Abstract
Background:
Lifestyle factors may contribute to the development of Parkinson’s disease, but little is known about factors that influence progression. The objective of the current study was to examine whether caffeine or alcohol consumption, physical activity, or cigarette smoking is associated with progression and survival among PD patients.
Methods:
We assessed lifelong coffee, tea, and alcohol consumption, smoking, and physical activity in a prospective community-based cohort (n = 360). All patients were passively followed for mortality (2001–2016); 244 were actively followed on average ± SD 5.3 ± 2.1 years (2007–2014). Movement disorder specialists repeatedly assessed motor function (Hoehn & Yahr) and cognition (Mini-Mental State Exam). We used Cox proportional hazards models and inverse probability weights to account for censoring.
Results:
Coffee, caffeinated tea, moderate alcohol consumption, and physical activity were protective against at least 1 outcome. Smoking and heavy alcohol consumption were associated with increased risks. Coffee was protective against time to Hoehn & Yahr stage 3 (hazard ratio, 0.52; 95% confidence interval, 0.28–1.01), cognitive decline (hazard ratio, 0.23; 95% confidence interval, 0.11,0.48), and mortality (hazard ratio, 0.47; 95% confidence interval, 0.32–0.69). Relative to moderate drinkers, those who never drank liquor and those who drank more heavily were at an increased risk of Hoehn & Yahr 3 (hazard ratio, 3.48; 95% confidence interval, 1.90–6.38; and hazard ratio, 2.16; 95% confidence interval, 1.03, 4.54, respectively). A history of competitive sports was protective against cognitive decline (hazard ratio, 0.46; 95% confidence interval, 0.22–0.96) and Hoehn & Yahr 3 (hazard ratio, 0.42; 95% confidence interval, 0.23–0.79), as was physical activity measured by metabolic-equivalent hours. Current cigarette smoking was associated with faster cognitive decline (hazard ratio, 3.20; 95% confidence interval, 1.02–10.01).
Conclusions:
This population-based study suggests that lifestyle factors influence PD progression and mortality.
Keywords: alcohol, coffee, cognition, Parkinson’s disease, progression
Motor and nonmotor dysfunction in Parkinson’s disease (PD) is progressive and highly heterogeneous.1 Predicting and eventually preventing progression is important to patients. Understanding how lifestyle factors contribute may be a first step toward stabilizing or preventing PD progression, especially as current treatments do not halt or reverse disease, but primarily address and ameliorate motor symptoms.
Epidemiologic studies have identified lifestyle factors that may influence the development of PD. Coffee and moderate alcohol consumption, physical activity, and cigarette smoking have all been inversely associated with PD onset.2–4 How or whether these factors may affect disease progression is unclear, but some smaller or short-term patient cohorts found neither smoking nor coffee consumption to influences PD motor pro- gression.5–7 Whether coffee or alcohol consumption and cigarette smoking influence cognitive decline in PD is even less studied. Nonpharmacologic therapies for PD, including physical activity, are recommended based on evidence that exercise interventions benefit physical functioning, balance, and gait and may protect PD patients against dementia.8,9
The aim of the present study was to examine whether coffee or alcohol consumption, physical activity, or cigarette smoking habits in adults before PD diagnosis influences motor progression, cognitive decline, and survival in a population-based PD patient cohort.
Methods
All procedures described were approved by the University of California at Los Angeles (UCLA) Human Subjects Committee, and informed consent was obtained from all participants.
Subjects
PD patients enrolled early in the disease (within 3 years of diagnosis; average, 2.1 years) were recruited as part of the population-based Parkinson’s Environment and Gene study in central California (2001–2007).10,11 Patients were recruited through medical groups, neurologists, and public service announcements. Patient inclusion criteria included having received a PD diagnosis within 3 years and living in 1 of 3 central California counties (Kern, Fresno, and Tulare). Briefly, 473 eligible patients were seen by movement disorder specialists, 94 did not meet published criteria for idiopathic PD12 at baseline, 6 had incomplete data and could not be recontacted, and 13 were reclassified as not suffering from idiopathic PD during follow-up.
