Abstract
Patterning of the vertebrate eye into optic stalk, retinal pigment epithelium (RPE) and neural retina (NR) territories relies on a number of signaling pathways, but how these signals are interpreted by optic progenitors is not well understood. The primary cilium is a microtubule-based organelle that is essential for Hedgehog (Hh) signaling, but it has also been implicated in the regulation of other signaling pathways. Here, we show that the optic primordium is ciliated during early eye development and that ciliogenesis is essential for proper patterning and morphogenesis of the mouse eye. Ift172 mutants fail to generate primary cilia and exhibit patterning defects that resemble those of Gli3 mutants, suggesting that cilia are required to restrict Hh activity during eye formation. Ift122 mutants, which produce cilia with abnormal morphology, generate optic vesicles that fail to invaginate to produce the optic cup. These mutants also lack formation of the lens, RPE and NR. Such phenotypic features are accompanied by strong, ectopic Hh pathway activity, evidenced by altered gene expression patterns. Removal of GLI2 from Ift122 mutants rescued several aspects of optic cup and lens morphogenesis as well as RPE and NR specification. Collectively, our data suggest that proper assembly of primary cilia is critical for restricting the Hedgehog pathway during eye formation in the mouse.
Keywords: Hedgehog, Cilia, Neural retina, Retinal pigment epithelium, IFT172, IFT122
1. Introduction
The developing vertebrate eye contains uncommitted progenitors with the capacity to differentiate into optic stalk, retinal pigment epithelium (RPE), or neural retina (NR) (Chow and Lang, 2001). How these cells commit to a particular fate is not fully understood. While much effort has been focused on identifying the signaling factors governing eye formation, relatively little is known about how these signals are integrated within optic progenitors to promote distinct cellular behaviors (Adler and Canto-Soler, 2007; Fuhrmann, 2010; Yang, 2004).
One mechanism might involve the primary cilium. This microtubule-based organelle is essential for proper Hedgehog (Hh) signaling and has been implicated in the transduction of a number of other signals (e.g., Wnt, PCP, RTK, TGF-β, PDGFα, mTOR and Notch) (Boehlke et al., 2010; Christensen et al., 2016; Ezratty et al., 2011; Goetz and Anderson, 2010; May-Simera and Kelley, 2012; Schneider et al., 2005; Umberger and Caspary, 2015). Indeed, eye defects have been observed in several mouse ciliogenesis mutants, yet there has been no detailed investigation of this phenomenonnor any insight into the underlying mechanism (Gorivodsky et al., 2009; Huangfu and Anderson, 2005; Ko et al., 2010; Qin et al., 2011; Snouffer et al., in press).
Intraflagellar transport (IFT) is critical for proper cilia formation and function. IFT involves anterograde transport of cargo primarily via IFT-B complex proteins and kinesin-2, as well as retrograde transport primarily by via IFT-A complex proteins and cytoplasmic dynein-2 (Taschner et al., 2012). IFT172 is an IFT-B component that is required for anterograde IFT; Ift172 mouse mutants completely fail to produce cilia in the tissues examined (Gorivodsky et al., 2009; Huangfu et al., 2003). In contrast, IFT122, an IFT-A component, is required for retrograde IFT; Ift122 mutant cilia are bulbous and accumulate cargo at the tips primarily due to defective retrograde IFT (Cortellino et al., 2009; Qin et al., 2011). In this study, we investigated Ift172 and Ift122 mouse mutants to gain insight into the role of the primary cilium in early eye formation.
2. Materials and methods
2.1. Mouse lines
All mice were on a C3Heb/FeJ background and were genotyped using DNA isolated from tail or yolk sac tissue. For harvesting embryos, noon on the day of finding a vaginal plug was considered embryonic day 0.5 (E0.5). Ift122sopb is a recessive null allele (Qin et al., 2011) and mice were genotyped by PCR followed by restriction digest using the forward and reverse primers 5’-AACTCATGCGCGGTTCATTG-3’ and 5’-CGCTTTGTCTCTCCACGTCA-3’, respectively. The amplified region of the mutant allele is digested with Hpy99I, producing 125 bp and 35 bp fragments, while the wild-type allele is an uncut 160 bp fragment. Ift172wim embryos were obtained from Tamara Caspary (Emory University) and genotyped by PCR followed by restriction digest with forward and reverse primers 5’-CACTGTGCTGATGA-AAGACTGGAATAGCC-3’ and 5’-TCTGCAGGGAGTAACTGGGTGTG-GCGGAAG-3’, respectively. The amplified region of the WT allele is digested with EarI, producing 163 and 25 bp products, while the amplified mutant allele is an uncut 188 bp product. Gli2zfd mice were obtained from Alexandra Joyner (Sloan Kettering Institute) and genotyped as previously described (Matise et al., 1998). Gli3Xt-J mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and genotyped as previously described (Hui and Joyner, 1993).
2.2. Immunohistochemistry
Embryos were dissected in PBS, fixed for 2 h with 4% PFA at 4°C, washed 4 times for 5 min each with PBS, and incubated in 15% sucrose/PBS and 30% sucrose/PBS at 4 °C for 2 h each. Embryos were then embedded in O.C.T. (Tissue-Tek), flash frozen, and sectioned in the coronal plane at 12 μm with a Leica CM1850 cryostat. Slides were washed 3 times for 10 min each in 0.2% Triton X-100/PBS, blocked in 10% serum/0.2% Triton X-100/PBS for 1 h, and incubated overnight at 4 °C in 1% serum/0.2% Triton X-100/PBS with primary antibodies at the following concentrations: rabbit anti-PAX2 (1:450, Covance Research Products), mouse anti-PAX6 (1:50, Developmental Studies Hybridoma Bank), sheep anti-CHX10 (1:600, Exalpha Biologicals), mouse anti-MITF (1:1500, Abcam), rabbit anti-OTX2 (1:600, Upstate Biotechnology), mouse anti-COUPTFII (1:300, R & D Systems), goat anti-SOX1 (1:100, R & D Systems), rabbit anti-SOX2 (1:300, EMD Millipore), mouse anti-γ-TUBULIN (1:1000, Sigma-Aldrich), rabbit anti-ARL13B (1:3000, T. Caspary, Emory University). Slides were washed 3 times for 20 min each (as above) and incubated for 1 h at room temperature in with Alexa Fluor 488/555–conjugated secondary antibodies (Invitrogen) and Cy2/Cy3/Cy5–conjugated secondary antibodies (Jackson ImmunoResearch) at 1:300. Slides were washed 3 times for 20 min each (as above) and mounted with 90% glycerol/PBS. Images were taken with either a Zeiss Axioplan 2 or a Keyence BZ-X710 fluorescence microscope. High-magnification images of cilia are maximum intensity projections from z-stacks taken on a Zeiss LSM510 confocal microscope.
