Abstract
Breast cancer (BC) is one of the most important neoplasias among women. Many patients receive radiotherapy (RT), which involves radiation exposure of the thoracic zone, including the heart and blood vessels, leading to the development of cardiovascular disease (CVD) as a long-term side effect. The severity of CVD-related pathologies leads research on assessing novel CVD biomarkers as diagnostic, prognostic or therapeutic agents. Currently, the possible candidates include blood microRNAs (miRNAs). Previous studies have supported a role for miRNA-146a, -155, -221, and -222 in the progression of CVD. Our purpose was to evaluate the RT-induced modulation of the expression of these miRNAs in the blood of women with BC. Pre-RT control and post-RT blood samples were collected, and after miRNA isolation and reverse transcription, the levels of the selected miRNAs were measured by real-time PCR. Our results showed that miRNA-155 exhibited the lowest expression, while miRNA-222 exhibited the highest expression, followed by miRNA-221. The expression of each individual miRNA was positively correlated with that of the others both pre-RT control and post-RT and inversely correlated with age before RT. Furthermore, RT promoted the overexpression of the selected miRNAs. Their levels were also affected by CVD-linked clinical parameters, treatment and BC side. Modulation of the expression of the selected miRNAs together with other risk factors might be associated with the development of future cardiovascular pathologies. Further confirmatory studies are needed to assess their potential as possible biomarkers in the progression of or as therapeutic targets for RT-induced CVD in BC patients.
Introduction
Breast cancer (BC) is one of the most important neoplasias among women. In Spain, 1 in 8 women will develop BC, and approximately 26,000 new cases per year are diagnosed [1]. According to the Instituto Nacional de Epidemiología, there were 6,213 BC deaths in 2014 [2]. Currently, fewer cases in UE and North America are diagnosed because of both early detection and efficient systemic therapies. Nevertheless, it is still the most common cause of death from cancer in less developed countries and the second cause in more developed countries, subsequent to lung cancer [3,4].
The treatment of BC involves a combination of therapies such as surgery, chemotherapy, hormonotherapy, targeted therapy and radiotherapy (RT) [5]. RT can be omitted in some patients with a low-risk profile, which reduces the risk of local recurrence and mortality [6]. RT treatment involves radiation exposure of the thoracic zone, including the heart and blood vessels. Despite RT-related benefits, epidemiological data support a relationship between RT and cardiovascular disease (CVD) [7–9].
Patients with left-sided BC exhibit greater cardiotoxicity than those with right-sided tumours due to RT exposure to the heart and left coronary artery [10–12]. In a retrospective study, Darby et al. [12] observed that for every 1 Gy increase in radiation to the heart, the risk of cardiac damage increased 7.4% in patients with left-sided BC. These authors also reported similar results following ionizing radiation to the heart during RT of BC. Cheng et al. [13], who examined 1,191,371 patients from 39 studies, supported that RT applied to patients with left-sided breast tumours caused major coronary heart failure and cardiac death compared with patients with right-sided BC. They also stated that the risk of CVD begins within the first ten years after RT and that heart mortality increases in the second and third decades. Although the RT-CVD association is lower than that established by risk factors such as smoking, hypertension, diabetes or hyperlipidaemia, the risk of death due to CVD after this therapy exceeds the increase in RT-contributed survival. The CVDs associated with RT in BC patients are coronary heart disease [13,14] and atherosclerosis [15].
The severity of these CVD-related pathologies leads research on assessing novel CVD biomarkers. Currently, the possible candidates as suitable biomarkers in diagnostic, prognostic or therapeutic agents of this pathogenesis are microRNAs (miRNAs) [16–19].
miRNAs are small noncoding RNAs of approximately 22 nucleotides in length that act as posttranscriptional regulators, thus modulating gene expression [20,21]. Approximately 1,500–2,000 human miRNAs have been identified, and it is estimated that over 60% of protein-coding genes are directly regulated by miRNAs. One miRNA targets several mRNAs, and one mRNA could be regulated by different miRNAs. Furthermore, miRNAs are ubiquitously expressed, non-cell-specific and can be released into the circulation through microvesicles (i.e., exosomes and apoptotic bodies), lipoproteins (i.e., HDL), or as a protein complex (i.e., RISC and necrotic tissue) [22]. Blood miRNAs are useful as biomarkers for disease diagnosis because the extraction method is noninvasive, they are sensitive, disease-specific, and stable in body fluids for a long time and, finally, freeze-thaw cycles do not appear to affect their structure [23–25]. In fact, blood miRNAs have recently become attractive biomarkers in pathologies including CVD due to their role as disease regulators [26,27].
Previous studies have supported a role for miRNA-146a, -155, -221, and -222 in the progression of CVD, mainly by regulating inflammation, oxidative stress, apoptosis, and angiogenesis in atherosclerotic plaques [28–31]. It is supported that all these miRNAs are overexpressed in human atherosclerotic lesions [32–34]. miRNA-146a is an atheroprotective and anti-inflammatory miRNA that acts in oxidized LDL-activated macrophages, reducing lipid uptake and cytokine release [28,35], but its absence paradoxically suppresses atherosclerosis development in mice [32]. Moreover, miRNA-155 regulates immune and inflammatory processes and induces lipid uptake in monocytes and macrophages [29,36]. Finally, both miRNA-221 and 222 exhibit antiproliferative and proapoptotic actions in endothelial cells, whereas they promote proliferation and migration and inhibit apoptosis in smooth muscle cells [37,38].
Therefore, the aim of our study was to evaluate the expression of these miRNAs in the blood of pre-RT control and post-RT patients with BC.
Materials and methods
Participant treatment
The study was approved by the Ethics Committee (Institutional Review Board) of the Hospital Universitari de Sant Joan (Reference: CEIM 014/2017). All patients signed a written informed consent according to the declaration of Helsinki and a written informed consent for publication.
In the study, 136 women (mean age: 56 years, range: 27–84 years) diagnosed with BC were included. They were patients at the Department of Radiation Oncology of Hospital Sant Joan (Reus, Spain). All patients had a Karnofsky Index <70 and were classified as 0 or 1 on the Eastern Cooperative Oncology Group scale [39]. The protocol for metastatic BC patients (2.94%) was to proceed to RT following complete response of the metastases to primary systemic treatment [39].
The applied treatment for these women was as follows. First, the tumour was extirpated by surgery. Then, according to their risk factor status, they received RT alone or RT combined with other therapies. On the one hand, some patients received adjuvant chemotherapy (55.2%) and/or targeted therapy (17.6%), which were administered postsurgery, for approximately 4 or 5 months and was concluded between 1 and 2 months before RT. On the other hand, adjuvant hormone therapy commenced 1 to 2 months postsurgery and was usually administered simultaneously with RT (78.7% of patients).
Radiotherapy schedule
All patients received RT for approximately 2 months. The radiation scheduled could be normofractionated RT (50 Gy at 2 Gy/day on the affected breast and 16 Gy at 2 Gy/day on the tumour bed for 5 days/week) or hypofractionated RT (40 Gy at 2.67 Gy/day for 5 days/week). The dose rate of all schedules was 4.5 Gy/min (400 MU/min) [40]. Additionally, some patients received irradiation to the regional lymph nodes depending on risk factor status [41]. During RT, a weekly acute toxicity assessment was performed using the criteria of the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer [42].
