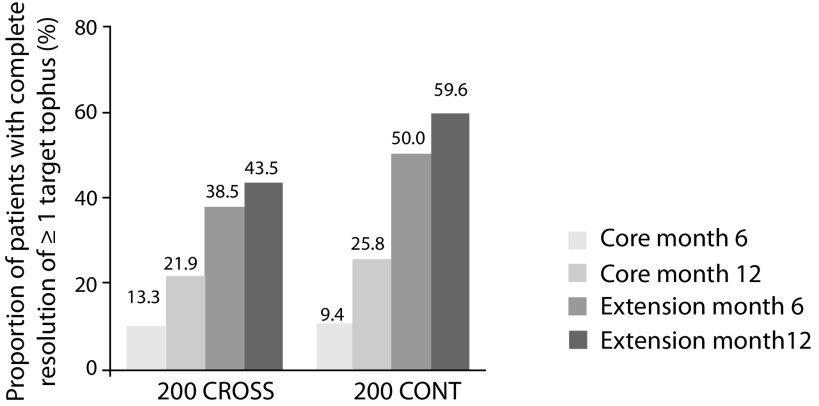Abstract
The aim of this review is to present current evidence about the efficacy and safety of lesinurad in combination with xanthine oxidase inhibitors (XOIs) in the treatment of hyperuricemia in patients with gout. Gout is the most common inflammatory form of arthritis. It is caused by an elevated concentration of serum uric acid (UA) that leads to the formation of monosodium urate crystals in joints and different tissues. The goal of therapy is to maintain serum UA levels at <6 mg/dL (0.36 mmol/L), to prevent the formation and deposition of monosodium urate crystals, and to dissolve existing crystals. Lesinurad, a new uricosuric, increases renal urate excretion by selectively inhibiting the renal uric acid transporter 1 (URAT1). Lesinurad is indicated in adults, in combination with a XOI, for the adjunctive treatment of hyperuricemia in patients with gout (with or without tophi) who have not achieved target serum UA levels with an adequate dose of a XOI alone. With the combination strategy, serum UA targets could be reached with the consequence of inhibiting formation of new crystals and promoting dissolution of existing crystals and, therefore, inducing improvement of outcomes such as flares and tophi. The approval of lesinurad was based on data from three pivotal phase III studies (CLEAR 1, CLEAR 2, and CRYSTAL). These clinical studies assessed lesinurad 200 and 400 mg doses. As only lesinurad 200 mg/day dose was finally approved and commercialized, it will be the focus of this paper. In the pivotal clinical trials, the target serum UA level was achieved by significantly more patients in lesinurad 200 mg plus allopurinol group (CLEAR 1 and CLEAR 2 trials) or lesinurad 200 mg plus febuxostat group (CRYSTAL study) compared with patients who received either XOI alone. In these trials, the safety profile of lesinurad 200 mg plus a XOI was comparable to allopurinol or febuxostat alone. Lesinurad, in combination with a XOI, is an effective and safe treatment that covers unmet needs in adults with gout who have not achieved target serum UA levels with a XOI alone.
Keywords: allopurinol, febuxostat, gout, hyperuricemia, lesinurad, serum uric acid levels, urate lowering, uric acid transporter 1
Introduction
Gout is the disease caused by elevated serum uric acid (UA) levels, resulting in the deposition of monosodium urate crystals in the joints and other tissues, which leads to a recurrent inflammatory arthritis and tophi formation.
Gout is the most common cause of inflammatory arthritis in adults, and its prevalence, ranging from 1 to 4% in developed countries, has been increasing in the last decades despite the availability of effective therapies.1–5
Urate levels in the body are maintained by a balance between production and excretion. Excretion of urate occurs primarily in the gut and in the kidneys through a complex process of glomerular filtration, reabsorption, and secretion. Usually, about 90% of the glomerular-filtered urate is reabsorbed into the bloodstream and only 10% is excreted. However, most patients with gout show inefficient renal excretion of urate, leading to hyperuricemia. It is considered that inefficient renal excretion of urate is observed in over 90% of patients with gout.6
Known risk factors for hyperuricemia and gout are diverse: genetic predisposition; male gender; aging; renal impairment; postmenopausal status in females; dietary factors, including alcohol consumption; and the use of a variety of drugs, including diuretics (Table 1).3–5
Table 1.
Common causes of drug-induced hyperuricemia.4
|
As well as the typical arthritis and tophi due to gout, further clinical manifestations are renal manifestations such as urolithiasis and progredient worsening of renal function. Gout is associated with comorbidities such as hypertension, chronic kidney disease, diabetes mellitus, increased risk of suffering cardiovascular disease,7,8 and increased risk of premature mortality.9 If not properly managed, continued elevated serum UA can result in formation of monosodium urate crystals in joints, causing both acute and chronic inflammation.10 Therefore, untreated or insufficiently treated gout can lead to chronic manifestation of the disease, such as persistent inflammation, increased number of flares, development of tophi, and structural joint damage.2–6,9,10
In general, there is evidence-based consensus that the target for serum UA levels is <6 mg/dL for mild-to-moderate gout and <5 mg/dL for severe gout.5,11–14 The European League Against Rheumatism (EULAR) recommends to achieve serum UA levels of <6 and <5 mg/dL for severe gout in a recent evidence-based recommendations report for the management of gout.14 In Spain, guidelines of the Spanish Society of Rheumatology (SER, Sociedad Española de Reumatología) also proposed similar targets for serum UA levels, <6 mg/dL for gout and probably less for severe gout.13
Typically, a xanthine oxidase inhibitor (XOI), either allopurinol or febuxostat, is recommended as first-line therapy to inhibit urate production. Nonetheless, in clinical studies, only 40% of patients treated with allopurinol 300 mg/day achieved serum UA levels of <6.0 mg/dL.15 With febuxostat 80–240 mg/day, 48–69% of subjects achieved a serum UA level of <6.0 mg/dL; however, there was still approximately a third of patients entering to clinical trials who did not reach or maintain the serum levels goal.16
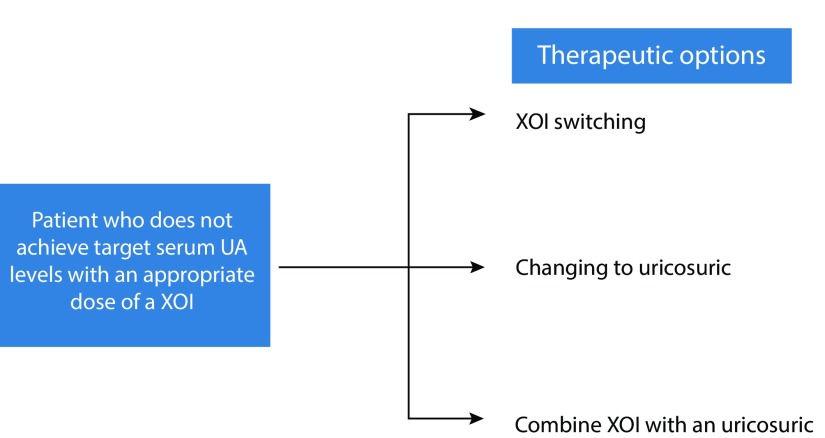
The EULAR recommended that if target serum UA levels cannot be achieved with an appropriate dose of a XOI, there is a need for additional treatment options such as XOI switching, changing to a uricosuric, or combining a XOI with a uricosuric drug (Figure 1).14 Lesinurad combined with a XOI offers a dual mechanism for serum UA lowering by means of a blockade of renal urate reabsorption and a reduction in urate production. Lesinurad, therefore, is a novel uricosuric that covers unmet needs for patients with inadequate response to XOIs.5,11–14
Figure 1.
