Version Changes
Revised. Amendments from Version 1
We updated Dataset 1 and the accompanying web resource, along with variant counts and statistics in the manuscript:
- We re-examined insertions and deletions (indels) with the new version of the Shannon pipeline (SP). Major updates to this version of SP include:
- Binding sites adjacent to indels are now correctly localized.
- Cryptic splice acceptor sites in the first exon and cryptic donor sites in the last exon of a gene are no longer reported.
- Information models derived from splicing regulatory and transcription factor binding sites can now be utilized.
Genomic and transcriptomic data from the International Cancer Genome Consortium (ICGC) became available to us in January 2019. This dataset was evaluated using the same methods as those used for TCGA.
Differences in the expression of predicted mutant splice forms between two distinct BAM files were discovered from the same tumour of the same patient. We reanalyzed and replaced results for all variants in the TCGA-ESCA dataset using a second set of RNAseq BAM files.
DNAStack is acknowledged by including a citation of their recent article describing beacons. We discuss:
Comparison of results obtained from the TCGA and ICGC datasets. Figure 2 summarizes this.
Comparison of gene expression values in patients with mutations validated by Veridical vs predicted mutations in the same gene that did not achieve statistical significance using this algorithm.
Comparison of Veridical-flagged, cryptic splicing mutations with results of another study which evaluated cryptic splicing in TCGA ( Jayasinghe et al., 2018).
Evaluation of a large set of validated splicing mutations (n=1050, Cheung et al., 2019).
The analyses reported in this paper are being adopted by the genomics community. Since the version 1 publication (07 Dec 2018), the internet resource described has been queried by the global Beacon Network 6906 times and through the ValidSpliceMut website 8523 times.
Abstract
We present a major public resource of mRNA splicing mutations validated according to multiple lines of evidence of abnormal gene expression. Likely mutations present in all tumor types reported in the Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (ICGC) were identified based on the comparative strengths of splice sites in tumor versus normal genomes, and then validated by respectively comparing counts of splice junction spanning and abundance of transcript reads in RNA-Seq data from matched tissues and tumors lacking these mutations. The comprehensive resource features 341,486 of these validated mutations, the majority of which (69.9%) are not present in the Single Nucleotide Polymorphism Database (dbSNP 150). There are 131,347 unique mutations which weaken or abolish natural splice sites, and 222,071 mutations which strengthen cryptic splice sites (11,932 affect both simultaneously). 28,812 novel or rare flagged variants (with <1% population frequency in dbSNP) were observed in multiple tumor tissue types. Single variants or chromosome ranges can be queried using a Global Alliance for Genomics and Health (GA4GH)-compliant, web-based Beacon “Validated Splicing Mutations” either separately or in aggregate alongside other Beacons through the public Beacon Network, as well as through our website.
Keywords: RNA Splice Sites, Single Nucleotide Polymorphism, Genome, Mutation, Chromosomes, Neoplasms, Information Theory, Next Generation Sequencing, Validation
Introduction
Next generation sequencing continues to reveal large numbers of novel variants whose impact cannot be interpreted from curated variant databases, or through reviews of peer-reviewed biomedical literature 1. This has created a largely unmet need for unequivocal sources of information regarding the molecular phenotypes and potential pathology of variants of unknown significance (VUS); in cancer genomes, such sources are critically needed to assist in distinguishing driver mutations from overwhelming numbers of bystander mutations. VUS classification criteria highlight the limitations in genome interpretation due to ambiguous variant interpretation. Of the 458,899 variant submissions in NCBI’s ClinVar database with clinical interpretations, nearly half (n=221,271) are VUS (as of November 5th 2018). Only 10,784 variants in ClinVar have been documented to affect mRNA splicing at splice donor or acceptor sites, with 1,063 of these being classified as VUS, and cryptic mRNA splicing mutations are not explicitly described. The current ACMG criteria 2 for variant pathogenicity prevent clinical classification of most VUS. Functional evidence that VUS either disrupt or abolish expression of genes has been sought to improve classification and provide insight into the roles, if any, of individual VUS in predisposing or causing disease. We present a comprehensive data repository for a relatively common mutation type (cis-acting variants that alter mRNA splicing). Mutations are predicted with information theory-based analyses 3 , and supported with functional evidence that variants in tumor genomes are specifically associated with abnormally spliced mRNAs that are infrequent or absent in transciptomes lacking these variants 4.
Information theory (IT) has been proven to accurately predict impact of mutations on mRNA splicing, and has been used to interpret coding and non-coding mutations that alter mRNA splicing in both common and rare diseases 3, 5– 15. We have described an IT-based framework for the interpretation and prioritization of non-coding variants of uncertain significance, which has been validated in multiple studies involving novel variants in patients with history or predisposition to heritable breast and/or ovarian cancer 11– 15.
The Cancer Genome Atlas (TCGA) Pan-Cancer Atlas (PCA) is a comprehensive integrated genomic and transcriptomic resource containing data from >10,000 tumors across 33 different tumor types 16. Here, we utilized IT-based tools for assessment of high quality sequenced variants in TCGA patients, as well as patients from tumor datasets provided by the International Cancer Genome Consortium (chronic lymphocytic leukemia, esophageal adenocarcinoma, malignant lymphoma, pancreatic cancer endocrine neoplasms, as well as liver, ovarian, and renal cell cancers), for their potential impact on mRNA splicing. The accuracy of predicted mutations was evaluated with the algorithm we previously developed 4 that compares transcripts from individuals carrying these variants with others lacking them. The results of these genome-wide analyses are presented using an online internet resource, ValidSpliceMut, which can also be queried through the Beacon Network 17, 18.
Methods
TCGA and ICGC data acquisition and processing
Controlled-access data was obtained with permission from the Data Access Committee at NIH for TCGA and from the International Cancer Genomics Consortium. Patient RNA sequencing BAM files (tumor and normal, when available) and their associated VCF files (GRCh37) were initially obtained from the CancerGenomeHub (CGhub). Files were later downloaded through Genomic Data Commons using the GDC Data Transfer Tool (version 1.3.0), as CGhub was decommissioned mid-project. Genomic data from ICGC was downloaded through the Score client (version 1.5.0). Variants in VCF files which did not pass quality control (QC) were not analyzed.