2.3. In situ hybridization
Embryos were dissected in PBS, fixed overnight with 4% PFA at 4 °C, washed 4 times for 5 min each with depc-treated PBS, and then dehydrated through a dilution series in methanol. Whole mount in situ hybridization was performed as previously described, with the modification of extended wash times (Lauter et al., 2011). Embryos were sectioned following whole mount staining using methods described above (see Immunohistochemistry). Riboprobes for Vax2 (Mui et al., 2002), Shh (Echelard et al., 1993), Nkx2.1 (Long et al., 2009) and Gli1 (Hui et al., 1994) were generated by in vitro transcription using a dig-UTP labeling mix (Roche) following manufacturer’s specifications.
2.4. Three-dimensional reconstruction
Embryos were dissected in PBS, fixed overnight with 4% PFA, washed 4 times for 5 min each with PBS, and then dehydrated through a dilution series in ethanol followed by two washes in xylene. Embryos were embedded in paraffin wax and 10 μm sagittal sections were cut with a Leica RM2155 microtome. Hematoxylin and eosin (H & E) staining was performed according to standard protocols (Fischer et al., 2008). Three-dimensional reconstruction was performed by stacking tracings of the optic neuroepithelium from serial sagittal sections stained with H & E using Surf Driver™ 3.5.3 software (Surfdriver).
3. Results
3.1. IFT172 and IFT122 regulate ciliogenesis in optic progenitors
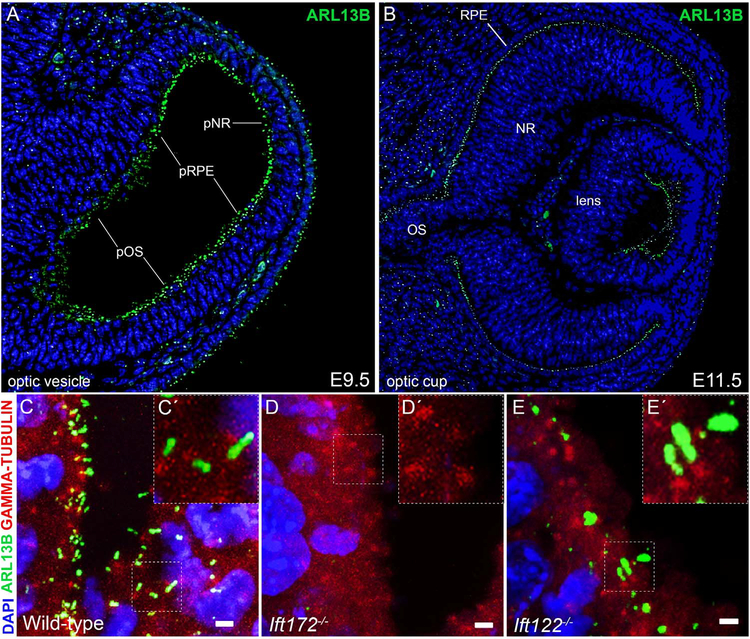
To understand how primary cilia contribute to development of the eye, we first asked which cells of the developing eye were ciliated. We used antibodies against ARL13B (Caspary et al., 2007) and γ-TUBULIN (Muresan et al., 1993) to visualize the cilium and basal body, respectively. Primary cilia occupied the surface of cells along the optic neuroepithelium, surface ectoderm and periocular mesenchyme in wild-type embryos during the optic vesicle and optic cup stages (Fig. 1A–C). Ift172−/− embryos lacked ARL13B localization distal to the γ-TUBULIN+ basal body, confirming optic progenitors in these mutants failed to produce cilia (Fig. 1D). By contrast, optic progenitors in Ift122−/− embryos produced cilia that exhibited a swollen, bulbous morphology, resembling cilia previously characterized in Ift122−/− spinal neural progenitors (Qin et al., 2011; Fig. 1E). These data indicate IFT172 and IFT122 are required for proper cilia formation in optic progenitors, consistent with previous reports regarding the roles of these proteins in ciliogenesis in other embryological contexts (Cortellino et al., 2009; Gorivodsky et al., 2009; Huangfu et al., 2003; Qin et al., 2011).
Fig. 1.
Optic progenitor cells are ciliated and require IFT122 and IFT172 for proper cilia assembly. (A-B) Sections through a wild-type E9.5 optic vesicle (A) and a wild-type E11.5 optic cup (B). Cilia are visualized with an antibody against ARL13B (green) and DNA counterstained with DAPI (blue). Abbreviations for optic progenitor territories: pOS, presumptive optic stalk; pRPE, presumptive retinal pigment epithelium; pNR, presumptive neural retina; OS, optic stalk; RPE retinal pigment epithelium; NR, neural retina. (C-E) Maximum intensity projections of confocal z-stacks of wild-type (C), Ift172 mutant (D) and Ift122 mutant (E) eyes in the presumptive optic stalk at E10.5 stained with antibodies against ARL13B (green) and γ -TUBULIN (red); DNA counterstained with DAPI (blue). Scale bars are 2 μm. Insets (C’-E’) are magnifications of the area marked by the dotted box in the corresponding image (C-E).