Therapeutic algorithm for patients who do not achieve target serum UA levels with an appropriate dose of a xanthine oxidase inhibitor (XOI). Medically appropriate dose of XOI is recommended for individual patients.
The approval of lesinurad for the treatment of hyperuricemia in patients with gout was based on data from three pivotal phase III studies (CLEAR 1, CLEAR 2, and CRYSTAL). These clinical studies evaluated two doses (200 and 400 mg). The 400 mg dose was not submitted to regulatory authorities for approval based on careful considerations of safety and efficacy. Therefore, only the 200 mg concentration was approved by agencies such as the US Food and Drug Administration (FDA) and European Medicines Agency (EMA), so it will be the primary focus of this study.
Pharmacological and clinical properties of lesinurad
Mechanism of action
Lesinurad is a selective inhibitor of the uric acid transporter URAT1, which is responsible for most reabsorption of urate anion in the renal tubular system. By inhibiting URAT1, lesinurad increases the net UA excretion and reduces serum UA levels. Lesinurad inhibits OAT4 as well, an organic anion transporter involved in luminal urate reabsorption and diuretic-induced hyperuricemia.17,18
Pharmacodynamic effects
In healthy volunteers, lesinurad 200 mg reduced serum UA levels and improved renal clearance and fractional excretion of UA. Mean serum UA decreases following lesinurad 200 mg administration alone were approximately 46 and 26% at 6 and 24 hours post dose, respectively.17,18 When lesinurad 200 mg was combined with a XOI, additional 25 and 19% of serum UA reductions were observed at 6 and 24 hours post dose, respectively.17,18
Pharmacokinetic properties
Lesinurad is rapidly absorbed after oral administration. The absolute bioavailability of lesinurad is close to 100%.17–18 After the administration of a single oral dose of lesinurad in either the fed or fasted state, maximum plasma concentrations (Cmax) were reached within 1–4 hours. Cmax and AUC exposures of lesinurad increased proportionally with increasing single oral doses of lesinurad. In clinical trials, lesinurad was administered with food, because the serum UA-lowering effect was improved under fed conditions, and the Cmax peak slowed.17,18 It is therefore recommended to take lesinurad in the fed state, after food and fluid intake.17,18 Lesinurad is highly bound to proteins in plasma, approximately 98%, mostly to albumin. Plasma protein binding is not significantly changed in patients with renal or hepatic impairment.17,18
Lesinurad undergoes oxidative metabolism chiefly via cytochrome P450 (CYP) 2C9, with minimal contribution from CYP1A1, CY2C19, and CYP 3A. Metabolites are not known to contribute to the UA-lowering effects of lesinurad.17,18
Renal clearance of lesinurad is high (25 mL/minute; 1.5 L/hour), showing that active secretion of lesinurad has an important role in its renal excretion.17,18 Most of the radioactivity recovered in urine occurred in the first 24 hours. The elimination half-life (t½) of lesinurad was in the order of 5 hours following a single dose and the drug did not accumulate following multiple doses.17–20
Clinical studies of lesinurad
Lesinurad has been given FDA and EMA approval for combined therapy with a XOI. Pivotal phase III studies that led to regulatory approvals of lesinurad were CLEAR 1 (Combining Lesinurad with Allopurinol Standard of Care in Inadequate Responders) and CLEAR 2, both in combination with allopurinol, and CRYSTAL (Combination Treatment Study in Subjects with Subcutaneous Tophaceous Gout with Lesinurad and Febuxostat), in combination with febuxostat. After completing the core studies, open extension studies with 24 months of follow-up assessed the efficacy and safety of lesinurad in long-term treatment.21–27
Efficacy results from pivotal studies
CLEAR 1 study
A 12-month, multicenter, randomized, double-blind, placebo-controlled phase III study (CLEAR 1) was conducted in the United States to evaluate oral lesinurad (200 or 400 mg/day) plus allopurinol versus placebo plus allopurinol in patients with elevated serum UA concentrations despite allopurinol monotherapy. Included patients were receiving at least 300 mg of allopurinol (200 mg allowed in patients with moderate renal impairment) and had serum UA levels of >6.5 mg/dL at screening and two or more gout flares during the previous year.21 Previously, lesinurad combined with allopurinol has shown superior reductions in serum UA than allopurinol alone in phase I and II trials.4,20–22
The primary endpoint in CLEAR 1 was the percentage of patients achieving a serum UA level of <6.0 mg/dL at month 6. Key secondary endpoints included the mean gout flare rate requiring treatment from month 7 to month 12 and the proportions of subjects with complete resolution of one target tophus at month 12. Safety and tolerability assessments included adverse events (AEs), clinical laboratory data, vital signs, physical examination, and electrocardiogram readings. Safety assessments of particular interest included renal and cardiovascular (CV) events.21
Of 2377 patients evaluated at the screening visit, 607 were double-blind randomized to receive lesinurad 200 mg, lesinurad 400 mg, or placebo in a 1:1:1 ratio in 138 study sites; 603 patients received at least 1 dose of study medication. Patients received gout flare prophylaxis for the first 5 months starting from day 14 of screening randomization.
Study subjects were mostly male (94.0%) and white (76.3%), with a mean ± SD age of 51.90±11.28 years. Patients had longstanding symptomatic gout, with a mean ± SD time since diagnosis of 11.84±9.37 years. Patients were receiving an allopurinol dosage of 306.6±59.58 mg/day. The mean ± SD baseline serum UA level was 6.94±1.27 mg/dL. Demographic data and disease characteristics were similar between treatment groups at baseline.