Information analysis and RNA-Seq validation of splicing variants
We used the Shannon Pipeline software (SP; which applies IT to rapidly perform high-throughput, in silico prediction of the impacts of variants on mRNA splicing) 19 to analyze all QC-passing variants in VCFs from TCGA and ICGC (>168 million TCGA and >41 million ICGC variants) to evaluate their potential impact on splice site binding strength (changes in information content, R i, measured in bits). Variants which were predicted to strengthen known natural sites or weaken cryptic splice sites were excluded from all subsequent analyses. We also required novel cryptic splice sites to be strengthened by ≥ 2 bits (at least 4 fold), and with final strengths (R i,final) exceeding that of the nearest natural site of the same polarity.
To validate the potential impact of SP-flagged mutations, Veridical software analyzed genomic variants (including insertions and deletions) by comparing the RNA-Seq alignment in the region surrounding the variant with the corresponding interval in control transcriptomes (normal and tumor tissue of the same type) lacking the variant 4, 20. The Veridical algorithm: a) counts abnormally spliced reads in RNA-Seq data (categorized as either cryptic site use, exon skipping, or intron inclusion [containing or adjacent to the flagged mutation]), b) applies the Yeo-Johnson transformation to these results, and c) determines the null hypothesis probability (p-value) that the transformed read count corresponds to normal splicing. In tumor types where normal controls were not available, a set of RNA-Seq datasets from 100 different normal tissues from TCGA were used (e.g. a combination of 5 tissue types: BRCA, BLCA, LUAD, KIRC, PRAD). Veridical results that were not significant for a particular variant (p-value > 0.05 for all evidence types, or evidence of altered splicing consisting of fewer than 2 reads) were not further analyzed. After this analysis, Veridical validated 341,486 unique mutations for their direct impact on mRNA splicing ( Table 1). The Shannon pipeline-flagged and Veridical-filtered results were combined into a single large table ( Dataset 1 21) that contains the source data for the ValidSpliceMut SQL database and the associated Beacon application.
Table 1. Unique Flagged Variants by TCGA and ICGC Tumor Tissue Type *.
| TCGA-ACC | TCGA-BLCA | TCGA-BRCA | TCGA-CESC | TCGA-CHOL | TCGA-COAD | TCGA-DLBC |
| 1,717 | 9,865 | 24,181 | 25,822 | 9,817 | 7,512 | 6,036 |
| TCGA-ESCA | TCGA-GBM | TCGA-HNSC | TCGA-KICH | TCGA-KIRC | TCGA-KIRP | TCGA-LAML |
| 19,361 | 935 | 2,840 | 26,519 | 6,711 | 4,892 | 19,503 |
| TCGA-LGG | TCGA-LIHC | TCGA-LUAD | TCGA-LUSC | TCGA-MESO | TCGA-OV | TCGA-PAAD |
| 1,346 | 12,461 | 18,262 | 2,628 | 303 | 88,136 | 1,585 |
| TCGA-PCPG | TCGA-PRAD | TCGA-READ | TCGA-SARC | TCGA-SKCM | TCGA-STAD | TCGA-TGCT |
| 90 | 944 | 3,083 | 20,024 | 12,515 | 20,245 | 467 |
| TCGA-THCA | TCGA-THYM | TCGA-UCEC | TCGA-UCS | TCGA-UVM | ICGC-CLLE | ICGC-ESAD |
| 56,962 | 16,599 | 28,524 | 10,716 | 2,498 | 2,041 | 61 |
| ICGC-LIRI | ICGC-MALY | ICGC-OV | ICGC-PACA | ICGC-RECA | ||
| 2,255 | 2,652 | 2,818 | 3,182 | 4,255 |
*The number of Veridical-flagged mutations in each TCGA and ICGC cancer data sets. Variants shared between multiple tissue types were counted for each category. Variant and RNA-Seq data were provided by The Cancer Genome Atlas Pan-Cancer Analysis Project 16. TCGA: ACC [Adrenocortical carcinoma], BLCA [Bladder Urothelial], BRCA [Breast Cancer], CESC [Cervical Squamous Cell Carcinoma], CHOL [Cholangiocarcinoma], COAD [Colon Adenocarcinoma], DLBC [Lymphoid Neoplasm Diffuse Large B-cell Lymphoma], ESCA [Esophageal Cancer], GBM [Brain Glioblastoma Multiforme], HNSC [Head and Neck Squamous Cell Carcinoma], KICH [Kidney Chromophobe], KIRC [Kidney Renal Clear Cell Carcinoma], KIRP [Kidney Renal Papillary Cell Carcinoma], LAML [Acute Myeloid Leukemia], LGG [Brain Lower Grade Glioma], LIHC [Liver Hepatocellular carcinoma], LUAD [Lung Adenocarcinoma], LUSC [Lung Squamous Cell Carcinoma], MESO [Mesothelioma], OV [Ovarian Serous Cystadenocarcinoma], PAAD [Pancreatic Cancer], PCPG [Pheochromocytoma and Paraganglioma], PRAD [Prostate Adenocarcinoma], READ [Rectum Adenocarcinoma], SARC [Sarcoma], SKCM [Skin Cutaneous melanoma], STAD [Gastric Adenocarcinoma], TGCT [Testicular Germ Cell Tumors], THCA [Head and Neck Thyroid Carcinoma], THYM [Thymoma], UCEC [Uterine Corpus Endometrial Carcinoma], UCS [Uterine Carcinosarcoma], UVM [Uveal Melanoma]. ICGC: CLLE [Chronic Lymphocytic Leukemia], ESAD [Esophageal Adenocarcinoma], LIRI [Liver Cancer], MALY [Malignant Lymphoma], OV [Ovarian Cancer], PACA [Pancreatic Cancer Endocrine Neoplasms], RECA [Renal Cell Cancer].