3.2. Loss of IFT172 leads to patterning defects consistent with elevated Hedgehog signaling
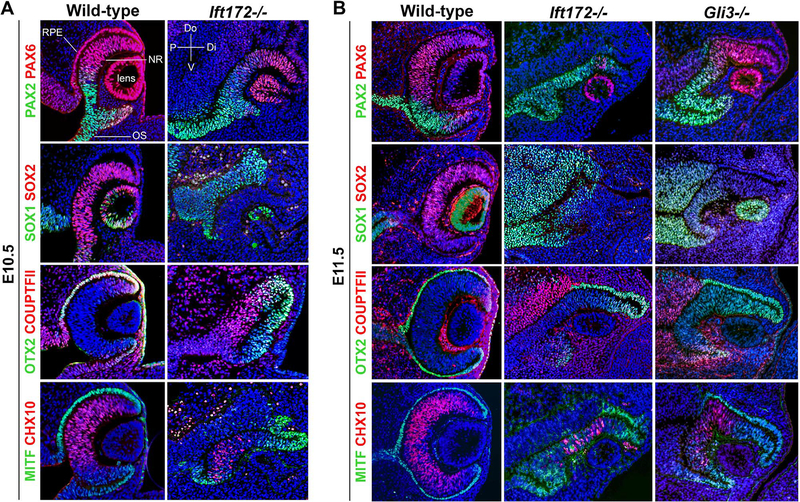
To determine how the complete loss of primary cilia would impact eye formation, we examined cell fate specification in Ift172 mutants at optic cup stages (E10.5 and E11.5). PAX2 is a marker for proximal (optic stalk) fate, whereas PAX6 is expressed distally in the optic cup and lens (Schwarz et al., 2000). Ift172 mutants expressed PAX2 in a distally-expanded domain at the expense of the neuroepithelial PAX6 domain at E10.5 (Fig. 2A). This PAX2 and PAX6 expression pattern was exacerbated by E11.5 (Fig. 2B). SOX1 is normally expressed in the optic stalk of wild-type embryos (Wood and Episkopou, 1999). Like PAX2, its expression was expanded distally in Ift172 mutants at both stages. These data suggest that the optic stalk domain is expanded in Ift172 mutants (Fig. 2A,B). COUPTFII is expressed in the dorsal optic stalk, RPE and extraocular mesenchyme (Tang et al., 2010). We detected no change in COUPTFII expression in Ift172 mutants. In wild-type embryos, OTX2 and MITF are expressed in the outer optic cup (RPE), whereas CHX10 is expressed in the inner optic cup (NR) (Horsford et al., 2005; Martínez-Morales et al., 2003; Rowan et al., 2004). In Ift172 mutants, MITF and OTX2 expression extended abnormally into the inner layer of the optic cup at both E10.5 and E11.5, which was accompanied by a reduction in the CHX10 domain (Fig. 2A,B). Additionally, SOX2 was expressed in the NR of controls, but its expression was absent in the Ift172−/− optic cup (Fig. 2A,B). These data suggest the RPE domain is expanded at the expense of the NR in Ift172−/− embryos.
Fig. 2.
IFT172 is required for patterning and morphogenesis of the optic cup. (A) Sections through E10.5 wild-type and Ift172 mutant eyes stained with markers for cell fates. Note the dorsal expansion of PAX2, dorsal restriction of PAX6, expansion of MITF and OTX2 into the inner optic cup, reduction in CHX10+ cells and loss of SOX2 expression in the Ift172 mutant. Abbreviations: OS, optic stalk; RPE, retinal pigment epithelium; NR, neural retina; P, proximal; Di, distal; Do, dorsal; V, ventral. (B) Sections through E11.5 wild-type, Ift172 mutant and Gli3 mutant eyes stained with markers for cell fates. Note the dorsal expansion of PAX2, dorsal restriction of PAX6, expansion of MITF and OTX2 into the inner optic cup, reduction of CHX10+ cells, distal expansion of SOX1, and loss of SOX2 expression in both Ift172 and Gli3 mutants.
The patterning defects we observed in Ift172 mutants are remarkably similar to those observed when the Hh pathway is elevated in other vertebrates (Amato et al., 2004; Ekker et al., 1995; Macdonald et al., 1995; Perron et al., 2003; Sasagawa et al., 2002; Wang et al., 2015; Zhang and Yang, 2001). This prompted us to compare the phenotype of Ift172 mutants to that of Gli3−/− embryos. As GLI3 is the main transcriptional repressor of the Hh pathway, its loss results in increased Hh pathway activity (Persson et al., 2002; Rallu et al., 2002; Tole et al., 2000). We found optic patterning of Gli3 mutants was strikingly similar to Ift172 mutants at E11.5 (Fig. 2B). The optic stalk markers PAX2 and SOX1 were distally expanded, the RPE markers MITF and OTX2 were expressed in the inner optic cup, and the domains of the NR markers CHX10 and SOX2 were reduced. Together, these data suggest that IFT172 is required for preventing ectopic activation of the Hh pathway in the eye.
3.3. Loss of IFT122 leads to patterning defects and prevents optic cup and lens formation
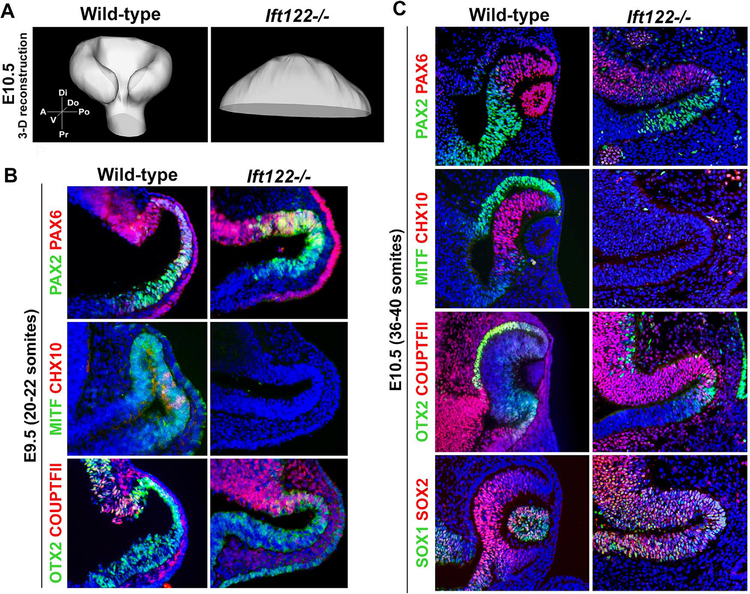
We next asked whether loss of another IFT protein, IFT122, would lead to a phenotype similar to Ift172 mutants. Through our analyses of Ift122−/− embryos at the optic vesicle–optic cup transition stages, it was evident that these mutants failed to make an optic cup or induce lens formation. We performed three-dimensional (3-D) reconstruction of this eye structure at E10.5, which confirmed that no invagination of the optic vesicle to produce an optic cup occurred in Ift122−/− embryos (Fig. 3A). We focused our subsequent analyses on patterning of the Ift122 mutant optic primordium.
Fig. 3.
IFT122 is required for optic cup and lens formation and for specification of the RPE and NR. (A) 3-D reconstruction of wild-type and Ift122 mutant eyes at E10.5. Compass abbreviations: A, anterior; Po, posterior; Di, distal; Pr, proximal; V, ventral; D, dorsal. (B) Sections through E9.5 (20–22 somite stage) wild-type and Ift122 mutant optic vesicles stained with markers for cell fates. Note the dorsal expansion of PAX2 and the loss of MITF and CHX10 expression in the Ift122 mutant. (C) Sections through E10.5 (36–40 somite stage) wild type and Ift122 mutant eyes stained with markers for cell fates. Note the loss of MITF and CHX10 expression, reduction of OTX2+ cells, and the distal expansion of SOX1 expression in the Ift122 mutant.
