Abstract
There has been considerable progress in elucidating the molecular mechanisms that contribute to memory formation and the generation of circadian rhythms. However, it is not well understood how these two processes interact to generate long-term memory. Recent studies in both vertebrate and invertebrate models have shown time-of-day effects on neurophysiology and memory formation, and have revealed a possible role for cycling molecules in memory persistence. Together, these studies suggest that common mechanisms underlie circadian rhythmicity and long-term memory formation.
Circadian rhythms are basic biological phenomena that exist throughout phylogeny. They are influenced by zeitgebers and regulate various physiological events that include the cell cycle, body temperature, metabolism, feeding and, perhaps most notably, the sleep–wake cycle. Far less well understood is the relationship between circadian rhythm biology and memory formation. The impact of time-of-day effects and of circadian rhythms on cognitive performance in humans1–3 and on memory in animals4–7 have been studied for decades, and there has been a renewed interest in this topic in light of an increased understanding of the genetic, molecular and systems-level events that underlie these complex processes8. Recent discoveries have shown a high level of integration between cellular signalling cascades (such as the cyclic AMP–mitogen-activated protein kinase (MAPK)-cAMP-responsive element-binding protein (CREB) pathway) that regulate circadian rhythms and memory processing. Disruption of circadian rhythms or specific signalling cascades that undergo time-of-day-dependent cycling, by behavioural, environmental, genetic or pharmacological means, has negative consequences on memory and cognitive performance in various tasks and in several species. Given that modern society is becoming less dependent on the natural 24-hour light–dark cycle, an increased understanding of the functional relationship between circadian rhythms and cognitive function has broad implications for public health9.
Here, we summarize studies that have shown a time-of-day effect on memory formation and compare the emerging common themes in various invertebrate and vertebrate species. We first describe the molecular pathways and time-of-day-dependent neuronal activity patterns that are conserved in circadian pacemaker cells in flies and rodents. Next, we present work that shows circadian alterations on neurophysiological processes that involve synaptic plasticity (such as long-term potentiation) and on memory formation in nocturnal (night-active), diurnal (day-active), and crepuscular model systems. On the basis of the cycling pattern of molecular cascades that are involved in memory formation, we address whether the cyclical reactivation of these cascades over the 24-hour day is essentially independent from inputs of the core time-keeping cells that are known to contribute to locomotor rhythm output. This Review expands on previously understood circadian effects on memory at the behavioural and physiological level, by focusing on recent data that show a possible involvement of circadian cycling of specific molecular pathways in long-term memory formation. Further background information has been published elsewhere on circadian rhythms9,10 and memory formation11,12.
Are clock genes memory genes?
The initial characterization of the molecular players involved in the generation of circadian rhythms was carried out in the Drosophila melanogaster model. Over three decades ago, work on fruitflies showed that the periodic timing of the eclosion rhythm was dependent on the strain of fly. This suggested a genetic basis for the circadian regulation of this process, prompting a forward mutagenesis screen that identified the first clock gene, period (per)13. This gene was eventually cloned independently by separate laboratories14,15. Levels of per mRNA and protein were shown to cycle in a circadian manner in flies and mammals and to be a part of a phylogenetically conserved transcriptional auto-regulatory feedback loop (FIG. 1) that is necessary for the synchronized expression of the circadian rhythm of locomotor activity16,17. In D. melanogaster, mutations in per result in differences in the length of the eclosion rhythm, and include long (perL), short (perS), and arrhythmic (per0) phenotypes. Interestingly, these mutations cause correlative changes in the periodicity of the circadian locomotor activity rhythm in adult flies. Under constant dark (DD) conditions, pers flies have a shortened circadian rhythm, perL have a lengthened circadian rhythm, and per0 are arrhythmic. This evidence suggests that the single clock gene per has pleiotropic effects on the timing of two separate processes at different developmental stages. Do clock genes have a role in the time-of-day effects on memory formation? Curiously, in contrast to wild-type flies, in mutant per0 flies and tim01 flies — which have a mutation in the gene encoding Timeless (TIM), the binding partner of PER proteins — there is no time-of-day effect on short-term olfactory avoidance memory under DD conditions18. In addition, as measured in a courtship conditioning assay, per0 flies are defective in long-term memory (LTM) formation — a phenotype that can be rescued with a wild-type copy of the per gene in the per0 background19. Overexpression of per in this paradigm has even been shown to enhance LTM19 despite these flies retaining rhythmic locomotor and mating activities under free-running conditions. This suggests that per regulates memory independently of its role in eclosion or in the generation of circadian rhythms.
Figure 1 |. Phylogenetic conservation of the core molecular clock.
The molecular clock in flies and mammals is composed of transcriptional and translational feedback networks. In flies, CLOCK (CLK) and CYCLE (CYC) heterodimerize and activate transcription of the period (per) and timeless (tim) genes by binding to E-box elements in their promoters. The protein products PER and TIM heterodimerize and enter the nucleus following phosphorylation (P) by proteins such as doubletime (DBT) or casein kinase II (CKII), and repress the transcriptional activity of CLK-CYC. In mammals, the circadian clock comprises a similar feedback network, including CLOCK and the CYC homologue brain and muscle ARNT-like (BMAL), which activate the transcription of per and cryptochrome (cry) genes via E-box elements. PERs and CRYs heterodimerize in the cytoplasm following phosphorylation by proteins such as casein kinase Iε (CKIε), and enter the nucleus where they inhibit CLOCK-BMAL transcriptional activation. Mitogen-activated protein kinase (MAPK) phosphorylates BMAL146, repressing BMAL-CLOCK activity. Putative mechanisms linking melatonin (MEL) rhythms and the circadian clock include repression of adenylate cyclase (AC) and protein kinase A (PKA), a pathway known to influence cAMP-responsive element (CRE)-binding protein (CREB) activation. A second mechanism is thought to activate the MAPK-CREB cascade147,148 through Ca2+ influx, leading to transcriptional activity through CRE elements in the per promoters22. An analogous putative pathway is shown for Drosophila melanogaster, in which MAPK phosphorylation represses CLK-CYC149 or activates CREB, leading to per transcrption.
Previous work has shown that there is a role for another transcription factor, CREB, in the core circadian clock of flies20 and mammals21. In addition, a functional cAMP-responsive element (CRE) site in the promoter of mouse per genes that binds CREB has been described22, suggesting a link between CREB activity and PER activity in circadian rhythm generation (FIG. 1). A functional relationship between CREB activity and per expression was also shown in D. melanogaster20. Flies that carry a luciferase reporter downstream of the per gene promoter (per-luc) have a disrupted and reduced amplitude of circadian transcriptional activity in a CREB-mutant background, indicating a functional link between CREB activity and circadian gene expression in D. melanogaster. In addition, per expression affects the cycling of CRE-mediated activity. Flies that carry a luciferase reporter downstream of three CRE sites (CRE-luc) normally show a circadian rhythm of luminescence under conditions of 12-hour light followed by 12-hour dark (LD) as well as under DD conditions. This CRE-luc cycling is coordinately altered in per mutants: in perL flies, the CRE-luc cycling pattern is lengthened, whereas it is shortened in pers flies, compared with wild-type controls, and in per0 flies the CRE-luc activity pattern is arrhythmic across the day20. A mutation in the Creb2 gene of D. melanogaster also produces a shortened circadian cycle of locomotor activity, suggesting that CREB regulates normal circadian behaviour in flies20. These data support a reciprocal relationship between CREB- and PER-mediated transcriptional regulation, with functional relationships in the generation of circadian rhythms. The precise relationship between CREB- and PER-mediated transcriptional activity (for example, through the cAMP-MAPK-CREB cascade) in the time-of-day-dependent regulation of memory is still unclear.