Development of the ValidSpliceMut database and Beacon
We created a publicly accessible Application Programming Interface (API) ( https://beacon.cytognomix.com) that can be utilized to programmatically query variants passing filter thresholds described above ( Dataset 1 21). It was built in accordance with the GA4GH Beacon v1.0.0 specification, which describes a Representational State Transfer (REST) API for genetic data sharing 22. A Beacon accepts queries using an HTTP request and returns JavaScript Object Notation (JSON). Our Beacon implementation is coded in PHP 7.0 and utilizes a MySQL database (version: 5.7.24) with indexes applied to variant ID, chromosome, and coordinate fields (GRCh37). The returned JSON object reports whether the variant was found within our Beacon dataset as well as metadata including splice site coordinate, splice type, site type, the IT-based measures R i,initial and R i,final, affected individual IDs, tumor type, Veridical evidence by type annotated with significance level, and, if known, the corresponding rsID with its average heterozygosity (dbSNP 150). The metadata for each variant sent to the Beacon Network is a concise subset of available results in our database. It includes the first relevant database entry, meaning that if the variant exists within multiple individuals only the first will contribute fields to the metadata. However, among this metadata is a hyperlink to our local website containing results for any remaining tumors.
We developed the website, ValidSpliceMut ( Figure 1) to serve as a local interface to our Beacon, allowing users to manually search for a variant, by gene name or genome coordinate range. ValidSpliceMut automatically queries our Beacon, and formats the results of the search, if any. This website provides a complete view of variants, including Veridical-based evidence on all data related to every affected individual. If a variant is associated with multiple splice sites, the user is presented with a brief overview of all affected sites and must select a desired site to continue. To obtain the coordinate of the queried variant in gene-centric notation, a link is provided which queries the Mutalyzer API and generates coordinates for all available transcripts. ValidSpliceMut only reports transcripts for the gene affected by the variant.
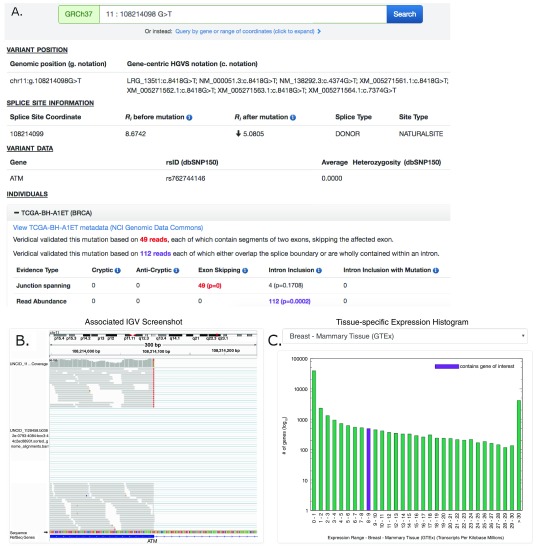
Figure 1. Screenshot of ATM:g.108214098G>T Results Provided By ValidSpliceMut Website.
( A) The ‘Variant Position’ heading displays the variant of interest in g. notation, and provides a link which queries the Mutalyzer API to obtain the variant coordinate in a gene-centric c. mutation format. Variant-specific and splice site-specific tabular results are presented under the headings “Splice Site Information” and “Variant Data”. Results are organized by TCGA and ICGC sample IDs harboring the mutation within a series of expandable panels. A link is provided to patient tumor metadata on the GDC data portal. Each panel consists of read counts and p-values by Veridical evidence type. Significant p-values (≤ 0.05) are highlighted in bold. Evidence types deemed “strongly corroborating” (Viner et al. 2014) are color coded and correspond to the dynamically generated text appearing above the table. ( B) An integrative genome viewer (IGV) image showing alignment of expressed sequence reads. IGV screenshots are provided only for mutations present <1% of population (in dbSNP 150), with ≥ 5 junction-spanning reads, and are highly significant (p < 0.01) for cryptic splicing, exon skipping, and/or intron inclusion with mutation. A specific IGV screenshot for this sample captures the region surrounding the mutation. Here, several RNA-Seq reads show skipping of the affected exon. ( C) A dynamically generated histogram presents expression levels of all genes for a selected normal tissue type. Genes are grouped into bins based on expression level, denoted on the x-axis. The number of genes present in each bin is shown on the y-axis (log 10 scale). The histogram key indicates the expression range of the variant-containing gene. Tissue type can be changed via a drop-down list.
A results page presents variant-specific data in tabular format and an expandable list of panels describing the affected individuals. Each of these panels contains Veridical output in tabular format for the selected tumor, a link to the tumor metadata at US National Cancer Institute (by querying the GDC API to obtain a UUID which is used to construct a link to the GDC data portal), an Integrative Genome Viewer (IGV) screenshot containing the variant (IGV screenshots are available for selected variants, see below), and a histogram which presents the expression levels of the variant-containing gene compared to all other gene expression levels across a selected normal tissue type (created dynamically using gnuplot 5.0). The tissue expression data is provided by GTEx (downloaded on 10/22/18). However, several tumor types did not have a GTEx equivalent (TCGA: CHOL, DLBC, MESO, READ, SARC, THYM and UVM; ICGC: MALY). The GNF Expression Atlas 2 23 was downloaded from the UCSC Genome Browser and was used for expression data for both lymph nodes (DLBC; MALY) and the thymus (THYM). For the remaining tissues, expression data from the following studies were obtained from the Genome Expression Omnibus (GEO): GSE76297 (CHOL), GSE2549 (MESO), GSE15781 (READ) GSE44426 (SARC), and GSE44295 (UVM).
To generate IGV images presented on the webpage, a bash script was written to automatically load the RNA-Seq BAM file of a patient with a mutation of interest into IGV, set the viewing window within the region of interest (300nt window, centered on the variant), sorted to bring reads containing the variant of interest to the top of the screen (to increase chance of visualizing mutant splice form), followed by a screen capture. The generation and storage of IGV images for all patient-mutation pairs would be prohibitive due to limitations in time and server space requirements. Therefore IGV images showing evidence of splicing abnormalities were generated only for patient-mutation pairs which met the most stringent criteria: the mutation was required to be flagged for junction-spanning cryptic site use, exon skipping, or intron inclusion (with mutation); the flagged category must include 5 or more reads in this category; if the variant is present in the dbSNP database (release 150), the frequency was required to be < 1% of the population; and the Veridical results, in which the mutations flagged were required to exhibit p ≤ 0.01 for at least one form of evidence of a splicing abnormality. In some cases, the splicing event observed by Veridical may not be present within the image window as the automated procedure used to create these images does not present all evidential sequence reads due to limitations on the number of reads that are shown. Additionally, reads appearing as exon skipping may instead indicate a pre-existing cryptic site outside of the viewing window (for examples, see Table 2; FAT1:g.187521515C>A [c.11641-1G>T] and SMAD3:g.67482748C>G [c.1155-3C>G]).