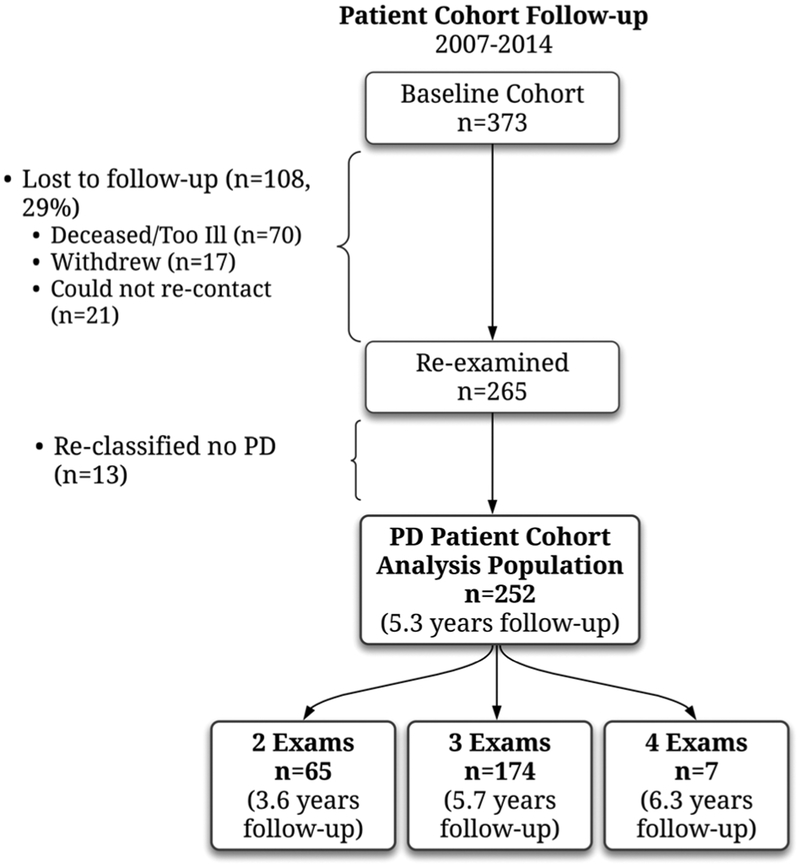
All patients (n = 360) with confirmed clinically probable or possible PD by a UCLA movement disorder specialist are included in survival analyses; 252 of these patients were assessed for signs of progression over follow-up (2 to 4 examinations; conducted in 2007–2014). At first attempted recontact, 108 of the initial patients could not be reexamined (64 were deceased, 6 too ill, 17 withdrew, and 21 could not be contacted). Of the remaining 252 patients, 8 did not provide the data necessary for at least 1 progression analysis. More detail on the longitudinal patient cohort has been pub- lished.13 Figure 1 details the patients’ follow-up. The majority of patients had 3 or more examinations (n = 181; mean follow-up, 5.8 years), but 64 had only 2 examinations (mean follow-up, 3.6 years). Most patients lost to follow-up had died or were too ill.
FIG. 1.
Details of the patient cohort follow-up.
In addition, 341 population controls from the same communities, having lived in California for at least 5 years and marginally matched to patients on age, sex, and race were recruited and interviewed during the same interval (2001–2007); more information about these community controls has been published.10,11 Controls were only passively followed, with no further contact with these participants, but we assessed mortality through vital statistics data searches. Thus, controls only contributed to mortality analyses.
Lifestyle Factors
Trained research staff conducted structured telephone interviews to obtain detailed information, including self-reported lifetime histories of (1) smoking; (2) aver- age caffeinated coffee, caffeinated tea, and alcohol consumption across 4 age periods; (3) overall physical activity level across 4 age periods; and (4) participation in competitive sports.
Participants were asked if they ever drank certain beverages (caffeinated coffee, caffeinated tea, beer, wine, and liquor) and, if so, at what age they started and stopped. Those who reported consumption were asked to report average frequency, that is, how much (cup, bottle, can, glass, etc.) they usually drank per day (or per week/per month/rarely [<1/month]), during 4 periods of adulthood, 18–24, 25–44, 45–64, and ≥ 65 years. For each beverage, we calculated a weighted average of drinks per day from age 18 to diagnosis (patients) or interview date (controls). We classified participants as never drinkers, drinking less than or at/above the beverage-specific median intake per day (for patients and controls separately).
Participants were also asked to report the average number of days per week and hours per day they engaged in mild, moderate, or vigorous physical activity at work and leisure time during each age period. These data were used to derive a weekly physical activity score in metabolic-equivalent hours (MET-h/wk), which accounts for duration and intensity.14 We calculated the sum of the MET-h/wk for every year of adulthood before the index date and divided it by the total number of adult years to derive an average lifetime activity score. Participants were also asked whether they ever participated in competitive sports; this included basket- ball (20.6% of participants), baseball (18.1%), football (18.1%), track and field (12.5 %), and softball (8.3%).
For each lifestyle factor, long-term exposure was based on each participant’s reported habits prior to diagnosis (or interview for controls). Interviews took place shortly after enrollment, within 3 years of PD diagnosis, to track the progression starting early in the disease.
Clinical Outcomes
UCLA movement disorder specialists conducted physical examinations at each examination to assess PD progression and assigned patients to one of the Hoehn & Yahr (H&Y) stages (1–5) based on clinical descriptions of each stage.15 If possible, patients were examined functionally while not receiving PD medications (off-score; 82% at the baseline examination and 80% at follow- up). We analyzed time to conversion to H&Y stage 3 (H&Y3). Patients scoring ≥H&Y3 at baseline (n = 31; Table 1) were excluded from these analyses.
TABLE 1.
