SUMMARY
Medically induced loss of consciousness (mLOC) during anesthesia is associated with a macroscale break-down of brain connectivity, yet the neural microcircuit correlates of mLOC remain unknown. To explore this, we applied different analytical approaches (t-SNE/watershed segmentation, affinity propagation clustering, PCA and LZW complexity) to two-photon calcium imaging of neocortical and hippocampal microcircuit activity and local field potential (LFP) measurements across different anesthetic depths in mice, and to micro-electrode array recordings in human subjects. We find that, in both cases, mLOC disrupts population activity patterns by i) a reduction of discriminable network microstates and ii) a reduction of neuronal ensembles. Our results indicate that local neuronal ensemble dynamics could causally contribute to the emergence of conscious states.
Graphical Abstract
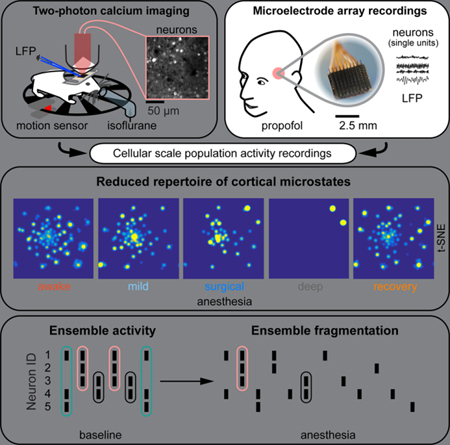
eTOC blurb:
Wenzel et al. use cellular resolution recording techniques to monitor local activity dynamics of neuronal populations in the cortex of mice and humans, and show that the available repertoire of discriminable patterns of activity (‚microstates’) as well as neuronal ensembles are reversibly reduced during medically induced loss of consciousness (mLOC). This study provides a micro-anatomical basis for how the cortex could contribute to the generation, loss or re-gain of consciousness in a distributed complex cerebral system.
INTRODUCTION
Medically induced LOC is generated via general anesthesia enabling life-saving surgical procedures or critical care, and is also a hallmark of incompletely understood disorders such as vegetative state. Despite its fundamental importance, the neural circuit and network mechanisms underlying LOC have remained unclear. Several brain areas have been implicated in causing the loss or recovery of consciousness, such as the hypothalamus (Herrera et al., 2016), thalamus (Castaigne et al., 1981), basal ganglia and claustrum (Crick and Koch, 2005; Mhuircheartaigh et al., 2010), or the brain stem (Minert et al., 2017; Moruzzi and Magoun, 1949; Penfield, 1954). Although thalamocortical connections have been central in LOC research (Flores et al., 2017; Herrera et al., 2016; Penfield, 1954; Steriade, 2003), the role of the cortex itself remains debated (Merker, 2007). Early on, animal studies by Lashley (Lashley, 1929) and Pavlov (Pavlov, 1927), or surgical procedures on epilepsy patients (Penfield, 1954) described pivotal role of the cortex in cognition, yet the removal of even expansive cortical areas resulted in no apparent quantitative change of consciousness (Lashley, 1929; Pavlov, 1927; Penfield, 1954; Scoville and Milner, 1957). However, diffusely damaged cortex, for example after hypoxia, might contribute to LOC (Jennett, 2002). Recently, fMRI studies investigating large-scale networks during mLOC have identified a break-down of functional connectivity between cortical areas (Barttfeld et al., 2015; Hudetz et al., 2015; Lewis et al., 2012). In addition, it has been reported that local network dynamics during mLOC remain surprisingly similar to those found in the conscious state (Hudetz et al., 2016; Lewis et al., 2012), impliying that LOC arises from discoordination of neural activity across brain areas. Yet, to date, no study has employed techniques with sufficient spatial resolution to properly examine the basic neural signatures of LOC at the scale of cortical microcircuits (Tononi et al., 2016).
A theoretical framework suggests that consciousness depends on the brain’s ability to discriminate between a specific sensory input and a large set of alternatives (Tononi, 2008). In basic agreement, several studies have identified a rich set of discriminable resting states of cortical activity at the macroscale (Barttfeld et al., 2015; Hudetz et al., 2015). With this in mind, along with the transmission rate of information depending logarithmically on the set of symbols used (Nyquist, 1924), we hypothesized that an individual’s ability to discriminate between a set of alternatives at any moment should be rooted in discriminable micro-patterns of activity (microstates) at the level of local neuronal ensembles, which have been postulated to represent functional building blocks of neural circuits (Hebb, 1949; Hopfield, 1982). If this is the case, LOC could arise from alterations in the local microcircuit, which would secondarily generate macroscale connectivity deficits. As a consequence, microstate dynamics across anesthetic depth and recovery could provide mechanistic insights into the basic building blocks of LOC. Using in vivo two-photon calcium (Ca2+) imaging, we find in mice that across primary sensory and higher order cortex, isoflurane-induced mLOC is indeed associated with a decreased cortical repertoire of discriminable microstates. Our results further indicate that local ensemble activity patterns undergo fragmentation during anesthesia. Eventually, based on micro-electrode array (MEA) single unit recordings, we find that the same phenomena occur during propofol-induced LOC in two human subjects. Consistent with the hypothesis that coactive neuronal ensembles are building blocks of cortical function, and in conjunction with previous studies at coarser anatomical scales (Barttfeld et al., 2015; Hudetz et al., 2015; Lewis et al., 2012; Schroeder et al., 2016), this suggests that functional connectivity of the cortex breaks down across both micro-and macroscale during mLOC.
RESULTS
Reduction of cortical microstates during mLOC in mice
We first monitored the activity of neuronal populations by combining LFP and fast two-photon Ca2+ imaging in primary sensory cortex (Yang and Yuste, 2017; Yuste and Denk, 1995; Yuste and Katz, 1991) in head-restrained Thy1-GCaMP6F mice (Dana et al., 2014), allowed to move on a running wheel (Fig. 1A,B). To ensure seamless transitions between anesthetic depths, we used a custom tube for delivery of the inhalatory anesthetic isoflurane. In 7 mice, we assessed 5 consecutive conditions (10min each) matched across animals based on hallmark LFP patterns (Fig. 1C): wakefulness, light sedation (slowed LFP yet similar to wakefulness), surgical anesthesia (continuously increased LFP delta [1–4Hz] spectral power), deep anesthesia (burst-suppression LFP), and recovery from anesthesia (isoflurane off). To further index the animals’ level of consciousness, we assessed clinical parameters (breath-rate, reaction to tail pinching), and locomotion recorded by an infra-red sensor at the running wheel (Fig. 1D). During surgical or burst-suppression anesthesia, mice were unresponsive to tail-pinching. Ca2+ transients of imaged neurons were extracted from frame series (Fig. 1B, E), and binarized (Fig. 1F, see methods). To detect similar patterns of population activity from the spike raster matrices, we performed t-distributed stochastic neighbor embedding (t-SNE (van der Maaten, 2008), Fig. 1G) leading to a 2-D embedding space from which clusters of groups of neurons with similar neural activity, or “microstates”, could be identified by watershed segmentation (WS, Suppl. Fig. 1A). Identified clusters were confirmed by comparing intra-and inter-cluster distances (Suppl. Fig. 1B). Across 7 mice, a total of 851 neurons (122 ± 12 s.e.m.), and 34,321 active frames (4,903 ± 929 s.e.m.) participating in 425 microstates (61 ± 9 s.e.m.) were analyzed. To rule out spontaneous fluctuation of microstate presence as an experimental bias, we first examined their stability. We found that identified discriminable microstates remained present over at least 24 hours (n=4 exp.; microstates per exp. [d1 vs. d2,]: 55 vs 54, 61 vs 61, 75 vs 63, 55 vs 51; MW test [100 ± 0% vs 93.73 ± 3,59%]: p=0.143; Suppl. Fig. 1C). Across all mice, locomotion was strongly reduced during mild anesthesia and recovery, and no locomotion was detected during surgical or burst-suppression anesthesia (Fig. 1H). To examine if locomotion acts as a bias by increasing the number microstates, we determined the fraction of locomotion frames contributing to each identified microstate. Out of the 425 microstates across 7 mice, merely one state (0.24%) exclusively, and one additional state (0.24%) predominantly contained activity during locomotion (Suppl. Fig. 1D,E). While not in disagreement with evidence that locomotion changes cortical gain (stimulus-driven response amplitude) (Fu et al., 2014; Niell and Stryker, 2010; Polack et al., 2013; Saleem et al., 2013), this finding is in line with previous work showing stimulus-driven ensemble activity patterns to recur spontaneously, also in the absence of any stimulus (Han et al., 2008; Kenet et al., 2003; Miller et al., 2014).