Table 2. Representative Validated Splicing Mutations in COSMIC Cancer Gene Census genes.
| Gene | Splice Mutation | R i(bits) | Tumor | Observed Splicing Event |
|---|---|---|---|---|
| CASC5 |
15:40942786G>A
(c.6212+5G>A) |
4.8 > 1.7
(Natural Site) |
AML | The natural donor site of
CASC5 exon 19 (NM_144508.4) is weakened,
leading to a significant increase in intron inclusion. |
| DNMT3A |
2:25467022A>G
(c.1851+2T>C) |
3.6 > -3.5
(Natural Site) |
AML | The natural donor site of
DNMT3A exon 15 (NM_022552.4) is abolished,
resulting in a significant increase in total exon skipping and intron inclusion. |
| STAG2 |
X:123176495G>A
(c.462G>A) |
6.5 > 3.5
(Natural Site) |
BLCA | The natural donor of
STAG2 exon 6 (NM_006603.4) is weakened, and a
significant amount of exon 6 skipping is observed. |
| STAG2 |
X:123200024G>A
(c.2097-1G>A) |
19.5 > 8.6
(Natural Site) |
BLCA | The natural acceptor of
STAG2 exon 21 (NM_006603.4) is weakened,
resulting in a significant increase in exon 21 skipping. |
| ATM |
11:108214098G>T
(c.8418G>T) |
8.7 > 5.1
(Natural Site) |
BRCA | A natural donor site is weakened, leading to a significant increase in
ATM exon 57 (NM_000051.3) skipping events. Some reads with mutation depict wildtype, leaky splicing. |
| BARD1 |
2:215645882A>T
(c.716T>A) |
0.9 > 3.1
(Cryptic Site) |
BRCA | The mutation strengthens a cryptic site within
BARD1 exon 4
(NM_000465.2). Reads which use this activated cryptic site contain the mutation (one exception). Some reads with mutation depict wildtype, leaky splicing. |
| GATA3 |
10:8115701G>C
(c.1048-1G>C) |
0.9 > -10.7
(Natural Site) |
BRCA | The mutation abolishes the natural acceptor of
GATA3 exon 6
(NM_002051.2). This both increases the use of a pre-existing exonic cryptic splice site (4.2 > 5.6 bits; leads to an 8nt deletion) and significantly increases overall intron inclusion. |
| TP53 |
17:7577609C>T
(c.673-1G>A) |
6.0 > -4.9
(Natural Site) |
BRCA | A natural acceptor site is abolished, activating a cryptic site 49nt
upstream (R i=5.2 bits) of TP53 exon 7 (NM_000546.5). |
| POLD1 |
19:50920353A>G
(c.3119A>G) |
8.6 > 6.1
(Natural Site) |
COAD | The natural donor of
POLD1 exon 25 (NM_002691.3) is weakened,
leading to a significant increase in overall exon skipping. |
| SMAD3 |
15:67482748C>G
(c.1155-3C>G) |
11.9>3.1|-4.0 > 7.7
( Natural | Cryptic) |
COAD | This mutation weakens the natural acceptor of
SMAD3 exon 9
(NM_005902.3) and predicts a cryptic site that does not appear to be used. A significant number of intron inclusion reads are observed. A distant pre-existing cryptic acceptor (9.6 bits; 3598nt from natural acceptor) was used in multiple reads. |
| PIK3R1 |
5:67591246A>G
(c.936-2A>G) |
7.5 > -7.3
(Natural Site) |
GBM | The natural acceptor of
PIK3R1 exon 8 (NM_181504.3) is abolished,
which promotes a significant increase in exon 8 skipping. |
| FAT1 |
4:187521515C>A
(c.11641-1G>T) |
5.3 > -2.4
(Natural Site) |
HNSC | The natural acceptor of
FAT1 exon 22 (NM_005245.3) is abolished,
resulting in both intron inclusion (overall intron inclusion and the use of a 2.3 bit cryptic site 82nt upstream of natural acceptor) and use of two exonic cryptic sites (237nt and 234nt from the natural acceptor; R i=1.0 bits and -0.2 bits, respectively). |
| TGFBR2 |
3:30729875G>A
(c.1397-1G>A) |
8.4 > -2.5
(Natural Site) |
HNSC |
TGFBR2 exon 6 natural acceptor (NM_003242.5) is abolished, leading
to multiple splicing events: intron inclusion, use of three cryptic sites (35nt exonic [R i=3.7 bits], 30nt and 972nt intronic [R i=0.4 bits and 11.2 bits, respectively]), and exon 6 and 7 skipping (activates a novel pseudo exon ~55kb downstream of exon 7). |
| PBRM1 |
3:52682355C>G
(c.813+5G>C) |
6.8 > 2.9
(Natural Site) |
KIRC | The natural donor of
PBRM1 exon 8 (NM_018313.4) is weakened, which
leads to a significant increase in exon 8 skipping. |
| PBRM1 |
3:52685756A>G
(c.714+2T>C) |
7.7 > 0.7
(Natural Site) |
KIRC | The natural donor of
PBRM1 exon 7 (NM_018313.4) is abolished,
resulting in a significant increase in exon skipping. |
| SETD2 |
3:47079269T>A
(c.7239-2A>T) |
9.8 > 2.1| 6.4 > 9.0
( Natural | Cryptic) |
KIRC | This mutation both significantly weakens the natural acceptor of
SETD2
exon 18 (NM_014159.6) while strengthening a 4nt exonic cryptic site, which is used. |
| RB1 |
13:49027249T>A
(c.1814+2T>A) |
4.9 >-13.7
(Natural Site) |
LUAD | The natural donor of
RB1 exon 18 (NM_000321.2) is abolished, leading
to a significant increase in both exon skipping and intron inclusion. All intron inclusion reads contain the mutation of interest. |
| RBM10 |
X:47006900G>T
(c.17+3G>T) |
7.8 > 4.1
(Natural Site) |
LUAD | The natural donor of
RBM10 exon 2 (NM_005676.4) is weakened,
leading to a significant increase in exon 2 skipping. |
| RBM10 |
X:47028898G>T
(c.201+1G>T) |