Anatomical relay of circadian centres
It is well established that certain molecules with cycling activity patterns influence circadian rhythms, but what is known about how neural networks generate the ultimate behavioural output? Considerable progress has been made in elucidating the cellular components and neuronal pathways that are responsible for the generation of circadian rhythms, and some similarities have been found across many species. Across phylogeny, clock-containing circadian pacemaker cells in the central nervous system receive photic input and can drive changes in locomotor rhythms over the course of the day. For example, in mammals, photic activation of non-image-forming retinal ganglion cells, which contain the photo-responsive pigment melanopsin, send light information to the central pacemaker of circadian rhythms — the suprachiasmatic nucleus (SCN)23 — via the retinohypothalamic tract (RHT)24. The core of the SCN receives photic input from the RHT and relays it to the subparaventricular zone (sPVz), which in turn relays the information to other hypothalamic structures (FIG. 2). These hypothalamic structures are known to regulate many physiological processes, including thermoregulation, hormone secretion, feeding behaviour and arousal- sleep states. The SCN is therefore thought to regulate the circadian timing of these processes. Pathways connecting the SCN to limbic structures that are involved in memory processing, such as the hippocampus and amygdala, have been shown25,26. Other indirect connections — such as through hypocretin-expressing cells in the lateral hypothalamus27 or through superior cervical ganglion-stimulated melatonin release from the pineal gland28 — could relay SCN-derived circadian input to the hippocampus (FIG. 2). Whether these connections are responsible for the time-of-day-dependent expression of memory and/or synaptic plasticity is not known.In D. melanogaster, photic input entrains a circadian rhythm in circadian pacemaker cells (of which there are ~150) through at least three pathways29: the eyes30, the Hofbauer-Buchner eyelets31–33 and/or the blue-light photo pigment cryptochrome (CRY)30,34. Photoreceptive cells in the optic lobe are thought to project to the lateral neuronal cells via the posterior optic tract35 (POT) (FIG. 2). This has been supported by recent findings that describe functional connectivity between the contralateral optic lobe and the large ventral lateral neurons (LNv) via the POT36. Additionally, the Hofbauer-Buchner eyelets send projections to the accessory medulla37, where they synapse with the small LNv pacemaker clock neurons38,39. Anatomical connections among pacemaker cells that receive and relay this photic input to other brain regions — such as the mushroom bodies, a pair of neuropil structures in the insect brain known to regulate sleep40,41 and memory formation42,43 — in D. melanogaster are not well characterized. Although the small LNv cells have been shown to project to terminals near the mushroom bodies44, a direct functional connection between pacemaker cells and cells that are responsible for memory formation is still lacking. The genetically tractable model of D. melanogaster will be valuable in determining the neural circuitry that is responsible for the integration of these two complex processes.
Figure 2 |. Anatomical circadian pathways in flies and mice.
a | In fruitflies (Drosophila melanogaster), various light-receiving cells are involved in functional neuroanatomical connections, such as those in the Hofbauer-Buchner (H-B) eyelets and ocelli (OC), or from the optic lobes (OL). These project to circadian pacemaker cells, the lateral neurons (LN), via the posterior optic tract (POT). LN subtypes include the large, small, and 5th small ventral LN (LNv), as well as the dorsal LN (LNd). Little is known about the functional connectivity between these pacemaker cells and other clock cells, such as the dorsal neurons (DN1, DN2 and DN3 subtypes) the lateral posterior neurons (LPN) or cells that are involved in sleep and memory formation, such as the pars intercerebralis (PI) and mushroom bodies (MB). DNs and LNs comprise the ~150 cells of the clock network in the fly brain. b | In the mouse (Mus musculus), the suprachiasmatic nucleus (SCN) receives photic input through the retinohypothalamic tract (RHT). The SCN projects to the dorsal medial hypothalamus (DMH) through the subparaventricular zone (sPVz), which projects to various regions in the hypothalamus, including the ventrolateral preoptic area (VLPO), the lateral hypothalamus (LH) and the paraventricular nucleus (PVN). There are reciprocal connections between the VLPO and the tuberomammilary nucleus (TMN), which are thought to be partly responsible for the proper timing of sleep-wake rhythms. Functional connections between a circadian centre and a memory forming-centre, such as the hippocampus, are not well known. They may be partially gated through hypocretin- or orexin-expressing cells of the LH, or by melatonin secretion from the pineal gland following signalling from the PVN to the superior cervical ganglion (SCG)150. Note that C57BL/6J mice lack melatonin. OB, olfactory bulb.
Time-of-day effects on neurophysiology
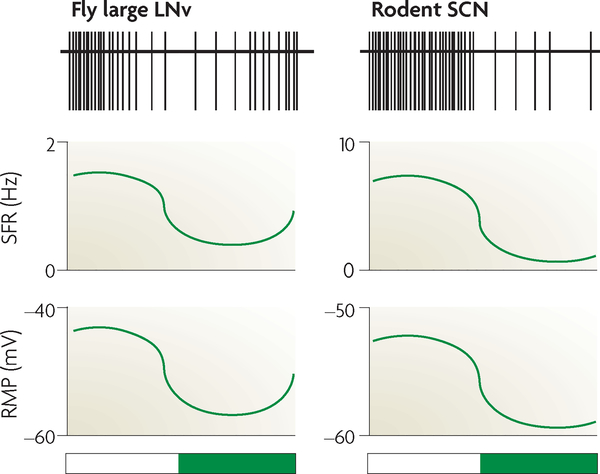
It is unclear whether a time-of-day-dependent relay exists between clock pacemaker neurons and memoryforming cells, such as between the SCN and the hippocampus of mammals or between the lateral neurons and the mushroom bodies of flies. However, cyclical changes over 24-hour periods have been observed in baseline physiological properties of central pacemaker cells in both mammals and flies. In nocturnal rodents, sCN neurons show circadian changes in spontaneous firing rate (SFR) and resting membrane potential (RMP), with an elevated SFR and more depolarized RMP in the light phase than the dark phase45–50 (FIG. 3). A similar effect was observed in the large LNv pacemaker cells in crepuscular D. melanogaster36,51,52. Taken together, these data further support a phylogenetically conserved mechanism of circadian neurophysiology in pacemaker cells (FIG. 3). This conserved mechanism may influence time-of-day-dependent expression of physiological events downstream of central pacemaker cells, such as stimulation of brain regions that are involved in learning and memory.
Figure 3 |. Time-of-day-dependent neurophysiology in pacemaker cells.
Fruitflies, which are crepuscular, have a higher spontaneous firing rate (SFR) and resting membrane potential (RMP) in their clock cells, such as the large ventral lateral neurons (LNv), near the dark-to-light transition and during the daytime, than in the light-to-dark transition and during the night-time36,52. Similar to fly clock cells, neurons in the nocturnal rodent suprachiasmatic nucleus (SCN) have an elevation in SFR and RMP during the light period compared with the dark period, despite a difference in the locomotor activity rhythm of these two species49,50. Top traces show a schematic representation of spike trace frequency over the day-night cycle. White bars represent ‘lights on’; dark green bars represent ‘lights off’.
Melatonin is a signalling molecule that is widely expressed throughout phylogeny and is secreted in a time-of-day-dependent manner53. Recently, melatonin synthesis has been suggested to interact with core circadian mechanisms54. The temporal release of melatonin is regulated over the 24-hour day in humans55 and many other species, including zebrafish56 (Danio rerio), sea slugs57 (Apylsia californica), mice58 (Mus musculus) and flies59 (D. melanogaster). Interestingly, melatonin affects the firing rate of the mammalian SCN60–62, suggesting that the time-of-day-dependent regulation of hormonal secretion may alter the firing patterns of circadian pacemaker cells. Melatonin also affects the firing rate of hippocampal CA1 neurons63. Therefore, circadian hormonal modulation of neuronal firing could be a general mechanism throughout the brain. It would be interesting to know whether there are circadian fluctuations in the baseline SFRs and RMPs in hippocampal or mushroom body neurons, and whether these oscillations occur in phase with those of the SCN and large LNvs, respectively. If these circadian firing patterns occur, an important next step will be to address whether they result from functional connectivity between pacemaker cells and memory-encoding regions, or whether they are caused by autonomous cycling molecules or circulating hormones such as melatonin. Further studies are needed to examine the functional connectivity between these brain regions that are involved in circadian rhythms and memory, and to compare baseline neurophysiological properties.
