulista, Presidente Prudente, São Paulo, Brazil
Degree
Mr
Research interests
Metabolism, exercise, inflammation, and health.
E-mail:caiquefigueiredo22@gmail.com

Barbara Moura ANTUNES
Employment
Exercise and Immunometabolism Research Group, Department of Physical Education, Universidade Estadual Paulista, Presidente Prudente, São Paulo, Brazil
Degree
PhD
Research interests
Low-grade chronic inflammation, training, immunometabolism, physical activity, and health
E-mail: ba.antunes2@gmail.com

Thaís Roque GIACON
Employment
Departament of Physioterapy, Universidade Estadual Paulista (UNESP), Presidente Prudente, São Paulo, Brazil.
Degree
MSc
Research interests
Cardiorespiratory physiotherapy, prevention and risk factors, and heart rate variability
E-mail: thaisgiacon@hotmail.com

Luiz Carlos Marques VANDERLEI
Employment
Depart. of Physioterapy, Universidade Estadual Paulista (UNESP), Presidente Prudente, São Paulo, Brazil.
Degree
PhD
Research interests
Cardiorespiratory physiotherapy, prevention and risk factors, and HRV
E-mail: lcmvanderlei@fct.unesp.br

Eduardo Zapaterra CAMPOS
Employment
Department of Physical Education, Federal University of Pernambuco
Degree
PhD
Research interests
Sport training, Sports science, and exercise physiology
E-mail: zacampos@yahoo.com.br
Fernando Pierin PERES
Employment
Universidade do Oeste Paulista, Presidente Prudente, São Paulo, Brazil
Degree
Mr
Research interests
Cardiology
E-mail: fernandopierin@outlook.com

Nicolas William CLARK
Employment
Univ. of Central Florida | UCF Institute of Exercise Physiology and Wellness
Degree
Mr
Research interests
Physiology, and Exercise
E-mail: nicolas.clark@knights.ucf.edu

Valéria Lemes Gonçalves PANISSA
Employment
Researcher, School of Physical Education and Sport, Univ. of São Paulo
Degree
PhD
Research interests
Sport and Exercise Physiology, Appetite and Exercise, High Intensity Intermittent Exercise
E-mail: valeriapanissa@gmail.com