8.7 > -9.9
(Natural Site) |
LUAD |
RBM10 exon 3 (NM_005676.4) natural donor is abolished. Reads
which overlap the exon-intron junction are observed (all reads contain mutation). Use of cryptic donor (61nt upstream of donor; R i=1.7 bits) is observed as well. |
| DDX5 |
17:62500098 TACAG>T
(c.441+2delACAG) |
-1.3 > 5.4
(Cryptic Site) |
PRAD | The mutation creates a 5.4 bit cryptic donor within
DDX5 exon 4
(NM_004396.3), which would lead to a 4nt deletion of exon 4. Note that wildtype splicing is still the dominant isoform observed. |
| PTEN |
10:89690802G>A
(c.210-1G>A) |
8.5 > -2.3
(Natural Site) |
PRAD | The natural acceptor of
PTEN exon 5 (NM_000314.4) is abolished,
leading to an increased amount of exon 5 skipping. |
| NRAS |
1:115258669A>G
(c.111+2T>C) |
8.1 > 1.1
(Natural Site) |
SKCM | The mutation abolishes the natural donor of
NRAS exon 2
(NM_002524.4), which promotes a significant increase in exon 2 skipping. |
| PPP6C |
9:127933364C>T
(c.171G>A) |
6.7 > 3.7
(Natural Site) |
SKCM | The mutation weakens
PPP6C exon 2 (NM_002721.4) natural donor,
leading to increased intron inclusion. All reads which cross the splice junction contain the mutation. An intronic cryptic site is also activated (110nt downstream; R i=6.4 bits). |
| PPP6C |
9:127923119C>G
(c.237+1G>C) |
6.8 > -11.8
(Natural Site) |
SKCM | This mutation abolishes the natural donor of
PPP6C exon 3
(NM_002721.4), resulting in a significant increase in exon 3 skipping. |
| BAP1 |
3:52442512T>C
(c.233A>G) |
1.9 > 5.1
(Cryptic Site) |
UVM | A pre-existing cryptic donor within
BAP1 exon 4 (NM_004656.3) is
strengthened, leading to a significant increase in its use. This mutation leads to a 27 nt deletion in the mutated exon 4 mRNA. |
Example mutations which alter splicing in tumor-associated genes found in patients with these tumor types. Mutations are hyperlinked to their ValidSpliceMut Beacon page, which provides additional material such as IGV images of the RNAseq evidence for the regions of interest. GRCh37 coordinates are indicated
Dataset validation and discussion
We have derived a GA4GH-standardized searchable resource for a large set of validated mRNA splicing mutations present in diverse tumor types. All variants passing QC in TCGA and ICGC cancer patients were analyzed with the Shannon pipeline 19. This revealed that 1,094,749 variants were predicted to have significant impacts on normal mRNA splicing (380,852 natural and 752,472 cryptic splice sites; 38,575 affecting both types). Subsequent RNA-Seq analysis with Veridical 4 provided evidence of abnormal gene expression specifically associated with a subset of these variant(s), identifying 341,486 unique mutations.
Our results contrast with an earlier TCGA study that investigated alternative mRNA splicing 24 and demonstrated a limited set of non-constitutive exon-exon junctions attributable to cis-acting splicing mutations (n = 32). The 3,311 novel or rare variants from TCGA patients that we report specifically activate abnormal cryptic splicing (significant ‘junction-spanning cryptic site use’ reads found by Veridical). This exceeds the number reported in cited study that analyzed all available TCGA tumor transcriptomes (n=1,914) 25. When ICGC datasets were included, a total of 3,650 variants were found to activate cryptic splicing. Comparing the validated cryptic splicing mutations we found with this previous report 25, 1,176 variants fulfilled our IT-based filtering criteria for constitutive splicing mutations. Veridical validated mRNA splicing effects for 824 of these variants (70.1%). The remaining 738 variants were reanalyzed for changes within the binding sites of regulatory splicing factors (SRF) that might affect normal mRNA processing. Together, including the effects on constitutive splicing, IT analysis of SRFs 6 (SRSF1, SRSF2, SRSF5, SRSF6, hnRNPA1, ELAVL1, PTB and TIA1) cumulatively identified changes in binding strength in 1746 (91.2%) sites affected by these variants.
Veridical validated splicing variants, which we define as mutations, were also tallied by tumor tissue type ( Table 1). 38.5% of unique mutations in TCGA (n=131,347) significantly weaken natural splice sites, while 65.0% (n=222,071) strengthen novel or pre-existing cryptic sites. 238,570 of these mutations (69.9%) are absent from dbSNP 150. 72,615 mutations (21.3%) are rare (found in <1% of the population), of which 28,812 (and those not present in dbSNP) were detected in multiple tumor types and individuals. Valid mutations lacking rsIDs, by definition, represent either novel or recently observed variants. This low level of dbSNP saturation in TCGA is consistent with the possibility that many currently unknown mRNA splicing mutations may yet be discovered through additional sequencing studies.
