Abstract
Objective:
Identification of prognostic biomarker candidates for stratification and long-term surveillance of oral leukoplakia progressing to cancer via a systematic literature review.
Materials and Methods:
Systematic searches with no date restrictions were conducted on March 29, 2018, targeting the databases PubMed (Ovid), EMBASE (Ovid), EBM (Ovid), and Web of Science (ISI). Bias was assessed using the Quality in Prognosis Studies tool. Biomarkers were stratified based on hallmarks of cancer.
Results:
Inclusion criteria were met by 25/3415 studies. A range of biomarkers were evaluated experimentally for risk stratification, prognosis, and surveillance of oral leukoplakia in tissue, blood, and saliva. However, the studies were highly heterogeneous and require further validation. Biomarkers reported in these studies included inflammatory or oxidative markers, growth factors, ion channels, genetic and cellular regulatory factors, and epigenetic biomarkers. Studies tended to include small sample sizes, under-reported or variably reported histopathological data, did not address potential confounding, reported limited/variable follow-up data or lacked a control group. Inclusion of subsets from chemoprevention trials may have introduced bias regarding reported malignant transformation rates and accuracy of prognostic biomarkers.
Conclusions:
This review identified insufficient longitudinal evidence to support validated prognostic biomarkers for oral leukoplakia. Further studies are needed to identify molecular targets with the potential to mitigate risk of malignant transformation.
Keywords: biomarkers, tumor, cancer progression, leukoplakia, neoplastic cell transformation, prognosis
INTRODUCTION
Oral squamous cell carcinoma (OSCC) continues to have significant clinical and economic impact at the international level. It is associated with a high rate of mortality, which often is a consequence of delayed diagnosis due to lack of screening programs, low health literacy relative to OSCC, and lack of access to care. The World Health Organization (WHO) projected 354,864 new cases of oral cavity and lip cancer in 2018 (Bray et al., 2018).
Oral potentially malignant disorders (OPMD), of which leukoplakia is one of several phenotypes, represent a group of conditions and lesions with variable propensity for oncogenic potential (Warnakulasuriya et al., 2007). In the era of precision medicine, there is burgeoning interest in defining and characterizing relative risk for oral cancer emergence in association with OPMD. As the understanding of the molecular pathogenesis of oral cancer continues to expand, there is active interest in identifying biomarkers that could provide ability for clinicians to longitudinally track key molecular signals associated with OPMD, and intervene prior to neoplastic transformation. The overarching aims of the systematic reviews performed by the Precision Medicine Group of World Workshop on Oral Medicine VII were to:
Assess if prognostic biomarkers could accurately stratify the risk of malignant transformation of oral leukoplakia.
Assess the relationship between prognostic biomarkers and the patient’s risk profile including lesion clinico-pathologic characteristics in addition to patient’s risk factors.
Evaluate whether biomarkers could independently predict malignant transformation of oral leukoplakia.
Establish the minimum follow-up intervals required for biomarkers to predict malignant transformation.
Assess the efficacy of the investigated biomarkers and management protocol. Formulate an algorithm that would help clinicians to provide the best supported evidence-based management protocol to patients with oral leukoplakia.
After oral submucous fibrosis, oral leukoplakia is the most common OPMD, with a worldwide prevalence of 4.11% (95% CI: 1.98–6.97) (Mello et al., 2018). Leukoplakia has been defined by the WHO as “a white plaque of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer” (Warnakulasuriya et al., 2007). Risk factors for oral leukoplakia are similar to those observed in oral cancer and include tobacco smoking, heavy alcohol consumption, areca nut chewing (especially in South Asian countries), immunosuppression (e.g., HIV/AIDS, post-organ transplantation), personal or family history of cancer (60–70%), ultraviolet light exposure (for lip lesions only) and selected syndromes (e.g., dyskeratosis congenita) (Villa & Woo, 2017), (Warnakulasuriya, 2018).
Leukoplakia is a clinical diagnosis, most commonly presenting in two main phenotypes: homogeneous and non-homogeneous leukoplakia. Proliferative verrucous leukoplakia represents a third, rarer, high risk subtype (Warnakulasuriya, 2018). Irrespective of type of oral leukoplakia, the gold standard for final diagnosis remains incisional biopsy. Risk of malignant transformation depends on the clinical form and the grade of dysplasia, although other clinical and histopathological parameters have been reported as drivers (Speight et al., 2018). Non-homogeneous leukoplakias carry a 20%–25% risk of cancer progression versus 0.6% – 5% in homogeneous cases (Napier & Speight, 2008, van der Waal & Axell, 2002, Reibel, 2003).
A key step to better understanding oral leukoplakia outcomes is to identify the molecular factors that drive malignant progression, as these factors may also represent attractive candidates for targeted therapies. With the advent of precision medicine, a growing evidence base has explored predictive and prognostic biomarkers for oral leukoplakia. This paper systematically reviewed longitudinal studies which specifically aimed to: 1) assess whether prognostic biomarkers could accurately stratify the risk of progression of oral leukoplakia to cancer, and 2) evaluate the reliability of biomarkers in long-term surveillance of oral leukoplakia. Future studies will focus on the other overarching aims as mentioned above.
MATERIALS AND METHODS
This study was conducted by the Precision Medicine Work Group within the World Workshop on Oral Medicine VII (WWOM VII). Results are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009b). PICO (Patients, Intervention/exposure/prognostic factor, Comparison group and Outcome) was used to formulate the research question.
Patients: patients with oral leukoplakia
Intervention/Exposure/Prognostic factor: Biomarkers in human specimens (saliva, blood, gingival crevicular fluid, oral tissues)
Comparison: healthy controls or patients with oral squamous cell carcinoma
Outcome: squamous cell carcinoma
Studies: longitudinal (prospective) studies
Study selection
Studies that were potentially eligible for inclusion evaluated biomarker expression in: (i) human specimens (saliva, blood, gingival crevicular fluid, oral tissues) with follow-up data (over time); (ii) patients with oral leukoplakia compared to healthy controls; or (iii) patients with oral leukoplakia compared to patients with oral squamous cell carcinoma. Studies were excluded if they were: 1) studies investigating only non-human tissues, 2) withdrawn/retracted studies, 3) reviews, 4) case reports, 5) commentaries, 6) opinion articles, 7) letters to the Editor, or 8) congress abstracts.
The selection of studies for review was conducted in the following five steps as illustrated in Figure 1.
Figure 1.

Selection of studies for systematic review of prognostic biomarkers for oral leukoplakia.
Step 1: Electronic literature searches were conducted on March 29, 2018, using the PubMed (Ovid), Embase (Ovid), Evidence Based Medicine (EBM) Reviews (Ovid), and Web of Science (ISI) databases with no publication year restrictions. The search strategies according to the syntax rules of each database are displayed in Supplementary Table S1. The identified citations were imported into the electronic database Endnote X8 (Clarivate Analytics, Philadelphia, PA, USA). De-duplication was achieved by the Endnote software and manually by the two reviewers (AVil, AC).
Each subsequent step was conducted by two blinded reviewers (AVil, AC) to exclude records ineligible for inclusion, based on sequential review of title only, title and abstract, and finally full text (Step 4). The applied exclusion categories are shown in Table 1. When more than one exclusion category would pertain to a study, the most important was selected. At each step, review discordance between the reviewers was resolved by discussion between the two reviewers (AVil, AC) under supervision of the senior reviewer (CSF).
Table 1.
