Abstract
Importance:
Many children with return of spontaneous circulation (ROSC) following cardiac arrest (CA) experience acute kidney injury (AKI). The impact of therapeutic hypothermia on the epidemiology of post-CA AKI in children has not been fully investigated.
Objective:
The study aims were to: 1) describe the prevalence of severe AKI in comatose children following out-of-hospital CA (OHCA), 2) identify risk factors for severe AKI, 3) evaluate the impact of therapeutic hypothermia on the prevalence of severe AKI, and 4) evaluate the association of severe AKI with survival and functional outcomes.
Design:
A post hoc secondary analysis of data from the Therapeutic Hypothermia after Pediatric Cardiac Arrest Out-of-Hospital (THAPCA-OH) trial.
Setting:
Thirty-six pediatric intensive care units in the United States and Canada.
Participants:
Of 282 eligible subjects with an initial creatinine obtained within 24 hours of randomization, 148 were randomized to therapeutic hypothermia and 134 were randomized to therapeutic normothermia.
Main Outcomes and Measures:
Primary outcome was prevalence of severe AKI, as defined by stage 2 and 3 Kidney Disease Improving Global Outcomes (KDIGO) consensus definition; secondary outcome was survival with a favorable neurobehavioral outcome. For this study, risk factors and outcomes were compared between those with/without severe AKI.
Results:
Of the 282 subjects enrolled, 180 (64%) developed AKI of which 117 (41% of all enrolled) developed severe AKI. Multivariable modeling found younger age, longer duration of chest compressions, higher lactate level at time of temperature intervention and higher number of vasoactive agents through day 1 of intervention associated with severe AKI. There was no difference in severe AKI between therapeutic hypothermia (39.9%) and therapeutic normothermia (43.3%) groups (p=0.629). Survival was lower in those with severe AKI at 28 days (21% vs no severe AKI 49%, p<0.001) and 12 months (21% vs no severe AKI 42%, p<0.001). One year survival with favorable functional outcome was lower in those with severe AKI.
Conclusions and Relevance:
Severe AKI occurs frequently in children with ROSC after OHCA, especially in younger children and those with higher initial lactates and hemodynamic support. Severe AKI was associated with worse survival and functional outcome. Therapeutic hypothermia did not reduce the incidence of severe AKI.
Keywords: Post-Cardiac Arrest, Acute Kidney Injury, Therapeutic Hypothermia, Neurologic Outcomes
Introduction
Return of spontaneous circulation (ROSC) following out-of-hospital cardiac arrest (OHCA) is frequently associated with the post-CA syndrome due to a systemic ischemia-reperfusion response manifesting as myocardial dysfunction, brain injury, and other organ dysfunction[1–3]. Severe acute kidney injury (AKI) occurs in nearly 50% of adult cardiac arrest subjects[4]. AKI commonly occurs in critically ill children, including over one-third following CA, and is associated with increased morbidity and mortality [5–8]. The American Heart Association recommends treating comatose subjects following CA with either therapeutic hypothermia or therapeutic normothermia for neuroprotection[9,10]. Studies of neonates with perinatal asphyxia suggest that AKI is a risk factor for adverse neurologic outcomes [11]. Little is known about the impact of therapeutic hypothermia on the development AKI or whether AKI worsens survival or neurologic outcomes following pediatric CA.
The recently completed Therapeutic Hypothermia after Pediatric Cardiac Arrest Out-of-Hospital randomized trial (THAPCA-OH) evaluated the efficacy of targeted temperature management following pediatric OHCA [12]. This study is a secondary analysis of THAPCA-OH to describe the epidemiology of CA-associated AKI. The aims of this study were to: 1) describe the prevalence of severe AKI in comatose children following out-of-hospital CA (OHCA), 2) identify risk factors for severe AKI, 3) evaluate the impact of therapeutic hypothermia on the prevalence of severe AKI, and 4) evaluate the association of severe AKI with survival and functional outcomes.
Methods
This study is a planned secondary analysis of the THAPCA-OH trial, which received Institutional Review Board approval. The THAPCA-OH trial was conducted from September 1, 2009 through December 31, 2012 in 36 enrolling pediatric intensive care units (PICU) in the United States and Canada[12]. The trial included children >48 hours and <18 years of age who sustained an OHCA, received ≥ 2 minutes of chest compressions, and were admitted to a PICU mechanically ventilated after ROSC. Major exclusion criteria were inability to consent and randomize within 6 hours of ROSC and a Glasgow Coma Scale motor response subscale of 5 or 6. Subjects were randomized 1:1 to either therapeutic hypothermia or therapeutic normothermia.