At the optic vesicle stage (E9.5), PAX2 expression was expanded distally throughout the vesicle of Ift122 mutants compared to somite-matched, wild-type controls (Fig. 3B). This PAX2 expansion was maintained at E10.5, whereas PAX6 and COUPTFII expression was only found in the dorsal optic vesicle at both stages (Fig. 3B,C). OTX2 expression, which was expressed normally at E9.5, was confined to a small domain of cells in the distal optic vesicle at E10.5, suggesting RPE specification might be compromised in Ift122 mutants (Fig. 3B,C). Consistent with this, we failed to detect any MITF expression at E9.5 or E10.5 (Fig. 3B,C). Additionally, we did not detect CHX10 expression at either stage, suggesting that Ift122 mutants failed to specify the NR (Fig. 3B,C). To give further insight into the identity of Ift122 mutant optic progenitors, we utilized the expression patterns of SOX1 and SOX2. Whereas both SOX1 and SOX2 are normally co-expressed throughout the presumptive optic stalk, only SOX2 is additionally expressed in the presumptive NR (Wood and Episkopou, 1999). In Ift122 mutants, both SOX1 and SOX2 expression was found throughout the entire optic vesicle at E10.5 (Fig. 3B,C), which, in addition to the expansion of PAX2, suggests this structure adopts an optic stalk-like identity.
3.4. Abnormal Hedgehog signaling in the Ift122 and Ift172 mutant optic vesicle
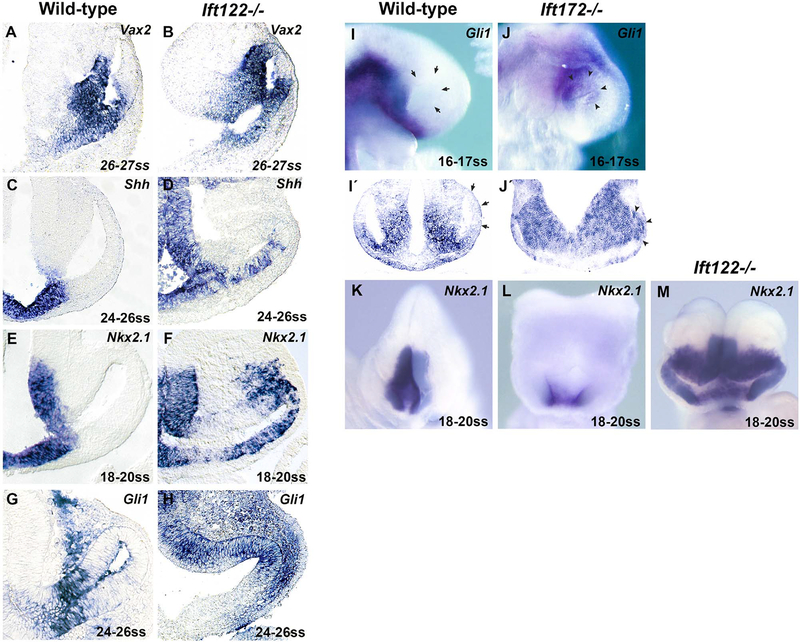
In comparison to the patterning changes in Ift172 mutants, the Ift122 phenotype described above was relatively severe. We performed in situ hybridization against Vax2 at E9.5, a gene expressed in the ventral optic vesicle, and found that its expression was expanded dorsally in Ift122 mutants compared to somite-matched controls (Fig. 4A,B). Dorsal expansion of Vax gene expression is a common effect of elevated Hh pathway activity (Sasagawa et al., 2002; Take-uchi et al., 2003; Wang et al., 2015; Zhang and Yang, 2001). Thus, we investigated Hh pathway activity directly in Ift122 mutants.
Fig. 4.
Elevated Hedgehog signaling in the Ift122 and Ift172 mutant optic vesicle. (A-H) Sections through the eyes of wild-type (A,C,E,G,) and Ift122 mutants (B,D,F,H) at the indicated somite stages (ss) following whole-mount in situ hybridization against Vax2 (A,B), Shh (C,D), Nkx2.1 (E,F) and Gli1 (G,H). Note the dorsal expansion of Vax2 and the distal expansion of Shh, Nkx2.1 and Gli1 expression in the Ift122 mutant optic neuroepithelium. (I-J) Whole-mount in situ hybridization against Gli1 on wild-type (I) and Ift172 mutants (J) at the indicated somite stages; (I’-J’) coronal sections of these embryos following whole-mount in situ hybridization. Note the wild-type distal optic vesicle is Gli1 negative (arrows), whereas the entire Ift172 optic vesicle expresses Gli1 (arrowheads). (K-M) Whole-mount in situ hybridization against Nkx2.1 on wild-type (K), Ift172 (L), Ift122 (M) mutants at the indicated somite stages. Note that Nkx2.1 expression is proximally restricted in Ift172 mutants, rather than distally expanded as in Ift122 mutants.
We analyzed the expression of Shh ligand and of the direct Hh pathway targets Gli1 and Nkx2.1 (Vokes et al., 2007) in the optic vesicle of Ift122 mutants at E9.5 and compared this to somite-matched controls. In this region of wild-type embryos, Shh is expressed along the ventral midline (Fig. 4C). Shh ligand is thought to spread laterally where it activates target gene expression in a concentration-dependent manner (Amato et al., 2004). Nkx2.1 expression is induced by high levels of Hh signaling and is expressed in the midline and along the peri-ocular diencephalon, in a domain largely overlapping with that of Shh (Fig. 4E). Gli1 expression is excluded from the midline and is found further distal along the optic neuroepithelium (Fig. 4G), indicating its expression is induced by moderate levels of Hh activity but suppressed by high Hh levels, as observed in other studies (Ribes et al., 2010). We found that Ift122 mutants expressed Shh in a distally-expanded domain along the optic neuroepithelium compared to wild-type controls (Fig. 4D). Nkx2.1 was expressed from the midline to the dorso-distal tip of the optic vesicle (Fig. 4F). We also observed ectopic Nkx2.1 expression in the dorsal extraocular mesenchyme, though we cannot rule out this phenomenon is a result of neuroepithelial delamination of Nkx2.1+ cells (Fig. 4F). Ift122 mutants also expressed Gli1 ectopically in the distal optic vesicle (Fig. 4H). We noted the proximo-ventral Gli1 negative domain was expanded distally in Ift122 mutants compared to controls, suggesting that the Hh pathway is elevated throughout this entire region. Together, these data provide direct evidence that the Hh pathway is strongly elevated in the optic vesicle of Ift122 mutants.