Fábio Santos de LIRA
Employment
Depart. of Physical Education, Univ. Estadual Paulista (UNESP), Presidente Prudente, São Paulo, Brazil
Degree
PhD
Research interests
Metabolism, immunological system, exercise, and nutrition
E-mail: fabio.lira@unesp.br
References
- Antunes B. M. M., Cayres S. U., Lira F. S., Fernandes R. A. (2016) Arterial thickness and Immunometabolism: the mediating role of chronic exercise. Current Cardiology Reviews 12(1), 47-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei P., Damirchi A., Mehdipoor M., Tehrani B. S. (2014) Long term habitual exercise is associated with lower resting level of serum BDNF. Neuroscience Letters 566, 304-308. [DOI] [PubMed] [Google Scholar]
- Bhati P., Moiz J. A., Menon G. R., Hussain M. E. (2018) Does resistance training modulate cardiac autonomic control? A systematic review and meta-analysis. Clinical Autonomic Research, 1-29. [DOI] [PubMed] [Google Scholar]
- Besnier F., Labrunée M., Pathak A., Pavy-Le Traon A., Galès C., Sénard J. M., Guiraud T. (2017) Exercise training-induced modification in autonomic nervous system: An update for cardiac patients. Annals of Physical and Rehabilitation Medicine 60(1), 27-35. [DOI] [PubMed] [Google Scholar]
- Brown L. E., Weir J. P. (2001) ASEP procedures recommendation I: accurate assessment of muscular strength and power. Journal of Exercise Physiology Online 4(3). [Google Scholar]
- Cabral-Santos C., Giacon T. R., Campos E. Z., Gerosa-Neto J., Rodrigues B., Vanderlei L. C. M., Lira F. S. (2016) Impact of high-intensity intermittent and moderate-intensity continuous exercise on autonomic modulation in young men. International Journal of Sports Medicine 37(6), 431-435. [DOI] [PubMed] [Google Scholar]
- Cabral-Santos C., Castrillón C.I., Miranda R.A., Monteiro P.A., Inoue D.S., Campos E.Z, Hofmann P., Lira F.S. (2016) Inflammatory Cytokines and BDNF Response to High-Intensity Intermittent Exercise: Effect the Exercise Volume. Frontiers Physiology 4, 7:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos G.E., Luecke T.J., Wendeln H.K., Toma K., Hagerman F.C., Murray T.F., Ragg K.E., Ratamess N.A., Kraemer W.J., Staron R.S. (2002) Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. European Journal of Applied Physiology 88(1-2), 50-60. [DOI] [PubMed] [Google Scholar]
- Cygankiewicz I., Zareba W. (2013) Heart rate variability. In: Handbook of Clinical Neurology Vol. 117 Elsevier; 379-39. [DOI] [PubMed] [Google Scholar]
- Delgado-Floody P., Espinoza-Silva M., García-Pinillos F., Latorre-Román P. (2018) Effects of 28 weeks of high-intensity interval training during physical education classes on cardiometabolic risk factors in Chilean schoolchildren: a pilot trial. European Journal of Pediatrics 177(7), 1019-1027. [DOI] [PubMed] [Google Scholar]
- de Sousa A. F., Medeiros A. R., Benitez-Flores S., Del Rosso S., Stults-Kolehmainen M., Boullosa D. A. (2018) Improvements in Attention and Cardiac Autonomic Modulation After a 2-Weeks Sprint Interval Training Program: A Fidelity Approach. Frontiers in Physiology 9, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halcox J., Misra A. (2015) Type 2 diabetes mellitus, metabolic syndrome, and mixed dyslipidemia: how similar, how different, and how to treat? Metabolic Syndrome and Related Disorders 13(1), 1-21. [DOI] [PubMed] [Google Scholar]
- Helkin A., Stein J. J., Lin S., Siddiqui S., Maier K. G., Gahtan V. (2016) Dyslipidemia part 1 — review of lipid metabolism and vascular cell physiology. Vascular and Endovascular Surgery 50(2), 107-118. [DOI] [PubMed] [Google Scholar]
- Kaess B.M., Preis S.R., Lieb W., Beiser A.S., Yang Q., Chen T.C., Hengstenberg C., Erdmann J., Schunkert H., Seshadri S., Vasan R.S., CARDIoGRAM (2015) Circulating Brain-Derived Neurotrophic Factor Concentrations and the Risk of Cardiovascular Disease in the Community. Journal of the American Heart Association 4(3), e001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler H. S., Sisson S. B., Short K. R. (2012) The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Medicine 42(6), 489-509. [DOI] [PubMed] [Google Scholar]
- Kuipers H., Verstappen F. T. J., Keizer H. A., Geurten P., Van Kranenburg G. (1985) Variability of aerobic performance in the laboratory and its physiologic correlates. International Journal of Sports Medicine 6(04), 197-201. [DOI] [PubMed] [Google Scholar]
- Lira F.S., Zanchi N.E., Lima-Silva A.E., Pires F.O., Bertuzzi R.C., Santos R.V., Seelaender M. (2009) Acute high-intensity exercise with low energy expenditure reduced LDL-c and total cholesterol in men. European Journal of Applied Physiology 107(2), 203-210. [DOI] [PubMed] [Google Scholar]
- Lira FS, Rosa JC, Lima-Silva AE, Souza HA, Caperuto EC, Seelaender MC, Damaso AR, Oyama LM, Santos RV. (2010) Sedentary subjects have higher PAI-1 and lipoproteins levels than highly trained athletes. Diabetology & Metabolic Syndrome 2(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magkos F., Tsekouras Y.E., Prentzas K.I., Basioukas K.N., Matsama S.G., Yanni A.E., Kavouras S.A., Sidossis L. S. (2008) Acute exercise-induced changes in basal VLDL-triglyceride kinetics leading to hypotriglyceridemia manifest more readily after resistance than endurance exercise. Journal of Applied Physiology 105(4), 1228-1236. [DOI] [PubMed] [Google Scholar]