Temperature intervention was maintained for 120 hours after randomization: therapeutic hypothermia 33°C (range 32–34°C) or therapeutic normothermia 36.8°C (range 36 to 37.5°C). Subjects randomized to therapeutic hypothermia were cooled to 33°C, maintained at 32–34°C for 48 hours, rewarmed over 16–24 hours to 36.8°C and maintained at 36 to 37.5°C through hour 120. Subjects randomized to therapeutic normothermia were actively maintained at 36.8°C through 120 hours. Urine output (mL/kg/hr) was calculated by calendar day from Day 1 to Day 5. Details of the study protocol were previously reported [12].
Inclusion criteria for the current study
Those with an initial serum creatinine obtained within 24 hours of randomization were eligible for analysis. Subjects were excluded if they had a pre-existing renal condition or supported with Extracorporeal Life Support (ECLS).
Definition of Acute Kidney Injury
We defined AKI using the Kidney Disease Improving Global Outcomes (KDIGO) creatinine-based consensus definitions (granular urine output data was unavailable [13],[6]). Because no subjects had an immediate pre-arrest value available, baseline creatinine was calculated in the 253 subjects with available height, as (0.413 × height in cm)/100[14]. For the 29 subjects with height unavailable, baseline creatinine was defined by subject age: 0.3 mg/dL for age < 5 years, 0.5 mg/dL for age 5–12 years, and 0.7 mg/dL for age > 12 years. The current analysis is focused on the impact of severe AKI, stage 2 or 3, as has previously been utilized by other investigators [7,15].
Outcome Measures
Outcomes included 12-month survival, 12-month survival with favorable neurobehavioral function, and 12-month survival without change in neurobehavioral function. Neurobehavioral functioning was assessed using the Vineland Adaptive Behavior Scales – Second Edition (VABS-II), a caregiver report measure of daily functioning. The VABS-II includes communication, daily living, socialization, and motor skills domains and provides an overall adaptive behavior composite score. Baseline VABS-II scores were obtained within 24 hours of OH-CA and were based on functioning prior to CA. Research coordinators assisted caregivers with the baseline ratings. Additionally, 12-month VABS-II scores were obtained over the telephone by trained interviewers from Kennedy Krieger Institute. The VABS-II has an age-corrected mean of 100 and standard deviation of 15. Favorable neurobehavioral functioning was defined two ways 1) survival with a 12-month VABS-II adaptive behavior composite score of ≥70 and 2) survival with a decrease in 12-month VABS-II by ≤15 points from baseline).
Statistical Analysis
Time from ROSC to peak AKI was calculated and described using histograms. Time to development of severe AKI is based on the time serum creatinine was obtained, or for cases where a subject was defined as having Stage 3 AKI due to use of renal replacement therapy (RRT) an estimated time of 12:00 (noon) on the day of RRT initiation. Time from ROSC to severe AKI or death was compared between treatment groups by Kaplan-Meier survival curves utilizing a log-rank test.
Univariate associations between the presence of severe AKI and subject characteristics or outcomes were examined using Fisher’s exact test (categorical variables) and Wilcoxon rank-sum test (continuous variables). Multivariable logistic regression was used to test the association of covariates with severe AKI at different time points, candidate variables with univariate associations with p-value < 0.10. Some variables were excluded from consideration due to excessively missing data, clinician preference when two factors were highly correlated (e.g. baseline versus peak lactate), or data occurring after day 1.
Predictor variables were grouped into four time periods in a stepwise model procedure: Step 1) pre-arrest, Step 2) at time of ROSC, Step 3) near time of start of temperature intervention, and Step 4) post intervention through day 1. Forward stepwise selection was performed in the four steps. In Step 1, only pre-arrest characteristics were considered. In Step 2, any characteristic kept by the model in Step 1 was included in the starting model and the candidate arrest characteristics were additionally considered. Step 3 and 4 followed similarly. In each step, a p-value < 0.1 was used as the criterion for entering the model while a p-value > 0.05 was used as the criteria for removing factors. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
Of 294 THAPCA-OH eligible subjects, 12 were excluded; eight for pre-existing renal condition, four for ECLS. Of 282 eligible subjects; 148 were randomized to therapeutic hypothermia and 134 to therapeutic normothermia. The median age at randomization was 2 years (IQR 0.4 to 8.8 years); 38% were <1 year. One hundred thirty (46%) of subjects had a pre-existing medical condition (Table 1). The most common etiologies of CA were respiratory related in 89 (31.6%), drowning in 71 (25.2%) and sudden unexplained infant death (SUID) in 45 (16.0%) (Table 1). Asystole was the first documented rhythm in nearly 60% of cases.