The fact that Ift172 mutants phenocopied Gli3 mutants with respect to eye development suggested Ift172 mutants exhibit elevated Hh levels in the optic vesicle. To test this directly, we analyzed Gli1 and Nkx2.1 expression in Ift172 mutants. Ectopic Gli1 expression was found distally in the Ift172−/− optic vesicle (Fig. 4I,J,I’,J’). We noted that Gli1 was expressed in the proximal optic vesicle of Ift172 mutants, whereas Gli1 expression was not observed in similar proximal regions of Ift122 mutants. This suggests that the ectopic Hh pathway activity in Ift172 mutants is not as high as in Ift122 mutants. In support of this, the Nkx2.1 expression domain was ventrally restricted in Ift172 mutants, rather than distally expanded as seen in Ift122 mutants (Fig. 4K–M). Collectively, these data indicate that the Hh pathway is indeed ectopically active in the distal optic primordium of both Ift172 and Ift122 mutants, but the degree of activity is much higher in the latter. This likely contributes to the difference in eye phenotypes of the two mutants.
3.5. Loss of GLI2 in Ift122 mutants rescues optic cup patterning and morphogenesis
In mice, the Hh pathway is mediated by the GLI family of transcription factors (GLI1–3). GLI1 has only activator function, which is redundant with GLI2 (Bai et al., 2002; Bai and Joyner, 2001). While GLI2 and GLI3 both have activator and repressor functions, GLI2 primarily acts as a Hh target transcriptional activator, whereas GLI3 acts primarily as a repressor (Bai et al., 2004; Buttitta et al., 2003; Dai et al., 1999; Lei et al., 2004; McDermott et al., 2005; Persson et al., 2002; Rallu et al., 2002; Sasaki et al., 1999; Yong et al., 2009). Thus, if the eye defects we observed in Ift122 mutants are indeed due to elevated Hh pathway activity, we hypothesized that simultaneous genetic ablation of Gli2 in Ift122 mutants should mitigate these defects and rescue aspects of eye formation. It has been previously reported that optic cup patterning and morphogenesis occur normally in Gli2 mutants, with the only defect being a slightly shortened optic stalk (Furimsky and Wallace, 2006). We first analyzed Gli2 mutants at E11.5 and confirmed that optic cup patterning and morphogenesis occurs similarly to wild-type controls (Fig. 5).
Fig. 5.
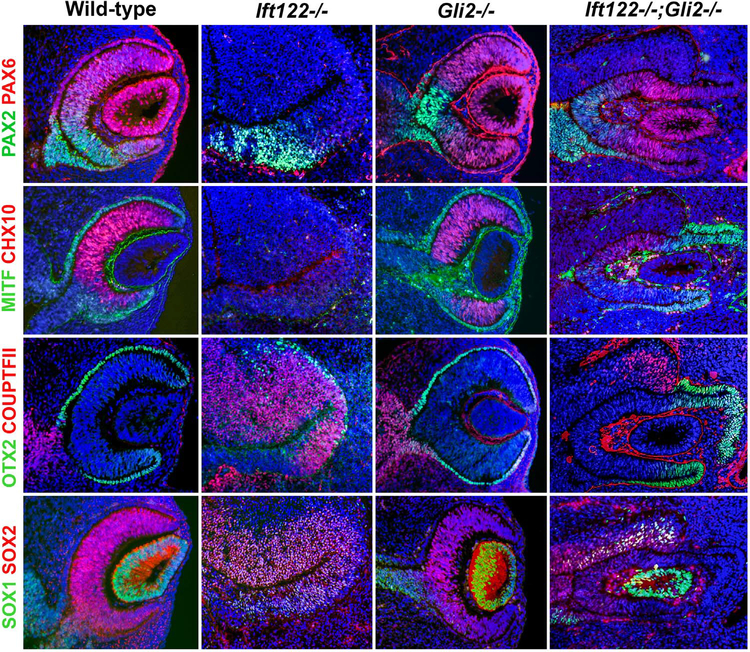
Loss of GLI2 in Ift122 mutants rescues optic cup patterning and morphogenesis. Sections through wild-type, Ift122 mutant, Gli2 mutant and Ift122;Gli2 double mutant eyes at E11.5 stained with markers for cell fates. Note the rescue of optic cup and lens formation, the rescue of PAX6, MITF and OTX2 expression in the distal optic cup as well as the rescue of CHX10 and SOX2 expression in the inner optic cup of the Ift122;Gli2 double mutant.
We found optic patterning of Ift122 mutants at E11.5 resembled that at E10.5, with a few exceptions. At E11.5, PAX2 expression did not extend to the distal edge of the optic vesicle, but rather was found proximo-ventrally, while PAX6 expression was completely absent from the optic primordium (Fig. 5). COUPTFII expression was found throughout the optic vesicle, whereas OTX2 was expressed in a few cells along the distal optic region. Similar to E10.5, CHX10 and MITF expression remained absent at E11.5, while the entire optic primordium co-expressed SOX1 and SOX2. These data indicate the RPE and NR are not specified in Ift122 mutants even at E11.5, arguing against the possibility of a developmental delay.
We then generated Ift122;Gli2 double mutants at this same stage. Remarkably, these embryos formed optic cup-like structures that expressed PAX6, MITF and OTX2 in the distal region of the optic cup (Fig. 5). CHX10 and SOX2 were expressed in the inner layer of the optic cup, while PAX2, COUPTFII and SOX1 were expressed in the proximal optic stalk region (Fig. 5). While all double mutants analyzed expressed these markers (N = 5/5), we noted variability in the extent of lens morphogenesis, with some having an obvious lens vesicle (N = 2/5; Fig. 5) and others having no apparent lens (N = 3/5; data not shown). Though we cannot rule out the possibility that a small lens was formed in these mutants, we propose this variability stems from differences in the ability of the optic vesicle to induce lens formation, or from differences in the competence of the surface ectoderm to respond to the inductive signals (Chow and Lang, 2001). Regardless, reducing Hh levels by removal of GLI2 was an effective strategy for rescuing specification of the NR and RPE as well as for the initiation of the optic cup development in Ift122 mutants, arguing that the eye defects due to loss of IFT122 largely, if not entirely, stem from elevated Hh pathway activity.