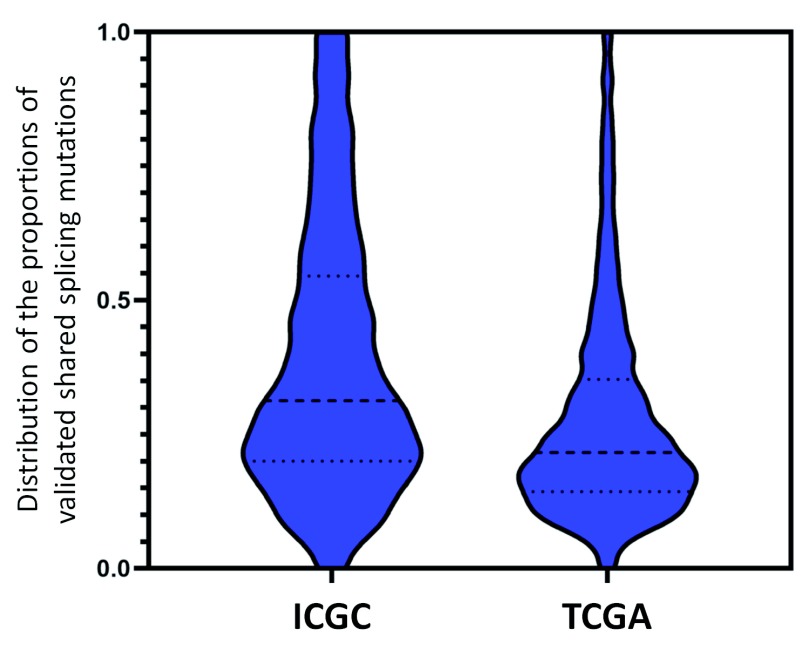
The ValidSpliceMut database consists of variants from both TCGA and ICGC, however the vast majority of variants were sourced from TCGA (329,758 of 341,597 flagged, unique variants were found only in TCGA patients; 96.5%). This had been expected, as the ICGC datasets were smaller (492 patients with available RNAseq data analyzed across 7 tumor types). There were 7,380 Veridical-flagged ICGC variants that were absent from TCGA patients; 4,459 variants were flagged in both TCGA and ICGC datasets (of which, 287 were not found in dbSNP 150). To evaluate the frequency of flagged TCGA and ICGC variants, we compared those shared between the two datasets that met SP criteria (n=9,485). We computed that for, a meaningful comparison (with 95% confidence interval [CI]), at least 9 ICGC and 24 TCGA patients should possess the shared mutation (typically, these correspond to common SNPs) and 1,379 shared variants met this criteria. We determined that on average, a higher average proportion of ICGC patients with shared mutations were flagged by Veridical compared to the TCGA cohort ( Figure 2). We observed that a higher fraction of SP-flagged variants are natural splice site alterations in the ICGC dataset compared to TCGA (49.7% to 38.3% of total SP-flagged variants, respectively), with fewer affecting cryptic sites (50.7% to 65.3%, respectively). A similar fraction of these sites were predicted to abolish natural splicing (16.8% ICGC and 14.4% TCGA of total SP-flagged variants). A higher percentage of ICGC variants compared to TCGA were confirmed with Veridical (49.1% to 30.9%, respectively), which may possibly be due to higher overall coverage in these regions in the RNAseq results for ICGC relative to TCGA. Interestingly, the fractions of novel variants i.e. not recorded in dbSNP, between TCGA and ICGC are inconsistent (70.0% vs. 42.4%, respectively). We speculate that this may be related to differences in sequence coverage, since TCGA variants were identified from a mixture of whole genome (WGS) and exomes 16 , while ICGC variants were exclusively derived by WGS.
Figure 2. Census of Recurrent Splicing Mutations Present in Multiple ICGC and TCGA Patients.
Predicted splicing mutations present in multiple tumors from the same dataset that cause splicing abnormalities were analyzed to determine validation rates, since such variants were less subject to technical artifacts, such as sequencing errors. Violin plots indicate the distributions of the fraction of predicted and validated splicing mutations present in multiple patients relative to the total number of tumours carrying those mutations in the TCGA and ICGA datasets. To achieve statistical significance (95% C.I.), distributions of 1,379 validated variants shared by both datasets and present in at least 9 ICGC (left) and 24 TCGA (right) patients were compared. A higher overall proportion of mutations are validated in the ICGC dataset (average of 38.6% for ICGC and 27.8% for TCGA). The dashed lines in each plot indicate the median (middle line), the upper and lower quartiles of the mutation fractions.
To assess the predictive accuracy of SP, we also analyzed an independent set of experimentally-validated splicing mutations that altered exon definition (1,050 variants), originally sourced from the Genome Aggregation Database; gnomAD 26 and validated with a high throughput, multiplexed splicing minigene reporter assay 27. Significant changes in constitutive splice site and/or (≥ 3 bit) SRF binding site strengths were found with SP for 1017 of these 1050 mutations (96.9%). Based on changes at constitutive splice sites alone, 447 variants were flagged (435 weaken natural sites, and 14 strengthen cryptic sites exceeding that of the most proximate natural splice site of the same polarity).
In Table 2, we highlight a representative subset of validated splicing mutations which were identified in known driver genes implicated in the COSMIC (Catalogue Of Somatic Mutations In Cancer) Cancer Gene Census catalog (CGC) 28. In total, 543 ‘Tier 1’ CGC genes exhibited at least one Veridical-flagged variant present in the ValidSpliceMut database. These mutations were associated with either increased exon skipping, intron inclusion, and/or cryptic site use. Mutations in Table 2 are hyperlinked to their corresponding ValidSpliceMut webpage, which provides additional information, including specific expression evidence for each of the tumors in the database that support predictions made by SP.
Many mutations generated multiple types of abnormal read evidence present in mis-spliced transcripts. Interestingly, a subset of TCGA mutations (n=33) produced evidence for every type of abnormal splicing reported by Veridical. Dataset 2 29 (see Data Availability) describes 11 representative mutations that simultaneously increase exon skipping, intron inclusion, and activate (or significantly increase use of) a strengthened cryptic site. In all but one instance, the mutation weakens the natural site, while simultaneously strengthening an adjacent cryptic site. The only exception involves the gene SAP30BP, where simultaneously occurring mutations in the same read (in linkage disequilibrium; separated by 4 nucleotides) independently cause two separate splicing changes: g.73702087G>A (c.661-1G>A; abolishes the natural acceptor of exon 10) and g.73702091G>A (c.664G>A; creates a weak cryptic acceptor site). The combined splicing impact of these variants is significant exon skipping, intron inclusion, and use of the activated cryptic site.