Figure 1.
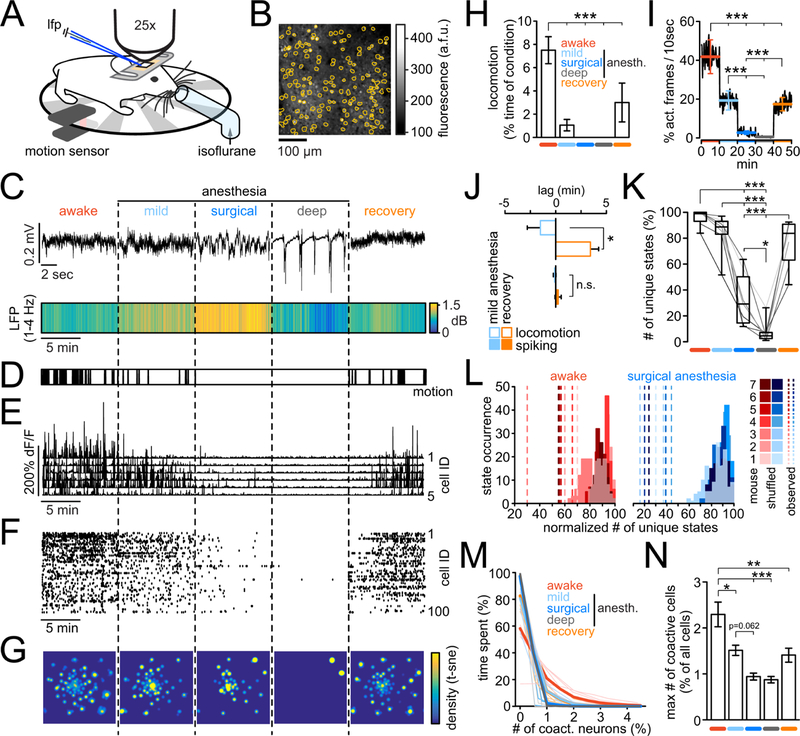
Monitoring microcircuit signatures of mLOC in mice. A) Awake, head-restrained mouse on a running wheel. Locomotion was measured by an infrared sensor. For seamless transitions across conditions, isoflurane was delivered through a custom tube placed right in front of the mouse. For LFP recordings, a pulled glass microelectrode was carefully inserted into the cortex at around 250µm depth through a small burr hole next to an implanted glass cover. Through the glass, two-photon calcium imaging was performed. B) Image of typical field of view and registered neuronal somata outlines (orange). C) Upper panel: Representative brief raw LFP traces across five conditions. Lower panel: Avg LFP delta range [1–4Hz] spectral power across all five 10min long conditions (n=7 animals). Note the continuously increased delta power during surgical anesthesia. D) Superimposed locomotion of all 7 mice across conditions. Note that movement is absent in surgical and burst-suppression anesthesia. E) Calcium transients of 5 representative registered neurons across all five conditions. F) Corresponding raster plot of all registered neurons. G) Density map of microcircuit states, visualized by t-SNE for the entire experiment, displayed per condition. Vectors representing the population activity at each time point were transformed into a two-dimensional space while preserving local structures, and a density map was generated from the scatter plot. H) Quantification of locomotion across the entirety of each experiment (exp.), displayed as % locomotion per condition (10min each); anesth.=anesthesia. I) Neural activity level across conditions, quantified as probability of detecting activity within a moving 10sec window in a yes or no fashion; errorbars represent means ± s.e.m. per condition; conditions colored as in Fig. 1H) J) Relationship between LOC and neural activity during mild anesthesia vs. recovery. Shown are the mean lags (n = 7 mice) of events (motion or spiking) with respect to the first (5 min), and second half (5 min) of mild anesthesia, or recovery. If motion or activity were evenly distributed throughout conditions, mean lags would be 0. Note how locomotion consistently stopped in the first half of the mild anesthesia condition, while it re-emerged exclusively in the second half the recovery condition (−90.6 ± 77.6 sec vs. 205 ± 48.6 sec, Mann Whitney test [n=7]; p=0.0105). Importantly, in both conditions neural activity levels were much more evenly distributed across both conditions (−6.2 ± 7.8 sec vs. 14,2 −18,3 sec, Mann Whitney test [n=7]; p=0.62). K) Boxplots of number of unique microstates (t-SNE/WS) across conditions as % of all identified unique microstates in a given experiment; line plots are individual experiments (grayscale) L) Total number of observed unique microstates (dashed lines) during wakefulness (red) or surgical anesth. (blue) vs. corresponding distributions of values from 100 randomized datasets. (n = 7 mice, each exp. max-normalized for purpose of visualization). No overlap of observed vs. random data (p<0.01). M) Number of co-active neurons (in %, for purpose of visualization) versus the relative time spent by imaged population containing such co-activity, displayed per condition. Thick lines represent means, thin lines individual exp. conditions L) Maximum number of co-active neurons (as % of all neurons per exp.) participating in a specific microstate, across conditions (colors as in M). Borderline statistical significance mild vs. surgical anesthesia p=0.062. Figure 1 H–L show data from n=7 mice; all errorbars represent mean ± s.e.m.; all boxes in boxplots represent 25–75%ile of the data, bars within boxes represent means. Except comparison between observed and randomized data (1L), and comparison of two groups (1J), all statistical analyses represent 1way-anova with Bonferroni post-test. *p<0.05, **p<0.01, ***p<0.001.
