Abstract
Objective:
We aimed to evaluate a possible association between the use of obesogenic medications and inadequate weight loss in a behavioral weight management program.
Methods:
This is a case-control, single-center, study of 666 adult patients within Veterans Health Administration health system that participated in the MOVE! behavioral weight loss program. We divided our cohort into responders (n=150), patients who achieved ≥5% total weight loss by the end of MOVE! program, and non-responders (n=516), <5% total
Results:
Approximately 62% (n = 411) of patients entering MOVE! had a prescription for obesogenic medications. Obesogenic medication use was associated with worse weight loss outcomes, and participants were 37% less likely to achieve a clinically meaningful (≥5% total weight loss) outcome at the end of the MOVE! program (odds ratio, 0.633; 95% CI, 0.427–0.937; adjusted P = 0.022). Patients who received 3 or more medications n=72) had the greatest difficulty achieving 5% weight loss compared to the control group (odds ratio, 0.265; 95% CI, 0.108–0.646; adjusted P = 0.003).
Conclusions:
The use of provider-prescribed obesogenic medications was associated with worse weight loss outcomes in a behavioral weight loss program. Closer scrutiny of patient medications is necessary to help improve outcomes of weight loss treatments.
Keywords: Obesity, Weight Loss, Behavior Therapy, Antiobesity, Pharmacotherapy
Introduction
The prevalence of obesity continues to increase, with most recent estimates suggesting that 51% of the US population will have obesity by 2030 (1). Veterans are particularly affected; up to 78% of veterans have overweight or obesity (2). The estimated annual medical cost for people who have obesity is $1429 higher than those with normal weight (3). To address this health problem, Veterans Health Administration (VHA) established in 2005 the MOVE! program. This is a patient-centered, comprehensive, evidence-based, multidisciplinary, weight management program. Behavioral weight loss programs are a cornerstone of obesity care and are offered as one of the first steps of obesity management. However, the weight loss outcomes from behavioral weight loss programs are quite variable (4, 5). Success rates of patients losing a clinically meaningful amount of weight by the end of the MOVE! 8-week program have been found to be 18–20% (6). Multiple factors have been implicated in the variability of these types of programs including poor adherence, and unrealistic patient expectations (7). Improving patient outcome is a high priority, not only for decreasing a modifiable risk factor for some of the leading causes of preventable death, but also decreasing the financial burden on a national level.
The etiology of obesity is multifactorial, including genetics; easy access to energy-dense, nutrient-poor, highly-palatable food; physical inactivity; and changes in sleep-wake cycle (8). A growing body of evidence shows that a number of frequently prescribed medications can cause weight gain and contribute to the obesity epidemic (9–12). The Endocrine Society Clinical Practice Guidelines advise that obesogenic medications should be avoided in patients with overweight and obesity (13). Notably, these drugs fall into various categories of medications, including some of the most frequently prescribed medications such as beta-blockers, anti-depressants, antipsychotics, anticonvulsants, anti-diabetic medications, antihistamines, and hormones (Table 1) (14). Given the frequent use of obesogenic medications and the mechanisms of their effects, provider-prescribed obesogenic medications could be a potential risk factor for poor weight loss outcomes in patients being treated for obesity.
Table 1. Obesogenic Medications.
Common physician-prescribed, obesongenic medications identified in Endocrine Society Guidelines (13, 14). The number of patients in our study who were taking these medications is also provided. Some medications identified in the Endocrine Society guidelines were not taken by any individuals in our cohort, mainly due to them not being on formulary in the VA Health Systems.