Biological samples
Blood samples of the patients were collected at two time-points: prior to RT (named as pre-RT control) and one month after the conclusion of RT (named as post-RT). Samples were collected into “PAXgene Blood RNA” tubes (Qiagen, Madrid, Spain) allowing the stabilization of intracellular miRNA, and they were stored at -80°C until use.
miRNA isolation
For the miRNA isolation, 2mL of blood samples were centrifuged for 10min at 4,000 g. Then, supernatant was discarded and pellet was resuspended with 2mL PBS. After 10min at 4,000g centrifugation, supernatant was discarded and the next steps were performed by following the miRCURY RNA Isolation Kit (Exiqon, BioNova, Madrid, Spain). Briefly, the pellet was resuspended with 350μL of lysis buffer mixed with β-mercaptoethanol (100:1 ratio). Then, 200μL of 100% ethanol was added and mixed by vortex. The content was transferred onto a column and centrifuged 1min at 3,500g. The flowthrough was discarded and the column was washed three times by adding 400μL of Wash Solution and subsequently centrifuging 1min at 14,000g. Then, the flowthrough was discarded and the column was dry off by centrifuging 2min at 14,000g. Finally, 50μL of Buffer Elution was added onto the column and sample was centrifuged twice for 2min at 200g and 1min at 14,000g. The flowthrough containing miRNA was stored into RNA-free tubes at -80°C until use.
cDNA synthesis
cDNA was synthesized using the miRCURY LNA Universal RT microRNA PCR, Polyadenylation and cDNA synthesis kit II (Exiqon, BioNova, Madrid, Spain). Briefly, 2μL of miRNA sample was mixed with 1μL Reverse Transcriptase enzyme, 2μL Reaction buffer and 5μL H2O. Then, GeneAmp PCR System 2700 Thermal Cycler (Applied Biosystems, Thermo Fisher Scientific, Waltham, Massachusetts, USA) was employed for the sample incubation. Using the thermocycler program, the miRNAs samples were reversing transcript for 60min at 42°C, the Reverse Transcriptase was inactivated for 5min at 95°C and the samples containing cDNA were maintained at 4°C and finally stored at -20°C.
Real-time PCR
To determine miRNA levels, real time PCR assays were performed. The assessed miRNAs were miRNA-146a, -155, -221 and -222. Levels of target miRNAs were normalized employing the snRNA-U6 as housekeeper. Briefly, the reactions were performed in a final reaction volume of 10μL, adding 3.5μL cDNA sample (previously diluted 1:40 with RNase-free water) and 6.5 μL mix containing 5μL ExiLENT SYBR Green Master Mix (Exiqon, BioNova, Madrid, Spain), 1 μL tested Primer (miRCURY LNA uniRT PCR primer mix, Exiqon), 0.2μL ROX (Passive reference RT-PARE-03, Exiqon, BioNova, Madrid, Spain) and 0.3 μL RNase-free water. Negative controls were also run for each assay. Real-time reactions were run by use of a 7900 HT Fast Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific, Waltham, Massachusetts, USA), performing dsDNA denaturation activation for 10min at 95°C and 40 cycles of 10 s at 95°C followed by 1min at 60°C for amplification. Finally, results were analysed using the 2.4 SDS Software (Applied Biosystems, Thermo Fisher Scientific, Waltham, Massachusetts, USA) and RQ Manager 1.2.1 (Applied Biosystems, Thermo Fisher Scientific, Waltham, Massachusetts, USA).
Statistical analysis
For the statistical analysis, 2-DCt of selected miRNAs was calculated for each sample. DCt was calculated as Cycle threshold (Ct) of each miRNA–Ct of snRNA-U6. Then, in order to normalize the variable we transformed into log (2-DCt) and in order to positivize it we added a 3 units constant to obtain log (2-DCt) + 3. Statistics of transformed variable log (2-DCt) + 3 did not differ from log (2-DCt) ones. First, ANOVA test was used to evaluate differences between their expressions and percentage of increase was calculated for each miRNA in comparison to the lower expressed one. Then, correlations between miRNAs expression and age at diagnose of patients were assessed employing Pearson correlation coefficient. Moreover, paired t-test was performed in order to assess the differences between pre-RT and post-RT expressions in blood of the selected miRNAs and their percentage of increase was calculated. Finally, single factors ANOVA tests were executed to evaluate the differences in their expression depending on CVD-linked clinical parameters, received treatment and BC side. The level of statistical significance for all tests was established at p<0.05. All the calculations were performed with IBM SPSS Statistics (SPSS version 25 for Windows, USA).
Results
Clinical characteristics of BC patients
The main clinical characteristics of the BC patients are shown in Table 1. On the one hand, when recluded before RT, some women presented CVD-related diseases, such as diabetes mellitus (8.1%), arterial hypertension (25.7%), dyslipidaemia (27.2%), cardiac pathology (2.9%) or hypothyroidism (7.4%). Furthermore, the majority were postmenopausal. On the other hand, prevalent tumour molecular classifications were luminal A and B types. Moreover, 55.1% of women received chemotherapy, 78.7% of women received hormonal therapy, and targeted therapy was applied to only 17.6% of patients. Lastly, the affected BC side was similarly right or left, whereas only 1.5% of women presented bilateral BC.
Table 1. Characteristics of BC patients.
| Variable | Total | Frequency (%) | |
|---|---|---|---|
| Diabetes mellitus | 11 | 8.1 | |
| Arterial hypertension | 35 | 25.7 | |
| Dyslipidemia | 37 | 27.2 | |
| Cardiac pathology | 4 | 2.9 | |
| Hypothyroidism | 10 | 7.4 | |
| Menopause state | |||
| Premenopausal | 29 | 21.3 | |
| Perimenopausal | 20 | 14.7 | |
| Postmenopausal | 85 | 62.5 | |
| Tumor molecular classification | |||
| Luminal A | 40 | 29.4 | |
| Luminal B | 50 | 36.8 | |
| Her2 positive | 27 | 19.9 | |
| Triple negative | 17 | 12.5 | |
| Ki67 antigen in tumor biopsy | |||
| <15 | 48 | 35.3 | |
| 15–50 | 62 | 45.6 | |
| >50 | 19 | 14.0 | |
| HER2 receptor in tumor biopsy | |||
| negative | 107 | 78.7 | |
| positive | 26 | 19.1 | |
| Estrogen receptors | |||
| negative | 22 | 16.2 | |
| positive | 113 | 83.1 | |
| Progesterone receptors | |||
| negative | 46 | 33.8 | |
| positive | 89 | 65.4 | |
| Chemotherapy | 75 | 55.1 | |
| Hormone therapy | 107 | 78.7 | |
| Targeted therapy | 24 | 17.6 | |
| BC location | |||
| Right | 66 | 48.5 | |
| Left | 68 | 50.0 | |
| Bilateral | 2 | 1.5 | |
BC, breast cancer; HER2, human epidermal growth factor receptor 2.