Consistent with previous studies, neural firing decreased promptly during mLOC (Fig. 1I) (Ishizawa et al., 2016; Lewis et al., 2012). In line with Lewis and colleagues who investigated non-steady state anesthesia using propofol boluses (Lewis et al., 2012), locomotion and response to tail pinching lagged behind neural activity levels during the recovery period, while, inversely, neural activity outlasted the arrest of locomotion during mLOC (Fig. 1J). In contrast to recent reports (Hudetz et al., 2016; Lewis et al., 2012), the number of discriminable microstates was strongly reduced during surgical and burst-suppression anesthesia (Fig. 1K). To investigate the potential influence of neuronal coactivity on the microstate classification, we repeated the analysis only using frames that contained no more than the maximum number of coactive cells observed during surgical anesthesia (2.6 ± 0.4 s.e.m., n=7 mice), finding consistent results (Suppl. Fig. 2A).
To explore if the effect of microstate reduction during deeper anesthesia could be explained by random coactivation of sparsely active neurons, we repeated t-SNE/WS on 100 randomized datasets derived from observed data through within-frame shuffling. This procedure preserved total per-frame-activity while disrupting within-frame patterns (Suppl. Fig. 2B,C). Importantly, in all mice and conditions, the number of randomly generated microstates was significantly higher than that found in the corresponding observed data (Fig. 1L, Suppl. Fig. 2C,D), except for the wake-up period in one experiment and burst-suppression anesthesia. This lack of significant difference could be explained by a critically low overall activity. These results indicated that observed microstates were non-random, given sufficient activity, and that they represented a much smaller set compared to all possible states.
To rule out that our results could be due to the t-SNE/WS approach, we applied several additional analytical approaches based on different algorithms. Affinity propagation clustering (APC) (Frey and Dueck, 2007) and principal component analysis (PCA) also showed a progressive and non-random reduction of discriminable patterns of microciruit activity across anesthetic depth (Suppl. Fig. 3A–C). Further, consistent results were obtained by calculating Lempel-Ziv-Welch complexity (LZWC, Suppl. Fig. 3D,E) (Welch, 1984; Ziv and Lempel, 1978), and the number of unique features (‘words’, or collectively, ‘dictionary’) encountered by LZW across conditions (Suppl. Fig. 3F). Expectedly, the number of discriminable activity patterns were not identical across approach. The intention here was not to test if our data fits a certain algorithm’s assumption the best or not, but instead to prove that the results can be reproduced from multiple algorithmic angles.
Microstate reduction during mLOC in mice is not restricted to primary sensory cortex
To exclude the possibility that our finding could be restricted to primary sensory areas, we repeated the experiments in higher order visual cortex (anterolateral/rostrolateral visual cortex) (Marshel et al., 2011), and hippocampal CA1 (stratum pyramidale, Suppl. Fig. 4 A,B) (Eichenbaum, 2017). As in our experiments in primary sensory cortex, mLOC was associated with a striking reduction of discriminable microstates with increasing depth of anesthesia, and recovery of states during the wake-up period (Suppl. Fig. 4 C). Again, in both secondary visual cortex and hippocampus, observed microstates were non-random given sufficient activity (data not shown), and the results were similar across analytical approaches (Suppl. Fig. 4 C). These experiments showed that microstate reduction during mLOC in mice is not restricted to primary sensory cortex, but can be found across cortical areas.
Neuronal ensemble fragmentation during mLOC in mice
While collecting data for this study, we became aware that changes in ensemble co-activity seemed to be associated with mLOC. To study this, we operationally defined an ensemble as a group of neurons that are coactive in any given frame (Miller et al., 2014). Indeed, neural co-activity within identified microstates was also consistently decreased with anesthetic depth (Fig. 1M,N, Suppl. Fig. 5 A). These neuronal ensembles were also not due to chance firing of cells, as within-cell shuffling, disrupting co-activity patterns while maintaining the same total activity per condition (Suppl. Fig 2B, lower panel), led to random distributions significantly smaller than the observed data (Suppl. Fig. 5 B,C). These results indicated a reduction of local ensembles (Miller et al., 2014) during mLOC, with a progression towards independent single neuron activity patterns during deeper anesthesia.
Reduction of cortical microstates and ensemble fragmentation during mLOC in humans
To investigate whether our mouse findings hold true in humans, we studied discriminable microstates and neural coactivity across anesthetic depth in two neurosurgical patients with pharmacoresistant epilepsy undergoing pre-surgical evaluation. In addition to subdural macro-electrode implantation for epileptic focus identification in the temporal lobe (Fig. 2A), these patients were implanted with a Utah microelectrode-array (MEA, 4×4mm, 96 electrodes, Fig. 2A,B) measuring both LFP (Fig. 2C), and single unit activity (SUA) in infragranular layers (Schevon et al., 2012) of the anterior middle temporal gyrus (Fig. 2D). This higher cortical area has been shown to be involved in semantic processing (Faroqi-Shah et al., 2018), retrieval of conceptual knowledge (Davey et al., 2015), or intersensory conflict detection (van Kemenade et al., 2018). In both patients, 3 anesthetic conditions (mild, surgical, and deep [burst-suppression] anesthesia) were examined by applying intravenous propofol. Again, anesthetic depth was matched across patients based on LFP signals (Fig. 2C). Since MEAs were implanted on the day clinical electrodes were removed, neither wakefulness nor the wake-up period could be recorded. As with the mouse analysis, neural firing was binarized (Fig. 2D), and microstates were first identified using t-SNE/WS (Fig. 2E, suppl. Fig. 1A). In the two human subjects, a total of number of 145 single units (72.5 ± 18.5 s.e.m.), and 6950 active frames (3475 ± 1147 s.e.m., 1 frame = 100ms epoch) participating in 89 microstates (44.5 ± 5.5 s.e.m.) were identified. As in mice, a general drop of neural activity was observed with increasing anesthetic depth (Fig. 2F). Likewise, our finding of a decrease of the number of discriminable t-SNE/WS microstates during anesthesia in mice held true in both patients (Fig. 2G). Again, applying AP clustering (Fig. 2H), PCA analysis (Fig. 2I), LZWC or LZW dictionary size (Fig. 2J,K) to the data as complementary analytical approaches led to consistent results. As before, the effects could not only be explained by a random coactivation of neurons, as observed results were significantly different from randomized datasets (Fig 2L). Thus, in line with our results in mice, the local population drew from an increasingly reduced repertoire of microstates with increasing anesthetic depth. Finally, in both patients, neural coactivity patterns non-randomly disappeared with increasing depth of anesthesia (Fig 2M–N, suppl. Fig. 5D).
Figure 2.