Reason for exclusion after full text assessment.
| Exclusion Category |
Reason for Exclusion | Number of Studies |
|---|---|---|
| N0 | Not English language | 17 |
| N1 | Not original study (review, guideline, editorial, conference abstract) | 2 |
| N2 | No human tissue or only immortalized human cell lines in vitro | 3 |
| N3 | No clinical diagnosis of oral leukoplakia, only dysplasia | 332 |
| N4 | Cancer patients only: resection margins/perilesional tissue in oral/oropharynx squamous cell carcinoma; history of cancer prior to or during study; undergoing chemo or radiation therapy | 6 |
| N5 | Predatory journals | 2 |
| N6 | Retracted articles, or title includes “expression of concern” | 3 |
| N7 | Other reason for not meeting inclusion criteria | 53 |
| All (N1-N7) | Reports ineligible for inclusion | 418 |
Step 2: Among unique records screened by title, only 1,006 out of 3,145 were retained. Cohen’s kappa statistic for inter-reviewer agreement was 0.95 (95% CI: 0.94–0.96) and the absolute agreement between the two reviewers was 96.7%.
Step 3: Screening by both title and abstract resulted in retention of 749 of 1,006 for full text review. The absolute agreement between the two reviewers was 96.7% and the kappa statistic was 0.92 (95% CI: 0.91–0.94).
Step 4: Based on review of the full text, 331 of 749 were retained based on identification of “leukoplakia” as the definitive diagnosis. The reasons for excluding the 418 studies are shown in Table 1.
Step 5: The remaining 331 papers were allocated to one or more of the following eligibility categories, which respectively assessed the efficacy of biomarkers in:
-
-
Y1: stratifying the risk of progression of oral leukoplakia to cancer (prospective longitudinal studies only).
-
-
Y2: long-term surveillance of oral leukoplakia (longitudinal studies only).
-
-
Y3: diagnosis of oral leukoplakia as an adjunct to oral examination (prospective case-control/cross-sectional studies only)
-
-
Y4: progression of oral leukoplakia in a retrospective data set (case-control/cross-sectional studies only)
-
-
Y5: differentiating oral leukoplakia from controls; correlation of differential biomarker(s) expression with diverse clinico-pathological parameters (case-control/cross-sectional studies only).
Inter-reviewer absolute agreement for Step 5 was 85.47% and the kappa statistic was 0.59 (95% CI: 0.50–0.68), but 100% absolute agreement was reached upon a second revision.
As per title, the current report was limited to the 25 longitudinal studies included in the categories Y1 regarding progression risk and Y2 on long-term surveillance. Consequently, 306 of 331 studies classified by Y categories that did not meet eligibility for inclusion in Y1 or Y2 categories due to either lack of eligibility for any of the Y category or because they were included into categories Y3 – Y5, were excluded from further review in the current report. Of note, all papers included in the Y2 category were also included in the Y1 category and the results will therefore be reported collectively.
The reviewer (AVil) and the consultant (CSF) extracted and entered into specifically developed forms (Microsoft Excel 2010, Redmond, Washington, USA) all relevant data from the selected papers. Risk of bias for the 25 included studies was assessed independently by one reviewer (AVil) and the consultant (CSF) using the ‘Quality in Prognosis Studies’ (QUIPS) tool (Hayden et al., 2013), which evaluates the six domains ‘study participation’, ‘study attrition’, ‘prognostic factor measurement’, ‘outcome measurement’, ‘study confounding’, and ‘statistical analysis and reporting’. Discord was resolved by consensus.
Statistical analysis
Absolute percent inter-reviewer agreement and Cohen’s kappa coefficient were calculated using IBM Statistics 23 (SPSS, Chicago, Illinois, USA). The heterogeneity of the studies and the high number of different biomarkers studied prevented any quantitative analysis of the results, so no meta-analysis was performed.
RESULTS
Of the 331 publications eligible for the categories Y1 – Y2 out of the originally identified 3,415 unique records, 25 studies were allocated to groups Y1 and Y2 and therefore included in this report (Figure 1). Results are reported by types of biomarkers stratified to the hallmarks of cancer (Hanahan & Weinberg, 2011). The main characteristics of the studies are presented in Table 2, and a detailed description of the biomarkers identified is reported in the Supplementary Results.
Table 2.
Findings from the 25 eligible studies using biomarkers with leukoplakia specimens.
| Author Year Country Study Design Duration |
Bio-marker* | Specimen Type |
Leukoplakia (n) |
Risk Factors: Leukoplakia No. of cases /smoking or alcohol use |
Age, (yr) Mean (±SD)** (Range) |
Sex #M/#F |
Histopathology No. of cases/type |
Site of lesion: No. of cases/location |
Outcomes/Results Biomarker~Malignanc y Link |
|---|---|---|---|---|---|---|---|---|---|
| Tissue invasion, metastasis and inflammatory markers | |||||||||
|
Chang et al. 2013 China Follow up: 28 months (only available for OSCC patients) |
IL-6 M-CSF TGF-β1 ICAM-1 E-selectin CRP SAA MMP-2 MMP-9 |
serum | 46 | NA |
Leukoplakia Mean 46.7 Range: (26–72) Healthy: Mean 48.3 Range: (27–82) |
44/2 | NA |
Leukoplakia samples:
4 tongue 30 buccal mucosa 2 hard palate 2 retromolar trigone 5 tongue+buccal mucosa 2 lip+buccal mucosa |
Significant difference: TGF- β1 (AUC: 0.756, p<0.001); CRP (AUC: 0.704, p<0.001) MMP-9; (AUC: 0.729, p<0.001) in leukoplakia vs. healthy controls. Sensitivity to detect leukoplakia or OSCC following evaluation of MMP-9, MMP-2, TGF-β1, CRP, and E-selectin: 67.4% and 90%, respectively. |
|
Massarelli et al. 2005
USA Follow up: median of 28 months |
p27 &
E-cadherin protein expression |
tissue (biopsy) | 31 |
Smokers: E-cadherin leukoplakia group (laryngeal/oral): 26 former 11 current 20 never p27 leukoplakia group (laryngeal/oral) 23 former 8 current 9 never |
E-cadherin group: Mean 58.3 p27 group: mean 57.3 |
E-cadherin group: 15/31 p27 group: 14/26 |
E-cadherin group: 5 hyperplasia/ hyperkeratosis Dysplasia: 13 mild 16 moderate 12 severe p27 group: 5 hyperplasia/ hyperkeratosis Dysplasia 13 mild 12 moderate 10 severe |
NA | 21 mo post follow up: Loss of E-cadherin expression 25/46 (54%) Development of OSCC: 14/23 (61%), No. who developed OSCC with: - leukoplakia (14/31 (45%)). - p27 loss (13/19 (68%)) - p27 retention: (7 /21 (33%)). p27 loss was an independent predictor of malignant transformation (log‐rank test; P=0.02). E‐cadherin and p27 loss: was associated with a decreased time to disease progression (log‐rank test; P=0.04). |