Time-of-day effects on synaptic plasticity
Given that circadian changes in molecular and neurophysiological properties of pacemaker cells are observed throughout the animal kingdom, an obvious question is whether neural correlates of plasticity-related neurophysiology are also regulated by circadian rhythms. In the hippocampus, long-term potentiation (LTP) — a form of synaptic plasticity (BOX 1) that is thought to underlie learning and memory64 — has been shown to change depending on the time of day in various nocturnal rodents65–70 (FIG. 4). These circadian effects on LTP can be considered a naturally occurring form of metaplasticity71, in that the synaptic efficacy for a given amount of stimulation varies based on the time of day. Circadian changes in plasticity may serve as a useful model with which to study the neurophysiological and molecular mechanisms of metaplasticity. It would also be interesting to compare plasticity-related mechanisms in the SCN with those in the hippocampus. Molecular pathways (FIG. 1) that are involved in establishing plastic changes in circadian neurophysiology may be more fundamental than previously appreciated and could be shared with other known plasticity-related brain regions, such as those involved in memory formation. Lastly, knowledge of how baseline conditions can be modulated by the time of day is crucial for understanding the effect on synaptic plasticity of other experimental manipulations.
Box 1 |. Physiological analysis of long-term potentiation.
Two examples of the methods used to study the physiological manifestations of long-term potentiation (LTP) are shown in the figure. Rodent brain slices are obtained at the level of the suprachiasmatic nucleus (SCN; a), or the hippocampus (b). Following stimulation (STIM) of the optic tract or the Schaffer collateral pathway, recordings are made from electrodes (REC) that are implanted in cells of the SCN or area CA1 of the hippocampus, respectively. Example graphs depicting representative traces of recordings following stimulation show LTP in the form of a population spike or a field potential. Example traces (inset) are shown for the pre-stimulation baseline (shown by the orange line) and post-stimulation LTP (shown by the green line). LTP is expressed as a percentage increase of the slope or amplitude of electrical potential from the baseline over time.

Figure 4 |. Time-of-day effects on synaptic plasticity.
Time-of-day-dependent changes in long-term potentiation (LTP) occur in various rodent species, including rats, hamsters and mice. a | Interestingly, in the nocturnal rat hippocampus, LTP is greater during the dark phase than in the light phase in the dentate gyrus (DG) but is greater during the light phase than in the dark phase in area CA1 (REF. 67). LTP is also time-of-day-dependent in the rat suprachiasmatic nucleus (SCN), with an elevation during the day phase compared to the night phase69. b | In hippocampal area CA1 of the hamster, LTP is higher when tissue is isolated during the light phase and tested during the dark phase (light > dark) and lower when tissue is isolated during the dark phase and tested during the light phase (dark > light)73. c | In area CA1 of the nocturnal mouse, LTP is lower during the light period, but enhancement of LTP (metaplasticity) occurs when tissue is harvested during the light period and tested during the dark period70. Taken together, these data suggest that there are endogenous cellular oscillators that continue to function when dissociated from the intact brain, driving time-of-day-dependent changes in synaptic plasticity.
Synaptic plasticity in the suprachiasmatic nucleus
Time-of-day-dependent changes in synaptic plasticity have been observed in the SCN. Stimulation of the optic nerve can elicit potentiated responses in the SCN that last for hours, analogous to those observed following Schaffer collateral stimulation of the CA1 region of the hippocampus (BOX 1). In the SCN of rats, following stimulation of the optic nerve and with changes recorded in field potentials at six time points over the course of the day (starting 1 hour after normal lights-on), a potentiation of synaptic strength was observed during the day phase of the circadian cycle69 (FIG. 4). However, this study was unable to detect the previously reported time-of-day-dependent changes in LTP in the hippocampus67, which raises questions about how time-of-day effects on LTP may be regulated.
Hippocampal long-term potentiation
Hippocampus-dependent tasks, such as inhibitory avoidance conditioning, are thought to elicit LTP-like responses following training72, suggesting a functional relationship between synaptic plasticity mechanisms and memory formation. In 1977, it was shown that synaptic excitability of the rat hippocampal dentate gyrus exhibited a circadian rhythm65. Previously, a meta-analysis examined over 170 studies for a circadian component of hippocampal LTP in rats, and found that the incidence and the magnitude of LTP were dependent on the time of day67. Interestingly, LTP was elevated during the dark phase in the dentate gyrus but was elevated during the light phase in area CA1 (FIG. 4). A clear influence of the time of day on LTP in the hippocampal CA1 region was also observed in the Syrian hamster, with increased LTP in animals that were killed during the light phase than during the dark phase73. However, unlike the rat studies analysed previously67, this study used tissue that was harvested during the opposite time of day from when the electrophysiology was completed. ‘Daytime’ hippocampal slices were prepared between zeitgeber time (ZT) phase 4.5–5.5, but LTP was not evaluated until the night-time (between ZT13.5–19.5). Conversely, ‘night-time’ hippocampal slices were prepared between ZT18.5–19.5, but LTP was not evaluated until ZT4. LTP in the CA1 was greater in hamster tissue that was harvested during the light period and then tested later during the dark period than in hamster tissue that was harvested and tested in the reverse conditions73 (FIG. 4). This prompted examination of whether the time-of-day effects on LTP are dependent on the time of tissue harvest or the time of testing70. In hippocampal CA1 tissue harvested from nocturnal C3H and C57Bl/6J strains of mice during the light period, LTP was greater in tissue that was examined during the dark period than during the light period70 (FIG. 4). These data are in contrast to the meta-analysis of nocturnal rat LTP67, but are consistent with data on nocturnal hamsters73, and together suggest that the time-of-day effects on LTP are dependent on the time of testing, and not the time of harvest. Furthermore, these results support the hypothesis that an independent circadian pacemaker controls the time-of-day-dependent changes in hippocampal plasticity and that arousal state or sleep per se are not necessary for circadian changes in LTP.
Hormonal regulation of long-term potentiation
Hormones have been shown to modulate LTP in the SCN and hippocampus. For example, melatonin has been shown to block LTP in both regions74–77. C57Bl/6J mice, which lack melatonin, undergo less dramatic time-of-day-dependent changes in hippocampal LTP than C3H mice, which express melatonin70. Melatonin also inhibits neocortex-dependent LTP and memory formation in rats78, suggesting a broad action of melatonin on synaptic plasticity in other brain regions. These data are interesting given the influence of melatonin on memory formation79 and the timing of melatonin peaks in the circadian rhythm of memory formation in various species (FIG. 5). In addition, studies in adrena-lectomized (ADX) rats have implicated hormonal modulation in the time-of-day effect on hippocampal LTP68. In ADX rats, the peak of LTP in the dentate gyrus shifted from the night-time to the daytime, suggesting that hippocampal LTP is regulated by other circulating hormones, such as those found in the adrenal gland. As the circadian regulation of adrenal corticosterone is under the influence of the SCN80, the altered cycling of hippocampal LTP that is observed in ADX rats may result from a disruption in signalling from the sCN to the hippocampus via the adrenal gland. Given that the time of testing, rather than the time of harvest, seems to affect hippocampal LTP, it is plausible that cycling hormones affect the ‘clock-time’ of hippocampal cells before tissue harvest. These data suggest that circulating hormones could operate as a zeitgeber, setting the clock in hippocampal cells such that they retain the rhythm during testing that was previously entrained by hormone signalling. Whether there is a relationship between rhythms of LTP and rhythms of memory formation remains to be determined.
Figure 5 |. Melatonin and circadian rhythms of memory.
Low levels of melatonin correlate with high levels of memory performance and are seemingly independent of the activity state in vertebrate and invertebrate species. In the diurnal zebrafish (Danio rerio) and sea slug (Aplysia californica), there is a time-of-day-dependent enhancement of memory formation during the ‘active phase’ (a) of their circadian rhythm. By constrast, in the nocturnal mouse (Mus musculus) and the crepuscular fruitfly (Drosophila melanogaster),there is memory enhancement during the ‘inactive phase’ (b) of their circadian rhythm. Each species, regardless of activity state, has a corresponding anti-phase relationship between memory performance and melatonin levels. White bars represent ‘lights on’; dark green bars represent ‘lights off’. Figure is based on data from REFS 56,57,59,79,88–90,93.
Circadian effects on memory formation
Time-of-day and circadian effects on cognitive performance and memory formation have been observed in various behavioural paradigms81–84. The influence of sleep and behavioural state on memory consolidation are reviewed elsewhere85–87.
