Abstract
Objective:
To measure components of the circulating transforming growth factor β (TGFβ) system in patients with Turner syndrome (TS) compared to relevant controls and to ascertain correlation with endocrine and cardiovascular parameters.
Methods:
TGFβl, TGFβ2 and endoglin (a vascular TGF receptor component) were measured using enzyme-linked immunoassays in the sera of 40 subjects with TS and 40 healthy volunteer women. The cardiovascular phenotype in TS subjects was extensively characterized by cardiac magnetic resonance (MR) and echo.
Results:
TGFβl levels were about 3-fold higher in TS while TGFβ2 levels were about 3. 5-fold higher in controls (P < 0. 000 1 for both). Soluble endoglin levels were 25% higher in TS (P < 0.000 1). Variation in TGFβ system components was not explained by age, blood pressure, platelet count, thyroid function, body proportions or cardiovascular phenotype.
Conclusion:
There is profound perturbation of the TGFβ system evident in the circulation of individuals with TS.
Keywords: Turner syndrome, Transforming growth factor beta, Cardiovascular diseases, X chromosome, Aorta
Abstract
目的:
观察转化生长因子 β (transforming growth factor-beta,TGFβ )在特纳综合征(Turner syndrome,TS )患 者血清中的变化及其与内分泌和心血管参数的关系。
方法:
选择TS患者和健康志愿者妇女(对照组)各40例,采 用酶联免疫方法测定血清中的TGFβl、TGFß2及转化生长因子受体(endoglin)。所有TS患者均行心血管系统磁共 振和超声检查。
结梁:
TS患者血清中TGFβl和endoglin水平均高于对照组(Ρ< 0.000 1),而TGFß2的水平则低于 对照组(Ρ<0.000 1)。TGFβ的改变与年龄、血压、血小板、甲状腺功能、体重指数或心血管疾病的分型无关。结
讼:
TS患者血清中TGFβ显著性改变对疾病的研究具有重要意义。
Keywords: 特纳综合征, 转化生长因子β, 心血管疾病, X染色体, 主动脉
Loss of a sex chromosome leading to monosomy X, or Turner syndrome (TS), occurs in ~ 1/200 conceptions. Most of these do not survive due mainly to circulatory failure. TS occurs in about 1/2000 live female births[1], with about 10% of these newborns manifesting severe congenital cardiovascular disease, commonly including left heart or aortic arch hypoplasia or coarctation and aortic valve disease. Aortic dilation and dissection are major causes of medical concern and contribute to premature morbidity and mortality in this syndrome[2–6].
The genetic cause(s) of the congenital heart disease in TS are unclear. It has been hypothesized that haploin-sufficiency for one or more of about two dozen pseudoautosomal genes located at the Xpter contribute to the major circulatory defect affecting most 45× gestations[7].
Recent advances have implicated transforming growth factor β (TGFβ) signaling the pathogenesis of the aortopathy of Marfan syndrome and other genetic isorders[8–9]. Activation of TGFβ-induced SMAD proteins is reported in aortic aneurysms, regardless of etiology[10]. In addition a specific vascular TGFβ coreceptor, endoglin, is implicated in congenital defects in vascular development in hereditary hemorrhagic telangiectasia and in murine models[11–12].TGFβ1 & 2 and a soluble form of endoglin are all produced by vascular cells and are relatively abundant in the circulation. TGFβ is also produced by the immune system and its dysregulation implicated in the pathogenesis of autoimmunity[13]. Thus, in this study we aimed to investigate circulating levels of the e three factors in patients with TS and female controls, and evaluate their abundance with respect to the Turner phenotype, including presence of congenital heart disease, aortic diameter and autoimmune thyroid disease.