| Medication Class (n) | Obesogenic Medications (n) | Mechanism of Weight Gain (reference) |
|---|---|---|
| Anticonvulsants (89) | Carbamazepine (3) Gabapentin (72) Pregabalin (12) Valproic Acid (2) |
Hypothalamic mediated increase in appetite and decrease in energy expenditure.18 |
| Antidepressants/Antianxiety (85) | Amitriptyline (19) Fluoxetine (33) Mirtazapine (18) Nortriptyline (5) Paroxetine (10) |
Appetite increase stimulated via serotonergic pathways.12 |
| Antihistamines (125) | Cetirizine (29) Diphenhydramine (18) Fexofenadine (2) Hydroxyzine (29) Loratadine (47) |
Increase appetite. Alters body weight regulation.28,29 |
| Antipsychotics (36) | Olanzapine (5) Quetiapine (17) Risperidone (11) Ziprasidone (3) |
Increased orexigenic and decreased anorexigenic neuropeptide expression in the hypothalamus.18,19 |
| Beta Blockers (205) | Atenolol (80) Carvedilol (24) Metoprolol (79) Propanolol (22) |
Inhibit sympathetic tone, decrease lipolysis, reduce exercise tolerance, increase fatigue, and reduce the resting energy expenditure.11 |
| Corticosteroids and Hormones (15) | Medroxyprogesterone (2) Oral contraceptives (2) Prednisone (11) |
Alters the energy intake and expenditure of the human body.10 |
| Diabetes medications (156) | Insulin (70) | Anabolic and adipogenic hormone, decreases daily energy expenditure.35 |
| Sulfonylureas (58) | Increase the secretion of insulin and cause water retention.36 | |
| Thiazolidinediones (28) | Act as insulin sensitizers, cause water retention.37 |
Here we present a retrospective case-control study that measures the impact of provider-prescribed obesogenic medications on weight loss outcomes of the VHA San Diego MOVE! program. In particular, we hope to answer the question: Do obesogenic medications affect the weight loss response of patients to a behavioral weight loss program? To do this, patients who participated in the MOVE! Program between 2011–2015 were split into those who responded to therapy (5% weight loss after the intervention) and those who did not. We then assessed whether exposure to any number of obesogenic medications affected whether a patient was a responder or not. Despite the retrospective study design, the short duration of the weight loss program made possible to collect homogenous data and correlate well the use of obesogenic medications and weight loss product.
Methods
Study Design, Data Source
This is a retrospective, single-center case-control study of adult patients older than 18 years old that completed the MOVE! program at the VHA San Diego. This study had the approval of the institutional review board of VHA San Diego. We identified patients that participated from 01/01/2011 to 12/31/2015. MOVE! program is available to veterans with body mass index (BMI) of ≥ 30 and are referred by their primary care provider or other health care provider for weight loss. Participants that did not completed the MOVE! program were excluded. Completion of the program is defined as having more than 75% participation in the program activities.
Study Population and Measurements
We divided our study population based on the ≥5 percent total weight loss (%TWL) at the end of the MOVE! program. This level of weight loss has shown to be associated with clinically meaningful outcomes, for example improvements in blood pressure, glycemia, and triglycerides (15–17). Responders (“cases”) are defined as patients who were able to lose 5% or more from their intake total weight at the end of MOVE! program. Non-responders (“controls”) lost less than 5% of their total weight by the end of the 8-week program. Secondary outcomes include the number of obesogenic medications prescribed, and the effects of specific medication classes (e.g., antidiabetic, beta-blockers, antidepressants, etc.) on 5%TWL, and %TWL as a continuous variable. Percent excess weight loss was calculated based on ideal body weight corresponding to BMI 25. Pharmacy data were recorded from the VHA electronic medical records through manual chart review. These data include prescription regimens for 28 obesogenic medications (Table 1; “exposure”), four leptogenic medications (Table S1) and two obesity pharmacotherapy drugs (Table S1). We defined obesogenic medications as drugs with a known side effect of weight gain of 2 kg or ≥5% from baseline, leptogenic medications as drugs where weight loss is a known side effect, and obesity pharmacotherapy as drugs approved for the treatment of obesity. Obesogenic medications were selected from recent practice guidelines (13, 14). Outpatient prescriptions of one month or more duration that were both filled and received by the patient were considered as medication use. For example, if during the intake visit at MOVE! program patient has an active prescription of one or more months duration, and it was filled by the VA pharmacy, this is considered as medication use. On the other hand, if based on pharmacy records a prescribed medication is not filled, it is not considered as used. Most prescriptions for nearly all the patients were filled by VHA pharmacies. To address potential non-VHA medication usage, primary care notes were screened for medications that were not included at the pharmacy records. By including all obesogenic medications without regard to duration, we are reducing potential confounds and over-interpretation of our data. Comorbidities and demographic data were also abstracted through manual electronic health record review.
MOVE! program
The MOVE! program consists of eight weekly group classes, 90 minutes in duration. Licensed providers lead these classes. During each session, instructors use tools including audio-visual materials, didactic instruction, and class exercises. Education is provided and covers topics such as health benefits of weight loss, goal setting, self-monitoring, identification of environmental cues that may affect eating and physical activity, mindful eating, food composition and dietary guidelines, portion control strategies, benefits of exercise, barriers to physical activity and other. Patients have their weight checked during each class using a standardized digital scale and this is recorded to their electronic medical records along with an electronic note. Participants are given a pedometer with instructions. Patients have the option to keep food logs weekly that are reviewed by the weight loss providers and patients receive a written feedback.