Characteristics of the patient cohort
| Lifestyle factor distribution by median level | |||||
|---|---|---|---|---|---|
| Characteristic | Mean (SD) or n (%) | Duration (y) | Never | <Median | ≥Median |
| PD patient cohort (n = 244) | |||||
| Age at diagnosis | 66.9 (9.9) | ||||
| Sex, male | 139 (57) | ||||
| European ancestry | 195 (80) | ||||
| PD duration, baseline exam (y) | 2.1 (1.5) | ||||
| Follow-up, years | 5.3 (2.1) | ||||
| Years of schooling | 13.7 (4.4) | ||||
| Caffeinated coffee | 40.1 (19.8) | 34 (14) | 104 (43) | 106 (43) | |
| Caffeinated tea | 31.1 (23.3) | 67 (27) | 80 (33) | 93 (39) | |
| Alcohol, ever | 221 (91) | ||||
| Alcohol, weekly | 159 (65) | ||||
| Beer (drinks/day) | 21.9 (21.4) | 94 (39) | 80 (33) | 70 (29) | |
| Wine (drinks/day) | 20.1 (20.4) | 104 (43) | 67 (28) | 72 (30) | |
| Liquor (drinks/day) | 21.2 (21.2) | 102 (42) | 74 (30) | 68 (28) | |
| Physical activity, MET-h/wk (IQR) | 1.01 (1.2) | N/A | 126 (53) | 113 (47) | |
| Competitive sports, ever | 117 (49) | ||||
| Smoking, never | 133 (55) | ||||
| Former | 101 (41) | ||||
| Current (at baseline) | 10 (4) | ||||
| Pack-years | 9.4 (19.6) | ||||
| MMSE cognition score (n = 242) | |||||
| Mean change on MMSE, per 5 years | −1.3 (4.8) | ||||
| Cognitive decline (≥4 points in score) | 50 (21) | ||||
| Observed time from baseline exam (y)a | 5.1 (2.2) | ||||
| Mean change on MMSE, per 5 years among those with cognitive decline | −6.4 (7.1) | ||||
| Hoehn & Yahr (n = 243) | |||||
| Baseline H&Y ≥ 3 | 31 (13) | ||||
| Conversion from <3 to ≥3 | 77 (32) | ||||
| Observed time from baseline exam (y)a | 4.7 (2.0) | ||||
| Survival | |||||
| Registered deaths — PD patients (n = 360) | 209 (58) | ||||
| Age at death/censor (y) | 78.3 (9.1) | ||||
| Registered deaths — controls (n = 341) | 67 (20) | ||||
| Age at death/censor (y) | 78.7 (11.5) | ||||
MMSE, Mini-Mental State Exam; H&Y, Hoehn & Yahr score; N/A, not applicable.
Observed time to event or censor, excluding those with event at baseline
Screening for global cognitive function was con- ducted at each examination with the Mini-Mental State Exam (MMSE). Studies investigating reliable change indices for the MMSE, which estimate the reliability that an individual’s change in test scores is related to an actual cognitive change, have suggested that a reliable change over long intervals is represented by a 3- to 4-point drop on the MMSE.16,17 As our mean follow-up was 5.3 years (Table 1), we defined cognitive decline as a 4-point decline from the baseline MMSE examination and time to event as the time between baseline examination and the follow-up examination in which a 4-point decline was first measured.
Continued mortality surveillance was carried out throughout the study (2001–2016), primarily through vital statistics data, review of public obituaries, and continued active follow-up with patients and their families.
Statistical Analysis
We used Cox proportional hazards regression models to evaluate associations between lifestyle factors and progression or survival. We controlled for age (diagnosis or interview), sex, European ancestry, years of schooling, smoking history, and occupational exposure to pesticides (job exposure matrix). For mortality analysis, we also controlled for health indicators, including a history of heart attack, stroke, cancer, chronic obstructive pulmonary disease/emphysema, and type 2 diabetes. We calculated Spearman correlation coefficients between all lifestyle factors.
We considered ever/never beverage consumption and a 3-level beverage measure. MET-h/wk, standardized by the interquartile range (IQR), was analyzed as a continuous measure and also dichotomized at the median. Smoking was analyzed categorically (never, ex, current smoker) and as pack-years, with 1 year corresponding to 1 pack per day for a year. We also analyzed progression and survival in a multiexposure model, including ever/never coffee, alcohol, competitive sports, physical activity (MET-h/wk), and smoking in the same model.
When evaluating PD progression (4-point MMSE decline or H&Y3), we restricted to the patient subset with follow-up data (n = 244), excluding those lost to follow-up after baseline (n = 108). To account for selection bias that may have occurred from this censoring, we used inverse-probability weights for censoring (IPCW).18 To determine which covariates to include in the IPCW, we considered several demographic characteristics, base- line signs of PD motor and cognitive dysfunction, and each lifestyle factor (Supplemental Table e-1).
We used SAS 9.4 (SAS Institute Inc., Cary, NC) for analysis.
Results
On average ± SD, the patient cohort was followed for 5.3 ± 2.1 years. A minority of patients reported never drinking coffee (n = 34; 14%), caffeinated tea (n = 67; 27%), or alcohol (n = 23; 9%), and 51% (n = 122) never participated in competitive sports (Table 1). Consumption of different types of alcohol (beer, wine, and liquor) were moderately correlated with each other (rho, 0.27–0.44), whereas physical activity measures (MET-h/wk and competitive sports) were not correlated with each other (rho, 0.02–0.23); see Supplemental Table e-2. Characteristics of patients lost to follow-up after baseline and of control subjects used in mortality comparisons are shown in Supplemental Table e-3.
In total, 209 PD patients (58%) and 67 controls (20%) died during follow-up (2001–2016); see Table 1. Of the 244 PD patients assessed for progression, 50 (21%) experienced a ≥ 4-point decline on the MMSE, and 77 (32%) progressed to H&Y ≥ 3 (Table 1). Progression outcome events were only moderately correlated (rho, 0.23; P = 0.0003).