4. Discussion
We find two IFT proteins, IFT172 and IFT122, are required for proper eye development in mice. Ift172 mutants lack optic primary cilia and show evidence of modest ectopic Hh activity in the distal optic vesicle, as revealed by Gli1 expression. Such mutants adopt a prox-imalized optic cup with an expanded RPE at the expense of the NR, defects that are consistent with elevated Hh signaling (this study; Amato et al., 2004; Ekker et al., 1995; Macdonald et al., 1995; Sasagawa et al., 2002; Zhang and Yang, 2001; Perron et al., 2003). Ift122 mutants, which have malformed optic primary cilia, do not specify RPE or NR cell types, nor do they form an optic cup or lens. Our in situ hybridization data indicate that both Ift172 and Ift122 mutant optic vesicles show ectopic Hh pathway activity. However, the dramatic expansion of the Nkx2.1 expression domain in Ift122 mutants suggests that they experience much higher Hh activity than Ift172 mutants, and therefore Ift122 mutants exhibit a much more severe optic phenotype. Consistent with the causative role of excessive Hh pathway activity in the eye phenotype of Ift122 mutants, we observed substantial rescue of eye formation in Ift122 mutants upon reducing Hh pathway activity through simultaneous genetic ablation of Gli2. While it is possible that both IFT172 and IFT122 have roles outside of the cilium, the most parsimonious interpretation of our data is that the eye defects in these mutants result from their inability to generate normally functioning cilia.
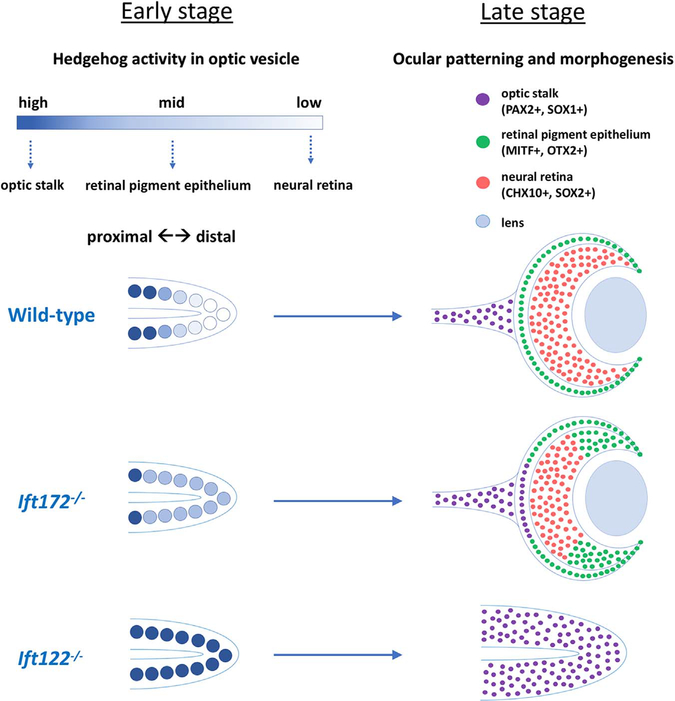
Based on these findings, we propose that the modest ectopic activation of the Hh pathway in Ift172 mutants leads to expansion of optic stalk and RPE fates at the expense of NR fates. In contrast, strong ectopic Hh pathway activity in Ift122 mutants completely blocks both formation of the optic cup and specification of the NR and RPE identities. The entire optic vesicle adopts an optic stalk-like identity. This model is summarized in Fig. 6. Of interest, our data provide some insight into the mechanism of RPE specification. In chick, Hh ligand overexpression leads to the expansion of RPE into the NR domain (Zhang and Yang, 2001), and our analysis of Gli3 and Ift172 mutants supports a role for Hh signaling in the promotion of RPE fate. In contrast, our data on Ift122 mutants indicate further elevation of Hh pathway activity completely suppresses RPE fate in the optic vesicle. This suggests Hh signaling can both promote and inhibit RPE specification depending on the level of activity.
Fig. 6.
Levels of Hedgehog activity bias optic progenitor cell fates. In wild-type, Hh activity forms a proximal-high, distal-low gradient within the optic vesicle. This leads to normal specification of the OS, RPE, and NR. In Ift172 mutants, Hh activity is moderately elevated within the optic vesicle, which results in the expansion of the OS into the proximal optic cup as well as the expansion of RPE into the inner optic cup. In Ift122 mutants, Hh activity is strongly elevated throughout the entire optic vesicle, which results in optic progenitors adopting an optic stalk-like identity.
In other contexts, primary cilia control Hh pathway activity by regulating the balance of GLI activator and repressor formation (Caspary et al., 2007; Chang et al., 2016; Huangfu et al., 2003; Huangfu and Anderson, 2005; Liu et al., 2005; Millington et al., 2017). Thus, loss of IFT172 or IFT122 could cause increased Hh activity within the eye through several possible mechanisms, which are not mutually exclusive. First, diminished GLI repressor (GLIrep) formation could lead to a de-repression of the Hh pathway. Indeed, both Ift172 and Ift122 mutants generate reduced levels of GLI3Rep (Huangfu and Anderson, 2005; Qin et al., 2011). Alternatively, constitutive activity of GLI activator (GLIAct) may lead to hyperactivation of the Hh pathway. Previous work suggests that GLIAct cannot be generated in the absence of cilia (i.e. in Ift172 or Ift88 mutants; Huangfu and Anderson, 2005; Ocbina and Anderson, 2008). However, genetic and molecular analysis of Ift122 mutants, which generate (abnormal) cilia, suggests that such mutants exhibit ligand-independent activation of GLI2 (Qin et al., 2011). Indeed, the observed rescue of eye development by removal of GLI2 in Ift122 mutants supports this argument with respect to eye development. Other findings indicate that Ift122 mutant cilia fail to localize the Hh pathway antagonist GPR161, which indirectly activates Protein Kinase A (Mukhopadhyay et al., 2013). Thus, Ift122 mutant cilia retain the ability to produce GLIAct, and the loss of ciliary GPR161 in such mutants allows this to occur independently of ligand-mediated pathway activation. As a result, we suggest that the high levels of ectopic Hh pathway activity in Ift122 mutants are caused by the combination of failure to inhibit GLIAct function together with failure to produce sufficient levels of GLI3Rep activity.