Statistical Analysis
We compared continuous and categorical outcomes across responders and non-responders using two-sample t-tests and Fisher’s exact test, respectively. We report adjusted odds ratios derived from multivariable logistic regressions with control for potential confounders including age of patient at the time of weight loss clinic intake visit, gender, the use of obesogenic medications (defined as prescription for one month or longer of an obesogenic medication during the 8-week weight loss intervention), leptogenic medications (defined as prescription for one month or longer of a leptogenic medication during the 8-week weight loss intervention), and obesity pharmacotherapy (defined as prescription for one month or longer of a medication used for obesity treatment during the 8-week weight loss intervention) and BMI.
Results
Patient Characteristics
The study population included 724 adult individuals (see Figure S1 for breakdown of patients in this case-control study). We excluded 58 patients who did not attend all the sessions or complete the MOVE! program. Of the remaining 666 patients in our cohort, 150 patients (22.5%) were responders, reaching 5%TWL or more at the end of MOVE! program. The remaining 516 (77.5%), considered non-responders, lost less than 5% of their total weight (Table 2). Responders were older (63.0 ± 0.8 vs 59.4 ± 0.5, respectively, P < 0.001), and had a lower percentage of women compared with the non-responders group (10.7 vs 18.4, P = 0.025). Age does not appear to correlate with weight loss (Figure S2). Responders had a higher percent excess weight loss after MOVE! compared with non-responders (27.1 ± 2.0 vs 3.0 ± 0.5, respectively, P < 0.001), and a higher %TWL at the end of MOVE! compared with non-responders (7.9 ± 0.5 vs 0.8 ± 0.1, respectively). Absolute weight loss at the end of the MOVE! program was also higher at responders compared with the non-responders, (21.0 ± 1.4 vs 1.9 ± 0.4, respectively, in pounds) (Table 2).
Table 2: Characteristics of Study Participants (total of 666 patients).
| All (n=666) | Responders (n = 150) | Non-respond(n = 516) | P-value | |
|---|---|---|---|---|
| Demographics; n (%) unless otherwise stated | ||||
| Age; mean (± SEM) | 60.2 (± 0.4) | 63.0 (± 0.8) | 59.4 (± 0.5) | <0.001 |
| Gender (female) | 111 (16.7) | 16 (10.7) | 95 (18.4) | 0.025 |
| White | 241 (36.2) | 45 (30.0) | 196 (38.0) | 0.152 |
| Black or African-American | 129 (19.4) | 21 (14) | 108 (20.9) | |
| Hispanic/Latino | 32 (4.8) | 8 (5.3) | 24 (4.7) | |
| American Indian / Alaskan Native | 12 (1.8) | 5 (3.3) | 7 (1.4) | |
| Asian American | 39 (5.9) | 7 (4.7) | 32 (6.2) | |
| Native Hawaiian / Pacific Islander | 7 (1.0) | 0 (0.0) | 7 (1.4) | |
| Other Ethnicity | 22 (3.3) | 4 (2.7) | 18 (3.5) | |
| Anthropomorphic; mean (± SEM) | ||||
| Intake weight; (pounds) | 256.1 (± 2.0) | 263.3 (± 4.1) | 254.1 (± 2.3) | 0.057 |
| Intake BMI (kg/m2) | 38 (± 0.3) | 38.9 (± 0.6) | 37.8 (± 0.3) | 0.076 |
| Intake Excess Weight, (pounds) | 87.6 (± 1.8) | 93.5(± 3.9) | 85.9 (± 2.0) | 0.079 |
| Total Weight Loss End of MOVE! (pounds) | 6.2 (± 0.5) | 21.0 (± 1.4) | 1.9 (± 0.4) | 0.014 |
| Total Weight Loss End of MOVE! (%) | 2.4 (± 0.2) | 7.9 (± 0.5) | 0.8 (± 0.1) | <0.001 |
| Excess Weight Loss at end of MOVE! (%) | 8.5 (± 0.7) | 27.1 (± 2.0) | 3.0 (± 0.5) | <0.001 |
| Co-Morbidities; n (%) | ||||
| Hypertension | 63.8 (425) | 98 (65.3) | 327 (63.4) | 0.700 |
| Type 2 Diabetes | 214 (32.1) | 42 (28.0) | 172 (33.3) | 0.234 |
| Dyslipidemia | 392 (58.9) | 95 (63.3) | 297 (57.6) | 0.221 |
| Major Depression | 302 (45.3) | 58 (38.7) | 244 (47.3) | 0.063 |
| Coronary Artery Disease | 83 (12.5) | 18 (12.0) | 65 (12.6) | 1.000 |
| Anxiety disorder | 100 (15.0) | 19 (12.7) | 81 (15.7) | 0.436 |
| Seizure disorder | 7 (1.0) | 2 (1.3) | 5 (1.0) | 0.658 |
| Psychoses | 26 (3.9) | 4 (2.7) | 22 (4.3) | 0.478 |
| Weight Affecting Medication; n (%) | ||||
| Obesogenic Medications | 411 (61.7) | 81 (54.0) | 330 (64.0) | 0.029 |
| Leptogenic Medications | 217 (32.6) | 39 (26.0) | 178 (34.5) | 0.060 |
| Anti-obesity Pharmacotherapy | 14 (2.1) | 2 (1.3) | 12 (2.3) | 0.746 |
NOTE: A Fisher Exact test was used for all comparisons except for all continuous variable comparisons (i.e. age, weight BMI), where a student’s unpaired t-test was used.