Expression of miRNA-146a, -155–221 and -222 at pre-RT control and post-RT
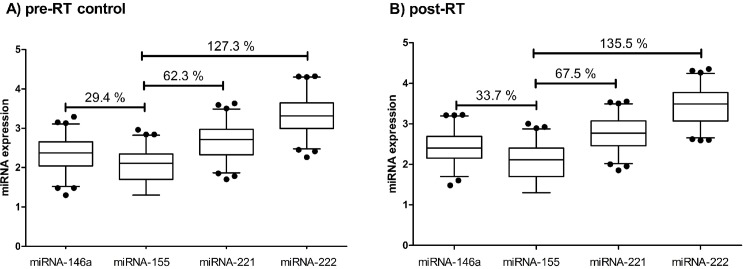
The levels of miRNA-146a, -155–221 and -222 in the blood of women with BC are shown in Fig 1A–1D. In the current study, miRNA-155 presented the lowest expression both pre-RT control and post-RT. When compared to this miRNA, the expression of miRNA-146a, -221 and -222 before RT was significantly higher (29.4%, 62.3% and 127.3%, respectively, p< 0.0001) (Fig 1A). In addition, similar differences in expression were maintained after RT, with significant increases of 33.7%, 67.5% and 135.5%, respectively (p< 0.0001) (Fig 1B).
Fig 1.
Expression of miRNA-146a, -155, -221 and -222 in blood of BC patients at pre-RT control (A) and post-RT (B) treatment. miRNA expression was calculated as log(2-DCt)+3. Results are expressed as percentile 2.5–97.5. Percentage of expression increase in comparison to miRNA-155 is indicated (%).
Correlations of miRNA-146a, -155, -221 and -222 expression at pre- RT control and post-RT
Table 2 shows the correlations between miRNA expression in the blood before and after RT in women with BC. The results revealed a significant positive correlation between miRNA expression both pre-RT control and post-RT; the Pearson correlation coefficient (r) was above 0.882 for all bivariate correlations.
Table 2. Correlations between expression of selected miRNAs in blood of BC patients at pre-RT control and post-RT.
| Pre-RT control | Post-RT | |||||
|---|---|---|---|---|---|---|
| miRNA-146a | miRNA-155 | miRNA-221 | miRNA-146a | miRNA-155 | miRNA-221 | |
| miRNA-155 | 0.927** | 0.924** | ||||
| miRNA-221 | 0.900** | 0.917** | 0.896** | 0.909** | ||
| miRNA-222 | 0.890** | 0.910** | 0.930** | 0.882** | 0.906** | 0.922** |
BC, breast cancer; RT, radiotherapy; miRNA, microRNA.
miRNA expression was calculated as log(2-DCt)+3. Pearson correlation coefficient (r) is shown.
**means significant differences at p<0.01 (bilateral).
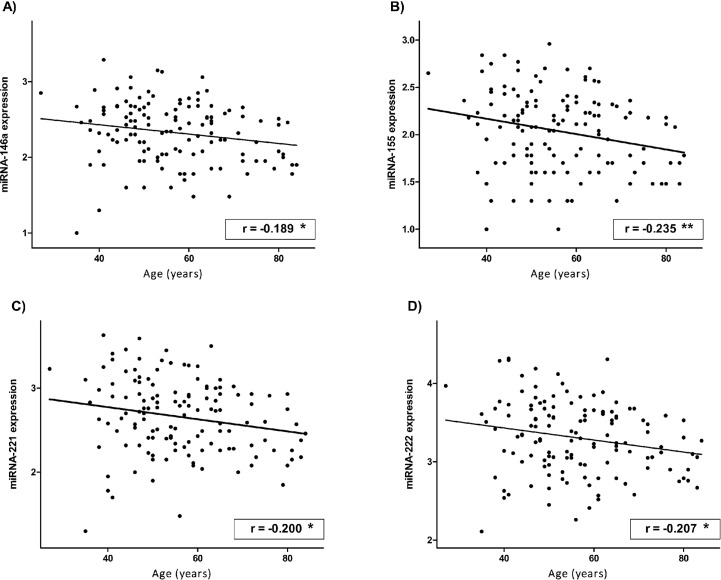
Moreover, the expression of all studied miRNAs pre-RT was inversely correlated with age at diagnosis (Fig 2). The results also showed that after RT, this significant correlation was lost, although the same tendency persisted (S1 Table of Supporting information).
Fig 2.
Correlations of the expression of miRNA-146a (A), -155 (B), -221 (C) and -222 (D) in blood of BC patients with age at diagnose at pre-RT control. miRNA expression was calculated as log(2-DCt)+3. Pearson correlation coefficient (r) is shown. -means inverse correlation. *means significant differences at p<0.05 (bilateral). **means significant differences at p<0.01 (bilateral).
Comparison of the expression of each miRNA between pre- RT control and post-RT time points
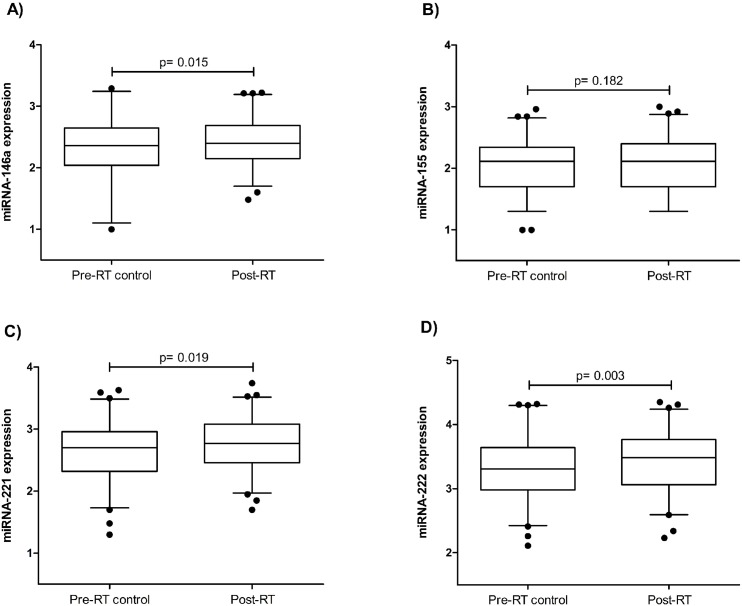
The expression of the selected miRNAs in the blood before and after RT in women with BC is depicted in Fig 3 (A-D). We observed that RT treatment induced an increase in the expression of the selected miRNAs whose levels were higher post-RT than pre-RT control. While miRNA-222 had the greatest statistically significant increase (12%, p = 0.003) (Fig 3D), miRNA-146a (Fig 3A) and -221 (Fig 3C) also increased significantly (7.6%, p = 0.015 and 9%, p = 0.019, respectively). Furthermore, miRNA-155 decreased by 3.8%, without statistical significance (p = 0.182) (Fig 3B).
Fig 3.
Comparison of each blood miRNA (miRNA-146a (A), -155 (B), -221 (C) and -222 (D)) of BC patients between pre-RT control and post-RT time-points. miRNA expression was calculated as log(2-DCt)+3.p value indicates differences between groups.
Evaluation of miRNA-146a, -155, -221 and -222 expression at pre- RT control and post-RT considering CVD-related clinical parameters, treatment and BC side
We assessed whether the expression of the selected miRNAs was associated with the radiation schedule, and we found no differences between miRNA levels in women who received normofractionated RT or hypofractionated RT at either time point (S2 Table of Supporting information).