| Serum oxidative markers | |||||||||
|
Rai et al. 2010
India Follow up: 181 days |
MDA 8-OHdG vitamin C vitamin E |
saliva serum |
25 | NA | Age range: 17–50 |
13/12 | NA | NA | Compared with healthy controls, - MDA and 8-OHdG levels were increased in oral leukoplakia, submucosal fibrosis and oral lichen planus cases, (p<0.05); serum and salivary vitamin C and E levels were decreased (p<0.05). Mean salivary and serum MDA, 8-OHdG, and vitamin C and E levels were significantly different in patients with oral leukoplakia, submucosal fibrosis and oral lichen planus before the intake of curcumin and after treatment (p<0.05). |
| Proliferation without exogenous stimulation | |||||||||
| Growth factors | |||||||||
|
Beenken et al. 1994
USA Follow up: 3 months |
EGFR
TGF-α HER-2/neu |
tissue (biopsy) | 9 | NA | NA | NA/NA |
Dysplasia:
5 mild 1 moderate 3 severe |
NA | Pretreatment expression scores for EGFR, TGF-a, and HER-2/neu in leukoplakia were increased when compared to their expression scores in normal-appearing mucosa. TGF-α expression ratios in leukoplakia were significantly reduced after treatment with 13-cis-retinoic acid. |
|
Beenken et al. 1999
USA Follow up: 1 month |
TGF-α EGFR |
tissue (biopsy) | 11 | NA | NA | NA/NA |
Dysplasia:
5 mild 3 moderate 3 severe |
NA | TGF- α and EGFR scores were increased in dysplastic oral leukoplakia specimens when compared with adjacent normal appearing mucosa. TGF-α expression scores in normal-appearing mucosa adjacent to dysplastic tissue were higher than those seen in healthy controls. |
|
Wan et al. 1999 USA Follow up: 1 month |
neu proteins | blood & oral mucosa cells | 25 | NA | NA | NA/NA | NA | NA | Changes in the protease activity in oral mucosal cells after therapy correlated with the changes in the neu protein levels in oral mucosal cells (p< 0.001) and serum (p< 0.001). In addition, the level of neu protein in oral mucosal cells correlated with the serum neu protein concentration in patients with oral leukoplakia before treatment (p< 0.001). |
|
Uehara et al. 2010
Japan Follow up: NA |
mean nuclear variation | tissue (biopsy) | 7 | NA | NA | 8/12 |
Leukoplakia samples:
epithelial dysplasias |
Leukoplakia samples:
4 tongue 1 hard palate 1 soft palate 1 buccal mucosa |
The mean nuclear areas: Before PDT: range: 54.1 to 158.8 μm2 (median=91.4 μm2, SD=23.0 μm2); After PDT: range: 24.6 to 106.8 μm2 (median=66.5 μm2, SD=21.7 μm2; p=0.0920). Significant differences in PCNA LI values before and after PDT were observed in: Recurrent grp (p=0.0496); non-recurrent grp (p =0.0188) with decreased PCNA LI values after PDT). |
| Cell signaling | |||||||||
|
Saintigny et al. 2011
USA Follow up: median of 7.5 years |
proteasome machinery, MYC ribosomal components | tissue (biopsy) | 35 |
Smokers: 22 current 35 former 29 never Alcohol use: 49 current 8 former 29 never |
median: 57.5 range: (23–90) | 45/41 | 32 dysplasia 54 hyperplasia |
NA | Gene sets associated with proteasome machinery, MYC upregulation as well as ribosomal components were associated with a high risk to develop OSCC. |
|
Saintigny et al. 2018
USA Follow up: The median follow-up of the 51 patients who did not develop OSCC was 6.08 years |
MET | tissue (biopsy) | 35 (pts who developed cancer) |
Smokers: 42 current 45 former 33 never Alcohol use: 70 current 10 former 40 never |
NA | 61/59 | 44 dysplasia 76 hyperplasia |
NA | No MET expression: Leukoplakia cases (26/120); 21.7%). MET expression: low (score 1), 34 (28.3% mild (score 2): 39(32.5%) high (score 3) 21(17.5%). Time-to-cancer-progression varied across 4 Met expression grps (score 0 through 3), p=0.001). Oral cancer–free survival -decreased in patients with high MET expression levels (p<0.001). MET overexpression in leukoplakia: HR: 3.84 (95%CI: 1.59–9.27, p<0.003) for malignant transformation. |
|
Sakata et al. 2017
Japan Follow up: NA |
SMAD4 | tissue (biopsy) | 127 (leukoplakias that did not transform) |
NA | median: 61.7 range: (19–87) | 67/60 |
Cases:
13 no dysplasia 7 mild dysplasia 3 moderate/ severe dysplasia Controls: 88 no dysplasia 30 mild dysplasia 9 moderate/ severe dysplasia |
64 (42.7%) gingiva 52 (34.7%) tongue 21 (14.0%) buccal mucosa 13 (8.7%) other oral cavity sites |
No. with malignant transformation: 23/150 (15.3%). Malignant transformation associated with: SMAD4 expression (p=0.0017) -lymphocyte infiltration (p= 0.0054). Low SMAD4 expression: -significant prognostic factor in pts with leukoplakia (hazard ratio, 2.632; p=0.043) -correlated with high lymphocyte infiltration (p=0.00035), -significant correlation between low SMAD4 expression and high lymphocyte infiltration (p=0.00027). |
| Insensitivity to antigrowth signals | |||||||||
| Cell cycle regulators | |||||||||
|
Lee et al. 2000
USA Follow up: 7 years (range: 0.2–10.6) |
p53
RAR-β CP LOH micro-nuclei |
tissue (biopsy) | 70 |
Smokers: 39 current 31 former/ never Tobacco chewers: 10 yes/60 no Alcohol use: 49 current 21 former/ never Cancer history: 11 yes/59 no |
NA | 33/37 | 9 moderate/ severe dysplasias 61 hyperplasia/ mild dysplasia | 27 tongue/FOM 43 others |
31.4% of patients developed OSCC. High chromosomal polysomy (CP) expression, p53 protein accumulation in the parabasal layer, and LOH at chromosome 3p or 9p: associated with higher cancer risk (p=0.0008). |
|
Nagao et al. 2017
Japan Follow up: NA |
p53
ki67 |
tissue (biopsy) | 4 |
Smokers: 1 former 3 never Alcohol use: 2 regular 2 never |
median: 66 | 2/2 |
Responders: dysplasia (1/4) no dysplasia (3/4) Non-responders: dysplasia (4/12) no dysplasia (5/12) |
Responders: tongue/FOM (4/4); Non-responders: (7/12), other (5/12) |
p53 expression: greater in basal layers vs. para-basal layers. Mean para-basal labeling index (LI) of p53 was higher in non-responders (26.0) than in responders (11.2) (p=0.028). Ki67 LIs were similar between groups. |
|
Tanić et al. 2009
Serbia Follow up: 4 years |
p53 |
Cases:
tissue (biopsy) Controls: blood |
32 | All smokers | NA | NA/NA | 30 mild/ no dysplasia 32 moderate/ severe dysplasia |
NA | p53 gene was mutated in 13 cases (40.6%). The majority of mutations were localized on exon 6. Four patients with moderate instability and mutated p53 underwent malignant transformation. |
|
Visioli et al. 2012
Brazil Follow up: 3–6 years |
p53 p21WAF1 | tissue (biopsy) | 36 | NA | Mean: 50.1 SD (±14.5) |
19/17 | NA | 11 gingiva 10 tongue 8 buccal mucosa 6 lip 1 palate |
Overexpression of p53 and p21WAF1: observed in dysplastic and non-dysplastic leukoplakias. |
| Loss of heterozygosity | |||||||||
|
Mao et al. 1996
USA Follow up: median time to OSCC progression: 63 months (range: 5–92) |