Effects of Obesogenic Medications on Excess Weight Loss
To investigate the effect of obesogenic medications on weight loss outcomes at the end of the MOVE! program, we measured the usage of these drugs in responders and non-responders. We found that more than 60% of the patients participating in the MOVE! program had at least one obesogenic medication prescribed (n=411, 61.7%) (Table 2). Obesogenic medications were less frequently prescribed in responders than non-responders (54% vs 64%, adjusted [adj] P = 0.029), suggesting that obesogenic medication use is associated with a poor weight loss outcome. Patients with any provider-prescribed obesogenic medications are 37% less likely than controls to achieve 5%TWL, (adj οdds ratio [OR], 0.633, 95% confidence interval [CI], 0.427–0.937, P = 0.022) (Figure 1).
Figure 1: Factors associated with worse weight outcome at the end of the MOVE! program.
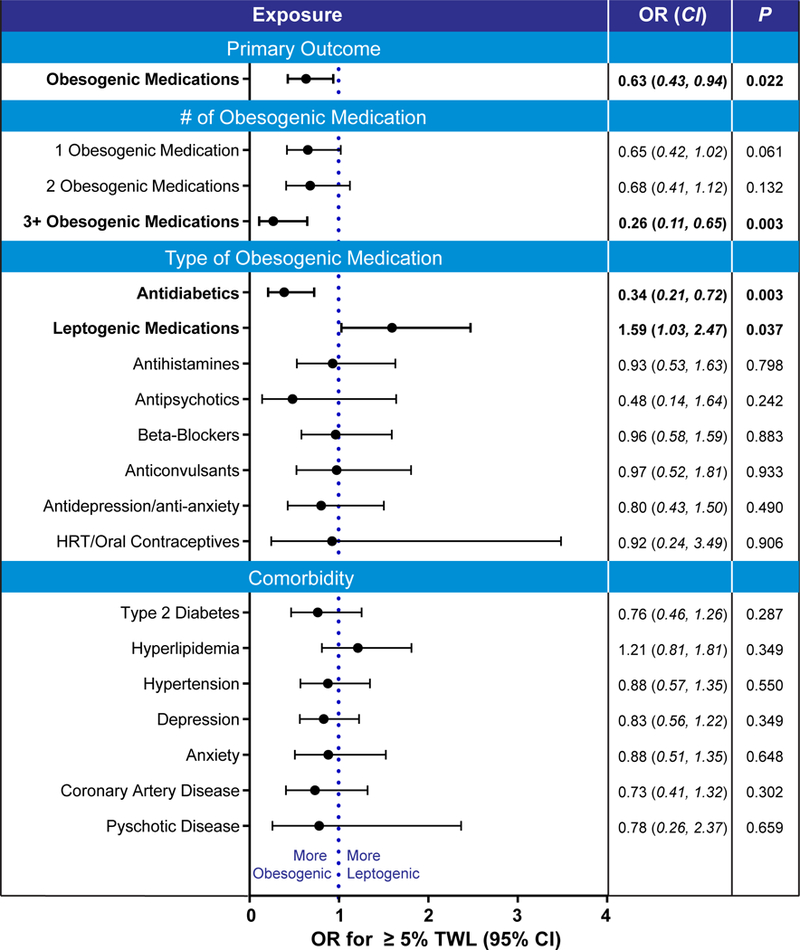
Odds ratio (OR) and 95% confidence interval (CI) of ≥5 percent total weight loss (%TWL) at the end of MOVE! program. P values from logistic regression adjusted for obesity pharmacotherapy and differences in baseline characteristics between responders and non-responders, including age, gender, obesogenic, Body Mass Index and leptogenic medications. Odds ration where P < 0.05 are in bold.