Table 3 shows the levels of miRNA-146a, -155, -221 and -222 in the blood of BC patients pre-RT control and post-RT stratified by dyslipidaemia, hypothyroidism, chemotherapy, targeted therapy and BC side. On the one hand, the expression of all miRNAs was significantly associated with dyslipidaemia both pre-RT control (miRNA-146a; p = 0.023, miRNA-155; p = 0.016, miRNA-221; p = 0.023, miRNA-222; p = 0.005) and post-RT (miRNA-146a; p = 0.023, miRNA-155; p = 0.033, miRNA-221; p = 0.018, miRNA-222; p = 0.009); dyslipidaemic patients had significantly lower levels than nondyslipidaemic patients. Similarly, hypothyroid women presented significantly lower levels than nonhypothyroid women before (miRNA-146a; p = 0.026, miRNA-155; p = 0.036, miRNA-222; p = 0.015) and after (miRNA-146a; p = 0.028, miRNA-155; p = 0.034, miRNA-221; p = 0.017, miRNA-222; p = 0.009) RT, excluding miRNA-221 pre-RT. In addition, the expression of some miRNAs was significantly associated with treatment. Women who received chemotherapy showed significantly increased levels of all the selected miRNAs pre-RT control (miRNA-146a; p = 0.003, miRNA-155; p = 0.000, miRNA-221; p = 0.000, miRNA-222; p = 0.001) and miRNA-155 (p = 0.025) and -221 post-RT (p = 0.030) compared to those not treated with chemotherapy. Moreover, regarding targeted therapy, the expression of miRNA-155, -221 and -222 prior to RT was significantly higher in treated women than in nontreated women (miRNA-155; p = 0.045, miRNA-221; p = 0.003, miRNA-222; p = 0.005). In contrast, the expression of these miRNAs did not differ significantly between women who received hormonal therapy and those in whom this therapy was not applied (S2 Table of Supporting information). Finally, the BC side was also significantly associated with miRNA expression. A tendency toward higher levels of all miRNAs was observed in women with left-sided BC compared to those with right-sided BC, which was significant for miRNA-146a both pre-RT control (p = 0.033) and post-RT (p = 0.025) and for miRNA-221 pre-RT control (p = 0.021).
Table 3. Expression of selected miRNAs expression in blood of BC patients at pre-RT control and post-RT depending on CVD-linked clinical parameters, received treatment and BC side.
| Pre-RT control | Post-RT |
|||||
|---|---|---|---|---|---|---|
| Dyslipidemia |
No (71.3%) |
Yes (27.2%) |
p value |
No (71.3%) |
Yes (27.2%) |
p value |
| miRNA-146a | 2.38±0.043 | 2.20±0.057 | 0.023 | 2.45±0.037 | 2.28±0.070 | 0.023 |
| miRNA-155 | 2.08±0.044 | 1.88±0.064 | 0.016 | 2.12±0.041 | 1.94±0.075 | 0.033 |
| miRNA-221 | 2.71±0.048 | 2.51±0.060 | 0.023 | 2.80±0.040 | 2.61±0.074 | 0.018 |
| miRNA-222 | 3.37±0.049 | 3.12±0.063 | 0.005 | 3.49±0.042 | 3.26±0.082 | 0.009 |
| Hypothyroidism |
No (91.2%) |
Yes (7.4%) |
p value |
No (91.2%) |
Yes (7.4%) |
p value |
| miRNA-146a | 2.35±0.037 | 2.06±0.1 | 0.026 | 2.43±0.034 | 2.15±0.144 | 0.028 |
| miRNA-155 | 2.05±0.039 | 1.76±0.10 | 0.036 | 2.07±0.037 | 1.80±0.141 | 0.034 |
| miRNA-221 | 2.68±0.041 | 2.38±0.10 | 0.450 | 2.77±0.036 | 2.45±0.146 | 0.017 |
| miRNA-222 | 3.33±0.042 | 2.96±0.10 | 0.015 | 3.46±0.039 | 3.07±0.173 | 0.009 |
| Chemotherapy |
No (39%) |
Yes (55.1%) |
p value |
No (39%) |
Yes (55.1%) |
p value |
| miRNA-146a | 2.20±0.053 | 2.42±0.047 | 0.003 | 2.34±0.045 | 2.45±0.050 | 0.119 |
| miRNA-155 | 1.86±0.054 | 2.14±0.048 | 0.000 | 1.97±0.053 | 2.15±0.051 | 0.025 |
| miRNA-221 | 2.46±0.056 | 2.78±0.050 | 0.000 | 2.65±0.053 | 2.81±0.050 | 0.030 |
| miRNA-222 | 3.13±0.055 | 3.41±0.055 | 0.001 | 3.34±0.055 | 3.49±0.055 | 0.069 |
|
Targeted therapy |
No (78.7%) |
Yes (17.6%) |
p value |
No (78.7%) |
Yes (17.6%) |
p value |
| miRNA-146a | 2.3±0.038 | 2.47±0.091 | 0.054 | 2.41±0.036 | 2.38±0.094 | 0.709 |
| miRNA-155 | 2.00±0.041 | 2.19±0.088 | 0.045 | 2.08±0.040 | 2.08±0.098 | 0.940 |
| miRNA-221 | 2.60±0.041 | 2.89±0.093 | 0.003 | 2.75±0.040 | 2.73±0.089 | 0.803 |
| miRNA-222 | 3.25±0.042 | 3.53±0.106 | 0.005 | 3.44±0.043 | 3.38±0.096 | 0.563 |
| BC side |
right (48.5%) |
left (50%) |
p value |
right (48.5%) |
left (50%) |
p value |
| miRNA-146a | 2.24±0.053 | 2.42±0.044 | 0.033 | 2.32±0.048 | 2.50±0.044 | 0.025 |
| miRNA-155 | 1.96±0.054 | 2.11±0.050 | 0.133 | 2.00±0.052 | 2.14±0.050 | 0.133 |
| miRNA-221 | 2.55±0.058 | 2.76±0.048 | 0.021 | 2.66±0.050 | 2.83±0.049 | 0.053 |
| miRNA-222 | 3.22±0.060 | 3.39±0.052 | 0.083 | 3.37±0.056 | 3.48±0.053 | 0.568 |
BC, breast cancer; RT, radiotherapy; miRNA, microRNA.
Mean ± SEM of the expression (log(2-DCt)+3) of selected miRNAs is shown. Statistics: Single factor ANOVA test. p value indicates differences between groups.
Discussion
In the present study, we evaluated the expression of miRNA-146a, -155, -221 and -222, which are involved in the development of CVD, in the blood of both pre-RT control and post-RT samples from women with BC. Our results showed that at both time points, miRNA-222 had the highest expression, followed by miRNA-221. Both miRNAs, miRNA-221 and 222, are involved in the regulation of development and progression of atherosclerosis, exhibiting antiproliferative and proapoptotic actions in endothelial cells [37]. In contrast, they play an opposite role in smooth muscle cells, inducing proliferation and migration and inhibiting apoptosis [38]. On the other hand, the expression of miRNA-155 was the lowest in both the pre-RT control and post-RT blood samples. This observation is in accord with Gezer et al. [43], who indicated that the circulating miRNA-221 levels were higher than those of miRNA-155 in BC patients. Furthermore, miRNA-155 was recently considered below the detection limit by Alunni-Fabbroni et al. [44], who was not able to detect the expression of this miRNA in the circulation of BC patients. In addition, our results showed that the expression of each of the four selected miRNAs was positively correlated with that of the others both pre-RT control and post-RT. Their correlation could be supported by a linked regulation of their expression, as a crosslink between these miRNAs has been described in several pathways in which they are involved, such as inflammatory processes that regulate atherosclerotic processes [31,45,46]. Similarly, Baldeon et al. [47] found a correlation between circulating miRNA-146 and -155 in diabetics and in healthy patients.