Interestingly, circadian rhythms of locomotor activity are not an accurate predictor of the timing of optimal performance in memory tasks over the course of the day. The circadian rhythms in memory formation in nocturnal, diurnal and crepuscular species do not show a clear correlation with their specific rhythm of locomotor activity. For example, peak performance in memory tasks occurs during the ‘active phase’ of the diurnal zebrafish79 (D. rerio) and sea slug18,88 (A. californica), but during the ‘inactive phase’ of the nocturnal house mouse89 (M. musculus) and the crepuscular fruitfly90 (D. melanogaster) (FIG. 5). This suggests that activity levels are not responsible for the changes in memory formation that occur over the course of 24 hours, as there is a clear dissociation of the rest-activity rhythm and cognitive performance in the various chronobiological models. This indicates that factors other than the behavioural state are involved, supporting the theory that specific cellular or molecular events that cycle over the 24-hour day are responsible for the enhancement of memory at specific times. Processes that could contribute to this memory enhancement include gene transcription and translation, epigenetic mechanisms, neurotransmitter release, synaptic excitability, neuronal activity and hormone secretion. In addition, each of these processes may have different effects on various phases (acquisition, consolidation and retrieval) of memory.
Does melatonin influence memory? A better predictor of the circadian variation of peak performance in memory tasks may be cycling molecules with a periodicity that follows the rhythm of performance — rather than the rhythm of locomotor activity — across species. One molecular correlate that seems to be a good candidate is melatonin. Interestingly, levels of melatonin are inversely related to cognitive function: peak melatonin release occurs during the lower periods of performance over the course of the day in humans8,91 and other species (FIG. 5). This suggests that levels of melatonin are good predictors of the nadir (lowest) period in the time-of-day cycling of memory.
Recently, the role of melatonin in the regulation of memory was investigated using an active-avoidance conditioning paradigm in zebrafish79. Fish were trained in a tank to make an ‘unsafe’ association in a dark compartment, in which they received electric shocks, and a ‘safe’ association within a lit compartment, in which there were no electric shocks. A clear time-of-day effect was observed in acquisition (learning) and memory formation, and both were improved during the daytime (active) period. These effects were maintained under DD — a condition that is necessary to evaluate whether an endogenous circadian system controls the time-of-day effect. Treatment of fish with melatonin before training had no effect on acquisition but significantly reduced LTM formation. Fish that were treated with melatonin, either following training or just before testing, did not show differences in LTM or retrieval, respectively. This finding supports a role for melatonin in the earlier stages of LTM formation. Furthermore, this phenotype was rescued by treating the fish with melatonin receptor antagonists, either simultaneously with melatonin during the daytime or alone during the night-time phase (when endogenous melatonin is high and LTM is low). These results suggest that melatonin signaling cascades influence the time-of-day effects on memory formation. However, the melatonin theory is controversial as C57B1/6J mice exhibit time-of-day-dependent changes in memory formation89 despite the absence of melatonin58,92,93, and so further studies are warranted.
Time of acquisition versus time of recall
Differences in time-of-day effects on memory acquisition versus recall have been observed on various tasks in various models79,88,89,94–96. In A. californica, the circadian rhythm of long-term sensitization (LTS) was examined88. LTS training consists of a series of shocks delivered to the side of the sea slug, which elicits a siphon withdrawal response, followed by a post-training electrical shock to the tail, which elicits a ‘sensitized’ siphon withdrawal. Memory is expressed as the ratio between pre-training and post-training durations of the siphon withdrawal behaviour. A time-of-day effect on LTS memory was observed, with peak responses during the ‘active period’ in both LD and DD conditions (FIG. 5). Time-of-day-dependent regulation of the baseline siphon withdrawal response or the withdrawal duration was not observed in either LD or DD conditions, suggesting that the memory results from an endogenous circadian mechanism. This time-of-day effect on LTS depended on the time of acquisition, rather than on the time of recall. The LTS response was greater in animals that were trained at circadian time 9 (CT9; when LTS is enhanced) and tested at CT21 (when LTS is suppressed) than in animals that were trained and tested at CT21. Animals that were trained at CT21 and tested at CT3 (when LTS is normally enhanced) did not show an elevated LTS response, suggesting that the time of training (learning) and not the time of testing (recall) is responsible for the circadian rhythm of LTS in A. californica. This is in contrast to the result in mice89, which suggested that optimal recall times are under circadian control.
The mouse inbred strains C3H and C57B1/6J have significant time-of-day effects on memory formation in a fear conditioning paradigm89. They display optimal memory recall during the ‘inactive phase’ (the light period) for contextual and cued fear conditioning. These mice also show a time-of-day effect on memory acquisition, with an elevation during the light period, at ZT3, compared with the dark period, at ZT15. A possible relationship between the effect of time of acquisition and the effect of the time of recall was also examined. Mice were trained during the day period, at ZT3, under LD conditions. They showed circadian cycling of memory that was maintained for at least 3 days, with a period of ~24 hours and a peak enhancement reoccurring at the same time as training. Interestingly, in DD conditions with training at CT3, mice displayed the same ~24-hour periodicity of peak memory, reoccurring at CT3 for 3 consecutive days89. This suggests that the peak time of acquisition may coincide with the peak time of recall. To test this, mice were trained during the time of poorer acquisition (ZT15), and examined for effects on recall. The circadian cycling of the peak time of recall (ZT3) was preserved to the third day of testing. To determine whether this effect was truly circadian, animals were trained in DD conditions at CT15 and, surprisingly, still showed memory enhancement at CT3 on subsequent days of testing. This suggests that the peak time of memory recall is under time-of-day-dependent control, that it is independent of the time of training and is regulated by an endogenous circadian system.
However, a time-of-day effect on recall not was observed in a subsequent study using fear conditioning in C57Bl/6J mice96. Specifically, there was no elevation in recall at ZT4 when animals were trained at ZT16 (REF. 96). However, this study had lower temporal resolution than the study described above89, which might account for the apparent conflict of results. It is also possible that variation in the strength of the training protocol could skew results, obscuring the circadian effects on memory processes. In addition, when animals are tested repeatedly89, testing would include combined effects of recall, reconsolidation and extinction, making it difficult to discern the circadian influence on solely the recall stage of memory. Further studies examining the role of several memory stages, such as acquisition versus recall, are required to determine the relative contributions of these factors to the time-of-day effects on memory formation.
Molecular oscillators and memory
The cAMP-MAPK-CREB pathway
The cAMP signalling cascade has a central role in memory formation97. For example, in D. melanogaster, overexpression of a repressor isoform of CREB (CREB2B) selectively abolishes LTM formation without affecting short-term memory98. By contrast, overexpression of an activator isoform of CREB (CREB2A) enhances LTM99, and this is dependent on the time of day100. When stimulated near the light-dark transition (before training), conditional expression of CREB2A enhances the formation of 4-day-long memory. This effect does not occur when CREB2A is stimulated in the middle of the daytime (before training), suggesting a time-sensitive window when the encoding of CREB-mediated enhancement can occur. Additionally, it was shown that time-of-day-dependent cycling of various components of the cAMP cascade is necessary for LTM formation in mice96. Circadian cycling of cAMP and MAPK phosphorylation paralleled time-of-day-dependent oscillations in RAS activity and the phosphorylation of MAPK kinase and CREB in the hippocampus. Disruption of the circadian rhythm of this cAMP-MAPK-CREB cascade in the hippocampus, by pharmacological approaches or by exposure of animals to constant light conditions, impaired memory formation.
In A. californica, although baseline activity of MAPK does not follow a circadian rhythm in the pleural ganglia, LTS training during different times of day produces different levels of activated MAPK101. For example, phosphorylation of MAPK increases following LTS training during the daytime as compared with the night-time, and this phosphorylation correlates with patterns of LTM enhancement. This poor performance during the night-time following LTS training can be rescued using compounds that activate MAPK activity or MAPK-dependent transcription. It therefore seems that, as in mice96, the circadian clock is also able to modulate LTM formation in A. californica through the MAPK cascade. Taken together, these data strongly suggest that cAMP-MAPK-CREB is a phylogenetically conserved pathway for the time-of-day dependence of memory.