Effect of Increased Number of Obesogenic Medications
To further examine the influence of obesogenic drugs in weight loss success, we calculated the OR’s of achieving 5%TWL for different numbers of obesogenic medications prescribed. Patients prescribed three or more obesogenic medications were least likely to achieve 5%TWL compared with the participants not using those drugs (adj OR 0.265, 95% CI, 0.108–0.646, P = 0.003) (Figure 1). This suggests that with a greater the number of obesogenic medications prescribed, it is more likely a patient to have a poor weight loss outcome. Moreover, patients with three or more obesogenic medications prescribed had the lowest absolute total body weight loss by the end of the program (Table 3).
Table 3. Absolute weight loss for different number of obesogenic medications prescribed.
| Number of Obesogenic Medications | n (%) | TBWL ± SEM |
|---|---|---|
| 0 | 255 (38.3) | 7.30 ± 0.66 |
| 1 | 204 (30.6) | 5.55 ± 0.91 |
| 2 | 135 (20.3) | 6.84 ± 1.71 |
| 3+ | 72 (10.8) | 2.90 ± 1.32 |
Obesogenic Medication Class Effects
The study assessed the effect of different classes of obesogenic drugs on weight loss outcomes. We found that compared to individuals who were not prescribed obesogenic drugs, participants who were on antidiabetic medications had lower odds of achieving an adequate weight loss of 5%TWL at the end of MOVE! (adj OR 0.387, 95% CI, 0.207–0.723, P = 0.003) (Figure 1).
Effect of comorbidities on weight loss outcome
We explored the possibility that comorbidities influence this weight loss outcome difference in patients using obesogenic medications (i.e. reverse causality). For instance, inadequate weight loss caused poor control of hypertension and that prompted the use of obesogenic medications. This is not supported from our data (Figure 2), as comorbidities are not different between responders and non-responders (Table 2). Furthermore, weight loss outcome was inferior for patients with a specific comorbidity using obesogenic medications when compared with patients with the same comorbidity not taking these medications, (Table 4). Furthermore, higher number of comorbidities is not correlated with inferior weight loss outcome (Table 5).
Figure 2: Comorbidities were not associated with worse weight outcomes and obesogenic effect cannot be attributed to increased comorbidities.
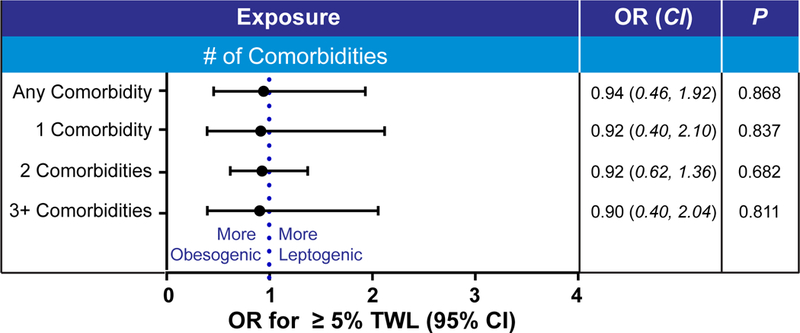
Odd ratio (OR) and 95% confidence interval (CI) of ≥5 percent total weight loss (%TWL) at the end of MOVE! program. P values from logistic regression adjusted for obesity pharmacotherapy and differences in baseline characteristics between responders and non-responders, including age, gender, obesogenic, Body Mass Index and leptogenic medications.