Table 1:
Demographics and DiagnosticData
| Severe AKI | ||||
|---|---|---|---|---|
| Overall (N = 282) |
Yes (N = 117) |
No (N = 165) |
P-value | |
| Age Group at Randomization | ||||
| <1 year | 106 (37.6%) | 70 (59.8%) | 36 (21.8%) | <.0011 |
| 1–4 years | 89 (31.6%) | 27 (23.1%) | 62 (37.6%) | 0.0131 |
| 5–12 years | 43 (15.2%) | 8 (6.8%) | 35 (21.2%) | <.0011 |
| > 13 years | 44 (15.6%) | 12 (10.3%) | 32 (19.4%) | 0.0451 |
| Male sex | 188 (66.7%) | 77 (65.8%) | 111 (67.3%) | 0.7991 |
| Preexisting conditions | ||||
| None | 152 (53.9%) | 63 (53.8%) | 89 (53.9%) | 1.0001 |
| Lung or airway disease | 63 (22.3%) | 27 (23.1%) | 36 (21.8%) | 0.8851 |
| Neurologic condition | 43 (15.2%) | 16 (13.7%) | 27 (16.4%) | 0.6151 |
| Gastrointestinal disorder | 36 (12.8%) | 19 (16.2%) | 17 (10.3%) | 0.1511 |
| Prenatal condition | 30 (10.6%) | 19 (16.2%) | 11 (6.7%) | 0.0171 |
| Congenital heart disease | 30 (10.6%) | 16 (13.7%) | 14 (8.5%) | 0.1751 |
| Other | 59 (20.9%) | 28 (23.9%) | 31 (18.8%) | 0.3031 |
| Primary etiology of cardiac arrest | ||||
| Cardiac | 35 (12.4%) | 13 (11.1%) | 22 (13.3%) | 0.7141 |
| ALTE/SUID | 45 (16.0%) | 32 (27.4%) | 13 (7.9%) | <.0011 |
| Drowning | 71 (25.2%) | 15 (12.8%) | 56 (33.9%) | <.0011 |
| Other Respiratory | 89 (31.6%) | 39 (33.3%) | 50 (30.3%) | 0.6051 |
| Other/Unknown | 42 (14.9%) | 18 (15.4%) | 24 (14.5%) | 0.8661 |
P-value is based on Fisher’s exact test.
Prevalence of AKI
One hundred eighty subjects (64%) had AKI. Of the 180 subjects, 63 had stage 1, 52 had AKI stage 2, and 65 had AKI stage 3 - we combined stage 2 and 3 to define severe AKI; therefore, 117 (41%) had severe AKI, including 10 subjects (3.5%) who received renal replacement therapy
Risk Factors for Severe AKI
Subjects who developed severe AKI were younger (mean, 3.0 years; SD, 4.9 vs mean, 6.1; SD, 5.6, p<0.001) with 70 of 117 (60%) less than one year of age. Subjects with a prenatal condition were more likely to develop severe AKI (Table 1). The primary etiology of the CA differed between subjects who developed severe AKI and those who did not (Table 1). Drowning subjects were least likely to develop severe AKI (odds ratio, 0.3; CI, 0.2 to 0.5) and SUID subjects were most likely (odds ratio, 4.4; CI, 2.2 to 8.8). SUID accounted for 27% (32/117) of the severe AKI cases. In a sensitivity analysis excluding subjects with CA etiology of SUID an association between severe AKI and age remained (Appendix Table 1).
The initial rhythm or bystander initiated chest compressions did not differ between severe AKI/no severe AKI groups (Table 1), however, the estimated duration of chest compressions and chest compressions on arrival to first hospital were associated with severe AKI. Children with severe AKI had a higher incidence of organ dysfunction as reflected by increased ALT, lactate and vasopressor requirements (Table 1). They were more likely to receive vancomycin and more doses of epinephrine (severe AKI: 4 [IQR 2, 6] doses vs no severe AKI: 3 [IQR 1, 4] doses).