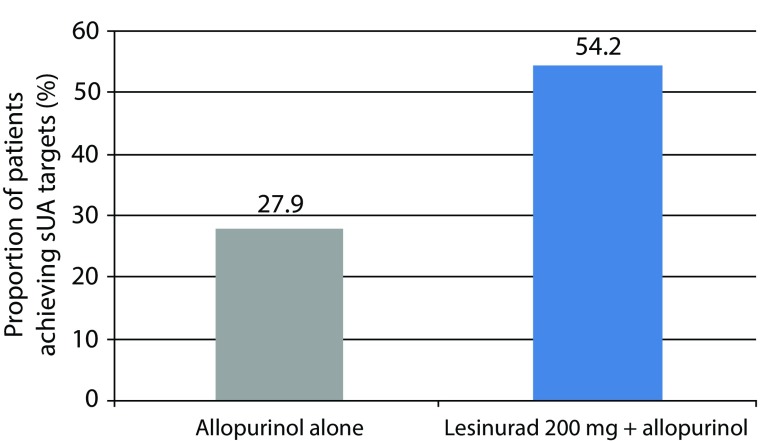
The proportions of patients achieving a serum UA level of <6.0 mg/dL by month 6 (the primary endpoint) were 27.9 and 54.2%, in the groups receiving allopurinol alone and lesinurad 200 mg plus allopurinol, respectively. At the primary endpoint, a significant difference was found for patients taking lesinurad 200 mg plus allopurinol as compared with only allopurinol (p<0.0001; Figure 2).
Figure 2.
CLEAR 1 Study. Proportion of patients achieving a serum UA level of <6.0 mg/dL by month 6 (primary endpoint). Difference between lesinurad 200 mg plus allopurinol group (n=140) versus allopurinol plus placebo group (n=149) was significant (p<0.0001).21
sUA, serum uric acid.
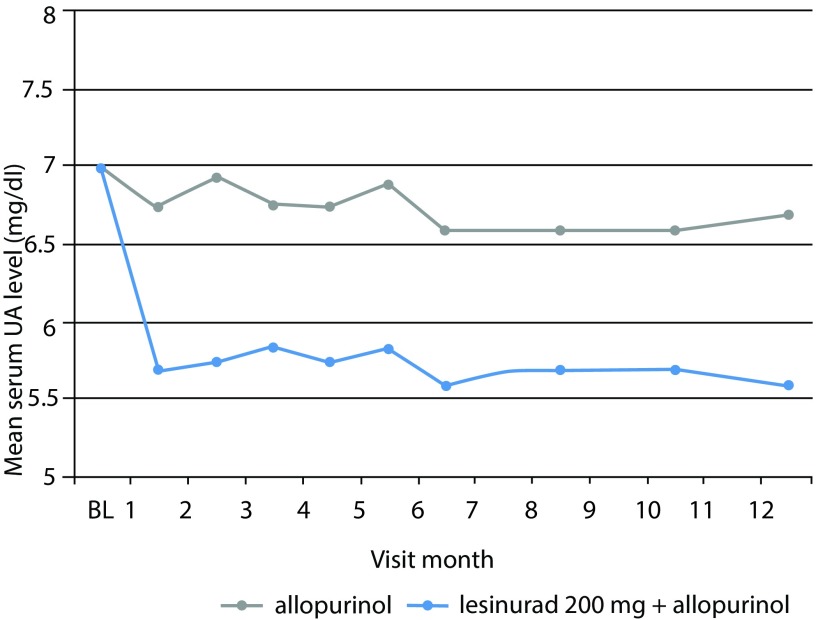
From month 1 to month 12, all monthly evaluations showed that the proportion of patients achieving a serum UA target level of <6.0 mg/dL was superior in both the lesinurad 200 mg plus allopurinol and the lesinurad 400 mg plus allopurinol groups than in the allopurinol-alone group (p<0.0001 for each comparison) (Figure 3).
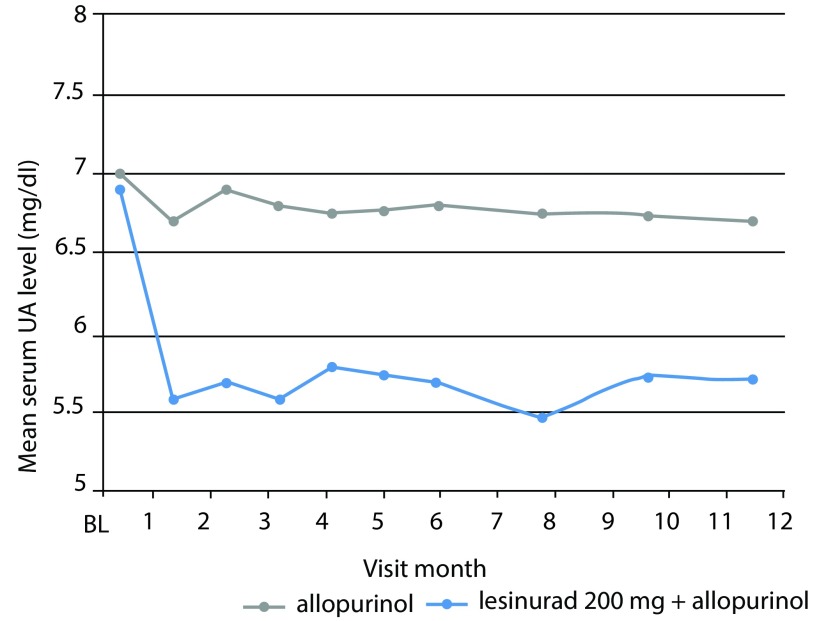
Figure 3.
CLEAR 1 study. Serum urate (UA) levels at each study visit throughout 12 months. The mean change from baseline (BL) for the group receiving lesinurad 200 mg plus allopurinol was significant as compared with allopurinol alone (p<0.0001) for each comparison at each timepoint tested. Adapted from Saag et al.21
UA, uric acid.
Subgroup analyses based on age, gender, race, baseline serum UA levels, comorbid conditions, renal function, and thiazide diuretic use provided results consistent with those from the primary analysis. The analysis of the key secondary endpoints, including rates of gout flare requiring treatment and complete resolution of tophi, did not demonstrate statistically significant treatment differences between groups along the duration of the 12-month trial. Related to key secondary endpoints, therapy may be needed for more than 12 months for the full effects to be appreciated.
CLEAR 2 study
CLEAR 2 was a duplicate study to CLEAR 1 conducted in North America, Europe, New Zealand, Australia, and South Africa. In CLEAR 2, of 2199 patients evaluated at the screening visit, 611 were double-blind randomized to lesinurad 200 mg, lesinurad 400 mg, or placebo in a 1:1:1 ratio in 152 study sites; 610 patients received at least 1 dose of study medication. Patients were predominantly male (96.2%) and white (79.0%), with mean (±SD) age 51.2±10.90 years, gout duration 11.5±9.26 years, and baseline serum UA of 6.9±1.2 mg/dL. At baseline, demographics data and disease characteristics were similar between treatment groups.