We reported that the expression of the selected miRNAs was inversely correlated with the age at diagnosis of patients before RT. Thus, it was observed that with age, the levels of the selected miRNAs decreased significantly. Human ageing is associated with an increase in age-related diseases, such as cancer, diabetes mellitus and CVD [48,49]. A number of investigations have suggested that longevity can be modulated by changes in the expression of particular genes [50]. It has been reported that changes in miRNA expression occur with human cellular senescence, which is defined as an irreversible decline in cell proliferation [51]. In this context, Noren Hooten et al. [52] analysed the expression of over 800 miRNAs in the blood of younger and older individuals. The authors identified several miRNAs, including miRNA-221 and miRNA-155, that were less expressed in older individuals than in younger individuals. Similarly, Fichtlscherer et al. [53] showed that the age of females was inversely correlated with the circulating miRNA-155 levels in coronary artery disease (CAD) patients and in the healthy population. Furthermore, our results showed that the correlation between miRNA expression and age was lost after RT treatment, which indicated that RT induced an increase in the levels of selected miRNAs, affecting mainly older patients.
We propose that the observed RT-induced modulation of blood miRNA-146a, -155, -221 and -222 might be involved in the progression of CVD and that BC patients usually develop CVD a few years after RT treatment [18,54]. Numerous studies have confirmed that all these miRNAs were overexpressed in atherosclerotic human lesions [32–34]. Thus, it would be expected to find high levels of these miRNAs in the circulation in CVD patients, as they are involved in this pathology. In fact, circulating miRNA-146a was overexpressed in CAD patients [55–57]. However, other studies have reported low levels of miRNA-146a, as well as miRNA-155, -221 and -222, in the circulation of patients with dyslipidaemia, CAD, coronary heart disease (CHD) or atherosclerosis [47,53,56,58,59]. The observed miRNA downregulation in these studies could be explained by the fact that miRNAs can be taken up by atherosclerotic lesions or perhaps a feedback mechanism could control their overactivation. In the current study, to assess the effect of RT on the selected miRNA levels, we compared the expression of each miRNA in the blood between pre-RT control and post-RT samples. We observed significant overexpression of miRNA-146a, -221 and -222 and an increased tendency of miRNA-155 in the blood one month after RT treatment. In previous investigations, miRNA-146a exhibited atheroprotective and anti-inflammatory properties by diminishing lipid uptake and cytokine release by oxidized LDL-activated macrophages [28,35]. Nevertheless, the absence of this miRNA paradoxically suppressed the development of atherosclerosis in mice [32]. On the other hand, it has been reported that miRNA-155 is highly expressed in activated immune cells and regulates immune and inflammatory responses and induces lipid uptake in monocytes and macrophages [29,36]. In a previous investigation carried out by Templin et al. [60], the effect of RT on blood miRNA profiles was analysed. The authors reported 45 differentially expressed miRNAs, including miRNA-221 and -222, that were overexpressed in the blood from patients in complete remission 1 or 2 after 4 hours of total body irradiation at a dose of 1.25 Gy. In contrast, Sochor et al. [61] stated that chemotherapy or RT did not affect the circulating levels of miRNA-155. The discordance between this study and ours could be due to differences between RT treatments, as their patients received two single RT sessions, and post-RT samples were taken at variable time points. Moreover, we observed that miRNA-222 had a higher increase. Although miRNA-221 and -222 contain the same seed [62] and both play a role in endothelial dysfunction and atherogenesis [37,63], miRNA-222 was demonstrated to be more biologically relevant for inflammation-mediated neovessel formation [64]. Therefore, our results, in conjunction with previous data, suggest that selected miRNAs could be overexpressed due to RT in BC patients after one month and might become downregulated many years later when developing CVD because they migrate into atherosclerotic lesions.
One goal of our study was to analyse differences in the expression of selected miRNAs considering CVD-linked clinical parameters, treatment received and BC side. First, we analysed dyslipidaemia and hypothyroidism, as both are well-known risk factors for CVD [65,66]. On the one hand, the results showed that women with BC who were dyslipidaemic had significantly lower expression of selected miRNAs than nondyslipidaemic women both pre-RT control and post-RT. Previous studies have assessed miRNA-146a levels in non-BC patients with dyslipidaemia. For instance, Baldeon et al. [47] found low levels of circulating miRNA-146a in the diabetes population, in which dyslipidaemia is a common feature. In contrast, this miRNA was overexpressed in the circulation in patients with hyperlipidaemia [67]. The disagreements between the results of both studies might be due to differences between the dyslipidaemic profiles of the patients studied. In the same way, our results showed that patients with hypothyroidism exhibited significantly lower miRNA levels than nonhypothyroid patients before and after RT, except for miRNA-221 pre-RT. In contrast, in a recent study enrolling 192 subjects, Quan et al. [68] found that circulating miRNA-146a was higher in patients with CHD and subclinical hypothyroidism than in those with only CHD, suggesting that hypothyroidism was responsible for miRNA-146a overexpression. It should be noted that the results of Quan et al. [68] differed from ours because their study examined not BC but rather CHD patients. Moreover, our results confirm the relationship between selected miRNA downregulation and CVD, as we showed that dyslipidaemic and hypothyroid patients had low levels of selected miRNAs.
On the other hand, we assessed whether treatment could lead to differential miRNA expression under both pre-RT control and post-RT conditions. It has been described that chemotherapy as well as targeted and hormonal therapies are involved in cardiopathologies but exhibit less severity than RT [69–72]. In the present study, some patients received chemotherapy at the pre-RT control time point. Our results established that women with BC who received chemotherapy overexpressed miRNA-146a and -222 pre-RT and miRNA-155 and -221 post-RT compared to those not treated with chemotherapy. Interestingly, other authors have found diminished circulating levels of miRNA-155 and -221 at the end of chemotherapy in BC patients [43,73], whereas Rigaud et al. [74] found increased miRNA-146a levels 3 weeks after the conclusion of chemotherapy in BC patients; the results are in agreement with ours. Furthermore, the differences in the expression of these miRNAs due to chemotherapy, as an established risk of cardiotoxicity [70,72], might support their role in CVD. In addition, our results indicated that blood miRNA-155, -221 and -222 levels were enhanced prior to RT in women who received targeted therapy compared to other women. Therefore, we suppose that this miRNA increase might be related to targeted therapy-associated CVD [69,71]. According to the data obtained in our study, we suggest that hormonal therapy, which is applied at the same time as RT in some women with BC, did not interfere with miRNA expression, because we did not find any differences between the miRNA levels in hormonal therapy-treated and nontreated patients either pre- or post-RT. Furthermore, miRNA-146a was overexpressed in patients with left-sided BC before and after RT and miRNA-221 post-RT when compared to patients with right-sided BC. Thus, a possible link between the observed modulation of miRNA expression and CVD might exist, as RT on left-sided tumours induced greater cardiotoxicity than that on right-sided tumours [10].