In multivariable modeling of prearrest and arrest periods variables (Step 1 and 2), only age and duration of chest compressions were associated with severe AKI. When additional variables were added reflecting a later point in time around temperature intervention (Step 3), lactate was associated with severe AKI development along with age but not duration of chest compressions. When variables beyond the intervention period were added through day 1 (Step 4), the number of vasoactive agents administered was associated with severe AKI (Table 2) along with age and lactate concentration. Of note, predictive ability of the model as assessed by area under the curve (AUC) improved only modestly from 70.9% to 78.9% when factors other than the child’s age were added.
Table 2:
Physiologic and Therapeutic Data
| Severe AKI | ||||
|---|---|---|---|---|
| Overall (N = 282) |
Yes (N = 117) |
No (N = 165) |
P-value | |
| Initial rhythm noted by EMS or hospital | ||||
| Asystole | 168 (59.6%) | 74 (63.2%) | 94 (57.0%) | 0.3251 |
| Bradycardia | 18 (6.4%) | 9 (7.7%) | 9 (5.5%) | 0.4681 |
| Pulseless electrical activity (PEA) | 40 (14.2%) | 16 (13.7%) | 24 (14.5%) | 0.8641 |
| Ventricular fibrillation or tachycardia | 22 (7.8%) | 5 (4.3%) | 17 (10.3%) | 0.0731 |
| Unknown | 34 (12.1%) | 13 (11.1%) | 21 (12.7%) | 0.7151 |
| Bystander witnessed cardiac arrest | 102/268 (38.1%) | 37/110 (33.6%) | 65/158 (41.1%) | 0.2501 |
| Chest compressions administered by | 178/272 | 67/112 | 111/160 | 0.1201 |
| bystander | (65.4%) | (59.8%) | (69.4%) | |
| Estimated duration of chest compressions in 272 patients (minutes) - Median (Q1, Q3) | 25.0 (17.0, 37.0) | 26.0 (20.0, 45.0) | 25.0 (15.0, 35.0) | 0.0142 |
| Chest compressions still required at | 189/276 | 89/114 | 100/162 | 0.0061 |
| time of arrival at first hospital | (68.5%) | (78.1%) | (61.7%) | |
| Total number of doses of epinephrine administered by EMS and at hospital in 253 patients - Median (Q1, Q3) | 3.0 (2.0, 5.0) | 4.0 (2.0, 6.0) | 3.0 (1.0, 4.0) | 0.0032 |
| Assigned to Hypothermia treatment | 148 (52.5%) | 59 (50.4%) | 89 (53.9%) | 0.6291 |
| Aminoglycoside (Day 0–1) | 3 (1.1%) | 2 (1.7%) | 1 (0.6%) | 0.5721 |
| Vancomycin (Day 0–1) | 93 (33.0%) | 48 (41.0%) | 45 (27.3%) | 0.0201 |
| Baseline Lactate3 in 273 patients (mmol/L) - Median (Q1, Q3) | 5.6 (3.2, 10.0) | 9.0 (4.5, 13.5) | 4.2 (2.8, 7.6) | <.0012 |
| Peak Lactate (Day 0–1) in 275 patients (mmol/L) - Median (Q1, Q3) | 6.5 (3.5, 10.7) | 9.7 (4.8, 14.1) | 4.8 (2.9, 7.9) | <.0012 |
| Baseline glucose in 258 patients concentration (mmol/L) - Median (Q1, Q3) | 250 (179, 321) | 241 (168, 324) | 252 (193, 317) | 0.7262 |
| Baseline ALT in 245 patients concentration (U/L) - Median (Q1, Q3) | 113 (59, 251) | 147 (80, 335) | 95 (51, 196) | 0.0022 |
| Peak ALT (Day 0–5) in 279 patients concentration (U/L) - Median (Q1, Q3) | 139 (71, 331) | 193 (99, 417) | 108 (60, 207) | <.0012 |
| Milrinone (Day 0–1) | 41 (14.5%) | 17 (14.5%) | 24 (14.5%) | 1.0001 |
| Vasopressin (Day 0–1) | 58 (20.6%) | 28 (23.9%) | 30 (18.2%) | 0.2951 |
| Vasoactive Agents (Day 0–1) | ||||
| Epinephrine | 139 (49.3%) | 71 (60.7%) | 68 (41.2%) | 0.0021 |
| Dopamine | 107 (37.9%) | 50 (42.7%) | 57 (34.5%) | 0.1731 |
| Norepinephrine | 48 (17.0%) | 26 (22.2%) | 22 (13.3%) | 0.0551 |
| Dobutamine | 14 (5.0%) | 7 (6.0%) | 7 (4.2%) | 0.5831 |
| Phenylephrine | 5 (1.8%) | 3 (2.6%) | 2 (1.2%) | 0.6521 |
| Number of Vasoactive Agents (Day 0–1) | 0.0011 | |||
| 0 | 99 (35.1%) | 26 (22.2%) | 73 (44.2%) | |
| 1 | 73 (25.9%) | 34 (29.1%) | 39 (23.6%) | |
| 2 | 90 (31.9%) | 48 (41.0%) | 42 (25.5%) | |
| 3 | 20 (7.1%) | 9 (7.7%) | 11 (6.7%) | |
P-valueis based on Fisher’s exact test.