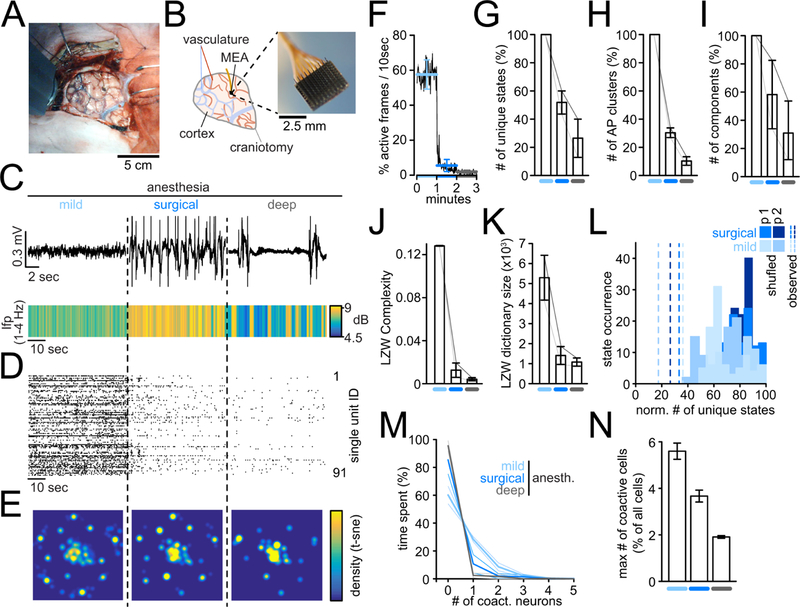
Reduced repertoire of cortical microstates and ensembles upon mLOC in humans. All errorbars in figure 2 represent mean ± s.e.m.. (n=2 patients). A) Photo of the craniotomy carried out on one patient in this study. B) Left: Drawing of craniotomy in A). craniotomy (gray); arteries (red); veins (blue), multi-electrode microarray (MEA, black). Right: close-up photo of micro-electrode array (4×4mm, 96 electrodes). C) Upper panel: Representative brief raw LFP traces across three anesthetic conditions in one patient. Lower panel: Avg LFP delta range [1–4Hz] spectral power across conditions, and patients. D) Raster plot of all single units in one patient. E) 2-dimensional density plot of same data after t-SNE, displayed per condition. Every dot represents a timepoint containing neural activity. F) Neural activity level across conditions, quantified as probability of detecting activity within a moving 10sec window in a yes or no fashion; conditions colored as in C). G) Number of unique microstates (t-SNE/WS) across conditions as % of all identified microstates per exp.; line plots are individual patients. H) Number of unique microstates (APC) across conditions as % of all identified microstates per exp.; line plots are individual patients. I) Number of PCA components across conditions as % of all identified components per exp.; line plots are individual patients. J) LZW complexity across conditions; line plots are individual patients. K) LZW dictionary size across conditions; line plots are individual patients. L) Number of observed unique microstates (dashed lines) during mild anesth. (light blue) or surgical anesth. (dark blue) vs. corresponding distributions of values from 100 randomized datasets, max-normalized for purpose of visualization. No overlap of observed vs. random data, equal to p<0.01; p½ represent patient ½ M) Number of co-active neurons versus the time spent by population in time points containing such co-activity per condition, displayed as mean across patients (thick lines). Thin lines represent conditions in individual patients. N) Maximum number of co-active neurons (as % of all neurons per exp.) participating in a specific microstate across conditions.
DISCUSSION
We used cellular scale population recording techniques in mice and humans to investigate changes in microscale activity patterns across anesthetic depth, and recovery from anesthesia. It is difficult to compare our results with a recent report by Schroeder and colleagues (Schroeder et al., 2016), where mLOC occurred despite unchanged levels of overall activity during the particular case of ketamine-induced dissociative anesthesia (Pender, 1971). Whereas most first line anesthetics decrease cerebral metabolism (Laaksonen et al., 2018; Pilge et al., 2014), ketamine differentially affects cerebral hemodynamics and neural firing with a net neutral effect (Cavazzuti et al., 1987; Laaksonen et al., 2018). Keeping in mind that functional brain imaging in the clinical setting relies on changes in cerebral metabolism as a proxy for neural firing (e.g. FDG-PET CT, or BOLD signal in fMRI), it is not surprising that Schroeder et al. found a preserved overall firing rate. Of note, our analysis of discriminable patterns of local network activity focused on the general presence of microstates, regardless of overall population activity.
The discrepancy between our findings and previous studies using similar anesthetics (Hudetz et al., 2016; Lewis et al., 2012) might be explained by the difference in recording techniques, and signal analysis. Using a 64-electrode microarray, Hudetz et al. (Hudetz et al., 2016) described that the repertoire of mesoscale cortical activity is not altered during anesthesia. Importantly, analysis was carried out on local field potentials (LFP) filtered at 4–60 Hz, which are dominated not by neural firing itself but the summed-up synaptic activity from thousands to millions of neurons. Hudetz et. al used negative LFP deflections as a proxy for local population activity. It is important to keep in mind that while such LFP deflections may correspond to local population firing, identical negative LFP deflections may not necessarily correspond to the firing of the identical local population, as dendritic arbors are much more spacially distributed and overlapping with dendritic arbors of neighboring neurons than neural somata. As a consequence, the spatial resolution blurs, and with it the detectable diversity of activity dynamics within local neural networks. By contrast, two-photon Ca2+ imaging and MEA unit recordings capture somatic firing of local groups of neurons in a spatially precise manner. In a recent study, two-photon imaging of interictal epileptiform activity indicated that seemingly identical LFP deflections (filtered similarly to Hudetz et al.) may be composed of varied patterns of local ensemble firing (Feldt Muldoon et al., 2013).
Using a 96-electrode MEA, Lewis and colleagues (Lewis et al., 2012) reported that, while macroscale connectivity broke down during mLOC, microscale activity persisted similarly to the conscious state. This conclusion was based on pairwise correlation of a small fraction (<15%) of recorded single units showing the highest firing rate in one human subject. This pre-selection is debatable, given that sparse neural firing (e.g. reviewed in Barth and Poulet, 2012), and weak pairwise correlation between local neurons (Cohen and Kohn, 2011) represent elementary constituents of cortical activity. These aspects neither preclude synchronous network states (Schneidman et al., 2006), nor computational dynamics of the network (Hopfield, 1982). In our human recordings, all units belonging to the recorded local network were included in the analysis across anesthetic depth.
To conclude, extending previous studies at the macroscale (Barttfeld et al., 2015; Hudetz et al., 2015; Lewis et al., 2012), our results provide evidence that, during mLOC, functional connectivity of the cortex also breaks down at the microscale. Regarding general brain function, a number of mechanistic studies on local cortical circuit activity (Afraz et al., 2006; Carrillo-Reid et al., 2018; Houweling and Brecht, 2008; Huber et al., 2008; Salzman et al., 1990) have substantiated hypothetical considerations that local neural ensembles serve as functional building blocks of cognition (Hebb, 1949; Hopfield, 1982). The precisely controlled simultaneous holographic activation of as few as two cortical neurons with pattern completion capabilities, meaning that their activation triggers an entire local neural ensemble, can alter performance in a visual task (Carrillo-Reid et al., 2018). This implies that behavior, which is inherently based on the integration of information processed across cortical areas, can be altered by fine-scaled local neural activity. It remains currently technically difficult to prove that the same microscale-macroscale circuit mechanisms hold true for mLOC as well, as one would need to locally interfere with a vast number of neural ensembles across the entire cortex simultaneously. Our results imply that as local microstates reversibly disappear and neural ensembles undergo fragmentation during mLOC, functional connectivity and integration of information at the macroscale should be altered, too. In sum, our study provides a micro-anatomical basis for how the cortex, along with subcortical areas in a distributed complex cerebral system, could contribute to the generation, loss or re-gain of consciousness (Koch, 2012).