The time to mortality results are summarized in Table 2 and PD progression outcomes in Table 3. Multiple factors influenced mortality among patients. Both ever coffee and caffeinated tea consumption (correlation rho, 0.15; P = 0.01) were protective against all-cause mortality (HR, 0.47; 95% CI, 0.32–0.69; HR, 0.67; 95% CI, 0.48–0.93, respectively). Alcohol consumption (vs never; HR, 0.72; 95% CI, 0.51–1.00) was also protective against mortality. This was seen specifically for beer and liquor, where relative to moderate drinkers (below the median drinks/day), those who never drank were at an increased risk (HR, 1.54; 95% CI, 1.07–2.22; HR, 1.46; 95% CI, 1.02–2.07, respectively). And a history of competitive sports was protective (HR, 0.71; 95% CI, 0.52–0.99). Smoking among both patients and controls was associated with an increased risk of mortality (Table 2). Similarly, among population controls, alcohol consumption was also protective of all-cause mortality, where relative to moderate drinkers those who never consumed wine and liquor were at an increased risk (Table 2). However, coffee consumption was only suggestively associated with mortality among controls (HR, 0.56; 95% CI, 0.28–1.12), although our sample size of never coffee drinkers was limited (n = 48 never consumers among controls).
TABLE 2.
Associations (hazard ratios [HRs] and 95% confidence intervals [CIs]) between lifestyle factors and mortality among PD patients and population controls
| Mortality | ||||
|---|---|---|---|---|
| PD patients (n = 360) | Controls (n = 341) | |||
| Lifestyle factor | HR (95% CI) | P | HR (95% CI) | P |
| Caffeinated coffee | ||||
| Ever (vs never) | 0.47 (0.32–0.69) | 9.9 × 10−05 | 0.56 (0.28–1.12) | 0.10 |
| Never vs < median (cups/day)a | 2.37 (1.57–3.58) | 4.5 × 10−05 | 1.23 (0.57–2.68) | 0.60 |
| ≥Median vs < median (cups/day)a | 1.30 (0.94–1.79) | 0.11 | 0.67 (0.38–1.19) | 0.18 |
| Caffeinated tea | ||||
| Ever (vs never) | 0.67 (0.48–0.93) | 0.02 | 0.91 (0.51–1.61) | 0.74 |
| Never vs < median (cups/day)a | 1.81 (1.23–2.65) | 0.003 | 1.01 (0.52–1.94) | 0.98 |
| ≥Median vs < median (cups/day)a | 1.41 (0.97–2.03) | 0.07 | 0.77 (0.42–1.44) | 0.42 |
| Alcohol | ||||
| Ever (vs never) | 0.67 (0.42–1.07) | 0.10 | 0.42 (0.20–0.92) | 0.03 |
| Weekly (vs never weekly) | 0.72 (0.51–1.00) | 0.05 | 0.82 (0.46–1.48) | 0.52 |
| Beer | ||||
| Ever (vs never) | 0.71 (0.51–0.99) | 0.04 | 0.88 (0.48–1.64) | 0.71 |
| Never vs < median (cups/day)a | 1.54 (1.07–2.22) | 0.02 | 1.21 (0.62–2.35) | 0.58 |
| ≥Median vs < median (cups/day)a | 1.39 (0.92–2.09) | 0.12 | 1.09 (0.57–2.09) | 0.79 |
| Wine | ||||
| Ever (vs never) | 0.66 (0.49–0.89) | 0.006 | 0.46 (0.27–0.76) | 0.003 |
| Never vs < median (cups/day)a | 1.35 (0.95–1.91) | 0.09 | 2.03 (1.11–3.73) | 0.02 |
| ≥Median vs < median (cups/day)a | 0.76 (0.51–1.13) | 0.17 | 0.72 (0.36–1.44) | 0.35 |
| Liquor | ||||
| Ever (vs never) | 0.73 (0.54–0.98) | 0.04 | 0.50 (0.29–0.84) | 0.009 |
| Never vs < median (cups/day)a | 1.46 (1.02–2.07) | 0.04 | 1.86 (1.00–3.48) | 0.05 |
| ≥Median vs < median (cups/day)a | 1.11 (0.75–1.63) | 0.60 | 0.75 (0.38–1.47) | 0.40 |
| Physical activity | ||||
| Competitive sports, ever (vs never) | 0.71 (0.52–0.99) | 0.04 | 1.11 (0.65–1.92) | 0.70 |
| MET-h/wk (IQR) | 0.93 (0.78–1.11) | 0.41 | 1.29 (0.97–1.72) | 0.08 |
| ≥Median MET-h/wk (vs < median) | 0.82 (0.60–1.12) | 0.20 | 1.70 (0.98–2.94) | 0.06 |
| Smoking | ||||
| Pack-years (per 10) | 1.12 (1.06–1.19) | 6.2 × 10−05 | 1.09 (0.98–1.21) | 0.12 |
| Former (vs never) | 1.47 (1.08–1.99) | 0.01 | 2.15 (1.21–3.81) | 0.009 |
| Current, at baseline (vs never) | 1.90 (0.84–4.30) | 0.12 | 2.32 (0.88–6.08) | 0.09 |
Median of lifetime weighted average drinks per day (>0) found in the baseline cohort; specific to patients or controls. <Median reference for never and ≥ median. Mortality associations were assessed in the entire baseline cohort.
Models control for age at diagnosis, sex, European ancestry, years of schooling, smoking history, a history of heart attack, stroke, cancer, COPD/emphysema, and type 2 diabetes, and occupational pesticide exposures (JEM).
TABLE 3.