P-value is based on the Wilcoxon rank-sum test.
First non-missing lactate value occurring ≤ 8 hours after randomization.
Impact of Hypothermia on Severe AKI
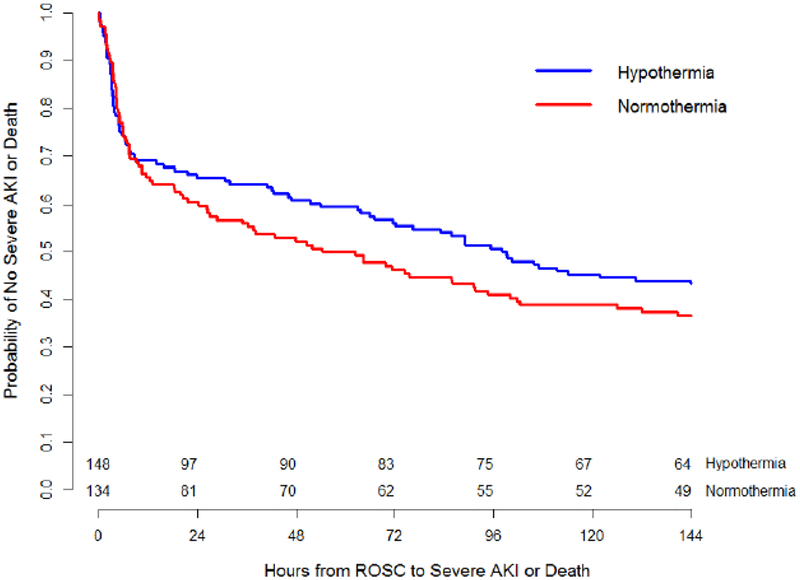
There was no difference in rates of severe AKI between the temperature treatment groups (therapeutic hypothermia 39.9% vs therapeutic normothermia 43.3%; p=0.629). Day-by-day rates of severe AKI were higher in the therapeutic normothermia group on Days 1 and 2 and the therapeutic hypothermia group on Day 5, but not significant different. Rates of a combined outcome of severe AKI or death did not differ over time in the therapeutic normothermia group versus the hypothermia group (Figure 1; p=0.27).
Figure 1:
Hours to Severe AKI or Death
The two lines represent Kaplan-Meier rates from 0 to 144 hours after ROSC for patients in each study arm (p=0.27 by log-rank test).