STAR Methods
Contact for Resource Sharing
Dr. Michael Wenzel (michaelwenzel2946@gmail.com) is the Lead Contact for resource sharing. All resource requests should be directed to the Lead author.
Experimental Model and Subject Details
Animal subjects
Experiments were conducted with care and accordance with the Columbia University institutional animal care guidelines. Experiments were carried out on Thy1-GCaMP6F (Dana et al., 2014) adult transgenic mice at postnatal age of 4–6 months. No animals were used for previous or subsequent experimentation. Food and water was provided ad libitum. All mice were kept at a 12 hour light/dark cycle.
Human subjects, and Ethics Statement.
Two human subjects were included in this study. Patient 1 was a 31-year-old male and patient 2 was a 64-year-old male. Both patients were undergoing neurosurgical resection of the anterior temporal lobe (patient 1: left; patient 2: right) in order to treat medically refractory mesial temporal lobe epilepsy. The University of Utah Institutional review board approved these experiments and both subjects provided informed consent prior to participating in the study.
Method Details
Animals, surgical procedures, and setup acclimatization.
Prior to the actual experiment, mice were anesthetized with isoflurane (initial dose 2–3% partial pressure in air, then reduction to 1–1.5%). Right before surgery, all mice received carprofen (s.c.), enrofloxacin (s.c.), and dexamethasone (i.m.). Under sterile conditions, a small flap of skin above the skull was removed and a titanium head plate with a central foramen (7×7mm) was attached to the skull with dental cement above the left hemisphere. A small cranial aperture (around 2×2mm) was established above left somatosensory (coordinates from bregma: x 2.5mm, y −0.24mm, z −0.2mm) or visual cortex (from lambda x 2.5mm, y −0.02mm, z −0.2mm, one exp. over lateral visual cortex: from Lambda x 3 mm y 2.4 mm z −0.2) using a dental drill. Then, the craniotomy was covered with a thin glass cover slip (3×3mm, No. 0, Warner Instruments), which was fixed in place with a slim meniscus of silicon around the edge of the glass cover and finally cemented on the skull using small amounts of dental cement around the edge. A small section of skull (1×1mm) was left blank next to the cemented glass cover. For hippocampal imaging, a small area of cortex (around 1.5×1.5mm) above left CA1 was removed by gentle suction down to the external capsule, as described previously (Dombeck et al., 2010). The site was repeatedly rinsed with sterile saline until no further bleeding could be observed. Then, a small UV-sterilized miniature glass plug (1.5×1.5mm, BK7 glass, obtained from BMV Optical), glued to the center of a thin glass coverslip (3×3mm, No. 0, Warner Instruments) with UV-sensitive glue, was carefully lowered onto the external capsule, and until the edges of the attached glass cover touched the skull surrounding the craniotomy. Finally, the plug was fixed in place with a slim meniscus of silicon around the edge of the glass cover and by applying small amounts of dental cement around the edge of the glass cover.
Post-operatively, all mice received carprofen daily for 3 days. Over the following days, mice were accustomed to the experimenter, and the experimental setup until no signs of distress were present. Mice usually became rapidly acclimatized to the microscope and running on a wheel under head-restrained conditions over the course of 2–3 acclimatization sessions lasting 30 minutes each. Around two weeks after the implant of the glass cover slip or glass plug, on the day of the actual experiment, mice underwent brief surgery again. Using isoflurane as described above, a small burr hole was established in the area that had been left blank next to the cemented glass cover for access by a glass micropipette for LFP measurements (find a more detailed description under electrophysiology, below). A reference electrode was place over the right frontal cortex. Thereafter, mice were transferred to the microscope for the experiment.
Experimental timeline in mice.
In each experiment, animals were kept in head-restrained conditions yet allowed to move freely on the running wheel. Throughout each experiment, in addition to local population imaging, cortical activity was recorded by LFP measurements serving as an additional proxy of anesthetic depth aside from clinical assessment (breath rate, locomotion, responsiveness to tail pinching). After the first image and LFP series during wakefulness, mild anesthesia was established by delivery of low concentrations of isoflurane through a plastic cylinder positioned right in front of the mouse’s nose (Fig. 1 A). During mild anesthesia/low levels of isoflurane (0,5% partial pressure in air – ‘ppa’), mice remained responsive to tail pinching. This responsiveness ceased completely once general anesthesia was achieved by increasing the dose of isoflurane to around 1,0% ppa. Then, burst suppression anesthesia was induced through another increase of isoflurane to around 1.5% , and maintained for 10 minutes. Finally, isoflurane delivery was halted, and imaging and lfp recordings continued while the animal was allowed to fully recover from anesthesia. Once the experiment was completed, animals were deeply anesthetized and sacrificed humanely.
Infrared locomotion detection in mice.
Locomotion was detected using an infrared sensor at the running wheel. The initially transparent running wheel was adapted to block the infrared light from passing through the wheel by using equally spaced strips of light absorbent tape (Fig. 1A). Thus, whenever the mouse would locomote, the light path between the light source underneath the wheel and the sensor on top of it would alternatingly get blocked or released rapidly. During each such transition (the longer the locomotion, the more transitions), the infrared sensor produced a large positive (from blocked to transparent) or negative (transparent to blocked) change in voltage that could be recorded at 1 kHz temporal resolution alongside the LFP using Prairie View Voltage Recording Software. Transitions could be easily extracted after an experiment to create a binary vector of locomotion or rest. For each event in the binary vector, 1 second of locomotion was counted, and the relative time of locomotion versus resting, for each experimental condition, was calculated.
Two-photon calcium imaging in mice.
Neural population activity was recorded by imaging changes of fluorescence with a two-photon microscope (Bruker; Billerica, MA) and a Ti:Sapphire laser (Chameleon Ultra II; Coherent) at 940 nm through a 25x objective (Olympus, water immersion, N.A. 1.05). Resonant galvanometer scanning and image acquisition (frame rate 30.206 fps, 512 × 512pixels, 150–250µm beneath the pial surface, or external capsule in the case of hippocampal imaging) were controlled by Prairie View Imaging software. Multiple datasets were acquired consecutively over the course of an experiment (in total 100,000–150,000 frames) with several momentary breaks interspersed for reasons of technical practicality.
Two-photon image processing.
Active cells were first identified visually in raw tiff movie files using ImageJ software (Schneider et al., 2012). 10 minutes of imaging during each of the five anesthetic conditions (matched across animals by raw LFP, spectral power, and clinical parameters) were concatenated into one large movie tiff file spanning the entire experiment. A list of cell centroid spatial coordinates was obtained and used to initialize the recently described constrained nonnegative matrix factorization algorithm (CNMF) to extract calcium transients of all registered cells in MATLAB (Pnevmatikakis et al., 2016; Yang et al., 2016). Prior to the initialization, tiff series were down sampled (averaged) from the original 30Hz temporal imaging resolution to 10Hz, and 512×512 pixel spatial resolution to 256×256 pixels. The CNMF algorithm finds spatiotemporal components based on pixels of high covariance around defined cell centroids while accounting for background fluorescence and minimizing signal noise. Based on the extracted fluorescence traces, ∆F/F signals are calculated for each cell using a sliding window (30 seconds). To derive binarized activity events from the ∆F/F signals, the ∆F/F is temporally deconvolved with the CNMF parameterized fluorescence decay. In addition, a temporal first derivative (slope) is independently obtained from the ∆F/F signals of individual cells. Then, the deconvolved signal and the derivative are thresholded at at least four standard deviations from the mean signal, respectively. At each time point, if both the devoncolved signal and first derivative exceed the threshold, a binary activity event is detected. The binary matrices obtained in this way, contained the recorded number of neurons across 30,000 frames of imaging (50 minutes), and represented the input matrices for t-SNE embedding/watershed segmentation, AP clustering, PCA, or Lempel-Ziv complexity analysis, as described below.