This resource presents a set of splicing abnormalities in which we have the highest confidence because expression validation is required. We anticipate that some correct predictions of the Shannon pipeline may have not been validated by Veridical due to the limitations of mRNA detection; for example, either low expression of the gene harboring the mutation or nonsense-mediated decay of the corresponding transcript could be consistent with the effects of a valid splicing mutation, but in the absence of a sufficient number of abnormal reads, the mutation could not be confirmed. Indeed, expression of genes in tumours with validated splicing mutations exhibited greater overall gene expression than in other variants that were not validated in the same gene. The overall difference in gene expression between these two variant groups was statistically significant for 69.3% of genes based on the Student’s t-test (90% C.I.). Furthermore, overall gene expression in the group with Veridical-flagged variants exceeded the non-flagged group for nearly all other genes (99.7%). Differences between expression levels of Veridical-validated splicing mutations and other predicted mutations suggests the possibility that unverified SP predictions may arise from lack of or low levels of gene expression of the genes containing this subset of variants.
The original SP version that processed most of the TCGA data did not report regulatory splicing variants which influence exon definition. The Automated Splice Site and Exon Definition Analysis (ASSEDA) server 6 analyzes individual variants for regulatory and constitutive IT changes. Due to time constraints, it was not feasible to perform a reanalysis with the upgraded SP of the entire set of ~209 million unique variants present in the TCGA and ICGC datasets. However, the SP upgrade did verify improvements in detection of both constitutive and regulatory splicing mutations using the set of validated mutations reported in a recent study 27. This version of SP (available through our MutationForecaster system) is capable of predicting mutations in splicing regulatory sequences at higher throughput than ASSEDA and, like ASSEDA, accounts for relative abundance of mRNA isoforms by exon definition.
The Validated Splicing Mutation resource should significantly contribute to reducing the number of outstanding VUS in tumor (and possibly some germline) genomes, and substantially increases the number of functional variants with previously unappreciated consequences to mRNA splicing, in particular, those which activate cryptic splice sites. In our earlier study 20 , a subset of the TCGA breast cancer patient data was evaluated with IT-based tools, identifying 988 mutations as significantly altering normal splicing by Veridical (19% of total mutations flagged by IT). This database greatly expands the size of the repository. Here, a higher ratio of rare or novel mutations have been validated by Veridical (31% of total mutations were flagged by IT). The higher yields seen here could be related to mutations present in multiple samples from the same tumor type and other tumor tissues, which would be expected to increase the probability of observing abnormally expressed splice forms for the same mutation.
bioRxiv
An earlier version this article is available from bioRxiv: https://doi.org/10.1101/474452 30
Software availability
Archived code and scripts used as part of this study are available:
Zenodo: Validated Splicing Mutations Beacon API https://doi.org/10.5281/zenodo.1579898 22
Zenodo: Validated Splicing Mutations Website https://doi.org/10.5281/zenodo.1579822 31
Zenodo: Expression Data Processing, Histogram input generation and IGV Bash Script Generating Programs https://doi.org/10.5281/zenodo.1582421 32
All software is licensed under a Creative Commons Attribution-Non Commercial-ShareAlike 4.0 International Public License
Data availability
Zenodo: Dataset 1. Validated natural and cryptic mRNA splicing mutations. Source data computed by the Shannon pipeline and Veridical, displayed on the ValidSpliceMut website ( https://validsplicemut.cytognomix.com/). DOI: https://doi.org/10.5281/zenodo.2586179 21
Zenodo: Dataset 2. Mutations which lead to multiple types of aberrant splicing. Representative set of mutations which significantly alter splicing in all evidence types analyzed by Veridical (i.e. cryptic splice site use, exon skipping, intron inclusion). Mutations are linked to their page on https://validsplicemut.cytognomix.com/, which provides additional material such as RNA-Seq images of the regions of interest. DOI: https://doi.org/10.5281/zenodo.1489941 29
License: CC0 1.0
Consent
Controlled-access TCGA and ICGC sequence data was approved by NCBI at the US National Institutes of Health (dbGaP Project #988: “Predicting common genetic variants that alter the splicing of human gene transcripts”; Approval Number #13930-11; PI: PK Rogan) and by the International Cancer Genome Consortium (ICGC Project #DACO-1056047; “Validation of mutations that alter gene expression”).
Acknowledgements
We acknowledge Coby Viner, Stephanie Dorman, William J. Phillips and Ujani Hazra for their contributions to the early stages of this project.