1. Patients and Methods
1. 1. Study subjects
Data were collected from consecutive participants in the “Turner Syndrome; Genotype and Phenotype” study (CH-00–0219, NCT 00006334) conducted at the Clinical Center of the National Institutes of Health (NIH). Study protocols were approved by the National Institute of Child Health and Human Development (NICHD) institutional review board, and a subjects gave written informed consent. Two groups were included in this study; women with Turner syndrome (TS, 45×, n = 40) and Control group which including women with spontaneous primary ovarian insufficiency (POI, n = 20), and normal volunteer women (NV, n = 20). The first group had a high resolution, G-banding 50-white blood cell (WBC) karyotype done. To qualify for the Turner protocol, more than 70% of cells had to be missing all or major parts of one sex chromo-some. In this group of 40 TS participants, 37 had 100% 45× cells; 1 had 90% 45× with 2nd cell line with small ring X,1 had 82% 45× with 2nd cell line having an isoYq chromosome and the last had 60% 45× with 2nd cell line containing a small ring Y chromosome. Thus all TS participants had 100% of peripheral WBC showing abnormal sex chromosome constitution, with no evidence of mosaicism for a normal cell line. To qualify for the primary ovarian insufficiency study, all 50 cells had to be 46, XX. Women with primary ovarian insufficiency have low estrogen levels, similar to TS women, and serve as a good control group to assess the effects of sex hormone deficiency. Per protocol, both TS and POI groups discontinued routine hormone replacement treatment 2 weeks prior to study. All participants had routine blood chemistries, thyroid function tests and complete blood counts. Forty women with TS participants included those evaluated between 2006–2009 for whom sufficient sera had been collected and stored per protocol.
1. 2. Enzyme linked immunosorbent assays (ELISA)
TGFβ1, TGFβ2 and endoglin concentrations were measured with Quantikine® Immunoassay kits manufactured by R&D systems (catalog numbers DB100B, DB250, DNDG00, respectively) according to the manufacturer protocol. Sample were activated before TGFβ assay by acidification using 1 mol/L HCl. All measurements were performed in duplicate. The intra-assay coefficients of variation in our assays were 5. 6 %, 6.1 % and 5. 2% for TGFβ1, TGFβ2 and endoglin respectively. The detection limits for TGFβ1, TGFβ2, and endoglin were 4.6 ng/L, less than 7 ng/L, and 6 ng/L respectively.
1. 3. Cardiovascular examination
Information on the presence of cardiovascular abnormalities was obtained by medical history (e. g., coarctation or valve repair) and prospective imaging using echocardiography and magnetic resonance imaging MRI) as described[14].
1. 4. Statistical analysis
Sample size was determined by considering major potential variants to be estrogen exposure (for differences between controls and TS) and presence or absence of congenital bicuspid aortic valve (within the TS cohort). Based on available data for the R&D TGFβ1 ELISA, we estimated that a sample size of 20 had a 90% probability of detecting a 10 μg/L difference between groups with P < 0.05. Data for continuous variables are shown as x ± s and are compared by ANOVA or * ANCOVA adjusted for age (and platelet count for TGFβ1) followed by Fisher’s t-test. All measured parameters [age, body mass index (BMI), Wood pressure (BP), peptide levels] were very similar in the POI and NV groups, so their data was pooled as “Controls”. Multiple regression analyses were used to determine effects of age, blood pressure, platelet count, aortic diameter and body mass index on peptide levels. Statistical significance was a P-value <0.05. Analyses were performed using Stat View for Windows, version 5.0.1 (SAS Institute Inc., Cary, NC).
2. Results
Study participants were in good general health. Analysis of blood pressure and ELISA data showed no differences between groups with karyotypically normal primary ovarian insufficiency and normal female volunteers, so data for these two groups were combined. Baseline features of the TS and control groups are summarized in Table 1. The women with TS were a few years younger, on average. Also, diastolic BP mean was slightly but significantly higher in the TS group. These factors were thus used as co-variants in comparing assay results.
Table 1.
Study subjects
| TS (n = 40) | Control (n = 40) | P | |
|---|---|---|---|
| Age (years) | 28.8 ± 8.0 | 34.2 ± 8.0 | 0.03 |
| BMI (kg/m2) | 27.2 ± 9.0 | 25.8 ± 6.0 | 0.40 |
| SBP (mmHg) | 113.0 ± 10.0 | 114.0 ± 11.0 | 0.70 |
| DBP (mmHg) | 72.2 ± 8.0 | 68.3 ± 8.0 | 0.03 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure. 1 mmHg = 0.133 kPa.
TGFβ1 and TGFβ2 levels are shown in Figure 1. TGFβ1 levels were significantly higher in the TS group compare to control group [(31.60 ± 16.67) μg/L vs. (11.94 ± 8.13) μg/L, P < 0.000 1], while TGFβ2 were significantly lower in TS group vs. control group [(421.55 ± 556.41) ng/L vs. (1 606.88 ± 450.59) ng/L, P < 0.000 1]. Control group is composed of women with POI (n = 20) and NV (n = 20). No significant differences of TGFβ1 and TGFβ2 levels between these two groups were found (data not shown).