Local field potential recordings in mice.
For LFP measurements, a sharp glass micropipette (2–5 M) containing a silver chloride wire, back-filled with saline, was diagonally advanced into the cortex (30° angle) under visual control. The pi pette was lowered through a burr hole next to the glass cover slip, as described above, to a depth of around 200 µm beneath the pial surface. A reference electrode was positioned over the contralateral frontal cortex. LFP signals were amplified by use of a Multiclamp 700B amplifier (Axon Instruments, Sunnyvale, CA), low-pass filtered (300Hz, Multiclamp 700B commander software, Axon Instruments), digitized at 1 kHz (Bruker) and recorded using Prairie View Voltage Recording Software along with calcium imaging.
Single Unit Activity and LFP data acquisition and pre-processing in humans.
Human electrophysiological data were acquired from a Utah-style microelectrode array implanted in each subject’s middle temporal gyrus, approximately 3 cm from the temporal pole. Detailed surgical methods are described in House et al (House et al., 2006). Data from these microelectrodes were acquired at 30,000 samples per second and pseudo-differentially amplified by 10 using an FDA-approved neural signal processing system (Blackrock Microsystems, Salt Lake City, UT). Continuous recordings were acquired from the microelectrode arrays while anesthesia was maintained at different anesthetic depths. Signals from the microelectrode arrays were segregated into two data streams: single unit activity (SUA), and local field potentials (LFP). SUA was acquired by first band-pass filtering the voltages recorded on each microelectrode between 300 and 3,000 Hz (4th order butterworth filter). This high pass filtered signal was thresholded at −3.5 times its root mean square, and 48 samples around each threshold crossing were retained for spike sorting. Spike sorting was carried out in a semi-supervised fashion on a feature space of the first three principal components, and clustering using the T-distributed expectation maximization algorithm (Shoham et al., 2003). The time stamps of well-isolated single units were retained for further analysis in a binary matrix in which one dimension represented the activity of a single unit and the other dimension represented time at 1000 samples per second. LFP data were acquired by non-causal low pass filtering (500 Hz fir filter) the voltage recorded on each microelectrode and averaging across channels. This mean LFP across the array was then down-sampled to 1000 samples per second.
Analysis of LFP spectral power in mice or human subjects.
LFP low frequency spectral power (1–4Hz) was calculated with a 1 Hz temporal resolution. A Fast-Fourier transform (FFT) was carried out, and the spectral power was calculated as the squared absolute value of the complex output of the FFT. Finally, spectral power was averaged across the low frequency range.
T-SNE and watershed segmentation (t-SNE/WS).
We identified cortical microstates of the neuronal population using an un-supervised nonlinear embedding method, t-Distributed Stochastic Neighbor Embedding (t-SNE) (van der Maaten, 2008). T-SNE was performed on active frames in population raster plots of neural activity derived from two-photon calcium imaging in mice, or microelectrode array SUA in humans, as described above. In both mice and humans, temporal down-sampling was used so that in all datasets, one “frame” corresponded to 100ms of neural activity. Using the perplexity value with the lowest optimization error for each dataset (a value range from ~5–200 was tested), and an initial reduction to 25 dimensions of activity using principal component analysis (PCA), t-SNE was applied across 1000 repititions to produce a robust two-dimensional embedding space that could be conveniently visualized (Fig. 1G). This 2D embedding space of an entire experiment, in which every data point represents the activity of the entire recorded neural population per individual frame, was used for watershed segmentation (WS) in order to separate clusters of similar activity (Suppl. Fig. 1A). To this end, a density map was generated. A probability density function was calculated in the embedding space by convolving the embedded points with a Gaussian kernel; the standard deviation of the Gaussian was chosen to be ¼0 of the maximum value in the embedding space. To segment the density map, local maxima were identified in the density map, a binary map containing peak positions was generated, and peak points were dilated by three pixels. A distance map of the binary image was generated and inverted, and the peak positions were set to be minimum. Watershedding was performed on the inverted distance map, and the boundaries were defined with the resulting watershed segmentation.
Advantages and shortcomings of t-SNE: It is a nonlinear method for dimensionality reduction that preserves local structures well, and is robust against noise. It is widely used in different fields including neuroscience and genetics. The method is well-suited for visualizing data. As it is a tool only for dimensionality reduction and visualization, it has to be combined with additional methods for further analysis (e.g. watershed segmentation). Depending on the size of the dataset to be analyzed, t-SNE can be resource heavy.
Affinity propagation clustering (APC).
Affinity propagation clustering is a method to cluster data points by identifying a subset of representative examples and iteratively refining their cluster members through optimizing an objective function that describes the net similarity (Frey and Dueck, 2007). This method operates on the pairwise similarity matrix between all pairs of data points, and identifies the exemplers based on an input preference vector, then automatically determines the number of clusters. Here, we used a Matlab module to perform affinity propagation in both mice and human data [https://www.psi.toronto.edu/index.php?q=affinity%20propagation]. As we had no a priori knowledge about pairwise similarity between any set of two datapoints, we ran the script without sparse capacity. In both observed and shuffled datasets, we set the preference vector to ones. For consistency with t-SNE, we removed all the frames with no active neurons before the clustering procedure. In this paper, a cluster identified by t-SNE/WS or APC, is called a “microstate”.
Advantages and shortcomings of APC: APC is a primary clustering method. It does not require a cluster number as input but defines the cluster number automatically. The preference vector is an intuitive parameter, and the method is robust against noise. Similarly to t-SNE/WS, it can be resource heavy depending on the size of the dataset.
Lempel-Ziv-Welch Complexity (LZWC).
The Lempel-Ziv Complexity, or sequence complexity, measures the complexity of a finite sequence of symbols by way of trying to compress the sequence (Ziv and Lempel, 1978). Here, the Lempel-Ziv-Welch (LZW) algorithm was used for this purpose, which is an encoding algorithm that works by creating a dictionary of common substrings (Welch, 1984). The Lempel-Ziv-Welch algorithm is an improved implementation over the initial algorithm proposed for calculating the LZC, in terms of computation cost. LZW was applied to raster firing matrices of recorded cellular calcium transients in mice or SUA in humans (Suppl. Fig. 3D). To arrive at its encoded form, first the binary matrix was transformed into a vector that was scanned by the LZW algorithm. A Matlab module was used to calculate LZWC [https://www.mathworks.com/matlabcentral/fileexchange/4899-lzw-compression-algorithm]. To make the results of the LZW algorithm comparable across experiments, and therefore independent of the number of imaged neurons per animal or single units recorded in the two human subjects, an upper and lower bound was established. This was done by running the LZW algorithm on a vector of the same size but with all zeros, as well as taking the average of random vectors of the same size. These were used to establish lower and upper bounds between complete order and randomness, respectively, and then normalize the length of the compressed vector of neural activity to arrive at a final value between 0 and 1, so that:
Advantages and shortcomings of LZWC:
The method is fast, and can be done online. It is not a clustering method, and does not in itself define microstates, as by the nature of its algorithm it exclusively measures the ‚compressionability’ ofa dataset. In comparison to t-SNE/WS, APC, and PCA, It is not too robust against noise, and is vulnerale to temporal segregation of recorded signals. It is a practical analytical approach, but should in the optimal case, depending on the question addressed, be combined with other neural activity feature extraction methods.