markers located at chromosomes
9p21 & 3p14 |
tissue (biopsy) | 37 | 22 smokers | 57.5 | 20/17 | 32 dysplasia 52 hyperplasia and/or hyper-keratosis |
NA | 8/37(22%) developed OSCC. LOH: (19/37) (51%). -Cases (37%) with LOH at either 9p21 or 3p14 (or both): developed cancer -One case (6%) without LOH underwent malignant transformation. Time to cancer development was shorter in LOH (p=0.039). |
| Ion channels | |||||||||
|
Fernandez-Valle et al. 2016 (A) Spain Follow up: median time to OSCC progression: 25.5 months (range: 7–308). |
Kv3.4 | tissue (biopsy) | 62 | 28 smokers (45%) of whom 14 (22.6%) also consumed alcohol | 61.1 SD: +12.41 Range: (30–85) |
35/27 | 43 hyperplasia 8 mild dysplasia 7 moderate dysplasia 4 severe dysplasia/ carcinoma in situ |
Cannot discriminate between leukoplakia and OSCC | Detection of Kv3.4+ immunostaining noted in: -hyperplastic leukoplakias 7/ 43 (16%) -leukoplakic lesions with dysplasia 8/16 (50%) (p=0.008). No correlation observed between Kv3.4 expression in oral leukoplakia compared to OSCC samples (p=0.86). Leukoplakias with Kv3.4 neg expression: 8/13 (61.5%) showed strong Kv3.4 immunostaining in after subsequent OSCC development |
|
Fernández-Valle et al. 2016 (B) Spain Follow up: median time to OSCC progression: 39 months (range: 7 −226) |
HERG1 protein expression | tissue (biopsy) | 62 | 28 smokers (45%) of whom 14 (22.6%) also consumed alcohol | Mean 61.1 SD +12.4 Range (30–85) |
27/35 | 43 hyperplasia 8 mild dysplasia 7 moderate dysplasia 4 severe dysplasia/ carcinoma in situ |
NA | HERG1 status of lesions on OSCC development: -HERG1-(+): 8/22 (36%); HERG1(-neg): 18/39 (46%) |
| Sustained angiogenesis | |||||||||
| Growth factors | |||||||||
|
Nayak et al. 2015
India Follow up: median time to OSCC progression: 29.41 months |
FGF-2
FGFR-2 FGFR-3 |
tissue (biopsy) | 43 | NA | NA | NA/NA | NA | NA | Expression of FGF-2 and FGFR-2: associated with HR 28.2 (95% CI: 3.29–241.72; p<0.002) and HR 11.5 (95% CI: 2.20–59.91; p<0.004) of cancer progression, respectively. |
|
Yang CZ et al. 2014
NA country Follow up: up to 5 yrs |
GDF15 | serum | 24 | NA | NA | NA/NA | NA | NA | Serum GDF15 level: increased leukoplakia and OSCC cases compared with healthy controls (p< 0.001). OSCC cases with GDF15 <346.9 ng/L had improved 3-year disease-free survival rate compared to cases withGDF15 ≥ 346.9 ng/L. (64.3% vs 56.5%) |
| Podoplanin | |||||||||
|
Kawaguchi et al. 2008
USA Follow up: 7.5 years |
podoplanin | tissue (biopsy) | 163 |
Smokers: 52 current 60 former Alcohol use: 85 current 18 former |
NA | NA/NA | 101 hyperplasias 49 dysplasias |
NA | Podoplanin (+) lesions: more common in older patients (p=0.016), females (p= 0.02), and those with dysplastic leukoplakias (p=0.04). 23% of the patients progressed to OSCC. Podoplanin was an independent risk factor for malignant transformation (HR=3.087; 95% CI, 1.530–6.231; p= 0.002). |
|
Saintigny et al. 2009
USA Follow up: median of 7.5 years |
ΔNp63 podoplonin | tissue (biopsy) | 162 |
Smokers: 56 current 65 former 41 never Alcohol use: 93 current 19 former 50 never |
NA | 85/77 | NA | NA | Oral cancer was associated with: -ΔNp63 and podoplanin status (p< 0.0001), -ΔNp63 status and intraepithelial inflammatory cell clusters (EIC) (p= 0.0003), -between podoplanin status and presence of EIC (p=0.0012). Presence of all three biomarkers was associated with dysplastic histology (p= 0.016) and older age (p=0.004). Cumulative incidence for developing oral cancer at 3 years of follow-up: 53% for cases with all three biomarkers compared to 11% of other patients. Patients with podoplanin-positive leukoplakia and those with ΔNp63-positive leukoplakia had a higher incidence of OSCC (p<0.0001). |
| Glucose regulated proteins | |||||||||
|
Lin et al. 2010
Taiwan Follow up: median of 5.4 years (range: 0.02–6.8) |
GRP-78 | tissue (biopsy) | 28 | NA | NA | NA/NA | NA | Over-expression of GRP78 was associated with increasing malignant potential in 14% of leukoplakia patients. Patients with GRP78 over-expression had a poorer 6-year same-site premalignancy-free survival rate than those without (44.0% vs 89.6%, respectively; p<0.002). | |
| Gene transcription modification | |||||||||
| miRNA expression | |||||||||
|
Xiao et al. 2012
China Follow up: median of 32 months (range: 20–47) |
miRNA | tissue (biopsy) | 7 (progressing leukoplakias) |
Smokers: 3 current 4 never/former Alcohol use: 4 current 3 never/former |
Mean: 55.4 SD±16.3 | 3/4 | Non-transformed leukoplakias: 11 no/mild dysplasia 5 moderate dysplasia 4 severe dysplasia |
NA | Leukoplakia cases who OSCC had -upregulation of: miR-31, miR-142–5p, miR-33a, miR-1259, miR-146b-5p, miR-886–3p, miR-886–5p, miR-519d, and miR-301a -downregulation of: miR-572, miR-611, miR-602, miR-675, miR- 585, miR-623, miR-637, and miR-1184. FISH analysis confirmed high miR-31 expression in leukoplakia that underwent malignant transformation. |
|
Yang Y et al. 2013
China Follow up: median time of 35 months (range: 26–46) |
miRNA | saliva | 15 |
Smokers: 7 current 3 former 5 never/former |
Mean 57.6 | 3/4 | All 15 samples were LGD (mild to moderate dys-plasia). Among those that progressed 5 were severe dysplasia (3/5 with SCC micro-foci), 2 CIS, 1 moderate to severe dysplasia SCC micro-foci |
5 lateral tongue 2 lateral palate 6 buccal mucosa 1 palate 1 lower lip |
There was up-regulation of miR-708, miR-10b, miR-19a, miR-30e, miR-26a, miR-660 and down-regulation of miR-99, miR-15a, miR-197, miR-145 and miR-150. miRNAs were differentially expressed in non-progressing low grade dysplasias with different progression potentials. miR-181c and miR-181b were under-expressed in progressing low grade dysplasias and over-expressed in non-progressive low grade dysplasias. |
| DNA methylation | |||||||||
|
Foy et al. 2015
USA Follow up: For the 12 patients who did not develop OSCC (median follow-up, 7.64 years) and the patients who did develop OSCC (group B, median follow-up, 2.15 years) |
DNA methylation | tissue (biopsy) | 24 | NA | NA | NA/NA | Reported in the supplementary material | NA | 86 candidate genes were identified. Methylation of PENK, FOXI2, and AGTR1 promoter regions were associated with the development of OSCC. |
Please see Table 4 for explanation of biomarkers;
Some studies reported the median and not the mean;
#: number; CIS: carcinoma in situ; F: females; FOM: floor or the mouth; leukoplakia (n): leukoplakia group sample size; LOH: loss of heterozygosity; M: males; NA: not available; OSCC: oral squamous cell carcinoma; PDT: photodynamic therapy; pt(s): patient(s); SCC: squamous cell carcinoma y: year(s).