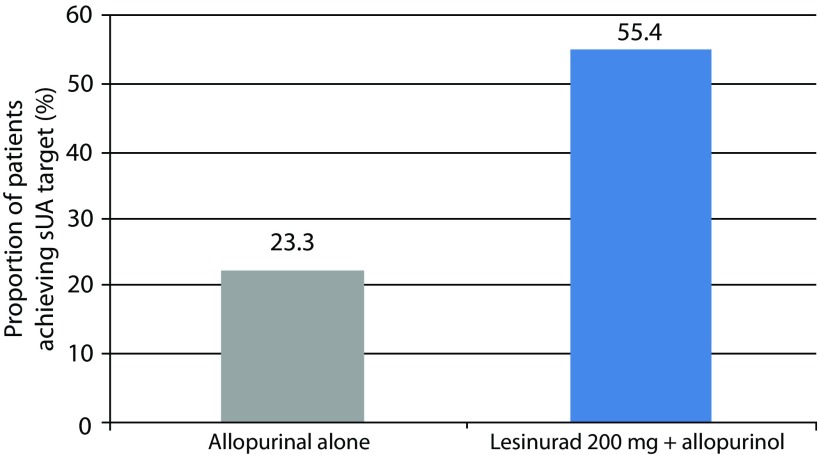
The proportions of patients achieving a serum UA level of <6.0 mg/dL by month 6 (the primary endpoint) were 23.3 and 55.4% in the allopurinol-alone and lesinurad 200 mg plus allopurinol group, respectively. A significant difference was found for patients taking lesinurad 200 mg plus allopurinol as compared with allopurinol alone by month 6 (primary endpoint; p<0.0001; Figure 4). The difference at the primary endpoint between lesinurad 400 mg plus allopurinol (66.5%) and allopurinol with placebo groups was also significant (p<0.0001).
Figure 4.
CLEAR 2 study. Proportion of patients achieving a serum UA level of <6.0 mg/dL by month 6 (primary endpoint). Difference between lesinurad 200 mg plus allopurinol group (n=162) versus allopurinol plus placebo group (n=154) was significant (p<0.0001).23
sUA, serum uric acid.
From month 1 to month 12, proportions of patients achieving the serum UA target were superior in the lesinurad groups compared to allopurinol-alone group at all monthly assessments (p<0.0001, for all comparisons). Mean serum UA levels were lower in both lesinurad plus allopurinol groups versus allopurinol-alone group at all timepoints through 12 months of treatment (p<0.001, for all comparisons) (Figure 5).
Figure 5.
CLEAR 2 study. Serum urate (UA) levels at each study visit for up to 12 months. Mean change from baseline (BL) for the group receiving lesinurad 200 mg plus allopurinol was significant as compared with allopurinol alone (p<0.0001) at each timepoint. Adapted from Bardin et al.23
UA, uric acid.
Regarding secondary endpoints, no statistically significant differences were found in the rates of gout flare requiring treatment or complete resolution of tophi, which occurred at low incidences at baseline and during study. With regard to key secondary endpoints, therapy may be needed for more than 12 months to observe full effects.
Long-term extension study
Saag and colleagues26 assessed the long-term safety and efficacy of lesinurad 200 mg plus allopurinol therapy in subjects enrolled in an extension from core studies CLEAR 1 and CLEAR 2.21,23
Efficacy was evaluated in patients completing the core study and either continuing core lesinurad 200 mg plus allopurinol treatment (200 CONT group) or crossing over from core allopurinol alone to lesinurad 200 mg plus allopurinol (200 CROSS group). In the open extension study, 200 CONT group (n=239) receiving treatment for up to 24 months and 200 CROSS group (n=121) receiving treatment for up to 12 months were assessed. Efficacy endpoints included the proportion of subjects with a target serum UA of <6.0 mg/dL and mean serum UA levels. The proportion of patients with serum UA <6.0 mg/dL during core and extension can be seen in Figure 6. Patients treated with lesinurad 200 mg plus allopurinol throughout 2 years continued to be at serum UA target. Patients crossing from allopurinol monotherapy demonstrated greater numbers that reached target. The safety profile during the extension study26 was consistent with that observed in the core studies.21,23
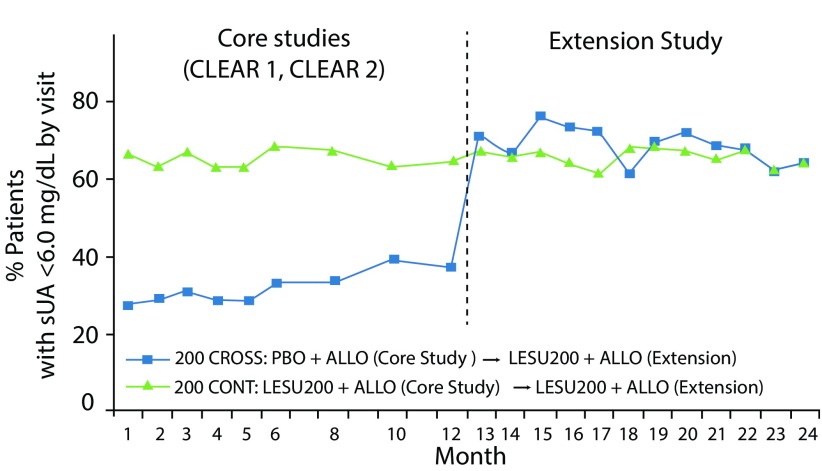
Figure 6.
Proportion of subjects with serum UA <6.0 mg/dL during core and extension studies. Patients continued core lesinurad 200 mg plus allopurinol treatment (200 CONT) or crossed over from core allopurinol alone to lesinurad 200 mg plus allopurinol (200 CROSS). Adapted from Saag et al.26
ALLO, allopurinol; LESU, lesinurad; PBO, placebo; sUA, serum uric acid.
CRYSTAL study
CRYSTAL was a 12-month, phase III, multicenter, randomized, double-blind, placebo-controlled trial conducted to assess the efficacy and safety of lesinurad in combination with febuxostat in patients with tophaceous gout. Previously, a phase Ib clinical trial of lesinurad plus febuxostat showed greater reduction in serum UA levels than febuxostat alone.24
The CRYSTAL study included adult tophaceous patients previously treated or not with a urate-lowering agent. Serum UA levels had to be ≥8.0 mg/dL in subjects not taking urate-lowering agents and ≥6.0 mg/dL in those already receiving urate-lowering therapy. The presence of at least one measurable tophus from 5 to 20 mm in maximal diameter either in the hands or in the feet was required for study entry.
Subjects were randomized 1:1:1 to take placebo plus febuxostat 80 mg, lesinurad 200 mg plus febuxostat 80 mg, or lesinurad 400 mg plus febuxostat 80 mg.