Epigenetic factors
Epigenetic factors provide an interesting link between the molecular mechanisms that underlie circadian rhythms and the formation of memory. In the mature nervous system these factors influence changes in synaptic plasticity and complex behaviour, such as drug addiction, memory and circadian rhythms102. Changes in the epigenetic state of specific cells may be a principal mechanism by which altered gene expression exerts a circadian influence on memory formation. Chromatin remodelling following stimulation occurs in hippocampal cells103 and is induced by light in SCN cells104. It is therefore possible that epigenetics is a common mechanism relaying the cyclical change in various memory-related processes. Rhythmic changes in chromatin states are altered in a circadian manner105–107. Furthermore, the protein product of the circadian gene CLOCK itself has histone acetyltransferase activity108 and has recently been shown — with its binding partner brain and muscle ARNT- like (BMAL) (FIG. 1) — to regulate LTM formation109. Memory formation elicits histone modifications110,111. It is possible that specific molecular pathways, such as the cAMP-MAPK-CREB cascade, may stimulate these epigenetic changes that ultimately drive LTM through circadian gene expression. Future study of the relationships between these molecular pathways and their influence on epigenetic mechanisms may provide new insight into the circadian regulation of memory persistence.
Central versus cell-autonomous oscillators
Which cells are responsible for driving the circadian regulation of memory? Is a central pacemaker required, or do cell-autonomous oscillators in the memory-encoding neurons themselves regulate the time-of-day effects on memory (FIG. 6)? Although SCN ablation inhibits cycling of Per2 in the amygdala and hippocampus of hamsters112, it does not prevent expression of the time-stamp memory in a conditioned place-avoidance task113. It is possible that the time stamp could be partially encoded by memory cells using the same molecular machinery that is involved in maintaining circadian rhythms within pacemaker cells. Furthermore, mouse knockouts of neuronal PAS domain protein 2 (NPAS2), which is not expressed in the sCN but is a binding partner of the essential clock protein BMAL, have LTM deficits in cued and contextual fear conditioning114. However time-place memory — a form of learning in which an association is formed between a specific location and the time of day — still requires the Cry genes in mice115 (FIG. 1). Together, these data suggest that the cycling of specific components of the core molecular oscillatory pathway is required, perhaps in the SCN or extra-SCN regions, for the expression of time-stamp memory. In addition, rhythms of passive avoidance conditioning in rats5,116 require an intact SCN117, supporting a role for central circadian pacemaker cells in the regulation of time-of-day effects on memory. Further work is therefore needed to determine how circadian transcriptional mechanisms in the core pacemaker (the SCN), versus the peripheral cells (extra-SCN), contribute to the persistence of LTM.
Figure 6 |. Models for the circadian regulation of memory.
Cellular and molecular correlates of circadian cycling exist in various regions of the brain. Such regions include the mammalian suprachiasmatic nucleus (the central clock), as well as other cell populations, including those in the mammalian hippocampus (the memory centre). The degree to which the central clock is responsible for directing oscillations of the memory circuit, versus autonomous control by the memory cells themselves, remains to be determined. An integrated model seems most likely, whereby peripheral oscillators have some control that is coordinated with inputs (either directly or indirectly) from a central pacemaker. Orange circles represent central pacemaker-driven oscillators; blue circles represent autonomous oscillators. Solid arrows indicate direct control; dashed arrows indicate an influence on cellular oscillations.
Conclusions and perspectives
The conservation of a time-of-day effect on memory in many species points towards a common molecular mechanism. This is likely to involve the cAMP-MAPK-CREB cascade, which is implicated in the generation of memory and circadian rhythm processes and is phylogenetically conserved. Given the circadian influence on the regulation of memory-related molecules, neurophysiology, synaptic plasticity and behaviour, it is crucial that neuroscientists consider the effect of time-of-day-dependent variation on the experimental design, analysis and interpretation of future studies. Further study is needed to examine the signalling pathways involving molecules that have been studied for roles in memory and circadian rhythms, such as fragile X protein. This protein has been shown to regulate circadian rhythms and memory in flies118–121 and mice122–124. In addition, levels of vasoactive intestinal peptide (VIP) cycle in the rodent SCN and contribute to the signalling of the cAMP-MAPK-CREB cascade23,125. Interestingly, vIP knockout mice, which lack a circadian locomotor rhythm, retain time-of-day effects on memory, albeit to a lesser extent than wild-type mice126. Further research is needed to determine whether these pleiotropic molecules exert circadian effects on memory through known core clock mechanisms or through secondary effects outside of the known circadian pathways.
What is the influence of core clock-controlled pacemaker cells on the neuronal networks that are responsible for memory formation? As circadian clocks are autonomous in many tissues127, it is possible that cell-autonomous clocks in different regions of the brain independently control the circadian timing of neurophysiology and memory-related processes. For example, in A. californica, time-of-day effects on LTS persist in the absence of an ocular circadian oscillator128, and in D. melanogaster, circadian rhythms in the olfactory system are autonomous from lateral neuron pacemaker cells129. Similarly, in rodents, ablation of SCN pacemaker cells does not affect circadian oscillations in gene expression in the olfactory bulb130,131 and daily oscillations of gene expression in brain regions outside of the SCN are anti-phase to those in the SCN130,132. Interestingly, Per2 expression oscillates in isolated hippocampal tissue133, suggesting that circadian gene expression in these extra-SCN regions may be autonomous. Further studies that analyse the contribution of endogenous oscillators in memory-forming brain regions (versus central pacemaker cells) to the time-of-day effects on memory are vital for our understanding of normal brain physiology. For example, does the learning event itself serve as a zeitgeber in memory-encoding regions, setting a memory ‘clock’ of its own such that the memory is better retrieved at certain times of day, as time-stamp conditioning suggests? Are the epigenetic mechanisms that are involved downstream of the light zeitgeber in the clock pacemaker cells (for example, the SCN) analogous to the epigenetic mechanisms that produce an engram in memory centres such as the hippocampus? Studies of functional connectivity between clock cells and learning and memory centres over circadian time following training, consolidation and retrieval in various organisms will improve our understanding of these systems-level relationships and verify the appropriate model (FIG. 6).
Studies that specifically examine how the circadian cycling of molecules may affect the persistence of memory are also needed. Recently, disruption of circadian locomotor behaviour by constant light conditions has been shown to disrupt the circadian rhythm of phosphorylation of MAPK in the hippocampus and to decrease the persistence of LTM in fear-conditioned mice96. In rats, constant light conditions induce retrograde amnesia following passive avoidance conditioning7 and impair hippocampus-dependent spatial memory performance in the Morris water maze134. However, chronic light exposure was unable to disrupt an aversive-conditioning memory in mice135. Furthermore, disruption of circadian rhythms by phase-shifting in mammals disrupts various forms of memory6,7,136–138, although it was not possible to impair memory in fear conditioning using this particular method137. There are data suggesting that circadian rhythms might have a role in other forms of memory, such as social recognition, although this is controversial84,139. As the hypothalamic-pituitary-adrenal axis is regulated by the circadian system140 and influences synaptic plasticity and memory141,142, the role of stress and stress-related hormones in the time-of-day effects on memory warrant further investigation. Future studies should also determine the involvement of circadian mechanisms in ageing-associated cognitive decline143,144. Studies to examine the influence of circadian clock machinery on memory and other complex behaviours will help to discern which behaviours are under time-of-day control, and whether the circadian cycling of molecules themselves function to facilitate memory persistence through ‘reverberating’ circuits or cascades145. This type of research is particularly relevant to public health as society is increasingly operating outside of the normal daily light-dark cycle, and disrupted circadian rhythms exacerbate poor cognitive function. Future work on the mechanisms that underlie time-of-day effects on cognitive performance and memory processes will undoubtedly guide treatments for various neurological and sleep-related disorders.
Zeitgeber.
A German word that means ‘time-giver’. It refers to an exogenous cue, such as the light-dark cycle, that entrains a circadian rhythm.
Circadian rhythm.
The regular cycling of biological processes in an organism over a ~24-hour period that occurs regardless of the zeitgeber.
Time-of-day effect.
The effect of the specific point in time during the day–night cycle on the biological processes of an organism. The effect can be dependent or independent of a zeitgeber.
Long-term potentiation.
A persistent increase in synaptic strength following high-frequency stimulation of a synapse.
Crepuscular.
Describes an organism that is active during twilight or during day-to-night or night-to-day transitions.
Eclosion rhythm.
The timing of the emergence of the adult fly from its pupal case, which usually occurs at dawn.
Clock gene.
A gene that regulates aspects of circadian rhythms.
Suprachiasmatic nucleus.
A hypothalamic bilateral structure that is the central pacemaker of circadian rhythms in mammals.
Melatonin.
A catecholamine hormone derived from serotonin.
Hofbauer-Buchner eyelets.
Photoreceptor cells that are located between the retina and the lamina of the fly eye.