In conclusion, our results showed that RT led to the overexpression of miRNA-146a, -155, -221 and -222, which are involved in the progression of CVD, in the blood of women with BC. The expression of each of the miRNAs was positively correlated with that of the others pre-RT control and post-RT and inversely correlated with age pre-RT control. Furthermore, CVD-linked clinical parameters, such as dyslipidaemia and hypothyroidism, as well as treatment with chemotherapy and targeted therapy and BC side, also affected their levels. Thus, the modulation of these miRNAs together with other risk factors might be associated with the development of future cardiovascular pathologies. Variation in the miRNA signature in a diseased peripheral circulatory system opens up a new avenue in the field of biomarker discovery. However, further confirmatory studies are needed to assess their potential in the progression of CVD or as therapeutic targets in the treatment of RT-induced CVD in BC patients.
Supporting information
(DOCX)
BC, breast cancer; RT, radiotherapy; miRNA, microRNA.
Mean ± SEM of the expression (log(2-DCt)+3) of selected miRNAs is shown. Statistics: Single factor ANOVA test. p value indicates differences between groups.
BC, breast cancer; RT, radiotherapy; miRNA, microRNA.
miRNA expression was calculated as log(2-DCt)+3. Pearson correlation coefficient (r) is shown. **means significant differences at p<0.01 (bilateral).
(DOCX)
Acknowledgments
We are especially grateful to Lluís Masana and Sandra Guaita for their useful comments that helped to improve the manuscript and to Roser Rosales for the technical assistance with the miRNA methods.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by grants from the Instituto de Salud Carlos III (PI 10/02547, PI030786), Centro de Investigación Biomédica en Red en Diabetes y Enfermedades Metabólicas asociadas (CIBER-DEM) and Fondo Europeo de Desarrollo Regional (FEDER). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.AECC. Asociación Española Contra el Cáncer. 2014. [Google Scholar]
- 2.INE. Instituto Nacional de Epidemiología. 2014. [Google Scholar]
- 3.Kim CS, Algan O. Radiation Therapy, Breast Cancer Early Stage. StatPearls. Treasure Island (FL)2018. [PubMed] [Google Scholar]
- 4.Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer biology & medicine. 2014;11(2):101–15. Epub 2014/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer biology & medicine. 2015;12(2):106–16. Epub 2015/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X YX, Chen J, Yang Z, Shao Z, Zhang Z, Guo X, Feng Y. Radiotherapy can improve the disease-free survival rate in triple-negative breast cancer patients with T1-T2 disease and one to three positive lymph nodes after mastectomy. The oncologist. 2013;18(2):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obi N, Eulenburg C, Seibold P, Eilber U, Thone K, Behrens S, et al. Associations between adjuvant radiotherapy and different causes of death in a German breast cancer cohort. Breast. 2018;38:75–80. Epub 2017/12/19. 10.1016/j.breast.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 8.de Vries S, Schaapveld M, van Nimwegen FA, Jozwiak K, Lugtenburg PJ, Daniels LA, et al. High burden of subsequent malignant neoplasms and cardiovascular disease in long-term Hodgkin lymphoma survivors. British journal of cancer. 2018. Epub 2018/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie X, Ouyang S, Wang H, Yang W, Jin H, Hu B, et al. Dosimetric comparison of left-sided whole breast irradiation with 3D-CRT, IP-IMRT and hybrid IMRT. Oncology reports. 2014;31(5):2195–205. Epub 2014/03/08. 10.3892/or.2014.3058 [DOI] [PubMed] [Google Scholar]
- 10.Palumbo I, Palumbo B, Fravolini ML, Marcantonini M, Perrucci E, Latini ME, et al. Brain natriuretic peptide as a cardiac marker of transient radiotherapy-related damage in left-sided breast cancer patients: A prospective study. Breast. 2016;25:45–50. Epub 2015/11/09. 10.1016/j.breast.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 11.Skytta T, Tuohinen S, Boman E, Virtanen V, Raatikainen P, Kellokumpu-Lehtinen PL. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol. 2015;10:141 Epub 2015/07/15. 10.1186/s13014-015-0436-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. The New England journal of medicine. 2013;368(11):987–98. Epub 2013/03/15. 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 13.Cheng YJ, Nie XY, Ji CC, Lin XX, Liu LJ, Chen XM, et al. Long-Term Cardiovascular Risk After Radiotherapy in Women With Breast Cancer. Journal of the American Heart Association. 2017;6(5). Epub 2017/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roos CTG, van den Bogaard VAB, Greuter MJW, Vliegenthart R, Schuit E, Langendijk JA, et al. Is the coronary artery calcium score associated with acute coronary events in breast cancer patients treated with radiotherapy? Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2018;126(1):170–6. Epub 2017/11/02. [DOI] [PubMed] [Google Scholar]
- 15.Raghunathan D, Khilji MI, Hassan SA, Yusuf SW. Radiation-Induced Cardiovascular Disease. Current atherosclerosis reports. 2017;19(5):22 Epub 2017/03/21. 10.1007/s11883-017-0658-x [DOI] [PubMed] [Google Scholar]
- 16.Costantino S, Camici GG, Mohammed SA, Volpe M, Luscher TF, Paneni F. Epigenetics and cardiovascular regenerative medicine in the elderly. International journal of cardiology. 2018;250:207–14. Epub 2017/10/11. 10.1016/j.ijcard.2017.09.188 [DOI] [PubMed] [Google Scholar]
- 17.Gacon J, Badacz R, Stepien E, Karch I, Enguita FJ, Zmudka K, et al. Diagnostic and prognostic micro-RNAs in ischaemic stroke due to carotid artery stenosis and in acute coronary syndrome: a four-year prospective study. Kardiologia polska. 2018;76(2):362–9. Epub 2018/01/20. 10.5603/KP.a2017.0243 [DOI] [PubMed] [Google Scholar]
- 18.Gangwar RS, Rajagopalan S, Natarajan R, Deiuliis JA. Noncoding RNAs in Cardiovascular Disease: Pathological Relevance and Emerging Role as Biomarkers and Therapeutics. American journal of hypertension. 2018;31(2):150–65. Epub 2017/12/01. 10.1093/ajh/hpx197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Rosa S, Eposito F, Carella C, Strangio A, Ammirati G, Sabatino J, et al. Transcoronary concentration gradients of circulating microRNAs in heart failure. European journal of heart failure. 2018. Epub 2018/01/10. [DOI] [PubMed] [Google Scholar]
- 20.Dlouha D, Hubacek JA. Regulatory RNAs and cardiovascular disease—with a special focus on circulating microRNAs. Physiological research. 2017;66(Supplementum 1):S21–S38. Epub 2017/04/06. [DOI] [PubMed] [Google Scholar]