Figure 1.
TGFβ levels in women with Turner syndrome (TS) and control women (CON)
The whiskers show the 10 th and 90 th centiles, the boxes the 25 th −75 th centiles and the dark horizontal bars within the boxes indicate the median.
Average molar ratio of TGFβl to TGFβ2 in controls was 7.64 ± 5.57 vs. 320.76 ± 381.47 in TS (P < 0.000 1). Because the ratios were not normally distributed, we also compared logarithmically transformed ratios [1.73 ± 0.88 (control) vs. 4.76 ± 1.79 (TS),P = < 0.000 1] illustrated in Figure 2.
Figure 2.
Relative excess of TGFβ1 vs. TGFβ2 in both groups
The graphs show the natural log of the TG Fβ1/TG Fβ2 (calculated in ng/L for both peptides) ratio for control (CON, 1.73 ± 0.88) and Turner syndrome (TS, 4.76 ± 1.79). P < 0.000 1.
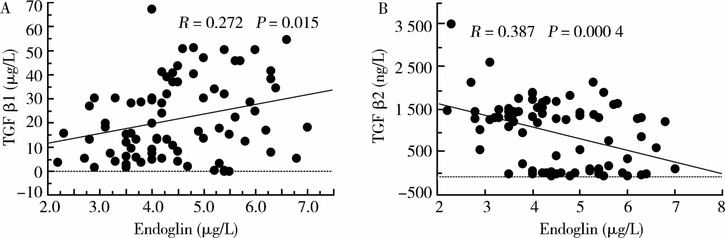
Endoglin levels (Figure 3) were significantly higher (P < 0.000 1) in the TS group compared to control group [(5.0 ± 1.5) μg/L vs. (3.0 ± 1.6) μg/L]. Correlation between TGFβs and endoglin are shown in Figure 4.
Figure 3.
Endoglin levels in women with Turner syndrome (TS) and control women (CON)
Interquartile ranges and median are shown in the boxes, n = 40 in both group.
Figure 4.
Correlations between TGFβl (A)/TGFβ2 (B) and endoglin
No significant difference of endoglin levels between women with POI (n = 20) and normal volunteer (n = 20) were found in the study (data not shown).
Multiple regression analyses were used to evaluate potential effects of age, systolic and diastolic blood pressure and BMI on peptide levels. The only significant effect identified by this approach was a positive correlation between diastolic BP and TGFβ1 levels for the combined control and TS groups (P = 0.008).
Platelets are a source of TGFβ 1 in the circulation, but platelet levels were similar in TS and control groups (P = 0.9). Moreover, comparison of TGFβ1, TGFβ2 and endoglin levels in the two groups using platelet level as a covariate yielded the same statistical results as shown in Figure 4.
Autoimmune thyroid disease is common in TS and in fact 21 of the TS group had Hashimoto’s thyroiditis, but there was no difference in TGF or endoglin levels in those with compared to those without thyroid autoimmunity within the TS group or vs. controls (data not shown). Nineteen of the 40 TS participants had a bicuspid aortic valve determined in a previous study[14] with variable degrees of dilation of the ascending aorta. However neither TGFβ1 or 2 nor endoglin levels were associated with the presencc of an abnormal valve or correlated with aortic diameters (Data not shown).
3. Discussion
TGFβl and TGFβ2 are closely related polypep-tides with pleiotropic effects in embryonic development, tissue homeostasis and disease processes. Both factors are involved in vascular development judging by abnormalities in targeted deletion mice[15]. TGFβ2 in particular seems essential for normal aortic arch formation, which is interesting in view of the aortic arch defects com monly seen in 45, X fetuses[16–17].
Specificity of TGFβ effects seems to be determined by local tissue availability and interaction with specific receptor or binding proteins. For example, while both ligands bind the Type I and Type II TGFβ receptors, only TGFβl binds the Type III receptor endoglin[18]. Interestingly, the present study found that TGFβ1 and endoglin were increased in the parallel in the TS group. Endoglin is a membrane glycoprotein that is strongly expressed in vascular endothelial cells during angiogenesis and injury. Soluble endoglin in the circulation is thought to derive from proteolytic release of vascular receptors, and may antagonize TGFβ1[s interaction with membrane receptors[19]. Haploinsufficiency for endoglin causes hereditary he-morrhagic telangiectasia (HHT), a disorder characterized by arteriovenous malformations and vascular fragi-lity in addition to telangiectasias[12]. Endoglin null mice die during midgestation due to cardiovascular defects[20], while heterozygotes have an HHT-like phenotype.