The primary efficacy endpoint was the proportion of subjects in each treatment group with a serum UA level of <5.0 mg/dL by month 6. The key secondary endpoint was the proportion of patients with total resolution of ≥1 target tophus by month 12. Other endpoints included mean serum UA levels recorded at each visit and the percentage change in total target tophi area. Safety evaluations included AEs, vital signs, clinical laboratory data, physical examination, and electrocardiogram readings. Assessments of particular interest included renal and CV events.
Of 1045 patients assessed at the screening in 102 study sites, 330 were randomized. From these, a total of 324 patients received at least 1 dose of randomized study medication. Subjects were mostly male (95.4%) and white (79.9%), with a mean ± SD age of 54.1±11.0 years. Mean time since gout diagnosis was 14.7±10.9 years. At screening, mean±SD serum UA levels were 8.7±1.6 and 5.3±1.6 mg/dL at baseline (randomization), after 3 weeks of treatment with febuxostat 80 mg. Demographic and baseline disease characteristics were similar between treatment groups.24
The proportion of patients who achieved a serum UA level of <5.0 mg/dL by month 6 was 46.8% in the febuxostat-alone group and 56.6% in the lesinurad 200 mg plus febuxostat group. The difference was not significant for the lesinurad 200 mg plus febuxostat group versus febuxostat alone (p=0.13).
In the prespecified analyses, a subgroup of subjects with serum UA levels of ≥5.0 mg/dL after 3 weeks of therapy with febuxostat (n=161) was included. In this subgroup, 23.5% of patients achieved the target by month 6 with febuxostat treatment alone, compared to 44.1% with lesinurad 200 mg plus febuxostat (p=0.024 versus febuxostat alone).
The difference in serum UA levels was not significantly different for the lesinurad 200 mg plus febuxostat group by month 6, but greater treatment effects were observed for this group at all other monthly assessments from month 1 to month 12 (p≤0.0281).
The proportion of patients with complete resolution of one or more target tophus was numerically greater in both the lesinurad 200 mg plus febuxostat (25.5%) and lesinurad 400 mg plus febuxostat (30.3%) groups in comparison with the febuxostat group (21.1%); however, the differences were not statistically significant. At month 12, the reductions in target tophi area in the lesinurad 200 mg plus febuxostat and lesinurad 400 mg plus febuxostat groups were 50.1 and 52.9%, respectively, compared with febuxostat alone (28.3%).
In an open extension of the CRYSTAL study, patients continuing on lesinurad plus febuxostat over 2 years continued to be at serum UA target levels, and attenuation of serum UA effect was not observed (Figure 7). Subjects demonstrated continued increases in complete resolution of tophi and additional tophi area reduction. In patients continuing core treatment (200 CONT), 59.6% showed complete resolution of ≥1 target tophus (Figure 8), with 68.3% reduction in the sum of areas for all target tophi, after 24 months of treatment.24
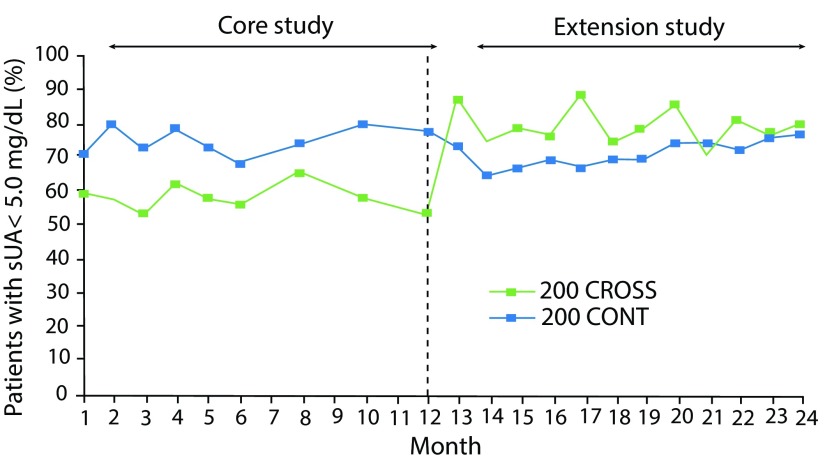
Figure 7.
CRYSTAL extension study. Proportion of subjects with serum UA <5.0 mg/dL during core and extension studies. Patients continued core lesinurad 200 mg plus febuxostat treatment (200 CONT) or crossed over from core febuxostat alone to lesinurad 200 mg plus febuxostat (200 CROSS).27
sUA, serum uric acid.
Figure 8.
Proportion of subjects with complete resolution of ≥1 target tophus in CRYSTAL extension study. Patients continued core lesinurad 200 mg plus febuxostat treatment (200 CONT) or crossed over from core febuxostat alone to lesinurad 200 mg plus febuxostat (200 CROSS).27
Safety of lesinurad 200 mg
Lesinurad 200 mg has shown a good safety profile in these three randomized clinical trials. In CLEAR 1 and CLEAR 2 studies, lesinurad was well tolerated, particularly at the 200 mg dose, and the AE profile was similar to that of allopurinol alone. The most frequent individual AEs in the groups receiving lesinurad 200 mg plus allopurinol and allopurinol alone were upper respiratory tract infection, hypertension, sinusitis, arthralgia, increased blood phosphokinase levels, increased blood creatinine levels, headache, and diarrhea. Most AEs were mild or moderate, and the incidence of serious AEs was comparable across lesinurad 200 mg and placebo.21,23
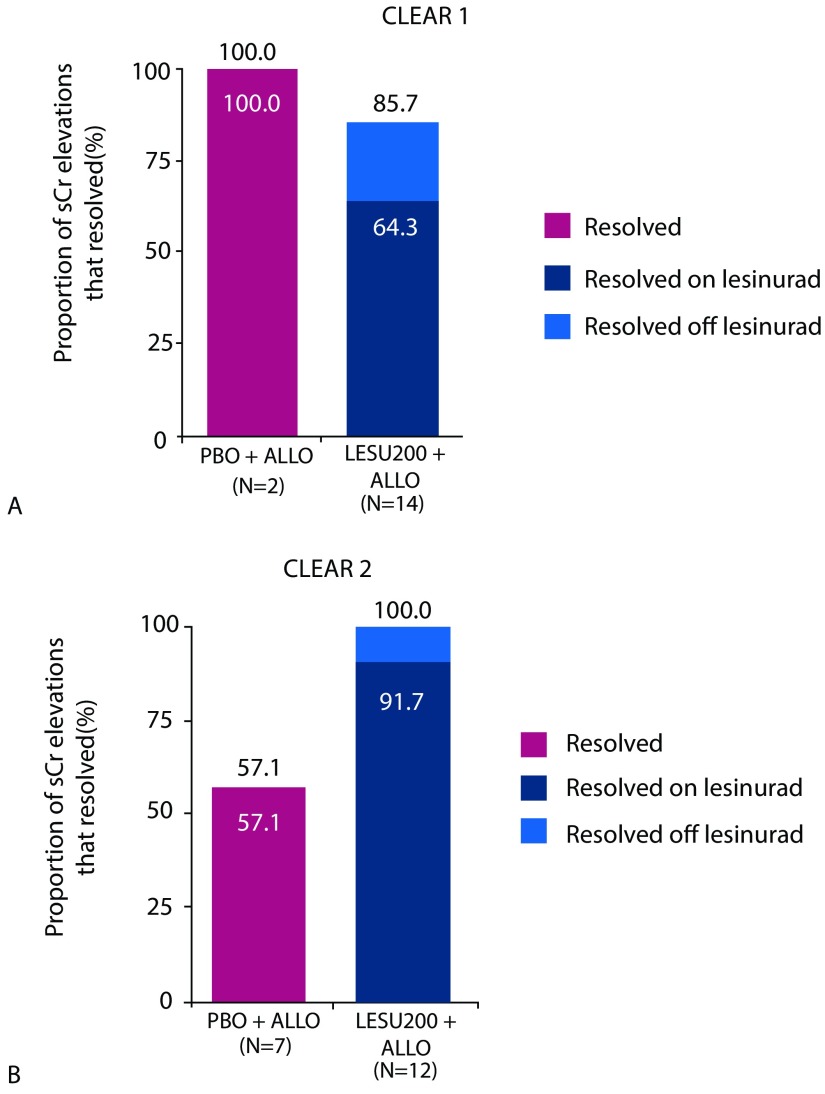
In a renal safety analysis, the incidence of renal-related AEs was comparable in the lesinurad 200 mg plus allopurinol and allopurinol-alone groups, except for higher incidences of predominantly reversible elevations of serum creatinine levels. Figure 9 shows the proportion of serum creatinine elevations that resolved in CLEAR 1 and CLEAR 2;23,26 overall results are shown in Table 2.