Types of biomarkers
Biomarkers reported in these studies (Table 2) included inflammatory or oxidative markers (Chang et al., 2013, Massarelli et al., 2005b, Rai et al., 2010), growth factors (Beenken et al. 1994, Beenken et al., 1999, Wan et al., 1999, Uehara et al., 2010) cell signaling biomarkers (Saintigny et al., 2011, Saintigny et al., 2018, Saintigny et al., 2018, Sakata et al., 2017) genetic and cellular regulatory factors (Lee et al., 2000, Nagao et al., 2017, Tanić et al., 2009, Visioli et al., 2012, Mao et al., 1996), ion channels (Fernandez-Valle et al., 2016a, Fernandez-Valle et al., 2016b) sustained angiogenesis factors (Nayak et al., 2015, Yang CZ et al., 2014, Kawaguchi et al., 2008, Saintigny et al., 2009, Lin et al., 2010, ) and epigenetic biomarkers (Xiao et al., 2012, Yang et al., 2013, Foy et al., 2015). Studies tended to include small sample sizes, under-reported or variably reported histopathological data, did not address potential confounding, reported limited/variable follow-up data or lacked a control group. Notably, 14 of the 25 studies were components of chemoprevention trials (Saintigny et al., 2009, Mao et al., 1996, Saintigny et al., 2011, Uehara et al., 2010, Massarelli et al., 2005b, Saintigny et al., 2018, Foy et al., 2015, Nagao et al., 2017, Kawaguchi et al., 2008, Rai et al., 2010, Lee et al., 2000, Yang et al., 2013, Wan et al., 1999, Beenken et al., 1994). Inclusion of subsets from chemoprevention trials may have introduced bias regarding reported malignant transformation rates and accuracy of prognostic biomarkers.
Quality assessment of the studies
Low risk of bias was observed in ‘statistical reporting’, ‘study participation’, ‘study attrition,’ and ‘outcome measurement’ (81%, 70%, 52% and 52% of included studies, respectively); while moderate risk of bias in ‘study confounding’ was found in 70% of studies and in ‘outcome measurement’, ‘prognostic factor measurement,’ and ‘study attrition’ in 48% of the studies. Percentage of studies with high risk of bias was relatively low and varied between 0 and 11% across the different domains (Figure 2; Table 3).
Figure 2.
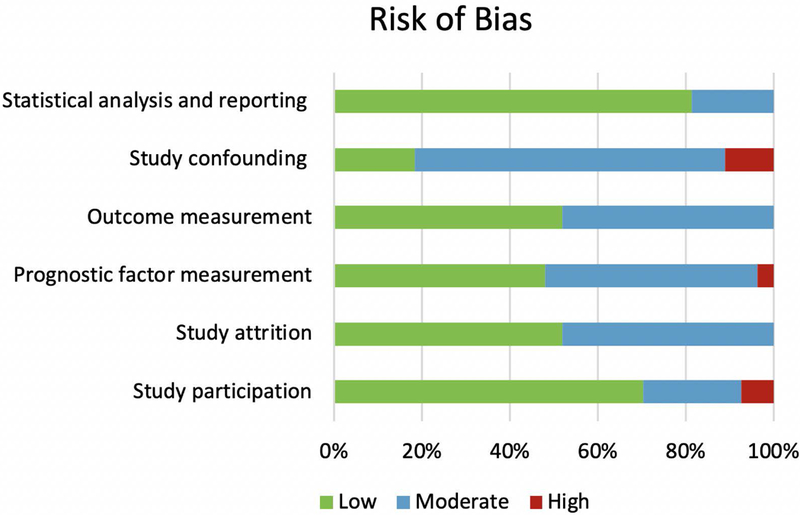
Risk of bias in the 25 included studies according to the Quality in Prognosis Studies (QUIPS) criteria (Hayden et al., 2013).
Table 3.
Quality assessment of the studies according to the Quality in Prognosis Studies (QUIPS) criteria (Hayden et a.l, 2013), illustrated in Figure 2.
| Author | Year | Study participation |
Study attrition |
Prognostic factor measurement |
Outcome measurement |
Study confounding |
Statistical analysis and reporting |
|---|---|---|---|---|---|---|---|
| Beenken S. et al. | 1994 | high | moderate | moderate | low | moderate | moderate |
| Beenken S. et al. | 1999 | high | moderate | moderate | low | moderate | moderate |
| Chang P. et al. | 2013 | moderate | moderate | high | moderate | moderate | low |
| Fernandez-Valle Á. et al. (A) | 2016 | low | low | low | low | low | low |
| Fernández-Valle Á. et al. (B) | 2016 | moderate | low | low | moderate | moderate | low |
| Foy JP. et al. | 2015 | low | low | low | low | moderate | low |
| Kawaguchi H. et al. | 2008 | low | low | low | moderate | moderate | low |
| Lee JJ. et al. | 2000 | low | low | low | low | moderate | low |
| Lin CY. et al. | 2010 | low | moderate | moderate | moderate | high | low |
| Mao L. et al. | 1996 | low | low | low | moderate | low | low |
| Massarelli E. et al. | 2005 | low | low | moderate | low | low | low |
| Nagao T. et al. | 2017 | moderate | low | moderate | moderate | moderate | moderate |
| Nayak S. et al. | 2015 | low | moderate | moderate | moderate | moderate | low |
| Rai B. et al. | 2010 | moderate | moderate | moderate | moderate | moderate | moderate |
| Saintigny P. et al. | 2009 | low | low | low | low | moderate | low |
| Saintigny P. et al. | 2011 | low | low | low | low | low | low |
| Saintigny P. et al. | 2018 | low | low | low | low | moderate | low |
| Sakata J. et al. | 2017 | low | low | low | low | low | low |
| Tanić N. et al. | 2009 | low | low | low | low | moderate | low |
| Uehara M. et al. | 2010 | low | moderate | moderate | moderate | moderate | low |
| Visioli F. | 2012 | low | low | moderate | moderate | moderate | low |
| Wan XS. | 1999 | low | moderate | moderate | moderate | moderate | moderate |
| Xiao W | 2012 | low | moderate | low | moderate | moderate | low |
| Yang CZ | 2014 | moderate | moderate | low | moderate | moderate | low |
| Yang Y | 2013 | moderate | moderate | moderate | low | moderate | low |
DISCUSSION
This systematic review was directed to identification of biomarkers that could predict the likelihood of malignant progression over time in patients affected by oral leukoplakia (Table 4). Therefore, only studies applying longitudinal designs were included. Overall, the 25 included studies documented a wide range of biomarkers derived from three different anatomic sources: serum, tissue, and saliva. Heterogeneity of biomarkers investigated across these studies precluded direct inter-study comparison in virtually all cases.
Table 4.