Table 4. Weight loss outcome for different comorbidity status.
| Comorbidity | Obesogenic Meds Group | Non-obesogenic Meds group | ||
|---|---|---|---|---|
| n | TBWL ± SEM | n | TBWL ± SEM | |
| HTN | 297 | 6.32 ± 0.89 | 128 | 7.25 ± 0.99 |
| HLD | 270 | 6.66 ± 0.99 | 122 | 8.35 ± 0.88 |
| Depression | 200 | 5.48 ± 1.24 | 102 | 6.47 ± 1.12 |
| CAD | 66 | 5.90 ± 1.50 | 17 | 7.62 ± 2.57 |
| Type 2 Diabetes | 173 | 6.73 ± 1.41 | 41 | 9.43 ± 1.91 |
| Anxiety | 64 | 5.05 ± 1.42 | 36 | 6.32 ± 1.74 |
| NAFLD | 27 | 6.54 ± 1.57 | 13 | 6.68 ± 2.46 |
| Psychoses | 20 | 3.10 ± 1.74 | 6 | 3.38 ± 3.34 |
Table 5. Weight loss outcome analysis for different number of comorbidities.
| Number of Comorbidities | n (%) | TBWL ± SEM |
|---|---|---|
| 0 | 54 (8.1) | 6.08 ± 1.49 |
| 1 | 110 (16.5) | 5.11 ± 1.32 |
| 2 | 188 (28.2) | 7.34 ± 0.82 |
| 3+ | 314 (47.1) | 5.92 ± 0.87 |
Discussion
In this retrospective case-control study, 22.5% of 666 patients achieved 5%TWL after the MOVE! behavioral weight loss program. Our study showed that the patients on any obesogenic medications were 37% less likely to have clinically meaningful weight loss by the end of the 8-week program. A stronger association was seen for those who were prescribed three or more obesogenic medications, where they had a 73% lower chance of achieving 5% TWL compared with controls. There is a need for larger studies that can determine whether there is a threshold of number of medications where the negative effects of obesogenic medications are realized, or if specific medications have a particularly strong effect on outcomes. In 2014 alone, patients spent approximately $2.5 billion on commercial or proprietary weight-loss services, and our study suggests that success rates may be impacted by a factor that was not previously considered; the use of provider-prescribed obesogenic medications (7).
Significant variability in weight loss product is noted after weight loss modalities, including behavioral programs, like MOVE! program and surgical interventions. In behavior-based weight control therapies a number of factors, like initial body weight, early weight loss, and other treatment factors and behavioral changes have been found to account for 20–30% of observed variance in weight loss success or failure. (18). These predictors include patient factors, like initial body weight, process variables such as early weight loss and other treatment factors and behavioral changes. Our main conclusion is bolstered by a recent publication which showed that exposure to obesogenic medications had a significant effect on post laparoscopic sleeve gastrectomy weight loss outcomes. More specifically, patients on obesogenic medications lost significantly less weight one year after bariatric surgery, compared with patients not using those drugs, 53.8% of excess weight lost vs 65.0%, respectively, P = 0.002 (19). Together, these studies show that regardless of patient population (i.e. predominantly male veterans, predominantly affluent women) and treatment modality (i.e. behavioral/dietary intervention, bariatric surgery), any exposure to obesogenic medication during treatment negatively affected weight loss outcomes. For both studies, weight loss outcomes were not associated with comorbidities. Hence, for all weight loss treatment options, addressing prescription of obesogenic medications could help to improve this observed variation in weight loss success.
Our data supports the 2015 published clinical practice guidelines of the Endocrine Society, the European Society of Endocrinology, and the Obesity Society who recommend leptogenic and weight-neutral medications as first- and second-line treatment in the management of patients with overweight and obesity with type II diabetes mellitus (13). Furthermore, identification of obesogenic medications in each clinical encounter is encouraged. Amongst the different classes of provider-prescribed obesogenic medications, patients on antihistamines, antidiabetic medications, and antipsychotics had significantly lower odds of achieving 5% weight loss in our study.
Understanding the obesogenic mechanism and impact of this drug class in the obesity epidemic and finding alternative agents could be important to improve weight loss efforts. In 2012 11.8% of the total prescriptions in the United States were for diabetes (20, 21). The 2016 guidelines by The American Association of Clinical Endocrinologists and The American College of Endocrinology recommend early use of leptogenic or weight-neutral drug options, such as metformin, pramlintide, sodium/glucose cotransporter 2 inhibitors, dipeptidyl peptidase-4 inhibitors or glucagon-like peptide-1 agonists before using obesogenic medications like insulin, sulfonylureas and thiazolidinediones (22–26). In a prospective study of 3234 patients, patients on metformin had greater weight loss compared to those in the placebo group, 2.1 kg vs 0.1 kg, P < 0.001 (27).