Figure 9.
Resolution of serum creatinine elevations. A. Proportion of serum creatinine elevations that resolved in CLEAR 1.24 B. Proportion of serum creatinine elevations that resolved in European study CLEAR 2.21
ALLO, allopurinol; LESU, lesinurad; PBO, placebo; sCr, serum creatinine.
Table 2.
Proportion of patients who achieved target serum uric acid levels (<6 mg/dL) with lesinurad in combination with allopurinol. Pooled data from CLEAR 1 and CLEAR 2. Reproduced from Zurampic et al.17
| Timepoint | Placebo + allopurinol N=407 | Lesinurad 200 mg + allopurinol N=405 | Lesinurad 200 mg versus placebo |
|---|---|---|---|
| Months 4, 5, 6 | 48 (12%) | 155 (38%) | 0.26 (0.21, 0.32) |
| Month 6 | 104 (26%) | 222 (55%) | 0.29 (0.23, 0.36) |
| Month 12 | 105 (26%) | 203 (50%) | 0.24 (0.18, 0.31) |
Lesinurad causes an increase in renal UA excretion. Potentially, this may provoke microcrystallization in the renal tubules and the urinary system and could manifest as kidney stones or acute UA nephropathy. In these studies, a history of kidney stones was reported globally in 9–14% of patients at baseline. However, a history of kidney stones was not an exclusion criterion. Therefore, patients with a history of kidney stones were permitted entry into the three pivotal studies of lesinurad in combination with a XOI.
In CLEAR 1, kidney stone treatment-emergent adverse effects (TEAEs) were reported in 2.0, 1.0, and 2.5% of patients of the allopurinol monotherapy, lesinurad 200 mg plus allopurinol, and lesinurad 400 mg plus allopurinol groups, respectively. In CLEAR 2, kidney stone TEAEs were reported in 0.5, 0 and 3.0% of patients taking allopurinol monotherapy, lesinurad 200 mg plus allopurinol, and lesinurad 400 mg plus allopurinol, respectively. In CRYSTAL, nephrolithiasis was reported in 3.7, 0.9, and 1.8% of the patients in the febuxostat, lesinurad 200 mg plus febuxostat, and lesinurad 400 mg plus febuxostat groups, respectively. Table 3 shows the rate of kidney stone TEAEs in the three pivotal studies.
Table 3.
Proportion of patients who were reported to suffer kidney stone TEAEs in the pivotal studies of lesinurad.
| XOI alone * | Lesinurad 200 + XOI | Lesinurad 400 + XOI | |
|---|---|---|---|
| CLEAR 1 (allopurinol) | 2.0% | 1.0% | 2.5% |
| CLEAR 2 (allopurinol) | 0.5% | 0% | 3.0% |
| CRYSTAL (febuxostat) | 3.7% | 0.9% | 1.8% |
TEAEs, treatment-emergent adverse effects; XOI, xanthine oxidase inhibitor.
Other agents that inhibit URAT1 have been associated with development of nephrolithiasis. The lack of increase in kidney stone numbers during lesinurad therapy in CLEAR 1 and CLEAR 2 studies is potentially related to concomitant use of allopurinol or febuxostat, which reduces serum urate levels, thus reducing the filtered load of UA to the kidney. The timing of lesinurad administration may also have been a factor to the low rate of nephrolithiasis, as once-daily dosing in the morning increases urinary UA at a time when urine volume and urine pH are highest and the potential for UA precipitation is lowest. Few kidney stone TEAEs were also reported during the CRYSTAL study.21–27
CV comorbidities and risk factors were present at baseline in a high proportion of patients, demonstrating the elevated rates of CV disease in patients with gout. The proportions of patients with TEAEs classified as cardiovascular events throughout the studies were low and comparable between groups.21,23,26
In CRYSTAL, lesinurad was well tolerated at the 200 mg dose. The most common AEs in the lesinurad 200 mg plus febuxostat and febuxostat groups were nasopharyngitis, hypertension, serum creatinine elevation, headache, extremity pain, and back pain. The incidence of overall AEs was higher in the lesinurad 200 mg plus febuxostat group in comparison with febuxostat alone, but most events were mild or moderate. The incidence of serious AEs was comparable across the treatment groups. Most serum creatinine elevations were transient and reversible and resolved by the time of the subsequent assessment without discontinuation of treatment.24,27
In these three pivotal studies, there were no substantial differences in vital signs, including blood pressure, between groups. Clinical laboratory test results, including hematology, serum chemistry (except for serum creatinine levels), and urinalysis, demonstrated no significant changes throughout the study of any group.21–27
Therapeutic indications, dosing, and clinical use
Lesinurad, in combination with a XOI, is indicated for the adjunctive treatment of hyperuricemia in gout patients (with or without tophi) who have not attained target serum UA levels with an adequate dose of a XOI monotherapy.17,18
The recommended dose of lesinurad is 200 mg once daily. Lesinurad should be taken in the morning with food and water, and patients should be instructed to stay well hydrated as both factors influence the pharmacokinetics of the drug (up to 2 L of liquid per day if needed). Lesinurad must be coadministered at the same time as the morning dose of a XOI. If treatment with the XOI is interrupted, then lesinurad administration must also be discontinued. No dose adjustment is needed for renal patients, because only a single daily dose of 200 mg is recommended.17,18
As shown for every urate-lowering drug during initiation of therapy, acute gout attacks may also occur after starting therapy with lesinurad. This is considered to be related with the reduction in serum UA levels, resulting in mobilization of urate from tissue deposits. Prophylaxis of gout attacks with colchicine or a nonsteroidal anti-inflammatory drug (NSAID) is recommended during first months (following recommendations) when starting lesinurad therapy. The gout flare should be managed as appropriate for the individual patient and lesinurad does not need to be discontinued because of a gout flare. Continuous therapy with lesinurad diminishes the occurrence of gout attacks.26,27
Discussion
Until now, uricosurics have never gone through extensive clinical trials.12 We have no data on long-term safety for other medications, although there is empirical evidence based only on expertise. Lesinurad is the first uricosuric with a specifically designed clinical development program.