Biomarkers used with leukoplakia specimens in the 25 studies displayed in Table 2.
| Biomarker Acronym |
Biomarker Name & Function |
|---|---|
| CP | chromosomal polysomy; |
| CRP | C-reactive protein; acute inflammatory marker |
| E-cadherin | molecule that makes cells adhere to each other (cadherin=“calcium-dependent adhesion”) |
| eGFR, EGFR | epidermal growth factor receptor |
| E-selectin | cell adhesion molecule only expressed on endothelial cells activated by cytokines; inflammatory marker |
| FGF | basic fibroblast growth factor and signaling protein |
| FGFR | fibroblast growth factor receptor |
| GDF | growth differentiation factor |
| GRP | glucose-regulated protein |
| HER | human epidermal growth factor receptor |
| HERG | human ether-à-go-go related gene |
| ICAM | intercellular adhesion molecule; inflammatory marker |
| IL | interleukin, cytokines (secreted proteins, signal molecules) |
| Ki-67 | antigen, nuclear protein |
| Kv3.4 | potassium voltage-gated channel gene |
| M-CSF | macrophage colony-stimulating factor; inflammatory marker |
| MET | mesenchymal epithelial transition |
| MDA | malonaldehyde; oxidative marker |
| miRNA | micro-ribonucleic acid |
| MMP | matrix metalloproteinase |
| MYC | family of regulator genes and proto-oncogenes that code for transcription factors (MyC: Myelocytomatosis) |
| ΔNp63 | Tumor protein p63 |
| 8-OHdG | 8-hydroxydeoxyguanosine |
| p21WAF1 | tumor protein, cell cycle protein regulators |
| p27 | protein that regulates the cell cycle |
| p53 | tumor protein, cell cycle protein regulators |
| podoplanin | mucin-type protein, specific lymphatic vessel marker |
| RAR-β | retinoic acid receptor-Beta |
| SAA | serum amyloid A; inflammatory marker |
| SMAD4 | Mothers against decapentaplegic homolog 4 gene |
| TGF | transforming growth factor; inflammatory marker |
Whereas selected biomarkers that were examined longitudinally seem promising, major limitations delineated in the current studies prevent definitive clinical application at this time. Most of the studies: 1) included small sample sizes with the largest comprising only 162 patients (Kawaguchi et al., 2008); 2) suffered from a paucity of histopathological data for leukoplakia and a lack of reporting of demographics and potentially confounding factors such as age, tobacco smoking, and alcohol consumption (Table 2); 3) reported on limited and variable follow-up data and/or did not include a control group. As such, no negative or positive predictive value was reported for the biomarkers. Each study included in the review evaluated distinct biomarkers, with the exception of podoplanin that was evaluated by two research groups (Saintigny et al., 2009, Kawaguchi et al., 2008), but continues to require further investigation. Thus, potential validity of biomarkers examined to date remains to be demonstrated in other studies. Several included papers reported on biomarkers examined in the context of chemoprevention trials (14/25 studies) where intervention may have potentially impacted the malignant transformation rates and accuracy of prognostic biomarkers relative to risk detection for malignant progression of oral leukoplakia (Saintigny et al., 2009, Mao et al., 1996, Saintigny et al., 2011, Uehara et al., 2010, Massarelli et al., 2005, Saintigny et al., 2018, Foy et al., 2015, Nagao et al., 2017, Kawaguchi et al., 2008, Rai et al., 2010, Lee et al., 2000, Yang et al., 2013, Wan et al., 1999, Beenken et al., 1994). Finally, most studies exhibited a moderate risk for bias in association with missing data, no consideration of potential confounding, and availability of limited follow up data (Table 3). Future studies should include a longer follow-up (the longest one reported in the studies included for this systematic review was 7.5 years; by Saintigny et al., 2009) to capture all cases of oral leukoplakia that undergo malignant transformation. This requires a larger effort with multiple centers and resources involved. Notably, biomarker assessment was not conducted in a blinded manner in any of the included studies, further introducing additional potential for bias.
Ten studies aimed to identify objectively measured molecular biomarkers for potential utility in identifying patients with oral leukoplakia at higher risk of malignant transformation. The remaining 14 studies that incorporated biomarker assessment as part of chemoprevention trials, had other objectives. We defined the potential role of each of the biomarkers reported in these studies based on their relational juxtaposition to essential hallmarks of cancer (Hanahan & Weinberg, 2011).
Based on evidence available to date, candidate biomarkers for cancer progression in patients affected by leukoplakia examined in this systematic review lacked substantive evidence as harbingers of risk for malignant transformation of oral leukoplakia. However, we only included those studies that specifically described “leukoplakia”; studies that described dysplasia only were excluded. Consequently, some biomarkers with prognostic value for oral dysplastic lesions (and possibly some leukoplakia cases) may have been missed. In addition, some of these biomarkers were captured in the Y3, Y4 or Y5 categories and will be reviewed separately in future publications by our group. One example of such a biomarker comes from the study by Zhang and colleagues who examined 296 oral premalignant lesions and showed that LOH at 3p and/or 9p was present in 20% of cases that underwent malignant transformation (Zhang et al., 2012), further supporting the role of LOH in malignant transformation of oral leukoplakia. Although LOH may indeed be a predictive biomarker for malignant transformation, this systematic review highlights that both clinical and histopathological descriptors of lesions should be included in future studies on the topic to ensure robustness of data, but also to allow subsequent meta-analyses should more than one study investigate the same marker or set of markers.
This systematic review disclosed that insufficient longitudinal evidence is currently available to support identification of biomarkers that could improve current methods for detection of leukoplakia and any subsequent malignant disease progression. Therefore, the strength of the current evidence precludes advancement of any candidate biomarkers examined to date into clinical practice. While a variety of technologies and laboratory techniques were employed to measure biomarker expression (including IHC, PCR, ELISA, and next-generation sequencing), outcomes of these studies based on the current body of evidence does not achieve the standards required to identify and validate candidate biomarkers investigated to date. While recent advancements in biomarker development using microarray technology, mass spectrometry, next-generation sequencing and proteomic technologies have facilitated accomplishment of relatively rapid screening in real time, one of the major challenges that remains unaddressed is the validation in large longitudinal trials to assess the true effectiveness of these biomarkers. Reliable, sensitive and specific biomarkers are needed to predict malignant transformation in patients affected by leukoplakia and inform physicians on correct treatment decisions. Thus, their relative value remains equivocal pending validation in future appropriately designed studies, with no advances currently observed. Well-designed randomized controlled studies with proper follow-up are required to propose and validate biomarkers for the purpose of establishing a new basis for their clinical and diagnostic utility. Consequently, clinicians continue to rely on tissue biopsy and histopathological assessment of oral dysplasia as the gold standard to determine the risk of malignant transformation and management strategies for patients with oral leukoplakia.
Limitations in tissue availability of leukoplakia samples (often small, formalin-fixed paraffin embedded) and the pre-invasive nature of leukoplakia may limit its systemic detectable expression in saliva and blood. Two promising biomarkers identified in this systematic review included podoplanin and cell cycle regulators. Podoplanin was identified as a possible independent predictor for cancer progression (hazard ratio = 3.1; 95% CI: 1.5–6.2) (Kawaguchi et al., 2008) and p27 loss was shown to be an independent factor for malignant transformation (p=0.02) (Massarelli et al., 2005a). However, despite the potential for greatly improving current clinical tools and collective recent advances, there is still insufficient longitudinal evidence to support the identification of these and other biomarkers that could improve the current methods for detection of leukoplakia and any subsequent malignant disease progression based on the 25 studies that met eligibility for inclusion in this systematic review. Thus, oral epithelial dysplasia on histopathological assessment of a biopsy specimen currently remains the best predictor for transformation to invasive squamous cell carcinoma.
Future directions
The past decade has seen an increase in availability and access to high-throughput profiling and sequencing technologies for genomic analysis and increased bioinformatic approaches to support rapid and comprehensive investigations of multi-omics data in the pre-cancer and cancer research domain. The growing capacity for integration and systems analysis of multi-omics data needs to be pursued to help elucidate interactions between genetic and epigenetic alterations being recognized in leukoplakia and promote identification of a prognostic biomarker or panel of biomarkers with improved potential for cancer prediction and simultaneously, definition of pathways involved in malignant transformation including candidate molecules which could be targeted by therapeutic interventions to impede progression of oral leukoplakia to invasive cancer. Notably, the era of precision medicine has seen achievement of this goal in the context of some cancers, including breast cancer. Achievement of this goal is further linked to definition of specific genetic alterations associated with leukoplakia and processes that drive malignant transformation. Increased understanding of these processes will also promote further identification of candidate biomarkers that may represent detectable molecular signals arising as a consequence of transformational processes. In the future, additional well-designed research that defines triggers of leukoplakia progression through integrated, ad-hoc computational analyses are needed to leverage genomic and other meta-data in defining appropriate candidate biomarkers and tailored molecular targets with translational potential to mitigate risk of malignant transformation associated with leukoplakia.