Dysregulation of the TGFβ system has b e n implicated in aortic aneurysm formation in a diverse collection of genetic disorders, including congenital bicuspid aortic valve, Marfan, Loeys-Dietz, Ehlers-Danlos and arterial tortuosity syndromes, and familial thoracic aortic aneurysm, as reviewed in reference [9]. Circulating TGFβ1 levels are increased in patients with Marfan syndrome[21] and decrease in response to medical treatment with losartan or beta-blocker[21] and also angiotensin inhibitor[22]. It appears that beneficial effects of angiotensin blockade on the Marfan aortopathy may be due to inhibition of TGFβ production or action [8,23]. These prior studies have not investigated potential involvement of other components of the TGFβ system such as TGFβ2 or endoglin.
We have found a marked perturbation of TGFβ system components in the circulation of women with TS compared to controls, including a 3-fold increase in TGFβ1 and > 3 -fold decrease in TGFβ2 levels, resulting in a profound alteration in TG Fβ1/TGFβ2 ratio. There was also a significant increase in the TGFβ1-specific receptor endoglin in the TS group. These alterations can % t be attributed to absent estrogen effect in TS, since our POI group had TGF and endoglin levels equal to the normal cycling controls. These differences in TGFβ system components were not related to platelet count or autoimmune thyroid disease in our study groups. However, perturbation of TGFβ system components was not selective for Turner patients with obvious aortic abnormality, in this case, bicuspid aortic valve. Comparison of the group with bicuspid aortic valves to the Turner group with apparently normal thoracic cardiovascular system did not reveal differences in any of the three TGFβ system components assayed. It is thus possible that these alterations are not related to the congenital defects in cardiovascular development in TS. Alternatively, it is possible that all of the Turner participants have a vasculopathy affecting vessels in general apart from valvular defects. There is some evidence for this, in that arterial diameter is generally increased in Turner patients compared to controls[24]. Additional support for this view is the recent observation that 1st degree relatives of (non-Turner) bicuspid aortic valve patients demonstrate dilation of the ascending aorta despite having normal aortic valve structure [25], suggesting a genetic aortopathy that includes frequent aortic valve abnormality but is not limited to that feature.
Further studies are essential to better understand the significance of our novel findings on TGFβ system dysregulation and their potential relevance to risk for vascular complications such as stroke or aortic dissection in TS. This will require longitudinal studies to determine if alterations in one or more of these components at baseline or over time predict vascular or valvular complications. In addition, we will investigate alterations in gene expression profiles in the TS group vs. controls and interrogate in regard to TGFβ system regulation. Finally, in vitro studies on TGFβ system expression and actions in derivatised vascular endothelial and smooth muscle cell may elucidate more fundamental aspects of TGFβ system dysregulation in TS.
Fund project:
Supported by National Institute of Child Health and Human Development
References
- [1].Stochholm K, Juul S, Juel K, et al. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome [J]. J Clin Endocrinol Metab, 2006, 91 (10) : 3897–3902. [DOI] [PubMed] [Google Scholar]
- [2].Lin AE, Lippe BM, Geffner ME, et al. Aortic dilation, dissection, and rupture in patients with Turner syndrome[J]. J Pediatr, 1986, 109 (5) : 820–826. [DOI] [PubMed] [Google Scholar]
- [3].Gravholt C, Landin-Wilhelmsen K, Stochholm K, et al. Clinical and epidemiological description of aortic dissection in Turner’s syndrome[J]. Cardiol Young, 2006, 16 (5) : 430–436. [DOI] [PubMed] [Google Scholar]
- [4].Gravholt CH, Juul S, Naeraa RW, et al. Morbidity in Turner syndrome [J]. J Clin Epidemiol, 1998, 51 (2) : 147–158. [DOI] [PubMed] [Google Scholar]