Lesinurad is a selective inhibitor of UA reabsorption approved for add-on treatment of gout in combination with a XOI for patients who did not achieve target serum UA levels using a XOI alone. Lesinurad was assessed in three randomized, double-blind, placebo-controlled, phase III clinical trials in combination with allopurinol (CLEAR 1 and CLEAR 2) or febuxostat (CRYSTAL) versus either XOI alone. The primary endpoint of CLEAR 1 and CLEAR 2 trials was the percentage of patients achieving a serum UA concentration of <6 mg/dL at month 6, while for CRYSTAL study with the focus on tophaceous gout patients, the primary endpoint was the percentage of patients achieving a serum UA concentration of <5 mg/dL at month 6.
Serum UA measurement is typically considered a biomarker and an adequate surrogate endpoint in gout management. It is used in daily clinical practice for following the target strategy recommended by numerous guidelines (<6 mg/dL) (comparable for the measurement of HbA1c in the monitoring of diabetes patients). Clinically relevant outcomes, like flares or tophi, can be difficult to study, as they require trials with larger sample sizes and, as has been shown by other trials, a longer treatment period and patient follow-up. Thus, there are obvious advantages using the evaluation of serum UA as a surrogate endpoint. A long-term sustained reduction in serum UA is regularly associated with a clinically relevant decrease of gout flares and reduction of tophi size and number.1–4,11–14
In the CLEAR 1 and CLEAR 2 studies, the proportion of patients who achieved a serum UA level of <6.0 mg/dL was significantly superior with lesinurad 200 mg plus allopurinol as compared with allopurinol monotherapy (p<0.0001), while in CRYSTAL the proportion of patients who attained a serum UA level of <5.0 mg/dL by month 6 was superior (although not significant) with lesinurad plus febuxostat versus febuxostat alone. In CLEAR 1 and CLEAR 2 studies, the dose of allopurinol was not fixed but individualized and was adjusted to medically appropriate doses before screening. Then, patients were required to be on stable dose of allopurinol for at least 8 weeks before randomization. Doses of allopurinol in these studies ranged from 200 to 900 mg.
Lesinurad was well tolerated in CLEAR 1, CLEAR 2, and CRYSTAL trials. The overall safety profile of the lesinurad 200 mg orally per day dose was similar to that of allopurinol or febuxostat alone, except for higher rates of reversible elevations of serum creatinine levels. Monotherapy is not recommended due to increased risk of adverse renal events.28
These three randomized, double-blind studies have shown that greater proportions of patients achieved serum UA targets at 6 and 12 months with lesinurad 200 mg plus a XOI compared with each XOI alone. Twelve months is an insufficient time frame to observe statistically significant differences in reduction of flares and tophi, which may be considered a limitation of these trials. However, subsequent extension studies evaluated long-term efficacy and safety up to 24 months with combined therapy, including evaluations of serum UA levels, tophi, and flares. Patients who completed 12 months of treatment in the core CLEAR and CRYSTAL studies were allowed to enroll in their respective uncontrolled extension trials.24–27
The CLEAR and CRYSTAL extension studies demonstrated that patients treated with lesinurad 200 mg plus a XOI for up to 24 months consistently maintained serum UA levels below target. Extension studies also demonstrated continued increases in the rate of complete resolution of tophi, reduction in tophi area, and reduction of gout flares rates. The safety profile during long-term therapy in the extension studies was consistent with that observed in the core trials.25–27
In conclusion, lesinurad was superior to XOI monotherapy in reducing the serum UA concentration under 6 or 5 mg/dL, when added to either allopurinol or febuxostat. The combination of lesinurad with a XOI provides a dual mechanism of action, inhibiting both renal UA reabsorption and urate production. Lesinurad represents an emergent treatment option for gout patients that need additional therapy to get UA target after receiving a XOI. Thus, it can be affirmed that lesinurad may be an effective and overall safe alternative for gout treatment when combined with a XOI.
Acknowledgements
Writing and editorial assistance in the preparation of this review article was provided by Content Ed Net (Madrid).
Footnotes
Contributions: All authors contributed equally to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: Dr Perez Ruiz reports personal fees from Menarini, personal fees from Grunenthal, and personal fees from Horizon, outside the submitted work. Dr Tim Jansen reports research support from Ardea/Olatec, lecture fees from Grunenthal, and consultancy fees from Abbvie/Astra-Zeneca/Celgene/Novartis. Mónica Juárez and Ravichandra Karra Gurunath are full-time employees in the Medical Affairs Department from Grünenthal Pharma. Dr Anne-Kathrin Tausche received personal fees from Berlin Chemie-Menarini, AstraZeneca/Ardea Biosciences, and Grünenthal Pharma. Dr Pascal Richette received fees from Ipsen, Menarini, Savient, Grunenthal, and AstraZeneca. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at http://www.drugsincontext.com/wp-content/uploads/2019/04/dic.212581-COI.pdf
Funding declaration: Editorial assistance was provided by Content Ed Net (Madrid) with funding from Grünenthal Pharma. There was no other funding associated with the preparation of this article.