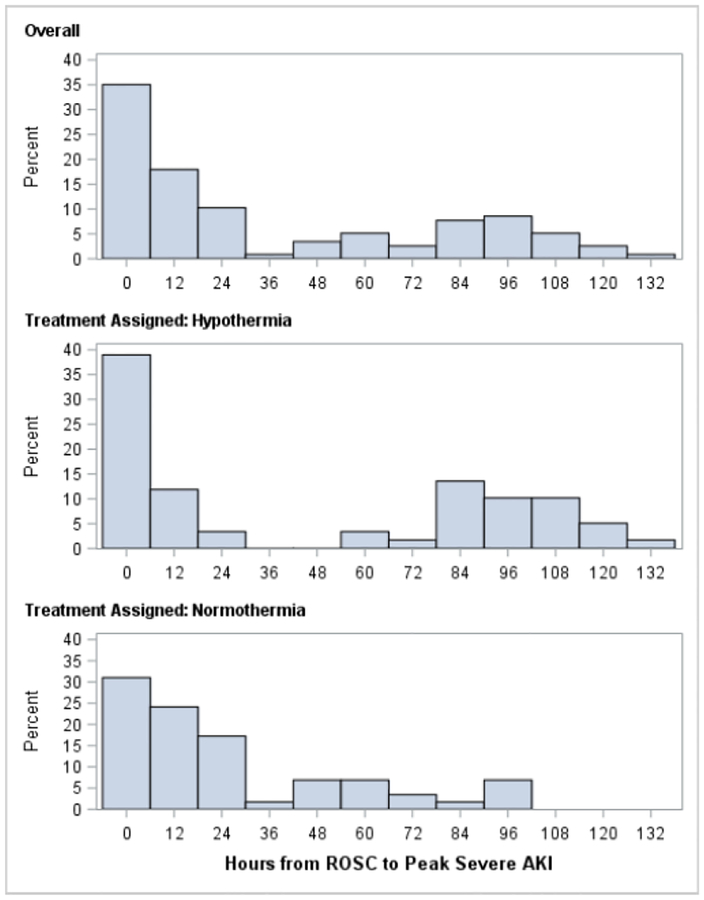
Half of subjects with severe AKI developed peak AKI within the first 24 hours after ROSC (Figure 2). A secondary peak of AKI occurred 96 hours after ROSC and was more commonly observed following rewarming in the hypothermia group (Figure 2).
Figure 2:
Hours from ROSC to Peak Severe AKI
Time in hours from ROSC to peak severe AKI did not differ between treatment groups (p=0.25, Wilcoxon rank-sum test).
Association of AKI and Outcomes
Rates of survival at 28 days and at 12 months were higher in those without severe AKI (severe AKI 21% vs no severe AKI 49% at 28 days, p<0.001, and severe AKI 21% and no severe AKI 42% at 12 months, p<0.001; Table 3). There was no difference in survival with VABS-II within 15 points of baseline at one year (p=0.099), however, there was a statistically significant association of lower one year survival with favorable neurobehavioral outcome in subjects who developed severe AKI (p=0.008; Table 3).
Table 3.
Logistic Regression Models for Severe AKI Outcome
| Through Step 1 Model AUC = 70.9% |
Through Step 2 Model AUC = 73.6% |
Through Step 3 Model AUC = 78.2% |
Through Step 4 Model AUC = 78.9% |
|||||
|---|---|---|---|---|---|---|---|---|
| Predictors1 | Odds Ratio (95% CI) |
P-value | Odds Ratio (95% CI) |
P-value | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) |
P-value |
| Step 1: Pre-arrest | ||||||||
| Age in years | <.001 | <.001 | <.001 | <.001 | ||||
| < 1 year | Reference | Reference | Reference | Reference | ||||
| 1 – 4 years | 0.22 (0.12, 0.41) | 0.23 (0.12, 0.42) | 0.26 (0.13, 0.51) | 0.23 (0.12, 0.47) | ||||
| 5 – 12 years | 0.12 (0.05, 0.28) | 0.11 (0.04, 0.27) | 0.13 (0.05, 0.34) | 0.12 (0.04, 0.30) | ||||
| > 13 years | 0.19 (0.09, 0.42) | 0.23 (0.10, 0.50) | 0.35 (0.15, 0.81) | 0.29 (0.12, 0.70) | ||||
| Step 2: At time of ROSC | ||||||||
| Estimated duration of chest compressions2 | 1.02 (1.00, 1.04) | 0.01 | 1.01 (0.99, 1.03) | 0.27 | 1.01 (0.99, 1.03) | 0.36 | ||
| Step 3: Post-arrest, near intervention start | ||||||||
| Baseline Lactate3 (mmol/L) | 1.14 (1.07, 1.22) | <.001 | 1.12 (1.05, 1.20) | 0.001 | ||||
| Step 4: Day 0–1 | ||||||||
| Number of Vasoactive Agents (Day 0–1) | 1.50 (1.09, 2.05) | 0.01 | ||||||
Predictors considered for logistic regression models: age, preexisting prenatal condition, etiology of arrest, initial rhythm, doses of epinephrine, estimated duration of chest compressions, chest compressions still required at time of arrival at first hospital, baseline lactate, vancomycin used (day 0 or 1), number of vasoactive agents used (day 0 or 1).
Replacement of ‘estimated duration of chest compressions’ with ‘total number of epinephrine doses’ in step 2 resulted in the selection of the same predictors and similar odds ratio estimates in steps 3 and 4.