We favor the hypothesis that Ift122 mutants generate significantly reduced levels of GLI3Rep as an explanation for the incomplete rescue of eye development in Ift122;Gli2 double mutants. We have no reason to believe that this aspect of the Ift122 mutant phenotype would be affected by loss of GLI2. Thus, although Ift122;Gli2 mutants may no longer be able to generate ectopic GLI2Act, the defect in GLI3Rep formation should still cause low levels of ectopic Hh activity in these double mutants. Indeed, the eye phenotype of Ift122;Gli2 double mutants (Fig. 5) is remarkably similar to that of Gli3 mutants (Fig. 2B), which lack GLI3Rep function. Nevertheless, we acknowledge there are other possible explanations for the lack of a complete rescue in the double mutants. Other signaling pathways associated with cilia (e.g. Wnt, Notch; Ezratty et al., 2011; Goetz and Anderson, 2010) could be directly affected by loss of IFT122, and such a defect would not be rescued by disruption of GLI2.
Our findings indicate that primary cilia play an important role in early mammalian eye development and that different aspects of ciliary assembly make unique contributions to the process. Such contributions are largely, if not entirely, a result of restricting Hh pathway activity to different extents and by different means. Appropriate control of Hh pathway activity, in turn, directs both morphogenesis and patterning of the eye primordium through mechanisms that are both known and yet to be understood.
Acknowledgements
The authors wish to thank Dr. Rongsun Pu (Kean University), who performed the initial characterization of the Ift122 mutant eye phenotype, Sadhana Durbha, who assisted with the analysis of cilia, and Drs. Julie Gordon and Kristina Buac (UGA), who provided technical advice. We thank Drs. Alyssa Bushey and Tamara Caspary (Emory University) for their generous help in generating Ift172 mutant embryos. The authors also wish to thank Dr. Muthugapatti Kandasamy at the UGA Biomedical Microscopy Core for his assistance with confocal microscopy as well as the husbandry and veterinary staff of the Coverdell Animal Resources Facility at UGA.
Funding
This work was supported by The National Institute of Child Health and Human Development (grant #R01HD050761, to JTE) and the Office of Vice President for Research at the University of Georgia (grant #FRGSE0027). JBB was supported by a T32 Training Grant (T32GM007103) from the National Institute of General Medical Sciences.
References
- Adler R, Canto-Soler MV, 2007. Molecular mechanisms of optic vesicle development: complexities, ambiguities and controversies. Dev. Biol 305, 1–13. 10.1016/j.ydbio.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato MA, Boy S, Perron M, 2004. Hedgehog signaling in vertebrate eye development: a growing puzzle. Cell. Mol. Life Sci. 61, 899–910. 10.1007/s00018-003-3370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL, 2002. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129, 4753–4761. 10.1242/dev.00115. [DOI] [PubMed] [Google Scholar]
- Bai CB, Joyner AL, 2001. Gli1 can rescue the in vivo function of Gli2. Development 128, 5161–5172. [DOI] [PubMed] [Google Scholar]
- Bai CB, Stephen D, Joyner AL, 2004. All mouse ventral spinal cord patterning by Hedgehog is Gli dependent and involves an activator function of Gli3. Dev. Cell 6, 103–115. 10.1016/S1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Gödel M, Müller K, Herbst M, Hornung M, Doerken M, Köttgen M, Nitschke R, Igarashi P, Walz G, Kuehn EW, 2010. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat. Cell Biol. 12, 1115–1122. 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttitta L, Mo R, Hui C-C, Fan C-M, 2003. Interplays of Gli2 and Gli3 and their requirement in mediating Shh-dependent sclerotome induction. Development 130, 6233–6243. 10.1242/dev.00851. [DOI] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, Anderson KV, 2007. The graded response to sonic hedgehog depends on cilia architecture. Dev. Cell 12, 767–778. 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chang C-F, Chang Y-T, Millington G, Brugmann SA, 2016. Craniofacial ciliopathies reveal specific requirements for GLI proteins during development of the facial midline. PLoS Genet. 12, e1006351 10.1371/journal.pgen.1006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA, 2001. Early eye development in vertebrates. Annu. Rev. Cell Dev. Biol. 17, 255–296. [DOI] [PubMed] [Google Scholar]
- Christensen ST, Morthorst SK, Mogensen JB, Pedersen LB, 2016. Primary cilia and coordination of receptor tyrosine kinase (RTK) and transforming growth factor β (TGF-β) signaling. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a028167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortellino S, Wang C, Wang B, Bassi MR, Caretti E, Champeval D, Calmont A, Jarnik M, Burch J, Zaret KS, Larue L, Bellacosa A, 2009. Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev. Biol 325, 225–237. 10.1016/j.ydbio.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S, 1999. Sonic hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J. Biol. Chem 274, 8143–8152. 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, Mcmahon JA, Mcmahon AP, 1993. Sonic Hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430. [DOI] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT,Beachy PA, 1995. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr. Biol 5, 944–955. 10.1016/S0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E, 2011. A role for the primary cilium in notch signaling and epidermal differentiation during skin development. Cell 145, 1129–1141. 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AH, Jacobson KA, Rose J, Zeller R, 2008. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc 3, 4986–4988. 10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, 2010. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol 93, 61–84. 10.1016/B978-0-12-385044-7.00003-5.Eye. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furimsky M, Wallace VA, 2006. Complementary Gli activity mediates early patterning of the mouse visual system. Dev. Dyn 235, 594–605. 10.1002/dvdy.20658. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV, 2010. The primary cilium: a signaling center during vertebrate development. Nat. Rev. Genet 11, 331–344. 10.1038/nrg2774.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorivodsky M, Mukhopadhyay M, Wilsch-Braeuninger M, Phillips M, Teufel A, Kim C, Malik N, Huttner W, Westphal H, 2009. Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev. Biol 325, 24–32. 10.1016/j.ydbio.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsford DJ, Nguyen M-TT, Sellar GC, Kothary R, Arnheiter H, McInnes RR, 2005. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development 132, 177–187. 10.1242/dev.01571. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV, 2005. Cilia and Hedgehog responsiveness in the mouse.Proc. Natl. Acad. Sci. USA 102, 11325–11330. 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Lui A, Rakeman AS, Murcia NS, Niswander L, Anderson KV, 2003. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87. [DOI] [PubMed] [Google Scholar]
- Hui C-C, Slusarski D, Platt KA, Holmgren R, Joyner AL, 1994. Expression of three mouse homologs of the drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev. Biol 162, 402–413. 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- Hui C, Joyner AL, 1993. A mouse model of Greig cephalo-polysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat. Genet 3, 241–246. [DOI] [PubMed] [Google Scholar]