Correct attribution: Copyright © 2019 Pérez-Ruiz F, Jansen T, Tausche A-K, Juárez-Campo M, Karra Gurunath R, Richette P. https://doi.org/10.7573/dic.212581. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/efficacy-and-safety-of-lesinurad-for-the-treatment-of-hyperuricemia-in-gout
Provenance: submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 3 March 2019
References
- 1.Ruoff G, Edwards NL. Overview of serum uric acid treatment targets in gout: why less than 6 mg/dL? Postgrad Med. 2016;128(7):706–715. doi: 10.1080/00325481.2016.1221732. [DOI] [PubMed] [Google Scholar]
- 2.Punzi L. Change gout: the need for a new approach. Minerva Med. 2017;108(4):341–349. doi: 10.23736/S0026-4806.17.05188-6. [DOI] [PubMed] [Google Scholar]
- 3.Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken) 2012;64(10):1447–1461. doi: 10.1002/acr.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham AQ, Doan A, Andersen M. Pyrazinamide-induced hyperuricemia. P T. 2014;39(10):695–715. [PMC free article] [PubMed] [Google Scholar]
- 5.Jones G, Panova E, Day R. Guideline development for the management of gout: role of combination therapy with a focus on lesinurad. Drug Des Devel Ther. 2017;11:3077–3081. doi: 10.2147/DDDT.S97959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Ruiz F, Calabozo M, Pijoan JI, Herrero-Beites AM, Ruibal A. Effect of urate-lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Rheum. 2002;47(4):356–360. doi: 10.1002/art.10511. [DOI] [PubMed] [Google Scholar]
- 7.Clarson LE, Chandratre P, Hider SL, et al. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22(3):335–343. doi: 10.1177/2047487313514895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–662. doi: 10.1038/nrrheum.2015.91. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Ruiz F, Martínez-Indart L, Carmona L, Herrero-Beites AM, Pijoan JI, Krishnan E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis. 2014;73(1):177–182. doi: 10.1136/annrheumdis-2012-202421. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Ruiz F, Castillo E, Chinchilla SP, Herrero-Beites AM. Clinical manifestations and diagnosis of gout. Rheum Dis Clin North Am. 2014;40(2):193–206. doi: 10.1016/j.rdc.2014.01.003. https://www.ncbi.nlm.nih.gov/pubmed/24703343. [DOI] [PubMed] [Google Scholar]
- 11.Perez Ruiz F, Sanchez-Piedra CA, Sanchez-Costa JT, et al. Improvement in diagnosis and treat-to-target management of hyperuricemia in gout: results from the GEMA-2 transversal study on practice. Rheumatol Ther. 2017 doi: 10.1007/s40744-017-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Ruiz F, Kandaswamy P, Karra Gurunath R, Burton H, Jansen T. A summary of clinical evidence for commonly used uricosurics for gout in Europe [abstract no. SAT0360] Ann Rheum Dis. 2018;77(Suppl 2):1045. doi: 10.1136/annrheumdis-2018-eular.3026. [DOI] [Google Scholar]
- 13.Pérez Ruiz F, Loza E, Yébenes MJG, et al. Sociedad Española de Reumatología (SER) Guía de Práctica Clínica para el Manejo de la Gota. [Accessed April 25, 2019]. https://www.ser.es/guia-de-manejo-de-la-gota/
- 14.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 15.Becker MA, Fitz-Patrick D, Choi HK, et al. An open-label, 6-month study of allopurinol safety in gout: the LASSO study. Semin Arthritis Rheum. 2015;45(2):174–183. doi: 10.1016/j.semarthrit.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Becker MA, Schumacher HR, Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 17.Zurampic, INN-lesinurad. EMASummary of Product Characteristics. [Accessed March 4, 2018]. https://ec.europa.eu/health/documents/community-register/2016/20160218134007/anx_134007_en.pdf.
- 18.Zurampic (lesinurad) tablets. FDA Full Prescribing Information. [Accessed March 4, 2018]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207988lbl.pdf.
- 19.Miner J, Tan PK, Hyndman D, et al. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res Ther. 2016;18(1):214. doi: 10.1186/s13075-016-1107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Z, Rowlings C, Kerr B, et al. Pharmacokinetics, pharmacodynamics, and safety of lesinurad, a selective uric acid reabsorption inhibitor, in healthy adult males. Drug Des Devel Ther. 2015;9:3423–3434. doi: 10.2147/DDDT.S85193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saag KG, Fitz-Patrick D, Kopicko J, et al. Lesinurad combined with allopurinol: a randomized, double-blind, placebo-controlled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study) Arthritis Rheumatol. 2017;69(1):203–212. doi: 10.1002/art.39840. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Ruiz F, Sundy JS, Miner JN, Cravets M, Storgard C RDEA594-203 Study Group. Lesinurad in combination with allopurinol: results of a phase 2, randomised, double-blind study in patients with gout with an inadequate response to allopurinol. Ann Rheum Dis. 2016;75(6):1074–1080. doi: 10.1136/annrheumdis-2015-207919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bardin T, Keenan RT, Khanna PP, et al. Lesinuradin combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinationalCLEAR2 study) Ann Rheum Dis. 2017;76(5):811–820. doi: 10.1136/annrheumdis-2016-209213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalbeth N, Jones G, Terkeltaub R, et al. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with febuxostat in patients with tophaceous gout: findings of a phase III clinical trial. Arthritis Rheumatol. 2017;69(9):1903–1913. doi: 10.1002/art.40159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardin T, Dalbeth N, Terkeltaub R, et al. Clinical response of tophus and flares to extended use of lesinurad in combination with a xanthine oxidase inhibitor in patients with gout [abstract] [Accessed March 2, 2018];Arthritis Rheumatol. 2016 68(suppl 10) http://acrabstracts.org/abstract/clinical-response-of-tophus-and-flares-to-extended-use-of-lesinurad-in-combination-with-a-xanthine-oxidase-inhibitor-in-patients-with-gout/ [Google Scholar]
- 26.Saag K, Becker MA, Storgard C, Fung M, Hu J, Bardin T. Examination of Serum Uric Acid (sUA) lowering and safety with extended lesinurad + allopurinol treatment in subjects with gout [abstract] [Accessed June 22, 2018];Arthritis Rheumatol. 2016 68(suppl 10) http://acrabstracts.org/abstract/examination-of-serum-uric-acid-sua-lowering-and-safety-with-extended-lesinurad-allopurinol-treatment-in-subjects-with-gout/ [Google Scholar]
- 27.Dalbeth N, Jones G, Terkeltaub R, et al. Efficacy and safety in patients with tophaceous gout receiving lesinurad and febuxostat combination therapy: interim analysis of an extension study [abstract] [Accessed June 22, 2018];Arthritis Rheumatol. 2015 67(suppl 10) https://acrabstracts.org/abstract/efficacy-and-safety-in-patients-with-tophaceous-gout-receiving-lesinurad-and-febuxostat-combination-therapy-interim-analysis-of-an-extension-study/ [Google Scholar]
- 28.Tausche AK, Alten R, Dalbeth N, et al. Lesinurad monotherapy in gout patients intolerant to a xanthine oxidase inhibitor: a 6 month phase 3 clinical trial and extension study. Rheumatology. 2017;56(12):2170–2178. doi: 10.1093/rheumatology/kex350. [DOI] [PubMed] [Google Scholar]