Quantification and Statistical analysis
Unless stated otherwise, all values reported in this paper represent means ± s.e.m.. To determine statistical significance of differences between mean values measured under different experimental conditions in mice (n=7 animals, 5 conditions: awake, mild anesthesia, surgical anesthesia, deep anesthesia, and wake-up), 1-way anova was carried out (4 degrees of freedom), followed by a multiple comparison Bonferroni correction. When two groups were statistically compared (Fig. 1J), a Mann-Whitney (MW) test was used. Regarding statistical differences between observed and corresponding randomized numbers of unique microstates across experimental conditions in both mice and human subjects, observed single values were compared to a distribution of 100 values derived from randomized surrogate datasets (performed for t-SNE/WS, APC, and PCA). If the observed value was smaller/bigger than 95% of the randomized values, statistical significance was reached (p<0.05). P<0.01 was reached, if the observed value was smaller/bigger than all 100 randomized values. In this paper, statistical significance levels are depicted as * for p<0.05, ** for p<0.01, or *** for p<0.001.
Additional Resources
MOCO (motion correction) is available on the Yuste lab website: http://www.columbia.edu/cu/biology/faculty/yuste/methods.html
Code availability
All custom code to identify discriminable patterns of neural activity was written in MATLAB (MathWorks). The code is available on: https://github.com/hanshuting/tsne_neural_states
Supplementary Material
Key Resource Table
| Experimental Models: Mouse lines | ||
|---|---|---|
| C57BL/6J-Tg(Thy1-GCaMP6f)GP5.11Dkim/J | Jackson Laboratory | RRID:IMSR_JAX:024276 |
| Recording device in human patients | ||
| Utah-style microelectrode array | Blackrock Microsystems | |
| Software and Algorithms | ||
| FDA-approved neural signal processing system | Blackrock Microsystems | |
| BIS Vista monitoring system | Aspect Medical Systems | |
| ImageJ | https://imagej.nih.gov/ij/ | |
| Moco | http://www.columbia.edu/cu/biology/faculty/yuste/methods.html | |
| MATLAB(R2014b) MathWorks | MathWorks | |
| Adobe Illustrator CS6 | Adobe | |
Highlights:
Cellular resolution population recordings of mLOC in mice and humans
mLOC is associated with a decreased cortical repertoire of discriminable microstates
Neuronal ensemble fragmentation towards independent single neuron activity during mLOC
Acknowledgements:
This work was supported by the NIMH (R01MH101218, R01MH100561) and NEI (DP1EY024503, R01EY011787). This material is also based upon work supported by, or in part by, the U. S. Army Research Laboratory and the U. S. Army Research Office under contract number W911NF-12–1-0594 (MURI). S.H. is a Howard Hughes Medical Institute International Student Research Fellow. We thank the Yuste lab members for input and constructive discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests
This manuscript contains supplementary information (SI, Figures S1 to S5).
References
- Afraz SR, Kiani R, and Esteky H (2006). Microstimulation of inferotemporal cortex influences face categorization. Nature 442, 692–695. [DOI] [PubMed] [Google Scholar]
- Barth AL, and Poulet JF (2012). Experimental evidence for sparse firing in the neocortex. Trends Neurosci 35, 345–355. [DOI] [PubMed] [Google Scholar]
- Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, and Dehaene S (2015). Signature of consciousness in the dynamics of resting-state brain activity. PNAS 112, 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reid L, Han S, Yang W, Akrouh A, and Yuste R (2018). Triggering visually-guided behavior by holographic activation of pattern completion neurons in cortical ensembles. biorxiv, doi: 10.1101/394999 [DOI] [Google Scholar]
- Castaigne P, Lhermitte F, Buge A, Escourolle R, Hauw JJ, and Lyon-Caen O (1981). Paramedian thalamic and midbrain infarct: clinical and neuropathological study. Ann Neurol 10, 127–148. [DOI] [PubMed] [Google Scholar]
- Cavazzuti M, Porro CA, Biral GP, Benassi C, and Barbieri GC (1987). Ketamine effects on local cerebral blood flow and metabolism in the rat. J Cereb Blood Flow Metab 7, 806–811. [DOI] [PubMed] [Google Scholar]
- Cohen MR, and Kohn A (2011). Measuring and interpreting neuronal correlations. Nat Neurosci 14, 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FC, and Koch C (2005). What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci 360, 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Chen TW, Hu A, Shields BC, Guo C, Looger LL, Kim DS, and Svoboda K (2014). Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS One 9, e108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J, Cornelissen PL, Thompson HE, Sonkusare S, Hallam G, Smallwood J, and Jefferies E (2015). Automatic and Controlled Semantic Retrieval: TMS Reveals Distinct Contributions of Posterior Middle Temporal Gyrus and Angular Gyrus. The Journal of Neuroscience 35, 15230–15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Harvey CD, Tian L, Looger LL, and Tank DW (2010). Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nature neuroscience 13, 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017). On the Integration of Space, Time, and Memory. Neuron 95, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroqi-Shah Y, Sebastian R, and Woude AV (2018). Neural representation of word categories is distinct in the temporal lobe: An activation likelihood analysis. Hum Brain Mapp doi: 10.1002/hbm.24334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldt Muldoon S, Soltesz I, and Cossart R (2013). Spatially clustered neuronal assemblies comprise the microstructure of synchrony in chronically epileptic networks. PNAS 110, 3567–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores FJ, Hartnack KE, Fath AB, Kim SE, Wilson MA, Brown EN, and Purdon PL (2017). Thalamocortical synchronization during induction and emergence from propofol-induced unconsciousness. PNAS 114, E6660–E6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey BJ, and Dueck D (2007). Clustering by passing messages between data points. Science 315, 972–976. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, and Stryker MP (2014). A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F, Caporale N, and Dan Y (2008). Reverberation of recent visual experience in spontaneous cortical waves. Neuron 60, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO (1949). The Organization of Behavior (Wiley, New York: ). [DOI] [PubMed] [Google Scholar]
- Herrera CG, Cadavieco MC, Jego S, Ponomarenko A, Korotkova T, and Adamantidis A (2016). Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat Neurosci 19, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ (1982). Neural networks and physical systems with emergent collective computational abilities. PNAS 79, 2554–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House PA, MacDonald JD, Tresco PA, and Normann RA (2006). Acute microelectrode array implantation into human neocortex: preliminary technique and histological considerations. Neurosurg Focus 20(5):E4. [DOI] [PubMed] [Google Scholar]