Associations (hazard ratios [HRs] and 95% confidence intervals [CIs]) between lifestyle factors and progression outcomes among PD patients
| Time to MMSE 4-point decline | Time to H&Y ≥ 3 | |||
|---|---|---|---|---|
| PD patients (n = 242) | PD patients (n = 212) | |||
| Lifestyle factor | HR (95% CI) | P | HR (95% CI) | P |
| Caffeinated coffee | ||||
| Ever (vs never) | 0.23 (0.11–0.48) | 1.10 × 10−04 | 0.52 (0.28–1.01) | 0.05 |
| Never vs < median (cups/day)a | 5.62 (2.42–13.03) | 5.70 × 10−05 | 2.09 (1.04–4.19) | 0.04 |
| ≥Median vs < median (cups/day)a | 1.23(0.86–3.32) | 0.13 | 1.23 (0.73–2.06) | 0.45 |
| Caffeinated tea | ||||
| Ever (vs never) | 0.56 (0.30–1.03) | 0.06 | 0.93 (0.51–1.71) | 0.82 |
| Never vs < median (cups/day)a | 2.33 (1.01–5.37) | 0.05 | 0.88 (0.45–1.70) | 0.70 |
| ≥Median vs < median (cups/day)a | 1.43 (0.64–3.20) | 0.38 | 0.64 (0.36–1.14) | 0.13 |
| Alcohol | ||||
| Ever (vs never) | 1.05 (0.40–2.74) | 0.93 | 0.76 (0.38–1.54) | 0.44 |
| Weekly (vs never weekly) | 0.95 (0.47–1.92) | 0.88 | 0.82 (0.48–1.42) | 0.48 |
| Beer | ||||
| Ever (vs never) | 0.93 (0.47–1.86) | 0.84 | 0.73 (0.43–1.22) | 0.23 |
| Never vs < median (cups/day)a | 1.20 (0.57–2.53) | 0.63 | 1.72 (0.96–3.08) | 0.07 |
| ≥Median vs < median (cups/day)a | 1.86 (0.88–3.90) | 0.10 | 1.86 (0.96–3.62) | 0.07 |
| Wine | ||||
| Ever (vs never) | 1.24 (0.68–2.25) | 0.49 | 0.85 (0.53–1.38) | 0.51 |
| Never vs < median (cups/day)a | 0.88 (0.44–1.75) | 0.71 | 1.41 (0.78–2.55) | 0.25 |
| ≥Median vs < median (cups/day)a | 1.18 (0.54–2.60) | 0.68 | 1.58 (0.83–2.99) | 0.16 |
| Liquor | ||||
| Ever (vs never) | 0.41 (0.23–0.72) | 1.83E-03 | 0.40 (0.25–0.64) | 1.40 × 10−04 |
| Never vs < median (cups/day)a | 2.36 (1.21–4.60) | 0.01 | 3.48 (1.90–6.38) | 5.41 × 10−05 |
| ≥Median vs < median (cups/day)a | 0.90 (0.37–2.23) | 0.82 | 2.16 (1.03–4.54) | 0.04 |
| Physical activity | ||||
| Competitive sports, ever (vs never) | 0.46 (0.22–0.96) | 0.04 | 0.42 (0.23–0.79) | 6.87 × 10−03 |
| MET-h/wk (IQR) | 0.71 (0.51–1.01) | 0.06 | 0.73 (0.53–1.00) | 0.05 |
| ≥Median vs < median (MET-h/wk) | 1.02(0.52–1.99) | 0.95 | 0.51 (0.28–0.91) | 0.02 |
| Smoking | ||||
| Pack-years (per 10) | 1.04 (0.89–1.22) | 0.62 | 1.10 (0.93–1.32) | 0.28 |
| Former (vs never) | 0.75 (0.40–1.42) | 0.38 | 1.04 (0.64–1.68) | 0.89 |
| Current, at baseline (vs never) | 3.23 (1.03–10.15) | 0.04 | 0.45 (0.05–4.29) | 0.49 |
Median of lifetime weighted average drinks per day (>0) found in the baseline cohort; specific to patients or controls. <Median reference for never and ≥ median. PD progression associations were assessed after applying IPCWs to the uncensored population.
Models control for age at diagnosis, sex, European ancestry, years of schooling, smoking history (excluded from smoking pack-years comparisons), and occupational pesticide exposures (JEM).
We considered the role of head trauma in competitive sports participation. Among patients, participating in competitive sports was associated with a history of reported head injury (OR, 2.66; 95% CI, 1.27–5.56). This association was not seen among controls (OR, 1.14; 95% CI, 0.43–3.00). Head injury was inversely associated with time from diagnosis to mortality among PD patients who participated in sports (participation: HR, 0.53; 95% CI, 0.28–1.04; no participation: HR, 0.93; 95% CI, 0.46–1.91), but head injury was not associated with mortality among controls.
A number of lifestyle factors were also associated with differences in signs of progression among the PD patients (Table 3). Ever coffee consumption was protective against both cognitive decline (HR, 0.23; 95% CI, 0.11, 0.48) and transition to H&Y3 (HR, 0.52; 95% CI, 0.28–1.01); see Table 3. For alcohol, those who never drank liquor were at increased risk of a 4-point decline on the MMSE (HR, 2.36; 95% CI, 1.21–4.60) and progression to H&Y3 (HR, 3.48; 95% CI, 1.90–6.38) relative to those who moderately consumed (below the median drinks/day). Although those who consumed liquor above the median were also at an increased risk of progression to H&Y3 (HR, 2.16; 95% CI, 1.03–4.54) relative to moderate consumers. The direction of association was similar for beer and wine, but they did not reach formal statistical significance (Table 3). Current (baseline) cigarette smoking was associated with a 3 times higher risk of a 4-point decline on the MMSE (HR, 3.23; 95% CI, 1.03–10.15), but not with progression to H&Y3.