Funding Statement
PKR is supported by The Natural Sciences and Engineering Research Council of Canada (NSERC) [RGPIN-2015-06290], Canadian Foundation for Innovation, Canada Research Chairs, and CytoGnomix. Compute Canada and Shared Hierarchical Academic Research Computing Network (SHARCNET) provided high performance computing and storage facilities.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 1 approved
References
- 1. Foley SB, Rios JJ, Mgbemena VE, et al. : Use of Whole Genome Sequencing for Diagnosis and Discovery in the Cancer Genetics Clinic. EBioMedicine. 2015;2(1):74–81. 10.1016/j.ebiom.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richards S, Aziz N, Bale S, et al. : Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caminsky N, Mucaki EJ, Rogan PK: Interpretation of mRNA splicing mutations in genetic disease: review of the literature and guidelines for information-theoretical analysis [version 1; referees: 2 approved]. F1000Res. 2014;3:282. 10.12688/f1000research.5654.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viner C, Dorman SN, Shirley BC, et al. : Validation of predicted mRNA splicing mutations using high-throughput transcriptome data [version 2; referees: 4 approved]. F1000Res. 2014;3:8. 10.12688/f1000research.3-8.v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mucaki EJ, Ainsworth P, Rogan PK: Comprehensive prediction of mRNA splicing effects of BRCA1 and BRCA2 variants. Hum Mutat. 2011;32(7):735–742. 10.1002/humu.21513 [DOI] [PubMed] [Google Scholar]
- 6. Mucaki EJ, Shirley BC, Rogan PK: Prediction of mutant mRNA splice isoforms by information theory-based exon definition. Hum Mutat. 2013;34(4):557–565. 10.1002/humu.22277 [DOI] [PubMed] [Google Scholar]
- 7. Rogan PK, Svojanovsky S, Leeder JS: Information theory-based analysis of CYP2C19, CYP2D6 and CYP3A5 splicing mutations. Pharmacogenetics. 2003;13(4):207–218. [DOI] [PubMed] [Google Scholar]
- 8. Rogan PK, Schneider TD: Using information content and base frequencies to distinguish mutations from genetic polymorphisms in splice junction recognition sites. Hum Mutat. 1995;6(1):74–76. 10.1002/humu.1380060114 [DOI] [PubMed] [Google Scholar]
- 9. Rogan PK, Faux BM, Schneider TD: Information analysis of human splice site mutations. Hum Mutat. 1998;12(3):153–171. [DOI] [PubMed] [Google Scholar]
- 10. Peterlongo P, Catucci I, Colombo M, et al. : FANCM c.5791C>T nonsense mutation (rs144567652) induces exon skipping, affects DNA repair activity and is a familial breast cancer risk factor. Hum Mol Genet. 2015;24(18):5345–5355. 10.1093/hmg/ddv251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mucaki EJ, Caminsky NG, Perri AM, et al. : A unified analytic framework for prioritization of non-coding variants of uncertain significance in heritable breast and ovarian cancer. BMC Med Genomics. 2016;9:19. 10.1186/s12920-016-0178-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caminsky NG, Mucaki EJ, Perri AM, et al. : Prioritizing Variants in Complete Hereditary Breast and Ovarian Cancer Genes in Patients Lacking Known BRCA Mutations. Hum Mutat. 2016;37(7):640–652. 10.1002/humu.22972 [DOI] [PubMed] [Google Scholar]
- 13. Yang XR, Devi BCR, Sung H, et al. : Prevalence and spectrum of germline rare variants in BRCA1/2 and PALB2 among breast cancer cases in Sarawak, Malaysia. Breast Cancer Res Treat. 2017;165(3):687–697. 10.1007/s10549-017-4356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dos Santos ES, Caputo SM, Castera L, et al. : Assessment of the functional impact of germline BRCA1/2 variants located in non-coding regions in families with breast and/or ovarian cancer predisposition. Breast Cancer Res Treat. 2018;168(2):311–325. 10.1007/s10549-017-4602-0 [DOI] [PubMed] [Google Scholar]
- 15. Burke LJ, Sevcik J, Gambino G, et al. : BRCA1 and BRCA2 5’ noncoding region variants identified in breast cancer patients alter promoter activity and protein binding. Hum Mutat. 2018;39(12):2025–2039. 10.1002/humu.23652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoadley KA, Yau C, Hinoue T, et al. : Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell. 2018;173(2):291–304.e6. 10.1016/j.cell.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Global Alliance for Genomics and Health: GENOMICS. A federated ecosystem for sharing genomic, clinical data. Science. 2016;352(6291):1278–1280. 10.1126/science.aaf6162 [DOI] [PubMed] [Google Scholar]
- 18. Fiume M, Cupak M, Keenan S, et al. : Federated discovery and sharing of genomic data using Beacons. Nat Biotechnol. 2019;37(3):220–224. 10.1038/s41587-019-0046-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shirley BC, Mucaki EJ, Whitehead T, et al. : Interpretation, stratification and evidence for sequence variants affecting mRNA splicing in complete human genome sequences. Genomics Proteomics Bioinformatics. 2013;11(2):77–85. 10.1016/j.gpb.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dorman SN, Viner C, Rogan PK: Splicing mutation analysis reveals previously unrecognized pathways in lymph node-invasive breast cancer. Sci Rep. 2014;4:7063. 10.1038/srep07063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mucaki EJ, Shirley BC, Rogan PK: Dataset 1. Validated natural and cryptic mRNA splicing mutations [Data set]. Zenodo. 2018. 10.5281/zenodo.2586179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shirley BC, Mucaki EJ, Rogan PK: Validated Splicing Mutations Beacon API (Version 1.0.0). Zenodo. 2018. 10.5281/zenodo.1579898 [DOI] [Google Scholar]
- 23. Su AI, Wiltshire T, Batalov S, et al. : A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101(16):6062–6067. 10.1073/pnas.0400782101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kahles A, Lehmann KV, Toussaint NC, et al. : Comprehensive Analysis of Alternative Splicing Across Tumors from 8,705 Patients. Cancer Cell. 2018;34(2):211–224.e6. 10.1016/j.ccell.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jayasinghe RG, Cao S, Gao Q, et al. : Systematic Analysis of Splice-Site-Creating Mutations in Cancer. Cell Rep. 2018;23(1):270–281.e3. 10.1016/j.celrep.2018.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lek M, Karczewski KJ, Minikel EV, et al. : Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–91. 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheung R, Insigne KD, Yao D, et al. : A Multiplexed Assay for Exon Recognition Reveals that an Unappreciated Fraction of Rare Genetic Variants Cause Large-Effect Splicing Disruptions. Mol Cell. 2019;73(1):183–194.e8. 10.1016/j.molcel.2018.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Futreal PA, Coin L, Marshall M, et al. : A census of human cancer genes. Nat Rev Cancer. 2004;4(3):177–183. 10.1038/nrc1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mucaki EJ, Shirley BC, Rogan PK: Dataset 2. Mutations which lead to multiple types of aberrant splicing. Zenodo. 2018. 10.5281/zenodo.1489941 [DOI] [Google Scholar]
- 30. Shirley BC, Mucaki EJ, Rogan PK: Pan-Cancer Repository of Validated Natural and Cryptic mRNA Splicing Mutations. bioRxiv. 2018; 474452. 10.1101/474452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shirley BC, Mucaki EJ, Rogan PK: Validated Splicing Mutations Website (Version 1.0.0). Zenodo. 2018. 10.5281/zenodo.1579822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mucaki EJ, Shirley BC, Rogan PK: Expression Data Processing, Histogram input generation and IGV Bash Script Generating Programs. Zenodo. 2018. 10.5281/zenodo.1582421 [DOI] [Google Scholar]