As more high-throughput technologies such as next-generation sequencing are utilized in the discovery of diagnostic, prognostic and predictive biomarkers, it is incumbent on researchers to better understand biomarker types and to better plan study designs to suit the biomarker type but also the question at hand. In the case of oral leukoplakia malignant transformation, assessing DNA tissue biomarkers through genome-wide association studies or even exome sequencing in affected individuals may provide insight into genetic mutations or aberrations of susceptibility in affected individuals, and if followed-up over time, may lead to discovery of a susceptibility profile for malignant transformation, as it is known that not all leukoplakia progress to malignancy. These biomarkers can be tested retrospectively on bio-banked samples, and would hasten biomarker discovery as patient recruitment for biomarker study design would not necessarily need to proceed prospectively. DNA tumor biomarkers of oral leukoplakia samples themselves may offer equally interesting insights, but would require a prospective study design. These biomarkers would be amenable for testing therapy response and pharmacodynamics, but may suffer from lack of reproducibility as different somatic mutations may be present in different parts of the lesion, thus highlighting the importance of biopsy site selection and multiple sampling of oral leukoplakia. Nonetheless, having this insight would dramatically increase our ability to discuss treatment options with patients, and would be invaluable prognostic predictors for therapy. Finally RNA, protein or metabolite biomarkers can be assayed as DNA tumor biomarkers can, but have the advantage of being tested in a variety of bio-samples including blood, saliva, gingival crevicular fluid, tissue, or cell scrapes, would equally be amenable to therapy testing and monitoring of response, would provide reproducibility at any one point in time, could be used in prospective study designs, but additionally would be suitable as surrogate markers of disease. Furthermore, their discovery can elucidate molecular pathways involved in malignant transformation and provide more comprehensive insights into this process.
Ideally, collaborative research, at the international level, strategically designed to systematically collect and analyze data is required in order to (i) achieve a robust, adequately- powered study population for follow-up of patients with leukoplakia and (ii) observe progression in a larger subset of patients than is normally possible in single-center studies due to the relatively low rate of malignant transformation. Patients with newly diagnosed leukoplakia should be included with subsequent longitudinal follow-up, and collection of structured data at specifically-defined time-points. Future studies should also focus on identifying biomarkers by integrating -omics data with environmental and lifestyle variables with the final goal of identifying more informative predictors of cancer progression that supersede dependence on histopathological diagnosis alone. Collection of these data into a centralized repository in the context of good histopathology data would further create a resource amenable to application of bioinformatics approaches and predictive modeling to identify the most informative variables for screening across time.
CONCLUSIONS
This review identified insufficient longitudinal evidence to support validated prognostic biomarkers for oral leukoplakia. Further studies are needed to identify molecular targets with the potential to mitigate risk of malignant transformation of oral leukoplakia to advance precision medicine approaches to management of patients with these lesions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors have no conflict of interest. The authors would like to thank Dr. Jim Berryman, Liaison Librarian, Brownless Biomedical Library, University Library, The University of Melbourne, for his guidance developing the literature search strategy.
The authors gratefully acknowledge the following organizations and companies that provided financial support for WWOM VII: American Academy of Oral Medicine, European Association of Oral Medicine, The British Society for Oral Medicine, The National Institute of Dental and Craniofacial Research, Colgate-Palmolive, Henry Schein Cares Foundation, AFYX, Unilever, Xerostom, Oral Diseases, and The World Dental Education Foundation. No additional external funding was received for conducting this study.
In addition, the authors express their sincere appreciation for the opportunity to collaborate with the WWOM VII Steering Committee. This committee provided the conceptual framework and logistical support to produce the WWOM VII Conference in September 2018 in Gothenburg, Sweden. In addition, the Steering Committee provided scientific and editorial critiques of this manuscript. The entire Steering Committee members were (in alphabetical order): Martin S. Greenberg (USA), Timothy A. Hodgson (UK), Siri Beier Jensen (Denmark), A. Ross Kerr (USA), Peter B. Lockhart (USA), Giovanni Lodi (Italy), and Douglas E. Peterson (USA).
REFERENCES
- Beenken SW, Huang P, Sellers M, Peters G, Listinsky C, Stockard C, … Grizzle W (1994). Retinoid modulation of biomarkers in oral leukoplakia/dysplasia. Journal of Cellular Biochemistry. Supplement, 19, 270–277. doi: N/A. [PubMed] [Google Scholar]
- Beenken SW, Sellers MT, Huang P, Peters G, Krontiras H, Dixon P, … Grizzle WE (1999). Transforming growth factor alpha (tgf-alpha) expression in dysplastic oral leukoplakia: modulation by 13-cis retinoic acid. Head & Neck, 21, 566–573. doi: . [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, & Jemal A (2018). Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians, 68, 394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Chang PY, Kuo YB, Wu TL, Liao CT, Sun YC, Yen TC, & Chan EC (2013). Association and prognostic value of serum inflammation markers in patients with leukoplakia and oral cavity cancer. Clinical Chemistry and Laboratory Medicine, 51, 1291–1300. doi: 10.1515/cclm-2012-0504. [DOI] [PubMed] [Google Scholar]
- D’Amico M, Gasparoli L, & Arcangeli A (2013).Potassium channels: novel emerging biomarkers and targets for therapy in cancer. Recent Patents on Anti-cancer Drug Discovery 8(1): 53–65. doi: 10.2174/15748928130106 [DOI] [PubMed] [Google Scholar]
- Fernandez-Valle A, Rodrigo JP, Garcia-Pedrero JM, Rodriguez-Santamarta T, Allonca E, Lequerica-Fernandez P, & de Vicente JC (2016). Expression of the voltage-gated potassium channel kv3.4 in oral leucoplakias and oral squamous cell carcinomas. Histopathology, 69, 91–98. doi: 10.1111/his.12917. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valle A, Rodrigo JP, Rodriguez-Santamarta T, Villaronga MA, Alvarez-Teijeiro S, Garcia-Pedrero JM, … de Vicente JC (2016). HERG1 potassium channel expression in potentially malignant disorders of the oral mucosa and prognostic relevance in oral squamous cell carcinoma. Head & Neck, 38, 1672–1678. doi: 10.1002/hed.24493. [DOI] [PubMed] [Google Scholar]
- Foy JP, Pickering CR, Papadimitrakopoulou VA, Jelinek J, Lin SH, William WN Jr., … Saintigny P (2015). New DNA methylation markers and global DNA hypomethylation are associated with oral cancer development. Cancer Prevention Research, 8, 1027–1035. doi: 10.1158/1940-6207.CAPR-14-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, & Weinberg RA (2011). Hallmarks of cancer: The next generation. Cell 144(5): 646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hayden JA, van der Windt DA, Cartwright JL, Cote P, & Bombardier C (2013). Assessing bias in studies of prognostic factors. Annals of Internal Medicine, 158, 280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, El-Naggar AK, Papadimitrakopoulou V, Ren H, Fan YH, Feng L, … Mao L (2008). Podoplanin: A novel marker for oral cancer risk in patients with oral premalignancy. Journal of Clinical Oncology, 26, 354–360. doi: 10.1200/JCO.2007.13.4072. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Hong WK, Hittelman WN, Mao L, Lotan R, Shin DM, … Lippman SM (2000). Predicting cancer development in oral leukoplakia: ten years of translational research. Clinical Cancer Research, 6, 1702–1710. doi: N/A. [PubMed] [Google Scholar]