Ever having engaged in competitive sports (HR, 0.46; 95% CI, 0.22–0.96) and higher lifetime average physical activity (MET-h/wk, HR per IQR, 0.71; 95% CI, 0.51–1.01) were protective against a 4-point decline on the MMSE during follow-up. Furthermore, competitive sports (HR, 0.42; 95% CI, 0.23–0.79), and MET-h/wk (HR per IQR, 0.73; 95% CI, 0.53–1.00) were also protective against a conversion to H&Y3.
When considering the progression results based on analyses that used the uncensored population with and without IPCW (Supplemental Table e-4), as expected, the loss to follow-up was found to likely bias associations toward the null. Bias toward the null occurs when a factor is related to loss to follow-up, and also the progression outcome and both associations have the same direction. This can be seen, for example, for caffeinated coffee, which was protective of loss to follow-up (OR, 0.41; 95% CI, 0.22–0.74; Supplemental Table e-1) and negatively associated with cognitive decline without IPCW (HR, 0.33; 95% CI, 0.14–0.77; Supplemental Table e-4). When applying IPCW, the results were stronger, suggesting selection bias toward the null (HR with IPCW, 0.23; 95% CI, 0.11–0.48; Table 2). There were no factors that resulted in selection bias away from the null, which would result if the factor was related to loss to follow-up in the opposite direction compared with the progression outcome.
When including caffeinated coffee, alcohol, competitive sports, physical activity (MET-h/wk), and smoking in the same model, most factors remained significantly associated with the outcomes among PD patients (Supplemental Table e-5). These included never coffee and alcohol consumption and smoking with mortality. Ever coffee consumption and physical activity (competitive sports and MET-h/wk) were protective against a 4-point decline on the MMSE, whereas smokers were at an increased risk, and a history of participation in competitive sports protected against conversion to H&Y3 (Supplemental Table e-5).
Given the strong associations between never coffee consumption and each of the outcomes among patients, we compared those who never drank caffeinated coffee with ever drinkers (Supplemental Table e-6). Those who reported never consuming coffee were younger at diagnosis (62.6 ± 10.9 vs 67.6 ± 9.5 years), had a longer PD duration at baseline (2.7 ± 2.2 vs 1.9 ± ± 1.4 years), and consumed less alcohol (44% vs 69% reported weekly drinking at some point). Other suggestive associations (P < 0.10) included that these patients more often dis- played a tremor-dominant PD subtype, smoked less, and less often took baby aspirin used for heart heath com- pared with ever coffee drinkers (Supplemental Table e-6).
Discussion
This community-based PD patient cohort study provides evidence that lifestyle factors may modify PD symptom progression, and that certain factors may also influence mortality risk among PD patients, several acting in a very similar manner as seen among elderly controls from the same communities. Lifestyle practices present modifiable factors that can improve health- related quality of life and are known to influence the occurrence of many chronic diseases, including cardiovascular events and cancers, and our study suggests this may also extend to the progression of PD.
In our patient cohort (mean age at diagnosis, 66.9 years; average disease duration at enrollment, 2.1 years; and 5.3 years of follow-up), we show that a history of prediagnosis consumption of caffeinated coffee, tea, and moderate alcohol consumption, and physical activity protects against disease progression. This is interesting, given that previous studies have suggested that all these factors may protect against PD onset, although because of the long prodromal period in PD, reverse causation has not been ruled out.19,20 Even if these lifestyles do not protect the patients from developing PD, it seems that aside from smoking, they might contribute to maintaining their cognition and physical function and prolong survival in those afflicted.
Our results for caffeine, cognitive decline, and survival among patients are consistent with previous findings that linked caffeinated drink consumption to a reduction in risk of cognitive decline or Alzheimer’s disease and all-cause mortality.21,22 Furthermore, the influence of caffeine seems to be stronger in PD patients, as we observed only a suggestive protective association with survival among controls for coffee (HR, 0.56; 95% CI, 0.28–1.12) and none for tea.
Best known as a psychoactive stimulant and antioxidant, caffeine increases alertness acutely. It may also protect against low-density lipoprotein oxidation and reduce oxidative DNA damage.23 Caffeine is also an A2A adenosine receptor antagonist, and selective A2A-antagonists have been shown to be neuroprotective and attenuate dopamine loss.24 In animal models of PD, treatment with caffeine ameliorated oxidative stress, restored the depletion of midbrain and striatal dopamine, prevented decline in motor activities and muscular strength, and improved norepinephrine level.25 All-cause mortality studies suggest that the inverse association with caffeine may be attributable to caffeine’s protection against cardiovascular disease and some cancers.22,26,27 We did not assess specific causes of death among patients because of sample size limitations, but we did control for these comorbidities when asses- sing all-cause mortality.
Two previous PD patient cohorts found no associations between caffeine and PD motor progression.6,7 However, the participants were younger at onset and more selected, having been recruited from a tertiary-care facility (mean age of onset ± SD of 58.1 ± 11.3 years)6or from the National Institute of Neurological Disorders and Stroke (NINDS) Exploratory Clinical Trials.7 NINDS participants tended to be well educated, not in need of care or requiring symptomatic therapy, with more than half still actively employed. Furthermore, their mean age at diagnosis was 60.8 years, and they were only followed for 1 year.7 Our population represents an older, community-based population from rural California, which may explain the differences in results and emphasizes the importance of studying life- style and other factors in community-based populations for prolonged periods.