Metaplasticity.
Alterations in the ability of the synapse to change in strength.
Acknowledgements
The authors thank C. F. Landry for his useful comments on this manuscript.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Folkard S, Wever RA & Wildgruber CM Multi-oscillatory control of circadian rhythms in human performance. Nature 305, 223–226 (1983). [DOI] [PubMed] [Google Scholar]
- 2.Monk TH et al. Task variables determine which biological clock controls circadian rhythms in human performance. Nature 304, 543–545 (1983). [DOI] [PubMed] [Google Scholar]
- 3.Monk TH, Weitzman ED, Fookson JE & Moline ML Circadian rhythms in human performance efficiency under free-running conditions. Chronobiologia 11,343–354 (1984). [PubMed] [Google Scholar]
- 4.Davies JA, Navaratnam V & Redfern PHA 24-hour rhythm in passive-avoidance behaviour in rats. Psychopharmacologia 32, 211–214 (1973). [DOI] [PubMed] [Google Scholar]
- 5.Holloway FA & Wansley RA Multiple retention deficits at periodic intervals after active and passive avoidance learning. Behav. Biol 9, 1–14 (1973). [DOI] [PubMed] [Google Scholar]
- 6.Tapp WN & Holloway FA Phase shifting circadian rhythms produces retrograde amnesia. Science 211, 1056–1058 (1981). [DOI] [PubMed] [Google Scholar]
- 7.Fekete M, van Ree JM, Niesink RJ & de Wied D Disrupting circadian rhythms in rats induces retrograde amnesia. Physiol. Behav 34, 883–887 (1985). [DOI] [PubMed] [Google Scholar]
- 8.Gerstner JR et al. Cycling behavior and memory formation. J. Neurosci 29, 12824–12830 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi JS, Hong HK, Ko CH & McDearmon EL The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature Rev. Genet 9, 764–775 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzog ED Neurons and networks in daily rhythms. Nature Rev. Neurosci 8, 790–802 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Kandel ER The biology of memory: a forty-year perspective. J. Neurosci 29, 12748–12756 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva AJ, Zhou Y, Rogerson T, Shobe J & Balaji J Molecular and cellular approaches to memory allocation in neural circuits. Science 326, 391–395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konopka RJ & Benzer S Clock mutants of Drosophila melanogaster. Proc. Natl Acad. Sci. USA 68, 2112–2116 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bargiello TA, Jackson FR & Young MW Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 312, 752–754 (1984). [DOI] [PubMed] [Google Scholar]
- 15.Reddy P et al. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38, 701–710 (1984). [DOI] [PubMed] [Google Scholar]
- 16.Panda S, Hogenesch JB & Kay SA Circadian rhythms from flies to human. Nature 417, 329–335 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Gallego M & Virshup DM Post-translational modifications regulate the ticking of the circadian clock. Nature Rev. Mol. Cell Biol 8, 139–148 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Lyons LC, Green CL & Eskin A Intermediateterm memory is modulated by the circadian clock. J. Biol. Rhythms 23, 538–542 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai T, Tamura T, Kitamoto T & Kidokoro Y A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc. Natl Acad. Sci. USA 101, 16058–16063 (2004).This study implicates a core clock gene in the regulation of LTM, reinforcing the pleiotropic nature of circadian genes in multiple behaviours, including memory.
- 20.Belvin MP, Zhou H & Yin JC The Drosophila dCREB2 gene affects the circadian clock. Neuron 22, 777–787 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neill JS, Maywood ES, Chesham JE, Takahashi JS & Hastings MH cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science 320, 949–953 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travnickova-Bendova Z, Cermakian N, Reppert SM & Sassone-Corsi P Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc. Natl Acad. Sci. USA 99, 7728–7733 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antle MC & Silver R Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 28, 145–151 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Hankins MW, Peirson SN & Foster RG Melanopsin: an exciting photopigment. Trends Neurosci. 31, 27–36 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Peng ZC & Bentivoglio M The thalamic paraventricular nucleus relays information from the suprachiasmatic nucleus to the amygdala: a combined anterograde and retrograde tracing study in the rat at the light and electron microscopic levels. J. Neurocytol 33, 101–116 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Moga MM, Weis RP & Moore RY Efferent projections of the paraventricular thalamic nucleus in the rat. J. Comp. Neurol 359, 221–238 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Peyron C et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci 18, 9996–10015 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perreau-Lenz S et al. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur. J. Neurosci 17, 221–228 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Ashmore LJ & Sehgal A A fly’s eye view of circadian entrainment. J. Biol. Rhythms 18, 206–216 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Stanewsky R et al. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95, 681–692 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Veleri S, Rieger D, Helfrich-Forster C & Stanewsky R Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J. Biol. Rhythms 22, 29–42 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Veleri S, Brandes C, Helfrich-Forster C, Hall JC Stanewsky R A self-sustaining, light-entrainable circadian oscillator in the Drosophila brain. Curr. Biol 13, 1758–1767 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Helfrich-Forster C, Winter C, Hofbauer A, Hall J & Stanewsky R The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron 30, 249–261 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Emery P, So WV, Kaneko M, Hall JC & Rosbash M CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95, 669–679 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Kaneko M & Hall JC Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J. Comp. Neurol 422, 66–94 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Cao G & Nitabach MN Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J. Neurosci 28, 6493–6501 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasuyama K & Meinertzhagen IA Extraretinal photoreceptors at the compound eye’s posterior margin in Drosophila melanogaster. J. Comp. Neurol 412, 193–202 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Helfrich-Forster C et al. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J. Neurosci 22, 9255–9266 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malpel S, Klarsfeld A & Rouyer F Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development 129, 1443–1453 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Pitman JL, McGill JJ, Keegan KP & Allada R A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 441, 753–756 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Joiner WJ, Crocker A, White BH & Sehgal A Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441, 757–760 (2006). [DOI] [PubMed] [Google Scholar]
- 42.McBride SM et al. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron 24, 967–977 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Pascual A & Preat T Localization of long-term memory within the Drosophila mushroom body. Science 294, 1115–1117 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Helfrich-Forster C The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc. Natl Acad. Sci. USA 92, 612–616 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green DJ & Gillette R Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 245, 198–200 (1982). [DOI] [PubMed] [Google Scholar]
- 46.Groos G & Hendriks J Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci. Lett 34, 283–288 (1982). [DOI] [PubMed] [Google Scholar]