- Houweling AR, and Brecht M (2008). Behavioural report of single neuron stimulation in somatosensory cortex. Nature 451, 65–68. [DOI] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, and Svoboda K (2008). Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451, 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG, Liu X, and Pillay S (2015). Dynamic repertoire of intrinsic brain states is reduced in propofol-induced unconsciousness. Brain Connect 5, 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG, Vizuete JA, Pillay S, and Mashour GA (2016). Repertoire of mesoscopic cortical activity is not reduced during anesthesia. Neuroscience 339, 402–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa Y, Ahmed OJ, Patel SR, Gale JT, Sierra-Mercado D, Brown EN, and Eskandar EN (2016). Dynamics of Propofol-Induced Loss of Consciousness Across Primate Neocortex. The Journal of Neuroscience 36, 7718–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennett B (2002). The Vegetative State: Medical Facts, Ethical and Legal Dilemmas (Cambridge: Cambridge University Press; ). [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, and Arieli A (2003). Spontaneously emerging cortical representations of visual attributes. Nature 425, 954–956. [DOI] [PubMed] [Google Scholar]
- Koch C (2012). In which I argue that consciousness is a fundamental property of complex things… (Cambridge, Massachusetts: MIT Press; ). [Google Scholar]
- Laaksonen L, Kallioinen M, Langsjo J, Laitio T, Scheinin A, Scheinin J, Kaisti K, Maksimow A, Kallionpaa RE, Rajala V, et al. (2018). Comparative effects of dexmedetomidine, propofol, sevoflurane, and S-ketamine on regional cerebral glucose metabolism in humans: a positron emission tomography study. Br J Anaesth 121, 281–290. [DOI] [PubMed] [Google Scholar]
- Lashley KS (1929). Brain Mechanisms and Intelligence: A Quantitative Study of Injuries to the Brain (University of Chicago Press; ). [Google Scholar]
- Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, Anderson WS, Hochberg LR, Cash SS, Brown EN, et al. (2012). Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. PNAS 109, E3377–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Garrett ME, Nauhaus I, and Callaway EM (2011). Functional specialization of seven mouse visual cortical areas. Neuron 72, 1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker B (2007). Consciousness without a cerebral cortex: a challenge for neuroscience and medicine. Behav Brain Sci 30, 63–81; discussion 81–134. [DOI] [PubMed] [Google Scholar]
- Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, and Tracey I (2010). Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. The Journal of Neuroscience 30, 9095–9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, Ayzenshtat I, Carrillo-Reid L, and Yuste R (2014). Visual stimuli recruit intrinsically generated cortical ensembles. PNAS 111, E4053–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minert A, Yatziv SL, and Devor M (2017). Location of the Mesopontine Neurons Responsible for Maintenance of Anesthetic Loss of Consciousness. The Journal of Neuroscience 37, 9320–9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi G, and Magoun HW (1949). Brain stem reticular formation and activation of the EEG. Electroencephalography and clinical neurophysiology 1, 455–473. [PubMed] [Google Scholar]
- Niell CM, and Stryker MP (2010). Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist H (1924). Certain factors affecting telegraph speed. Bell Syst Tech J 3, 324–346. [Google Scholar]
- Pavlov PI (1927). Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex (Oxford University Press; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender JW (1971). Dissociative anesthesia. JAMA 215, 1126–1130. [PubMed] [Google Scholar]
- Penfield WJ, H. H. (1954). Epilepsy and the Functional Anatomy of the Human Brain (Little, Brown, Boston: ). [Google Scholar]
- Pilge S, Jordan D, Kreuzer M, Kochs EF, and Schneider G (2014). Burst suppression-MAC and burst suppression-CP(5)(0) as measures of cerebral effects of anaesthetics. Br J Anaesth 112, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Pnevmatikakis EA, Soudry D, Gao Y, Machado TA, Merel J, Pfau D, Reardon T, Mu Y, Lacefield C, Yang W, et al. (2016). Simultaneous Denoising, Deconvolution, and Demixing of Calcium Imaging Data. Neuron 89, 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Friedman J, and Golshani P (2013). Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci 16, 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem AB, Ayaz A, Jeffery KJ, Harris KD, and Carandini M (2013). Integration of visual motion and locomotion in mouse visual cortex. Nat Neurosci 16, 1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman CD, Britten KH, and Newsome WT (1990). Cortical microstimulation influences perceptual judgements of motion direction. Nature 346, 174–177. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Weiss SA, McKhann G Jr., Goodman RR, Yuste R, Emerson RG, and Trevelyan AJ (2012). Evidence of an inhibitory restraint of seizure activity in humans. Nat commun 3, 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nature methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidman E, Berry MJ 2nd, Segev R, and Bialek W (2006). Weak pairwise correlations imply strongly correlated network states in a neural population. Nature 440, 1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder KE, Irwin ZT, Gaidica M, Nicole Bentley J, Patil PG, Mashour GA, and Chestek CA (2016). Disruption of corticocortical information transfer during ketamine anesthesia in the primate brain. Neuroimage 134, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, and Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, Fellows MR, and Normann RA (2003). Robust, automatic spike sorting using mixtures of multivariate t-distributions. J Neurosci Methods 127, 111–122. [DOI] [PubMed] [Google Scholar]
- Steriade M (2003). The corticothalamic system in sleep. Front Biosci 8, d878–899. [DOI] [PubMed] [Google Scholar]
- Tononi G (2008). Consciousness as integrated information: a provisional manifesto. Biol Bull 215, 216–242. [DOI] [PubMed] [Google Scholar]
- Tononi G, Boly M, Massimini M, and Koch C (2016). Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci 17, 450–461. [DOI] [PubMed] [Google Scholar]
- van der Maaten L, Hinton G (2008). Visualizing Data using t-SNE. Journal of Machine Learning Research 9, 2579–2605. [Google Scholar]
- van Kemenade BM, Arikan BE, Podranski K, Steinstrater O, Kircher T, and Straube B (2018). Distinct Roles for the Cerebellum, Angular Gyrus, and Middle Temporal Gyrus in Action-Feedback Monitoring. Cereb Cortex doi: 10.1093/cercor/bhy048 [DOI] [PubMed] [Google Scholar]
- Welch TA (1984). A Technique for High-Performance Data-Compression. Computer 17, 8–19. [Google Scholar]
- Yang W, Miller JE, Carrillo-Reid L, Pnevmatikakis E, Paninski L, Yuste R, and Peterka DS (2016). Simultaneous Multi-plane Imaging of Neural Circuits. Neuron 89, 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, and Yuste R (2017). In vivo imaging of neural activity. Nat Methods 14, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, and Denk W (1995). Dendritic spines as basic units of synaptic integration. Nature 375, 682–684. [DOI] [PubMed] [Google Scholar]
- Yuste R, and Katz LC (1991). Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron 6, 333–344. [DOI] [PubMed] [Google Scholar]
- Ziv J, and Lempel A (1978). Compression of Individual Sequences Via Variable-Rate Coding. Ieee T Inform Theory 24, 530–536. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