Consistent with a previous report, we found no associations between smoking and PD motor progression.5 However, our results do suggest that current smoking is associated with an increased risk of cognitive decline during follow-up; this is especially important given that it contrasts with the protective associations reported with PD onset.3 A history of smoking was also associated with an increased risk of all-cause mortality. As expected, we observed this among both patients and controls.
The influence of lifetime alcohol consumption on PD progression has not been widely studied. Based on general health literature,28 we hypothesized a J- or U-shaped association with alcohol, with both never drinkers and heavy drinkers at increased risk of symptom progression relative to moderate drinkers. In fact, this became apparent for all types of alcohol consumed by participants (beer, wine, and liquor) for time to H&Y3 conversion, although only the association with liquor consumption reached formal statistical significance. Patients never consuming liquor were at increased risk of H&Y3 conversion and cognitive decline, and among both patients and controls they were at increased mortality risk. Those who drank more heavily were also at increased risk of H&Y3 conversion. Unlike those who never drank caffeinated coffee, those who never drank liquor were quite similar to other PD patients. However, never drinking was associated with other factors we found to influence progression. Never liquor consumers were less likely to drink coffee, wine, or beer (data not shown). Thus, the protective associations may be because of a combination of coffee and moderate alcohol consumption. However, we did not see any evidence for statistical interactions between coffee and alcohol. Additional studies to elucidate the role of lifelong alcohol consumption on PD progression are needed.
Previous population-based studies have indicated that frequent moderate or vigorous physical activity (PA) in adulthood may reduce the risk of PD.4,14 Here we show that a history of competitive sports and more lifelong PA also predict slower motor and cognitive decline. If predisease activity level is not solely based on prodromal PD related-symptoms that reduce activity at this stage, our results suggest that PA prior to diagnosis may indeed slow down disease progression. Exercise- induced neuroprotection has been seen in animal models, and in PD patients, PA improved motor and cognitive function.29–31
A history of competitive sports was protective against all-cause mortality among patients, though not in controls. The types of sports most reported included football and basketball, and interestingly, participation was associated with a history of head trauma among patients, but surprisingly not in controls. Although competitive sports and head trauma were associated with PD risk in opposite directions, we found that patients who were younger at diagnosis reported more sports participation and also head trauma. The observation that head trauma in patients (but not controls) was protective against time to mortality from diagnosis may suggest residual confounding or reporting bias among younger patients who are searching for a cause for their disease. Importantly, however, sports participation was associated with a slower progressing sub- type of PD, especially in terms of cognitive decline.
Although previous studies have suggested leisure-time PA can reduce all-cause mortality,32 we did not find associations between lifelong PA and survival in patients or controls, measured by MET-h/wk. Leisure- time and work-related PA can have different effects on mortality, with some work-related PA imparting detrimental effects.33 In our agricultural study area, greater lifelong PA may mean more physically challenging work in farming, with harmful exposures. We con- trolled for occupational pesticide exposures based on a job exposure matrix, yet the paradoxical effect of high occupational PA or correlated work hazards that we were not able to control for may explain the MET-h/wk associations among controls, wjth higher PA suggestively associated with an increased mortality risk.
We selected our outcome cut points based on previous reports. Conversion to H&Y3 is a common cut point used for motor progression, as it signifies the point at which disability advances from mild to moderate, with the appearance of postural instability.15 We did not have clinical information on cognitive impairment or dementia for all participants; thus, we relied on the MMSE. It is recognized that not all changes in MMSE score reflect true clinical changes; thus, we used reliable change indices. Previous studies have shown that changes of 3–4 points on the MMSE over longer intervals provide sufficient confidence that an individual has experienced a functional cognitive change.16,17,34 Furthermore, patients with a 4-point decrease must have scored ≤26, a common cut point signifying cognitive impairment.
Our study is one of few population-based prospective PD patient cohorts. Although we were unable to follow all PD patients enrolled at baseline, primarily because of death and illness, we used IPCW methods to account for censoring. Although IPCW does not eliminate censoring, it ensures that censoring occurs randomly with respect to measured covariates.18 Still, this method only accounts for factors included in the censoring model, and some bias from unmeasured factors may remain. A notable strength of our study is the well-characterized PD population. All patients were examined by the same UCLA movement disorder specialists who standardized their clinical evaluations throughout the study, minimizing outcome misclassification. Also, follow-up began shortly after diagnosis, allowing us to track the natural history of progression. Because of our community-based design, our results are also likely more generalizable and reflective of average PD populations than patient cohorts assembled at tertiary-care centers.
Although replication is needed, our study suggests that multiple lifestyle factors potentially modify the rate of symptom progression, as measured by signs of dysfunction (H&Y3 status and 4-point MMSE decline), among PD patients. Identifying factors that prevent or slow progression may add to our understanding of neuroprotective mechanisms and ultimately inform future research and treatment.
Statistical analysis was conducted by Dr. Kimberly Paul, University of California, Los Angeles.