- 47.de Jeu M, Hermes M & Pennartz C Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. Neuroreport 9, 3725–3729 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Kuhlman SJ & McMahon DG Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur. J. Neurosci 20, 1113–1117 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Pennartz CM, de Jeu MT, Bos NP, Schaap J & Geurtsen AM Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature 416, 286–290 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Kononenko NI, Kuehl-Kovarik MC, Partin KM & Dudek FE Circadian difference in firing rate of isolated rat suprachiasmatic nucleus neurons. Neurosci. Lett 436, 314–316 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Park D & Griffith LC Electrophysiological and anatomical characterization of PDF-positive clock neurons in the intact adult Drosophila brain. J. Neurophysiol 95, 3955–3960 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Sheeba V, Gu H, Sharma VK, O’Dowd DK & Holmes TC Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J. Neurophysiol 99, 976–988 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandi-Perumal SR et al. Melatonin: nature’s most versatile biological signal? FEBS J. 273, 2813–2838 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Shimomura K et al. Genetic suppression of the circadian Clock mutation by the melatonin biosynthesis pathway. Proc. Natl Acad. Sci. USA 107, 8399–8403 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benloucif S et al. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J. Biol. Rhythms 20, 178–188 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Cahill GM Circadian regulation of melatonin production in cultured zebrafish pineal and retina. Brain Res. 708, 177–181 (1996). [DOI] [PubMed] [Google Scholar]
- 57.Abran D, Anctil M & Ali MA Melatonin activity rhythms in eyes and cerebral ganglia of Aplysia californica. Gen. Comp. Endocrinol 96, 215–222 (1994). [DOI] [PubMed] [Google Scholar]
- 58.Goto M, Oshima I, Tomita T & Ebihara S Melatonin content of the pineal gland in different mouse strains. J. Pineal Res 7, 195–204 (1989). [DOI] [PubMed] [Google Scholar]
- 59.Hintermann E, Grieder NC, Amherd R, Brodbeck D & Meyer UA Cloning of an arylalkylamine N-acetyltransferase (aaNAT 1) from Drosophila melanogaster expressed in the nervous system and the gut. Proc. Natl Acad. Sci. USA 93, 12315–12320 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McArthur AJ, Gillette MU & Prosser RA Melatonin directly resets the rat suprachiasmatic circadian clock in vitro. Brain Res. 565, 158–161 (1991). [DOI] [PubMed] [Google Scholar]
- 61.Rusak B & Yu GD Regulation of melatonin-sensitivity and firing-rate rhythms of hamster suprachiasmatic nucleus neurons: pinealectomy effects. Brain Res. 602, 200–204 (1993). [DOI] [PubMed] [Google Scholar]
- 62.Starkey SJ, Walker MP, Beresford IJ & Hagan RM Modulation of the rat suprachiasmatic circadian clock by melatonin in vitro. Neuroreport 6, 1947–1951 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Musshoff U, Riewenherm D, Berger E, Fauteck JD & Speckmann EJ Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus 12, 165–173 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Martin SJ, Grimwood PD & Morris RG Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci 23, 649–711 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Barnes CA, McNaughton BL, Goddard GV, Douglas RM & Adamec R Circadian rhythm of synaptic excitability in rat and monkey central nervous system. Science 197, 91–92 (1977). [DOI] [PubMed] [Google Scholar]
- 66.West MO & Deadwyler SA Circadian modulation of granule cell response to perforant path synaptic input in the rat. Neuroscience 5, 1597–1602 (1980). [DOI] [PubMed] [Google Scholar]
- 67.Harris KM & Teyler TJ Age differences in a circadian influence on hippocampal LTP Brain Res. 261, 69–73 (1983). [DOI] [PubMed] [Google Scholar]
- 68.Dana RC & Martinez JL Jr. Effect of adrenalectomy on the circadian rhythm of LTP. Brain Res. 308, 392–395 (1984). [DOI] [PubMed] [Google Scholar]
- 69.Nishikawa Y, Shibata S & Watanabe S Circadian changes in long-term potentiation of rat suprachiasmatic field potentials elicited by optic nerve stimulation in vitro. Brain Res. 695, 158–162 (1995). [DOI] [PubMed] [Google Scholar]
- 70.Chaudhury D, Wang LM & Colwell CS Circadian regulation of hippocampal long-term potentiation. J. Biol. Rhythms 20, 225–236 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abraham WC & Bear MF Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 19, 126–130 (1996). [DOI] [PubMed] [Google Scholar]
- 72.Whitlock JR, Heynen AJ, Shuler MG & Bear MF Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Raghavan AV, Horowitz JM & Fuller CA Diurnal modulation of long-term potentiation in the hamster hippocampal slice. Brain Res. 833, 311–314 (1999). [DOI] [PubMed] [Google Scholar]
- 74.Fukunaga K, Horikawa K, Shibata S, Takeuchi Y & Miyamoto E Ca2+/calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J. Neurosci. Res 70, 799–807 (2002). [DOI] [PubMed] [Google Scholar]
- 75.Collins DR & Davies SN Melatonin blocks the induction of long-term potentiation in an N-methyl-D-aspartate independent manner. Brain Res. 767, 162–165 (1997). [DOI] [PubMed] [Google Scholar]
- 76.Wang LM, Suthana NA, Chaudhury D, Weaver DR & Colwell CS Melatonin inhibits hippocampal long-term potentiation. Eur. J. Neurosci 22, 2231–2237 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ozcan M, Yilmaz B & Carpenter DO Effects of melatonin on synaptic transmission and long-term potentiation in two areas of mouse hippocampus. Brain Res. 1111, 90–94 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Soto-Moyano R et al. Melatonin administration impairs visuo-spatial performance and inhibits neocortical long-term potentiation in rats. Pharmacol. Biochem. Behav 85, 408–414 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Rawashdeh O, de Borsetti NH, Roman G & Cahill GM Melatonin suppresses nighttime memory formation in zebrafish. Science 318, 1144–1146 (2007).This study describes a time-of-day effect on memory formation in zebrafish, and shows that the hormone melatonin is necessary for the night-time suppression of memory.
- 80.Moore RY & Eichler VB Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42, 201–206 (1972). [DOI] [PubMed] [Google Scholar]
- 81.Valentinuzzi VS & Ferrari EA Habituation to sound during morning and night sessions in pigeons (Columba livia). Physiol. Behav 62, 1203–1209 (1997). [DOI] [PubMed] [Google Scholar]
- 82.Valentinuzzi VS, Menna-Barreto L & Xavier GF Effect of circadian phase on performance of rats in the Morris water maze task. J. Biol. Rhythms 19, 312–324 (2004). [DOI] [PubMed] [Google Scholar]
- 83.Valentinuzzi VS et al. Memory for time of training modulates performance on a place conditioning task in marmosets. Neurobiol. Learn. Mem 89, 604–607 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Moura PJ, Gimenes-Junior JA, Valentinuzzi VS & Xavier GF Circadian phase and intertrial interval interfere with social recognition memory. Physiol. Behav 96, 51–56 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Walker MP & Stickgold R Sleep, memory, and plasticity. Annu. Rev. Psychol 57, 139–166 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Frank MG & Benington JH The role of sleep in memory consolidation and brain plasticity: dream or reality? Neuroscientist 12, 477–488 (2006). [DOI] [PubMed] [Google Scholar]
- 87.Diekelmann S & Born J The memory function of sleep. Nature Rev. Neurosci 11, 114–126 (2010). [DOI] [PubMed] [Google Scholar]
- 88.Fernandez RI, Lyons LC, Levenson J, Khabour O & Eskin A Circadian modulation of long-term sensitization in Aplysia. Proc. Natl Acad. Sci. USA 100, 14415–14420 (2003).This paper describes the circadian control of a form of non-associative learning observed in sea slugs, which suggests that the circadian effects on memory processes are evolutionarily conserved.
- 89.Chaudhury D & Colwell CS Circadian modulation of learning and memory in fear-conditioned mice. Behav. Brain Res 133, 95–108 (2002).This paper describes in extensive detail, that both hippocampal and amygdala-dependent memory follow a circadian rhythm.
- 90.Lyons LC & Roman G Circadian modulation of short-term memory in Drosophila. Learn. Mem 16, 19–27 (2008).This study describes a time-of-day effect on olfactory avoidance-conditioning, and shows that this rhythm can be blocked by arrhythmic behaviour resulting from either clock mutants or constant lighting conditions.
- 91.Wright KP Jr, Hull JT, Hughes RJ, Ronda JM & Czeisler CA Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J. Cogn. Neurosci 18, 508–521 (2006). [DOI] [PubMed] [Google Scholar]
- 92.Roseboom PH. et al. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res. Mol. Brain Res 63, 189–197 (1998). [DOI] [PubMed] [Google Scholar]
- 93.Ebihara S, Marks T, Hudson DJ & Menaker M Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 231, 491–493 (1986). [DOI] [PubMed] [Google Scholar]
- 94.Decker S, McConnaughey S & Page TL Circadian regulation of insect olfactory learning. Proc. Natl Acad. Sci. USA 104, 15905–15910 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lyons LC, Rawashdeh O, Katzoff A, Susswein AJ & Eskin A Circadian modulation of complex learning in diurnal and nocturnal Aplysia. Proc. Natl Acad. Sci. USA 102, 12589–12594 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eckel-Mahan KL et al. Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nature Neurosci. 11, 1074–1082 (2008).This paper describes the pinnacle finding that the persistence of hippocampus-dependent memory relies on the circadian cycling of specific molecules.