- 21.Dragomir M, Chen B, Fu X, Calin GA. Key questions about the checkpoint blockade-are microRNAs an answer? Cancer biology & medicine. 2018;15(2):103–15. Epub 2018/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature cell biology. 2008;10(12):1470–6. Epub 2008/11/18. 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lucia C, Komici K, Borghetti G, Femminella GD, Bencivenga L, Cannavo A, et al. microRNA in Cardiovascular Aging and Age-Related Cardiovascular Diseases. Frontiers in medicine. 2017;4:74 Epub 2017/07/01. 10.3389/fmed.2017.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malla B, Zaugg K, Vassella E, Aebersold DM, Dal Pra A. Exosomes and Exosomal MicroRNAs in Prostate Cancer Radiation Therapy. International journal of radiation oncology, biology, physics. 2017;98(5):982–95. Epub 2017/07/20. 10.1016/j.ijrobp.2017.03.031 [DOI] [PubMed] [Google Scholar]
- 25.Kijima T, Hazama S, Tsunedomi R, Tanaka H, Takenouchi H, Kanekiyo S, et al. MicroRNA-6826 and -6875 in plasma are valuable noninvasive biomarkers that predict the efficacy of vaccine treatment against metastatic colorectal cancer. Oncology reports. 2017;37(1):23–30. Epub 2016/11/24. 10.3892/or.2016.5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Y, Wang HY, Li Y, Guo SH, Zhang L, Cai JH. Peripheral blood miRNAs as a biomarker for chronic cardiovascular diseases. Scientific reports. 2014;4:5026 Epub 2014/05/23. 10.1038/srep05026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Stefano V, Zaccagnini G, Capogrossi MC, Martelli F. microRNAs as peripheral blood biomarkers of cardiovascular disease. Vascular pharmacology. 2011;55(4):111–8. Epub 2011/08/19. 10.1016/j.vph.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 28.Economou EK, Oikonomou E, Siasos G, Papageorgiou N, Tsalamandris S, Mourouzis K, et al. The role of microRNAs in coronary artery disease: From pathophysiology to diagnosis and treatment. Atherosclerosis. 2015;241(2):624–33. Epub 2015/06/29. 10.1016/j.atherosclerosis.2015.06.037 [DOI] [PubMed] [Google Scholar]
- 29.Huang RS, Hu GQ, Lin B, Lin ZY, Sun CC. MicroRNA-155 silencing enhances inflammatory response and lipid uptake in oxidized low-density lipoprotein-stimulated human THP-1 macrophages. Journal of investigative medicine: the official publication of the American Federation for Clinical Research. 2010;58(8):961–7. Epub 2010/10/30. [DOI] [PubMed] [Google Scholar]
- 30.Liu G, Huang Y, Lu X, Lu M, Huang X, Li W, et al. Identification and characteristics of microRNAs with altered expression patterns in a rat model of abdominal aortic aneurysms. The Tohoku journal of experimental medicine. 2010;222(3):187–93. Epub 2010/10/30. [DOI] [PubMed] [Google Scholar]
- 31.Hulsmans M, De Keyzer D, Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25(8):2515–27. Epub 2011/04/22. [DOI] [PubMed] [Google Scholar]
- 32.Cheng HS, Besla R, Li A, Chen Z, Shikatani EA, Nazari-Jahantigh M, et al. Paradoxical Suppression of Atherosclerosis in the Absence of microRNA-146a. Circulation research. 2017;121(4):354–67. Epub 2017/06/24. 10.1161/CIRCRESAHA.116.310529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R, Dong LD, Meng XB, Shi Q, Sun WY. Unique MicroRNA signatures associated with early coronary atherosclerotic plaques. Biochemical and biophysical research communications. 2015;464(2):574–9. Epub 2015/07/15. 10.1016/j.bbrc.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 34.Xue Y, Wei Z, Ding H, Wang Q, Zhou Z, Zheng S, et al. MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1alpha in the progression of atherosclerosis. Atherosclerosis. 2015;241(2):671–81. Epub 2015/06/29. 10.1016/j.atherosclerosis.2015.06.031 [DOI] [PubMed] [Google Scholar]
- 35.Oner T, Arslan C, Yenmis G, Arapi B, Tel C, Aydemir B, et al. Association of NFKB1A and microRNAs variations and the susceptibility to atherosclerosis. Journal of genetics. 2017;96(2):251–9. Epub 2017/07/05. [DOI] [PubMed] [Google Scholar]
- 36.Chen T, Yan H, Li Z, Jing T, Zhu W, Ge J, et al. MicroRNA-155 regulates lipid uptake, adhesion/chemokine marker secretion and SCG2 expression in oxLDL-stimulated dendritic cells/macrophages. International journal of cardiology. 2011;147(3):446–7. Epub 2011/01/27. 10.1016/j.ijcard.2010.10.133 [DOI] [PubMed] [Google Scholar]
- 37.Celic T, Metzinger-Le Meuth V, Six I, Massy ZA, Metzinger L. The mir-221/222 Cluster is a Key Player in Vascular Biology via the Fine-Tuning of Endothelial Cell Physiology. Current vascular pharmacology. 2017;15(1):40–6. Epub 2016/09/17. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Cheng Y, Yang J, Xu L, Zhang C. Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. Journal of molecular and cellular cardiology. 2012;52(1):245–55. Epub 2011/12/06. 10.1016/j.yjmcc.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arenas M, Garcia-Heredia A, Cabre N, Luciano-Mateo F, Hernandez-Aguilera A, Sabater S, et al. Effect of radiotherapy on activity and concentration of serum paraoxonase-1 in breast cancer patients. PloS one. 2017;12(11):e0188633 Epub 2017/11/28. 10.1371/journal.pone.0188633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prades J, Algara M, Espinas JA, Farrus B, Arenas M, Reyes V, et al. Understanding variations in the use of hypofractionated radiotherapy and its specific indications for breast cancer: A mixed-methods study. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2017;123(1):22–8. Epub 2017/02/27. [DOI] [PubMed] [Google Scholar]
- 41.Arenas M, Montero A, de Las Penas MD, Algara M. The position and current status of radiation therapy after primary systemic therapy in breast cancer: a national survey-based expert consensus statement. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2016;18(6):582–91. Epub 2015/09/16. [DOI] [PubMed] [Google Scholar]
- 42.Bots WTC, van den Bosch S, Zwijnenburg EM, Dijkema T, van den Broek GB, Weijs WLJ, et al. Reirradiation of head and neck cancer: Long-term disease control and toxicity. Head & neck. 2017;39(6):1122–30. Epub 2017/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gezer U, Keskin S, Igci A, Tukenmez M, Tiryakioglu D, Cetinkaya M, et al. Abundant circulating microRNAs in breast cancer patients fluctuate considerably during neoadjuvant chemotherapy. Oncology letters. 2014;8(2):845–8. Epub 2014/07/11. 10.3892/ol.2014.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alunni-Fabbroni M, Majunke L, Trapp EK, Tzschaschel M, Mahner S, Fasching PA, et al. Whole blood microRNAs as potential biomarkers in post-operative early breast cancer patients. BMC cancer. 2018;18(1):141 Epub 2018/02/08. 10.1186/s12885-018-4020-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chamorro-Jorganes A, Araldi E, Suarez Y. MicroRNAs as pharmacological targets in endothelial cell function and dysfunction. Pharmacological research. 2013;75:15–27. Epub 2013/04/23. 10.1016/j.phrs.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feinberg MW, Moore KJ. MicroRNA Regulation of Atherosclerosis. Circulation research. 2016;118(4):703–20. Epub 2016/02/20. 10.1161/CIRCRESAHA.115.306300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baldeon RL, Weigelt K, de Wit H, Ozcan B, van Oudenaren A, Sempertegui F, et al. Decreased serum level of miR-146a as sign of chronic inflammation in type 2 diabetic patients. PloS one. 2014;9(12):e115209 Epub 2014/12/17. 10.1371/journal.pone.0115209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strain T, Kelly P, Mutrie N, Fitzsimons C. Differences by age and sex in the sedentary time of adults in Scotland. Journal of sports sciences. 2018;36(7):732–41. Epub 2017/06/18. 10.1080/02640414.2017.1339904 [DOI] [PubMed] [Google Scholar]