Supplementary Material
Funding agencies:
This work was supported by the National Institute of Environmental Health Science (grant numbers F32-ES028087, R01-ES010544, U54-ES012078), the American Parkinson’s Disease Association (APDA), the Veterans Administration Healthcare System (SW PADRECC), the Levine Foundation, and the Parkinson Alliance.
Footnotes
Relevant conflicts of interest/financial disclosures: Y. Bordelon has received speakers’ bureau funding from Teva Pharmaceuticals.
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Pfeiffer RF. Non-motor symptoms in Parkinson’s disease. Park Relat Disord 2016;22:S119–S122. [DOI] [PubMed] [Google Scholar]
- 2.Liu R, Guo X, Park Y, et al. Alcohol Consumption, Types of Alcohol, and Parkinson’s Disease. PLoS One 2013;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol 2002;52(3):276–284. [DOI] [PubMed] [Google Scholar]
- 4.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 2016;15(12): 1257–1272. [DOI] [PubMed] [Google Scholar]
- 5.Alves G, Kurz M, Lie SA, Larsen JP. Cigarette smoking in Parkinson’s disease: influence on disease progression. Mov Disord 2004; 19(9):1087–1092. [DOI] [PubMed] [Google Scholar]
- 6.Kandinov B, Giladi N, Korczyn AD. The effect of cigarette smoking, tea, and coffee consumption on the progression of Parkinson’s disease. Parkinsonism Relat Disord 2007;13(4):243–245. [DOI] [PubMed] [Google Scholar]
- 7.Simon DKDK, Swearingen CJCJCJ, Hauser RARA, et al. Caffeine and progression of Parkinson disease. Clin Neuropharmacol 2008; 31(4):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkin- son’s disease: A systematic review and meta-analysis. Mov Disord 2008;23(5):631–640. [DOI] [PubMed] [Google Scholar]
- 9.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med 2009;39(1):3. [DOI] [PubMed] [Google Scholar]
- 10.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol 2009;169(8):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. Eur J Epidemiol 2011;26(7):547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritz B, Rhodes SL, Bordelon Y, Bronstein J. α-Synuclein genetic variants predict faster motor symptom progression in idiopathic Parkinson disease. PLoS One 2012;7(5):e36199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih I-F, Liew Z, Krause N, Ritz B. Lifetime occupational and leisure time physical activity and risk of Parkinson’s disease. Parkin- sonism Relat Disord 2016;28:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology 1967;50(2):318–318. [DOI] [PubMed] [Google Scholar]
- 16.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry 2007;78(12): 1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tombaugh TN. Test-retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Arch Clin Neuropsychol 2005; 20(4):485–503. [DOI] [PubMed] [Google Scholar]
- 18.Hernán M, Robins J. Causal Inference. Boca Raton, FL: Chapman & Hall/CRC; 2017. [Google Scholar]
- 19.Ritz B, Lee PC, Lassen CF, Arah OA. Parkinson disease and smoking revisited: ease of quitting is an early sign of the disease. Neurology 2014;83(16):1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall CB. Caffeine and PD—time to consider other interventions. Neurology 2017:10–1212. [DOI] [PubMed] [Google Scholar]
- 21.Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging 2015;19(3):313–328. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Wu K, Zheng J, Zuo R, Li D. Association of coffee drinking with all-cause mortality: a systematic review and meta-analysis. Public Health Nutr 2015;18(7):1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mišík M, Hoelzl C, Wagner KH, et al. Impact of paper filtered coffee on oxidative DNA-damage: Results of a clinical trial. Mutat Res- Fundam Mol Mech Mutagen 2010;692(1–2):42–48. [DOI] [PubMed] [Google Scholar]
- 24.Chen JF, Xu K, Petzer JP, et al. Neuroprotection by caffeine and A (2A) adenosine receptor inactivation in a model of Parkinson’s dis- ease. J Neurosci 2001;21(10):RC143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khadrawy YA, Salem AM, El-Shamy KA, Ahmed EK, Fadl NN, Hosny EN. Neuroprotective and therapeutic effect of caffeine on the rat model of Parkinson’s disease induced by rotenone. J Diet Suppl 2017;14(5):553–572. [DOI] [PubMed] [Google Scholar]
- 26.Zulli A, Smith RM, Kubatka P, et al. Caffeine and cardiovascular diseases: critical review of current research. Eur J Nutr 2016;55(4): 1331–1343. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, Bao Z, Zou J, Dong J. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer 2011; 11(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166(22):2437–2445. [DOI] [PubMed] [Google Scholar]
- 29.Fisher BE, Li Q, Nacca A, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport 2013;24(10):509–514. [DOI] [PubMed] [Google Scholar]
- 30.Paillard T, Rolland Y, de Souto Barreto P. Protective Effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: a narrative review. J Clin Neurol 2015;11(3):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauzé M, Daneault J-F, Duval C. The effects of physical activity in Parkinson’s disease: a review. J Parkinsons Dis 2016;6(4):685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol 2011;40(5):1382–1400. [DOI] [PubMed] [Google Scholar]
- 33.Holtermann A, Hansen JV, Burr H, Søgaard K, Sjøgaard G. The health paradox of occupational and leisure-time physical activity. Br J Sports Med 2012;46(4):291–295. [DOI] [PubMed] [Google Scholar]
- 34.Iverson GL. Interpretation of mini-mental state examination scores in community-dwelling elderly and geriatric neuropsychiatry patients. Int J Geriatr Psychiatry 1998;13(10):661–666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.