- [5].Matura L, Ho V, Rosing D, et al. Aortic dilatation and dissection in Turner syndrome [J]. Circulation, 2007, 116 (15) : 1663–1670. [DOI] [PubMed] [Google Scholar]
- [6].Carlson M, Silberbach M. Dissection of the aorta in Turner syndrome : Two new cases and review of 85 cases in the literature [J]. J Med Genet, 2007, 44 (12) : 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bondy CA. Aortic dissection in Turner syndrome [J]. Curr Opin Cardiol, 2008, 23 (6)& 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome [J]. Science, 2006, 312 (5770) : 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jones J, Spinale F, Ikonomidis J. Transforming growth factor-beta signaling in thoracic aortic aneurysm development & a paradox in pathogenesis [J]. J Vasc Res, 2009, 46 (2) : 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gomez D, Zen AAH, Borges LF, et al. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway [J]. J Pathol, 2009, 218 (1) : 131–142. [DOI] [PubMed] [Google Scholar]
- [11].Gittenberger-de Groot AC, Azhar M, Molin DG. Transforming growth factor beta-SMAD2 signaling and aortic arch development [J]. Trends Cardiovasc Med, 2006, 16 (1) : 1–6. [DOI] [PubMed] [Google Scholar]
- [12].Lebrin F, Mummery CL. Endoglin-mediated vascular remodeling & Mechanisms underlying hereditary hemorrhagic telangiectasia [J]. Trends Cardiovasc Med, 2008, 18 (1) : 25–32. [DOI] [PubMed] [Google Scholar]
- [13].Rubtsov YP, Rudensky AY. TGF beta signaling in control of T-cell-mediated self-reactivity [J]. Nat Rev Immunol, 2007, 7 (6) : 443–453. [DOI] [PubMed] [Google Scholar]
- [14].Sachdev V, Matura L, Sidenko S, et al. Aortic valve disease in Turner syndrome [J]. J Am Coll Cardiol, 2008, 51 (19) : 1904–1909. [DOI] [PubMed] [Google Scholar]
- [15].Pardali E, Goumans MJ, ten Dijke P. Signaling by member of the TGF-beta family in vascular morphogenesis and disease [J]. Trends Cell Biol, 2010, 20 (9) : 556–567. [DOI] [PubMed] [Google Scholar]
- [16].Lacro RV, Jones KL, Benischke K. Coarctation of the aorta in Turner syndrome : a pathologic study of fetuses with nuchal cystic hygromas, hydrops fetalis, and female genitalia [J]. Pediatrics, 1988, 81 (3) : 445–451. [PubMed] [Google Scholar]
- [17].Surerus E, Huggon IC, Allan LD. Turner[s syndrome in fetal life [J]. Ultrasound Obstet Gynecol, 2003, 22 (3) : 264–267. [DOI] [PubMed] [Google Scholar]
- [18].Cheifetz S, Bellon T, Calés C, et al. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells [J]. J Biol Chem, 1992, 267 (27) : 19027–19030. [PubMed] [Google Scholar]
- [19].Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia [J]. Nat Med, 2006, 12 (6) : 642–649. [DOI] [PubMed] [Google Scholar]
- [20].Nomura-Kitabayashi A, Anderson GA, Sleep G, et al. Endoglin is dispensable for angiogenesis, but required for endocardial cushion formation in the midgestation mouse embryo [J]. Dev Biol, 2009, 335 (1) : 66–77. [DOI] [PubMed] [Google Scholar]
- [21].Matt P, Schoenhoff F, Habashi J, et al. Circulating transforming growth factor-beta in Marfan syndrome [J]. Circulation, 2009, 120 (6) : 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ahimastos AA, Aggarwal A, D’Orsa KM, et al. Effect of perindopril on large artery stiffness and aortic root diameter in patients with Marfan syndrome & A randomized controlled trial [J]. JAMA, 2007, 298 (13) : 1539–1547. [DOI] [PubMed] [Google Scholar]
- [23].Jimenez SA, Rosenbloom J. Angiotensin II blockade in Marfan’s syndrome [J]. N Engl J Med, 2008, 359 (16) : 1733–1734. [PubMed] [Google Scholar]
- [24].Ostberg JE, Donald AE, Halcox JPJ, et al. Vasculopathy in Turner syndrome & Arterial dilatation and intimai thickening without endothelial dysfunction [J]. J Clin Endocrinol Metab, 2005, 90 (9) : 5161–5166. [DOI] [PubMed] [Google Scholar]
- [25].Biner S, Rafique A, Ray I, et al. Aortopathy is prevalent in relatives of bicuspid aortic valve patients [J]. J Am Coll Cardiol, 2009, 53 (24) : 2288–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]