- Marosi K., Mattson M. P. (2014) BDNF mediates adaptive brain and body responses to energetic challenges. Trends in Endocrinology & Metabolism 25(2), 89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques L. R., Diniz T. A., Antunes B. M., Rossi F. E., Caperuto E. C., Lira F. S., Gonçalves D. C. (2018) Reverse Cholesterol Transport: Molecular Mechanisms and the Non-medical Approach to Enhance HDL Cholesterol. Frontiers in Physiology 9, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin M. D., St Jeor S. T., Hill L. A., Scott B. J., Daugherty S. A., Koh Y. O. (1990) A new predictive equation for resting energy expenditure in healthy individuals. The American Journal of Clinical Nutrition 51(2), 241-247. [DOI] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC). (2017) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. The Lancet 390(10113), 2627-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofuji Y.U., Suwa M., Moriyama Y., Nakano H., Ichimiya A., Nishichi R., Sasaki H., Radak Z., Kumagai S., (2008) Decreased serum brain-derived neurotrophic factor in trained men. Neuroscience Letters 437(1), 29-32. [DOI] [PubMed] [Google Scholar]
- Numakawa T., Kumamaru E., Adachi N., Yagasaki Y., Izumi A., Kunugi H. (2009) Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-γ signaling for glutamate release via a glutamate transporter. Proceedings of the National Academy of Sciences 106(2), 647-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistorio E., Luca M., Luca A., Messina V., Calandra C. (2011) Autonomic nervous system and lipid metabolism: findings in anxious-depressive spectrum and eating disorders. Lipids in Health and Disease 10(1), 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontzer H., Durazo-Arvizu R., Dugas L.R., Plange-Rhule J., Bovet P., Forrester T. E., Lambert E.V., Cooper R.S., Schoeller D.A., Luke A. (2016) Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Current Biology 26(3), 410-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Vélez R., Tordecilla-Sanders A., Téllez-T L.A., Camelo-Prieto D., Hernández-Quiñonez P.A., Correa-Bautista J.E., Garcia-Hermoso A., Ramirez-Campillo R., Izquierdo M. (2017) Similar cardiometabolic effects of high-and moderate-intensity training among apparently healthy inactive adults: a randomized clinical trial. Journal of Translational Medicine 15(1), 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F.E., Fortaleza A.C., Neves L.M., Buonani C., Picolo M.R., Diniz T.A., Kalva-Filho C.A., Papoti M., Lira F.S., Junior I.F.F. (2016) Combined training (aerobic plus strength) potentiates a reduction in body fat but demonstrates no difference on the lipid profile in postmenopausal women when compared with aerobic training with a similar training load. The Journal of Strength & Conditioning Research 30(1), 226-234. [DOI] [PubMed] [Google Scholar]
- Santos C.C., Diniz T.A., Inoue D.S., Gerosa-Neto J., Panissa V.L., Pimentel G.D., Campos E.Z., Hofmann P., Lira F. S. (2016) Influence to high-intensity intermittent and moderate-intensity continuous exercise on indices of cardio-inflammatory health in men. Journal of Exercise Rehabilitation 12(6), 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler S., Haugen O., Kuffel E. (2007) Autonomic recovery after exercise in trained athletes: intensity and duration effects. Medicine & Science in Sports & Exercise 39(8), 1366-1373. [DOI] [PubMed] [Google Scholar]
- Swift D. L., Houmard J. A., Slentz C. A., Kraus W. E. (2018) Effects of aerobic training with and without weight loss on insulin sensitivity and lipids. PloS One 13(5), e0196637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvainen M. P., Laitinen T.P., Lipponen J.A., Cornforth D.J., Jelinek H.F. (2014) Cardiac autonomic dysfunction in type 2 diabetes–effect of hyperglycemia and disease duration. Frontiers in Endocrinology 5, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsekouras Y. E., Magkos F., Kellas Y., Basioukas K. N., Kavouras S. A., Sidossis L. S. (2008) High-intensity interval aerobic training reduces hepatic very low-density lipoprotein-triglyceride secretion rate in men. American Journal of Physiology-Endocrinology and Metabolism 295(4), E851-E858. [DOI] [PubMed] [Google Scholar]
- Vanderlei L. C. M., Silva R. A., Pastre C. M., Azevedo F. M. D., Godoy M. F. (2008) Comparison of the Polar S810i monitor and the ECG for the analysis of heart rate variability in the time and frequency domains. Brazilian Journal of Medical and Biological Research 41(10), 854-859. [DOI] [PubMed] [Google Scholar]
- Vanderlei L. C. M., Pastre C. M., Hoshi R. A., De Carvalho T. D., de Godoy M. F. (2009) Noções básicas de variabilidade da frequência cardíaca e sua aplicabilidade clínica. Revista Brasileira de Cirurgia Cardiovascular 24(2), 205-217. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M. (2013) Endothelial dysfunction in obesity. In: Annales pharmaceutiques Francaises 71(1), 42-50. [DOI] [PubMed] [Google Scholar]
- Vlcek M., Radikova Z., Penesova A., Kvetnansky R., Imrich R. (2008). Heart rate variability and catecholamines during hypoglycemia and orthostasis. Autonomic Neuroscience 143(1-2), 53-57. [DOI] [PubMed] [Google Scholar]
- Zembron-Lacny A., Dziubek W., Rynkiewicz M., Morawin B., Woźniewski M. (2016) Peripheral brain-derived neurotrophic factor is related to cardiovascular risk factors in active and inactive elderly men. Brazilian Journal of Medical and Biological Research 49(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Li Y. F., Cornish K. G., Zucker I. H., Patel K. P. (2005) Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. American Journal of Physiology-Heart and Circulatory Physiology 288(5), H2332-H2341. [DOI] [PubMed] [Google Scholar]