- Lin CY, Chen WH, Liao CT, Chen IH, Chiu CC, Wang HM, … Cheng AJ (2010). Positive association of glucose-regulated protein 78 during oral cancer progression and the prognostic value in oral precancerous lesions. Head & Neck, 32, 1028–1039. doi: 10.1002/hed.21287. [DOI] [PubMed] [Google Scholar]
- Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, … Hong WK (1996). Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. 2, 682–685. doi: n/a. [DOI] [PubMed] [Google Scholar]
- Massarelli E, Brown E, Tran NK, Liu DD, Izzo JG, Lee JJ, … Papadimitrakopoulou VA (2005). Loss of E-cadherin and p27 expression is associated with head and neck squamous tumorigenesis. Cancer, 103, 952–959. doi: 10.1002/cncr.20879. [DOI] [PubMed] [Google Scholar]
- Mello FW, Miguel AFP, Dutra KL, Porporatti AL, Warnakulasuriya S, Guerra ENS, & Rivero ERC (2018). Prevalence of oral potentially malignant disorders: a systematic review and meta-analysis. Journal of Oral Pathology & Medicine. 47, 633–640. doi: 10.1111/jop.12726. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7): e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T, Warnakulasuriya S, Sakuma H, Miyabe S, Hasegawa S, Machida J, … Hashimoto S (2017). P53 and ki67 as biomarkers in determining response to chemoprevention for oral leukoplakia. Journal of Oral Pathology & Medicine, 46, 346–352. doi: 10.1111/jop.12498. [DOI] [PubMed] [Google Scholar]
- Napier SS, & Speight PM (2008). Natural history of potentially malignant oral lesions and conditions: an overview of the literature. Journal of Oral Pathology & Medicine. 37, 1–10. doi: 10.1111/j.1600-0714.2007.00579.x [DOI] [PubMed] [Google Scholar]
- Nayak S, Goel MM, Makker A, Bhatia V, Chandra S, Kumar S, & Agarwal SP (2015). Fibroblast growth factor (FGF-2) and its receptors FGFR-2 and FGFR-3 may be putative biomarkers of malignant transformation of potentially malignant oral lesions into oral squamous cell carcinoma. PLoS One, 10. doi: 10.1371/journal.pone.0138801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai B, Kaur J, Jacobs R, & Singh J (2010). Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. Journal of Oral Science, 52, 251–256. doi: 10.2334/josnusd.52.251. [DOI] [PubMed] [Google Scholar]
- Reibel J (2003). Prognosis of oral pre-malignant lesions: Significance of clinical, histopathological, and molecular biological characteristics. Critical Reviews in Oral Biology and Medicine, 14, 47–62. doi: 10.1177/154411130301400105. [DOI] [PubMed] [Google Scholar]
- Saintigny P, El-Naggar AK, Papadimitrakopoulou V, Ren H, Fan YH, Feng L, … Mao L (2009). Deltanp63 overexpression, alone and in combination with other biomarkers, predicts the development of oral cancer in patients with leukoplakia. Clinical Cancer Research, 15, 6284–6291. doi: 10.1158/1078-0432.CCR-09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saintigny P, William WN Jr., Foy JP, Papadimitrakopoulou V, Lang W, Zhang L, … Lippman SM (2018). Met receptor tyrosine kinase and chemoprevention of oral cancer. Journal of the National Cancer Institute, 110, 01. doi: 10.1093/jnci/djx186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saintigny P, Zhang L, Fan YH, El-Naggar AK, Papadimitrakopoulou VA, Feng L, … Mao L (2011). Gene expression profiling predicts the development of oral cancer. Cancer Prevention Research, 4, 218–229. doi: 10.1158/1940-6207.CAPR-10-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata J, Yoshida R, Matsuoka Y, Nagata M, Hirosue A, Kawahara K, … Nakayama H (2017). Predictive value of the combination of SMAD4 expression and lymphocyte infiltration in malignant transformation of oral leukoplakia. Cancer Medicine, 6, 730–738. doi: 10.1002/cam4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speight PM, Khurram SA, & Kujan O (2018). Oral potentially malignant disorders: Risk of progression to malignancy. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 125, 612–627. doi: 10.1016/j.oooo.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Tanic N, Tanic N, Milasin J, Vukadinovic M, & Dimitrijevic B (2009). Genomic instability and tumor-specific DNA alterations in oral leukoplakias. European Journal of Oral Sciences, 117, 231–237. doi: 10.1111/j.1600-0722.2009.00624.x. [DOI] [PubMed] [Google Scholar]
- Uehara M, Ikeda H, Nonaka M, & Asahina I (2010). Histopathological change of oral malignant tumour and epithelial dysplasia subjected to photodynamic therapy. Journal of Oral & Maxillofacial Research, 1, e5. doi: 10.5037/jomr.2010.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waal I, & Axell T (2002). Oral leukoplakia: A proposal for uniform reporting. Oral Oncology, 38, 521–526. doi: 10.1016/S1368-8375(01)00125-7 [DOI] [PubMed] [Google Scholar]
- Villa A, & Woo SB (2017). Leukoplakia - a diagnostic and management algorithm. Journal of Oral and Maxillofacial Surgery, 75, 723–734. doi: 10.1016/j.joms.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Visioli F, Lauxen IS, Sant’ana Filho M., & Rados PV (2012). Expression of the cell cycle regulation proteins p53 and p21WAF1 in different types of non-dysplastic leukoplakias. Journal of Applied Oral Science, 20, 369–375. doi: 10.1590/S1678-77572012000300013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan XS, Meyskens FL Jr, Armstrong WB, Taylor TH, & Kennedy AR (1999). Relationship between protease activity and neu oncogene expression in patients with oral leukoplakia treated with the Bowman Birk inhibitor. Cancer Epidemiology, Biomarkers & Prevention, 8, 601–608. doi: N/A. [PubMed] [Google Scholar]
- Warnakulasuriya S (2018). Clinical features and presentation of oral potentially malignant disorders. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology,125, 582–590. doi: 10.1016/j.oooo.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriya S, Johnson NW, & van der Waal I (2007). Nomenclature and classification of potentially malignant disorders of the oral mucosa. Journal of Oral Pathology & Medicine, 36, 575–580. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- Xiao W, Bao ZX, Zhang CY, Zhang XY, Shi LJ, Zhou ZT, & Jiang WW (2012). Upregulation of miR-31 is negatively associated with recurrent/newly formed oral leukoplakia. PLoS One, 7. doi: 10.1371/journal.pone.0038648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CZ, Ma J, Luo QQ, Neskey DM, Zhu DW, Liu Y, … Zhong LP (2014). Elevated level of serum growth differentiation factor 15 is associated with oral leukoplakia and oral squamous cell carcinoma. Journal of Oral Pathology & Medicine, 43, 28–34. doi: 10.1111/jop.12091. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li YX, Yang X, Jiang L, Zhou ZJ, & Zhu YQ (2013). Progress risk assessment of oral premalignant lesions with saliva miRNA analysis. BMC Cancer, 13, 129. doi: 10.1186/1471-2407-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Poh CF, Williams M, Laronde DM, Berean K, Gardner PJ, …Rosin MP (2012) Loss of Heterozygosity (LOH) Profiles – Validated Risk Predictors for Progression to Oral Cancer Cancer Prev Res (PhilA), 5, 1081–9. doi: 10.1158/1940-6207.CAPR-12-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