As obesogenic medications are frequently prescribed for common diseases, the use of alternatives is a challenging task requiring a multidisciplinary approach (28). Selecting a suitable alternative for disease management needs careful evaluation. Obstacles in the implementation of changes in prescription practices include exacerbation of a controlled disease and adverse effects from the new agent. Cost is also a considerable factor. For example, patients may require additional education and possibly extra care visits related to medication changes. Additionally, newer alternative agents could have increased cost compared with older agents, for example, on average pramlintide costs $250 for a months’ supply vs $20–30 cost for glipizide prescription. Nevertheless, the benefits of weight loss, considering the tremendous negative effect of obesity in public health as well as each individual’s morbidity and mortality, may outweigh possible risks and initial expenses. Previous experience from antibiotic safe prescription practices underlines that this multidisciplinary approach can be effective at different levels of the health care system, from societies/hospital policies to single providers. Change can be implemented in obesogenic drug use with a variety of strategies that focus on education and guidelines of safe practices, restricting agents based on indications, feedback from individual providers, computer assistance with information technology, and patient-specific recommendations (29, 30). It should be noted that although published effects on weight for a given obesogenic agent may be modest, its role should be considered as one of many factors that impact weight regulation and energy balance in a complex neurohormonal system, and efforts should be made to remove as many obstacles as possible for the difficult task of weight reduction.
Limitations of our study include a retrospective study design, unknown confounders that we could not identify and control, and data extraction from electronic records that were collected only for medical management purposes. Therefore, we do not have data for adherence to the prescribed regimen. In addition, dosing and duration of treatment were not recorded. Patients in our cohort had a VHA primary care physician and access to VHA pharmacies. Even though we cannot confirm that VHA pharmacy is the only source of medication dispensing, VHA pharmacies have a lower cost and, for most veterans, VHA is the single health care provider and use of outside pharmacies is limited (31). Nevertheless, primary care notes were screened for recorded medications prescribed outside of VHA. Moreover, we could not assess the use of over the counter drugs by our participants. However, over the counter drugs are more expensive than using VHA pharmacies and frequency of their use is likely small. Finally, we excluded patients who did not complete the MOVE! Program, some of whom may have quit the program due to lack of success. Whether these patients did not achieve 5% TWL due to obesogenic medication or lack of participation would not be clear and hence, their removal is justified. Of note, all of these factors would bias the results towards the null hypothesis. Our cohort of veterans consists mostly of older Caucasian men and our findings may not be applicable to the general population. The short nature of the MOVE! Program provides a unique cohort that is closely followed for 8 weeks. Inclusion in this study is dependent on both adequate participation, recorded intake weight, and recorded final weight upon completion of the program. Moreover, the short duration of the treatment program would bias the result toward the null hypothesis. Still, the effects of obesogenic medication on weight loss are robust enough to still be detected in this scenario.
More research is necessary to evaluate the true impact of obesogenic drugs on weight loss success after a behavioral weight loss program. A prospective study would more accurately describe the effect of obesogenic drugs on weight loss outcomes, such as randomization of patients to either continuation or discontinuation of obesogenic drugs prior to starting a weight loss program.
Conclusions
Despite growing evidence for medication-induced weight gain for numerous commonly prescribed medications, use of obesogenic medications in patients undergoing weight loss efforts is frequent, and the impact on weight loss outcomes has yet to be determined. This study is a retrospective, case-control study that examined the effect of obesogenic medications on weight loss after an evidence-based behavioral weight loss program. Obesogenic medication use was associated with a lower chance of achieving clinically meaningful weight loss. Our finding that higher numbers of obesogenic medications prescribed translates to worse weight loss outcomes further supports this association. Antidiabetics showed a stronger association between exposure and weight loss outcome. In summary, improvement of prescription practices, regarding commonly prescribed medications that cause weight gain, could facilitate weight loss efforts of patients undergoing behavioral weight loss programs.
Supplementary Material
Key Points.
Obesogenic medications are a diverse group of medications that can cause weight gain when used chronically
The association between obesogenic medications use and weight loss outcomes after a behavioral obesity treatment program has not been studied.
Our study shows that the use of commonly prescribed medications that have weight gain as a known side effect was associated with 37% lower chance of achieving clinically meaningful weight loss at the end of a nationally utilized behavioral obesity treatment program.
Anti-diabetics is the medication class with the strongest association with poor weight loss outcomes.
61.7% of patients starting a behavioral weight loss program were prescribed one or more obesogenic medications.
Acknowledgements
None
Funding:
Athanasios Desalermos received support from NIH T32 DK007202. Cecilia Leggett received support from UCSD Summer Research Training Program. Amir Zarrinpar received support from NIH K08 DK102902 and AASLD Liver Scholar Award. The project described was partially supported by the NIH UL1 TR001442.