- 97.Kandel ER The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Yin JC et al. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79, 49–58 (1994). [DOI] [PubMed] [Google Scholar]
- 99.Yin JC, Del Vecchio M, Zhou H & Tully T CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell 81, 107–115 (1995). [DOI] [PubMed] [Google Scholar]
- 100.Gonzales ED et al. dCREB2-mediated enhancement of memory formation. Soc. Neurosci. Abstr 35, 497.7, 3–4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lyons LC, Collado MS, Khabour O, Green CL & Eskin A The circadian clock modulates core steps in long-term memory formation in Aplysia. J. Neurosci 26, 8662–8671 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borrelli E, Nestler EJ, Allis CD & Sassone-Corsi P Decoding the epigenetic language of neuronal plasticity. Neuron 60, 961–974 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crosio C, Heitz E, Allis CD, Borrelli E & Sassone-Corsi P Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J. Cell Sci 116, 4905–4914 (2003). [DOI] [PubMed] [Google Scholar]
- 104.Crosio C, Cermakian N, Allis CD & Sassone-Corsi P Light induces chromatin modification in cells of the mammalian circadian clock. Nature Neurosci. 3, 1241–1247 (2000). [DOI] [PubMed] [Google Scholar]
- 105.Etchegaray JP, Lee C, Wade PA & Reppert SM Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 421, 177–182 (2003). [DOI] [PubMed] [Google Scholar]
- 106.Naruse Y et al. Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation. Mol. Cell. Biol 24, 6278–6287 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ripperger JA & Schibler U Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nature Genet. 38, 369–374 (2006). [DOI] [PubMed] [Google Scholar]
- 108.Doi M, Hirayama J & Sassone-Corsi P Circadian regulator CLOCK is a histone acetyltransferase. Cell 125, 497–508 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Kondratova AA, Dubrovsky YV, Antoch MP & Kondratov RV Circadian clock proteins control adaptation to novel environment and memory formation. Aging (Albany, N.Y) 2, 285–297 (2010).Using the open field paradigm in mice, this study describes circadian molecules as regulators of LTM formation, providing evidence of core clock components for memory processing in mammals.
- 110.Levenson JM et al. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem 279, 40545–40559 (2004). [DOI] [PubMed] [Google Scholar]
- 111.Levenson JM & Sweatt JD Epigenetic mechanisms in memory formation. Nature Rev. Neurosci 6, 108–118 (2005). [DOI] [PubMed] [Google Scholar]
- 112.Lamont EW, Robinson B, Stewart J & Amir S The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc. Natl Acad. Sci. USA 102, 4180–4184 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cain SW & Ralph MR Circadian modulation of conditioned place avoidance in hamsters does not require the suprachiasmatic nucleus. Neurobiol. Learn. Mem 91,81–84 (2009). [DOI] [PubMed] [Google Scholar]
- 114.Garcia JA et al. Impaired cued and contextual memory in NPAS2-deficient mice. Science 288, 2226–2230 (2000). [DOI] [PubMed] [Google Scholar]
- 115.Van der Zee EA et al. Circadian time-place learning in mice depends on Cry genes. Curr. Biol 18, 844–848 (2008).Using a novel time-place learning paradigm, this study describes cryptochrome genes as being necessary for proper time-place associations, and provides evidence for the molecular clock in time-stamp memory.
- 116.Holloway FA & Wansley R Multiphasic retention deficits at periodic intervals after passive-avoidance learning. Science 180, 208–210 (1973). [DOI] [PubMed] [Google Scholar]
- 117.Stephan FK & Kovacevic NS Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions. Behav. Biol 22, 456–462 (1978). [DOI] [PubMed] [Google Scholar]
- 118.McBride SM et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45, 753–764 (2005). [DOI] [PubMed] [Google Scholar]
- 119.Bolduc FV, Bell K, Cox H, Broadie KS & Tully T Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nature Neurosci. 11, 1143–1145 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dockendorff TC et al. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron 34, 973–984 (2002). [DOI] [PubMed] [Google Scholar]
- 121.Inoue S et al. A role for the Drosophila fragile X-related gene in circadian output. Curr. Biol 12, 1331–1335 (2002). [DOI] [PubMed] [Google Scholar]
- 122.Gu Y et al. Impaired conditioned fear and enhanced long-term potentiation in Fmr2 knock-out mice. J. Neurosci 22, 2753–2763 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao MG et al. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J. Neurosci 25, 7385–7392 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J et al. Fragile X-related proteins regulate mammalian circadian behavioral rhythms. Am. J. Hum. Genet 83, 43–52 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vosko AM, Schroeder A, Loh DH & Colwell CS Vasoactive intestinal peptide and the mammalian circadian system. Gen. Comp. Endocrinol 152, 165–175 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chaudhury D, Loh DH, Dragich JM, Hagopian A & Colwell CS Select cognitive deficits in vasoactive intestinal peptide deficient mice. BMC Neurosci. 9, 63 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ueda HR et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nature Genet. 37, 187–192 (2005). [DOI] [PubMed] [Google Scholar]
- 128.Lyons LC, Rawashdeh O & Eskin A Non-ocular circadian oscillators and photoreceptors modulate long term memory formation in Aplysia. J. Biol. Rhythms 21, 245–255 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tanoue S, Krishnan P, Krishnan B, Dryer SE & Hardin P E. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr. Biol 14, 638–649 (2004). [DOI] [PubMed] [Google Scholar]
- 130.Granados-Fuentes D, Prolo LM, Abraham U & Herzog ED The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J. Neurosci 24, 615–619 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Granados-Fuentes D, Tseng A & Herzog ED A circadian clock in the olfactory bulb controls olfactory responsivity. J. Neurosci 26, 12219–12225 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gerstner JR, Vander Heyden WM, Lavaute TM & Landry CF Profiles of novel diurnally regulated genes in mouse hypothalamus: expression analysis of the cysteine and histidine-rich domain-containing, zincbinding protein 1, the fatty acid-binding protein 7 and the GTPase, ras-like family member 11b. Neuroscience 139, 1435–1448 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang LM et al. Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro 1, e00012 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma WP et al. Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats. Neurosci. Res 59, 224–230 (2007). [DOI] [PubMed] [Google Scholar]
- 135.Castro JP et al. Effects of long-term continuous exposure to light on memory and anxiety in mice. Physiol. Behav 86, 218–223 (2005). [DOI] [PubMed] [Google Scholar]
- 136.Devan BD et al. Circadian phase-shifted rats show normal acquisition but impaired long-term retention of place information in the water task. Neurobiol. Learn. Mem 75, 51–62 (2001). [DOI] [PubMed] [Google Scholar]
- 137.Craig LA & McDonald RJ Chronic disruption of circadian rhythms impairs hippocampal memory in the rat. Brain Res. Bull 76, 141–151 (2008). [DOI] [PubMed] [Google Scholar]
- 138.Ruby NF et al. Hippocampal-dependent learning requires a functional circadian system. Proc. Natl Acad. Sci. USA 105, 15593–15598 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reijmers LG, Leus IE, Burbach JP, Spruijt BM & van Ree JM Social memory in the rat: circadian variation and effect of circadian rhythm disruption. Physiol. Behav 72, 305–309 (2001). [DOI] [PubMed] [Google Scholar]
- 140.Nader N, Chrousos GP & Kino T Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol. Metab 21, 277–286 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McEwen BS Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann. N.Y Acad. Sci 933, 265–277 (2001). [DOI] [PubMed] [Google Scholar]
- 142.Kim JJ & Diamond DM The stressed hippocampus, synaptic plasticity and lost memories. Nature Rev. Neurosci 3, 453–462 (2002). [DOI] [PubMed] [Google Scholar]
- 143.Gerstner JR The aging clock: to ‘BMAL’icious toward learning and memory. Aging (Albany, N. Y.) 2, 251–254 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rawashdeh O & Stehle JH Ageing or NOT, clock genes are important for memory processes: an interesting hypothesis raising many questions. Aging (Albany N. Y.) 2, 259–260 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Roth TL & Sweatt JD Rhythms of memory. Nature Neurosci. 11,993–994 (2008). [DOI] [PubMed] [Google Scholar]
- 146.Sanada K, Okano T & Fukada Y Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix-loop-helix-PAS transcription factor BMAL1. J. Biol. Chem 277, 267–271 (2002). [DOI] [PubMed] [Google Scholar]
- 147.Obrietan K, Impey S & Storm DR Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nature Neurosci. 1, 693–700 (1998). [DOI] [PubMed] [Google Scholar]
- 148.Dolmetsch RE, Pajvani U, Fife K, Spotts JM & Greenberg ME Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 294, 333–339 (2001). [DOI] [PubMed] [Google Scholar]
- 149.Weber F, Hung HC, Maurer C & Kay SA Second messenger and Ras/MAPK signalling pathways regulate CLOCK/CYCLE-dependent transcription. J. Neurochem 98, 248–257 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Richter HG et al. The circadian timing system: making sense of day/night gene expression. Biol. Res 37, 11–28 (2004). [DOI] [PubMed] [Google Scholar]