First non-missing lactate value occurring ≤ 8 hours after randomization.
Discussion
The current study shows that AKI occurs commonly in comatose pediatric subjects following OHCA, with 64% developing AKI and 41% developing severe AKI. Children with severe AKI were younger and had laboratory and clinical findings consistent with other severe end organ injury. Therapeutic hypothermia was not associated with decreased occurrence or severity of AKI.
AKI prevalence in our study was significantly higher than a recent report by Neumayr et al, who describe that 21.4% of subjects meet criteria for AKI after OHCA[8]. The higher prevalence of AKI in our cohort could be explained by different criteria to define AKI in the two studies, and differing inclusion and exclusion criteria. Our results are also consistent with the prevalence of AKI in neonates following perinatal asphyxia[11,16]. A recent publication investigating AKI and OHCA in adults showed a similar rate of AKI of 46%, which was associated with poor outcomes [17]. The lack of benefit of therapeutic hypothermia on AKI development is in contrast to Tanigasalam el al.’s findings that neonates who received therapeutic hypothermia had reduced occurrences of AKI and lower mortality [16]. Additionally, adult data regarding renal transplant from deceased organ donors demonstrate a protective benefit of hypothermia for the transplanted kidney [18]. The benefit of hypothermia in these studies may reflect a difference in the etiology of arrest, age and developmental differences, timing of cooling, and other factors.
Our findings demonstrate that severe AKI is associated with injury and dysfunction of other organs, consistent with the severe systemic ischemia/reperfusion injury following ROSC (Table 1). Higher lactate levels and more vasopressor medications are independent risk factors for post-OHCA AKI suggesting a key role of early hemodynamic insufficiency after ROSC in the development of severe AKI. The additional finding that SUID is associated with higher risk of AKI independent of age suggests that the duration of hemodynamic insufficiency may contribute to AKI as these individuals may be pulseless for an extended time prior compared to individuals with etiologies that occur near bystanders.
This secondary analysis of the THAPCA-OHCA trial represents the largest prospective cohort of children who experienced OHCA-associated AKI. We anticipate that randomization balanced known and unknown risk factors for AKI in the two temperature intervention groups, limiting confounding. One limitation of our analysis is the large proportion of early deaths may have resulted in underestimation of AKI at later time points. We attempted to correct for this by combining the outcomes of death and severe AKI, which did not demonstrate therapeutic hypothermia delayed the time to death or development of severe AKI compared to therapeutic normothermia (Figure 1). Another limitation is that the THAPCA-OH trial population excluded all cases that were not comatose at the time of randomization up to 6 hours following ROSC, which may have overestimated AKI for the overall OHCA population by selecting a cohort of subjects with more severe ischemia/reperfusion insult as result of CA. Other limitations are use of an estimate for baseline SCr, and we utilized modified KDIGO criteria, with SCr alone, to define AKI (sufficient urine output data not available). Finally, our data represents the OHCA cohort only. The association of AKI and In-hospital CA is currently being investigated.
In summary, our findings demonstrate that AKI frequently affects comatose children following OHCA, and therapeutic hypothermia does not decrease the rate of severe AKI. Younger children, longer duration of chest compressions, higher lactate concentration in the post arrest period, and high degree of hemodynamic support were independent risk factors for developing AKI post-OHCA. Development of severe AKI was associated with increased 28-day and 12-month mortality, and lower survival with favorable neurobehavioral outcome.
Supplementary Material
Table 4:
Association of Severe AKI with Outcomes
| Severe AKI | ||||
|---|---|---|---|---|
| Overall | Yes | No | P-value | |
| Survival at 12 months with VABS-II >= 70 | 40/251 (15.9%) | 9/106 (8.5%) | 31/145 (21.4%) | 0.0081 |
| Survival with VABS-II decreased no more than 15 points or improved | 35/273 (12.8%) | 10/115 (8.7%) | 25/158 (15.8%) | 0.0991 |
| Survival at 12 months | 91/275 (33.1%) | 24/116 (20.7%) | 67/159 (42.1%) | <.0011 |
| Survival at 28 days | 106/282 (37.6%) | 25/117 (21.4%) | 81/165 (49.1%) | <.0011 |
P-value is based on Fisher’s exact test.