- Ko HW, Norman RX, Tran J, Fuller KP, Fukuda M, Eggenschwiler JT, 2010. Broad-minded links cell cycle-related kinase to cilia assembly and hedgehog signal transduction. Dev. Cell 18, 237–247. 10.1016/j.devcel.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter G, Söll I, Hauptmann G, 2011. Two-color fluorescent in situ hybridization in the embryonic zebrafish brain using differential detection systems. BMC Dev. Biol 11, 43 10.1186/1471-213X-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Zelman AK, Kuang E, Li S, Matise MP, 2004. Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord. Development 131, 3593–3604. 10.1242/dev.01230. [DOI] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA, 2005. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132, 3103–3111. 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Long JE, Swan C, Liang WS, Cobos I, Potter GB, Rubenstein JLR, 2009. Dlx1 & 2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. J. Comp. Neurol 512, 556–572. 10.1002/cne.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW, 1995. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development 121, 3267–3278. 10.1016/0168-9525(95)90453-0. [DOI] [PubMed] [Google Scholar]
- Martínez-Morales JR, Dolez V, Rodrigo I, Zaccarini R, Leconte L, Bovolenta P, Saule S, 2003. OTX2 activates the molecular network underlying retina pigment epithelium differentiation. J. Biol. Chem 278, 21721–21731. 10.1074/jbc.M301708200. [DOI] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL, 1998. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development 125, 2759–2770, (doi:9655799). [DOI] [PubMed] [Google Scholar]
- May-Simera HL, Kelley MW, 2012. Cilia, Wnt signaling, and the cytoskeleton. Cilia 1,7 10.1186/2046-2530-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott A, Gustafsson M, Elsam T, Hui C-C, Emerson CP, Borycki A-G, 2005. Gli2 and Gli3 have redundant and context-dependent function in skeletal muscle formation. Development 132, 345–357. 10.1242/dev.01537. [DOI] [PubMed] [Google Scholar]
- Millington G, Elliott KH, Chang Y-T, Chang C-F, Dlugosz A, Brugmann SA, 2017. Cilia-dependent GLI processing in neural crest cells is required for tongue development. Dev. Biol 424, 124–137. 10.1016/j.ydbio.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui SH, Hindges R, O’Leary DDM, Lemke G, Bertuzzi S, 2002. The homeodomain protein Vax2 patterns the dorsoventral and nasotemporal axes of the eye. Development 129, 797–804. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK, 2013. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the sonic Hedgehog pathway via cAMP signaling. Cell 152, 210–223. 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Muresan V, Joshi HC, Besharse JC, 1993. Gamma-tubulin in differentiated cell types: localization in the vicinity of basal bodies in retinal photoreceptors and ciliated epithelia. J. Cell Sci. 104 (Pt 4), 1229–1237. [DOI] [PubMed] [Google Scholar]
- Ocbina PJR, Anderson KV, 2008. Intraflagellar transport, Cilia, and mammalian hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev. Dyn 237, 2030–2038. 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron M, Boy S, Amato MA, Viczian A, Koebernick K, Pieler T, Harris WA,2003. A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development 130, 1565–1577. 10.1242/dev.00391. [DOI] [PubMed] [Google Scholar]
- Persson M, Stamataki D, Welscher P. Te, Andersson E, Böse J, Rüther U, Ericson J, Briscoe J, 2002. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 16, 2865–2878. 10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT, 2011. Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proc. Natl. Acad. Sci. USA 108, 1456–1461. 10.1073/pnas.1011410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G, 2002Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development 129, 4963–4974. [DOI] [PubMed] [Google Scholar]
- Ribes V, Balaskas N, Sasai N, Cruz C, Dessaud E, Cayuso J, Tozer S, Yang LL, Novitch B, Marti E, Briscoe J, 2010. Distinct Sonic Hedgehog signaling dynamics specify floor plate and ventral neuronal progenitors in the vertebrate neural tube1186–1200. 〈 10.1101/gad.559910.diversity〉. [DOI] [PMC free article] [PubMed]
- Rowan S, Chen C-MA, Young TL, Fisher DE, Cepko CL, 2004Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development 131, 5139–5152. 10.1242/dev.01300. [DOI] [PubMed] [Google Scholar]
- Sasagawa S, Takabatake T, Takabatake Y, Muramatsu T, Takeshima K, 2002. Axes establishment during eye morphogenesis in Xenopus by coordinate and antagonistic actions of BMP4, Shh, and RA. Genesis 33, 86–96. 10.1002/gene.10095. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H, 1999. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126, 3915–3924. 10.1038/386735a0. [DOI] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST, 2005. PDGFRα signaling is regulated through the primary cilium in fibroblasts. Curr. Biol 15, 1861–1866. 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P, 2000. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development 127, 4325–4334. 10.1016/0092-8674(91)90434-z. [DOI] [PubMed] [Google Scholar]
- Take-uchi M, Clarke JDW, Wilson SW, 2003. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development 130, 955–968. 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- Tang K, Xie X, Park JI, Jamrich M, Tsai S, Tsai MJ, 2010. COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development 137, 725–734. 10.1242/dev.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner M, Bhogaraju S, Lorentzen E, 2012. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation 83, S12–S22. 10.1016/j.diff.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tole S, Ragsdale CW, Grove EA, 2000. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes. Dev. Biol 217, 254–265. 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- Umberger NL, Caspary T, 2015. Ciliary transport regulates PDGF-AA/alphaalpha signaling via elevated mammalian target of rapamycin signaling and diminished PP2A activity. Mol. Biol. Cell 26, 350–358. 10.1091/mbc.E14-05-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vokes SA, Ji H, McCuine S, Tenzen T, Giles S, Zhong S, Longabaugh WJR, Davidson EH, Wong WH, McMahon AP, 2007. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development 134, 1977–1989. 10.1242/dev.001966. [DOI] [PubMed] [Google Scholar]
- Wang X, Lupo G, He R, Barsacchi G, Harris WA, Liu Y, 2015. Dorsoventral patterning of the Xenopus eye involves differential temporal changes in the response of optic stalk and retinal progenitors to Hh signalling. Neural Dev. 10, 7 10.1186/s13064-015-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood HB, Episkopou V, 1999. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech. Dev 86, 197–201. 10.1016/S0925-4773(99)00116-1. [DOI] [PubMed] [Google Scholar]
- Yang XJ, 2004. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin. Cell Dev. Biol 15, 91–103. 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong P, Chengbing W, Wang B, 2009. Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev. Biol 326, 177–189. 10.1007/978-3-642-30406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Yang XJ, 2001. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev. Biol 233, 271–290. 10.1006/dbio.2000.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]