Footnotes
Competing Interests:
The authors declared no conflict of interest.
References
- 1.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med 2012; 42:563–70. [DOI] [PubMed] [Google Scholar]
- 2.Group TMoOaOW. Screening and Management of Overweight and Obesity. VA/DoD Clinical Practice Guideline 2014. [Google Scholar]
- 3.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009; 28:w822–31. [DOI] [PubMed] [Google Scholar]
- 4.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA 2007; 297:969–77. [DOI] [PubMed] [Google Scholar]
- 5.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 2007; 107:1755–67. [DOI] [PubMed] [Google Scholar]
- 6.Kahwati LC, Lance TX, Jones KR, Kinsinger LS. RE-AIM evaluation of the Veterans Health Administration’s MOVE! Weight Management Program. Transl Behav Med 2011; 1:551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med 2015; 162:501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaila B, Raman M. Obesity: a review of pathogenesis and management strategies. Can J Gastroenterol 2008; 22:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders KH, Igel LI, Shukla AP, Aronne LJ. Drug-induced weight gain: Rethinking our choices. J Fam Pract 2016; 65:780–8. [PubMed] [Google Scholar]
- 10.Kulkarni SK, Kaur G. Pharmacodynamics of drug-induced weight gain. Drugs Today (Barc) 2001; 37:559–71. [DOI] [PubMed] [Google Scholar]
- 11.Sharma AM, Pischon T, Hardt S, Kunz I, Luft FC. Hypothesis: Beta-adrenergic receptor blockers and weight gain: A systematic analysis. Hypertension 2001; 37:250–4. [DOI] [PubMed] [Google Scholar]
- 12.Harvey BH, Bouwer CD. Neuropharmacology of paradoxic weight gain with selective serotonin reuptake inhibitors. Clin Neuropharmacol 2000; 23:90–7. [DOI] [PubMed] [Google Scholar]
- 13.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015; 100:342–62. [DOI] [PubMed] [Google Scholar]
- 14.Domecq JP, Prutsky G, Leppin A, et al. Clinical review: Drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011; 34:1481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vilar-Gomez E, Friedman SL, Romero-Gomez M. Reply: To PMID 25865049. Gastroenterology 2015; 149:1988–9. [DOI] [PubMed] [Google Scholar]
- 17.Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring) 2015; 23:2319–20. [DOI] [PubMed] [Google Scholar]
- 18.Stubbs J, Whybrow S, Teixeira P, et al. Problems in identifying predictors and correlates of weight loss and maintenance: implications for weight control therapies based on behaviour change. Obes Rev 2011; 12:688–708 [DOI] [PubMed] [Google Scholar]
- 19.Leggett CB, Desalermos A, Brown SD, et al. The effects of provider-prescribed obesogenic drugs on post-laparoscopic sleeve gastrectomy outcomes: a retrospective cohort study. Int J Obes (Lond) 2018. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhaegen AA, Van Gaal LF. Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. J Endocrinol Invest 2017; 40:1165–74. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes A. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013; 36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2015; 21:438–47. [DOI] [PubMed] [Google Scholar]
- 23.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm−−2016 Executive Summary. Endocr Pract 2016; 22:84–113. [DOI] [PubMed] [Google Scholar]
- 24.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes--causes, effects and coping strategies. Diabetes Obes Metab 2007; 9:799–812. [DOI] [PubMed] [Google Scholar]
- 25.Ashcroft FM. Mechanisms of the glycaemic effects of sulfonylureas. Horm Metab Res 1996; 28:456–63. [DOI] [PubMed] [Google Scholar]
- 26.Fonseca V Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med 2003; 115 Suppl 8A:42S–8S. [DOI] [PubMed] [Google Scholar]
- 27.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ness-Abramof R, Apovian CM. Drug-induced weight gain. Drugs Today (Barc) 2005; 41:547–55. [DOI] [PubMed] [Google Scholar]
- 29.MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev 2005; 18:638–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J, Group AAS. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart--Then Focus. J Antimicrob Chemother 2012; 67 Suppl 1:i51–63. [DOI] [PubMed] [Google Scholar]
- 31.Shen Y, Hendricks A, Wang F, Gardner J, Kazis LE. The impact of private insurance coverage on veterans’ use of VA care: insurance and selection effects. Health Serv Res 2008; 43:267–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