Acknowledgments
Conflicts of Interest
Supported by the National Heart, Lung, and Blood Institute (NHLBI) grants HL094345 (to Dr. Moler) and HL094339 (to Dr. Dean). Support in part from the following federal planning grants contributed to the planning of the THAPCA Trials: HD044955 (to Dr. Moler) and HD050531 (to Dr. Moler). Additional in part support from the following research networks: Pediatric Emergency Care Applied Research Network (PECARN) from cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008; and the Collaborative Pediatric Critical Care Research Network (CPCCRN) from cooperative agreements U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012 and U01HD049934. Site support from P30HD040677, UL1TR000003UL1, RR 024986, and UL1 TR 000433.
Timothy T. Cornell receives funding for studies not related to this manuscript from National Institutes of Health – NHLBI (R01 HL119542)
David J Askenazi receives grant funding for studies not related to this manuscript from National Institutes of Health — National Institutes of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK, R01 DK103608 and NIH-FDA (R01 FD005092). Alexis Topjian is funded by 4K23NS075363.
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Topjian AA, French B, Sutton RM, Conlon T, Nadkarni VM, Moler FW, et al. Early Postresuscitation Hypotension Is Associated With Increased Mortality Following Pediatric Cardiac Arrest*. Crit Care Med 2014;42:1518–23. doi: 10.1097/CCM.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Topjian AA, Berg RA, Taccone FS. Haemodynamic and ventilator management in patients following cardiac arrest. Curr Opin Crit Care 2015;21:195–201 doi: 10.1097/MCC.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008;118:2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- [4].Geri G, Guillemet L, Dumas F, Charpentier J, Antona M, Lemiale V, et al. Acute kidney injury after out-of-hospital cardiac arrest: risk factors and prognosis in a large cohort. Intensive Care Med 2015;41:1273–80. doi: 10.1007/s00134-015-3848-4. [DOI] [PubMed] [Google Scholar]
- [5].Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013;8:1482–93. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Selewski DT, Cornell TT, Heung M, Troost JP, Ehrmann BJ, Lombel RM, et al. Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med 2014;40:1481–8. doi: 10.1007/s00134-014-3391-8. [DOI] [PubMed] [Google Scholar]
- [7].Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, AWARE Investigators. Epidemiology of Acute Kidney Injury in Critically III Children and Young Adults. N Engl J Med 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Neumayr TM, Gill J, Fitzgerald JC, Gazit AZ, Pineda JA, Berg RA, et al. Identifying Risk for Acute Kidney Injury in Infants and Children Following Cardiac Arrest. Pediatr Crit Care Med 2017:1–9. doi: 10.1097/PCC.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S465–82. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de Caen AR, Berg MD, Chameides L, Gooden CK, Hickey RW, Scott HF, et al. Part 12: Pediatric Advanced Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S526–42 doi: 10.1161/CIR.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Selewski DT, Jordan BK, Askenazi DJ, Dechert RE, Sarkar S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. The Journal of Pediatrics 2013;162:725–729.e1. doi: 10.1016/j.jpeds.2012.10.002. [DOI] [PubMed] [Google Scholar]
- [12].Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, et al. Therapeutic Hypothermia after Out-of-Hospital Cardiac Arrest in Children. N Engl J Med 2015;372:1898–908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- [14].Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;20:629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fitzgerald JC, Basu RK, Akcan-Arikan A, Izquierdo LM, Piñeres Olave BE, Hassinger AB, et al. Acute Kidney Injury in Pediatric Severe Sepsis: An Independent Risk Factor for Death and New Disability. Crit Care Med 2016;44:2241–50. doi: 10.1097/CCM.0000000000002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tanigasalam V, Bhat V, Adhisivam B, Sridhar MG. Does therapeutic hypothermia reduce acute kidney injury among term neonates with perinatal asphyxia? – a randomized controlled trial. The Journal of Maternal-Fetal & Neonatal Medicine 2015:1–4. doi: 10.3109/14767058.2015.1094785. [DOI] [PubMed] [Google Scholar]
- [17].Beitland S, Nakstad ER, Staer-Jensen H, Draegni T, Andersen G, Jacobsen D, et al. Impact of acute kidney injury on patient outcome in out-of-hospital cardiac arrest: a prospective observational study. Acta Anaesthesiol Scand 2016;60:1170–81. doi: 10.1111/aas.12753. [DOI] [PubMed] [Google Scholar]
- [18].Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M, et al. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N Engl J Med 2015;373:405–14. doi: 10.1056/NEJMoa1501969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.