- 49.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Frontiers in immunology. 2018;9:586 Epub 2018/04/25. 10.3389/fimmu.2018.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454(7208):1065–71. Epub 2008/08/30. 10.1038/nature07216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Scheiber MN, Neumann C, Calin GA, Zhou D. MicroRNA regulation of ionizing radiation-induced premature senescence. International journal of radiation oncology, biology, physics. 2011;81(3):839–48. Epub 2010/11/26. 10.1016/j.ijrobp.2010.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noren Hooten N, Abdelmohsen K, Gorospe M, Ejiogu N, Zonderman AB, Evans MK. microRNA expression patterns reveal differential expression of target genes with age. PloS one. 2010;5(5):e10724 Epub 2010/05/28. 10.1371/journal.pone.0010724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circulation research. 2010;107(5):677–84. Epub 2010/07/03. 10.1161/CIRCRESAHA.109.215566 [DOI] [PubMed] [Google Scholar]
- 54.Yang Z, Xie L, Han L, Qu X, Yang Y, Zhang Y, et al. Circular RNAs: Regulators of Cancer-Related Signaling Pathways and Potential Diagnostic Biomarkers for Human Cancers. Theranostics. 2017;7(12):3106–17. Epub 2017/08/26. 10.7150/thno.19016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo M, Mao X, Ji Q, Lang M, Li S, Peng Y, et al. miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunology and cell biology. 2010;88(5):555–64. Epub 2010/03/03. 10.1038/icb.2010.16 [DOI] [PubMed] [Google Scholar]
- 56.Yao R, Ma Y, Du Y, Liao M, Li H, Liang W, et al. The altered expression of inflammation-related microRNAs with microRNA-155 expression correlates with Th17 differentiation in patients with acute coronary syndrome. Cellular & molecular immunology. 2011;8(6):486–95. Epub 2011/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramkaran P, Khan S, Phulukdaree A, Moodley D, Chuturgoon AA. miR-146a polymorphism influences levels of miR-146a, IRAK-1, and TRAF-6 in young patients with coronary artery disease. Cell biochemistry and biophysics. 2014;68(2):259–66. Epub 2013/06/25. 10.1007/s12013-013-9704-7 [DOI] [PubMed] [Google Scholar]
- 58.Jia QW, Chen ZH, Ding XQ, Liu JY, Ge PC, An FH, et al. Predictive Effects of Circulating miR-221, miR-130a and miR-155 for Coronary Heart Disease: A Multi-Ethnic Study in China. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;42(2):808–23. Epub 2017/06/20. [DOI] [PubMed] [Google Scholar]
- 59.Bildirici AE, Arslan S, Ozbilum Sahin N, Berkan O, Beton O, Yilmaz MB. MicroRNA-221/222 Expression in Atherosclerotic Coronary Artery Plaque and Peripheral Blood. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals. 2018:1–19. Epub 2018/05/09. [DOI] [PubMed] [Google Scholar]
- 60.Templin T, Paul S, Amundson SA, Young EF, Barker CA, Wolden SL, et al. Radiation-induced micro-RNA expression changes in peripheral blood cells of radiotherapy patients. International journal of radiation oncology, biology, physics. 2011;80(2):549–57. Epub 2011/03/23. 10.1016/j.ijrobp.2010.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sochor M, Basova P, Pesta M, Dusilkova N, Bartos J, Burda P, et al. Oncogenic microRNAs: miR-155, miR-19a, miR-181b, and miR-24 enable monitoring of early breast cancer in serum. BMC cancer. 2014;14:448 Epub 2014/06/19. 10.1186/1471-2407-14-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C, Kang C, Wang P, Cao Y, Lv Z, Yu S, et al. MicroRNA-221 and -222 regulate radiation sensitivity by targeting the PTEN pathway. International journal of radiation oncology, biology, physics. 2011;80(1):240–8. Epub 2011/04/13. 10.1016/j.ijrobp.2010.12.049 [DOI] [PubMed] [Google Scholar]
- 63.Koroleva IA, Nazarenko MS, Kucher AN. Role of microRNA in Development of Instability of Atherosclerotic Plaques. Biochemistry Biokhimiia. 2017;82(11):1380–90. Epub 2017/12/11. 10.1134/S0006297917110165 [DOI] [PubMed] [Google Scholar]
- 64.Dentelli P, Rosso A, Orso F, Olgasi C, Taverna D, Brizzi MF. microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(8):1562–8. Epub 2010/05/22. 10.1161/ATVBAHA.110.206201 [DOI] [PubMed] [Google Scholar]
- 65.Turer CB, Brady TM, de Ferranti SD. Obesity, Hypertension, and Dyslipidemia in Childhood Are Key Modifiable Antecedents of Adult Cardiovascular Disease: A Call to Action. Circulation. 2018;137(12):1256–9. Epub 2018/03/21. 10.1161/CIRCULATIONAHA.118.032531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Decandia F. Risk factors for cardiovascular disease in subclinical hypothyroidism. Irish journal of medical science. 2018;187(1):39–43. Epub 2017/05/12. 10.1007/s11845-017-1617-9 [DOI] [PubMed] [Google Scholar]
- 67.Simionescu N, Niculescu LS, Sanda GM, Margina D, Sima AV. Analysis of circulating microRNAs that are specifically increased in hyperlipidemic and/or hyperglycemic sera. Molecular biology reports. 2014;41(9):5765–73. Epub 2014/06/15. 10.1007/s11033-014-3449-2 [DOI] [PubMed] [Google Scholar]
- 68.Quan X, Ji Y, Zhang C, Guo X, Zhang Y, Jia S, et al. Circulating MiR-146a May be a Potential Biomarker of Coronary Heart Disease in Patients with Subclinical Hypothyroidism. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2018;45(1):226–36. Epub 2018/01/23. [DOI] [PubMed] [Google Scholar]
- 69.Khosrow-Khavar F, Filion KB, Al-Qurashi S, Torabi N, Bouganim N, Suissa S, et al. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Annals of oncology: official journal of the European Society for Medical Oncology. 2017;28(3):487–96. Epub 2016/12/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zagar TM, Cardinale DM, Marks LB. Breast cancer therapy-associated cardiovascular disease. Nature reviews Clinical oncology. 2016;13(3):172–84. Epub 2015/11/26. 10.1038/nrclinonc.2015.171 [DOI] [PubMed] [Google Scholar]
- 71.Long HD LY, Zhang JJ, Zhong WZ, Zheng RN. Risk of Congestive Heart Failure in Early Breast Cancer Patients Undergoing Adjuvant Treatment With Trastuzumab: A Meta-Analysis. The oncologist. 2016;21(5):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koelwyn GJ LN, Ellard SL, Jones LW, Gelinas JC, Rolf JD, Melzer B, Thomas SM, Douglas PS, Khouri MG, Eves ND. Ventricular-Arterial Coupling in Breast Cancer Patients After Treatment With Anthracycline-Containing Adjuvant Chemotherapy. The oncologist. 2016;21(2):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun Y, Wang M, Lin G, Sun S, Li X, Qi J, et al. Serum microRNA-155 as a potential biomarker to track disease in breast cancer. PloS one. 2012;7(10):e47003 Epub 2012/10/17. 10.1371/journal.pone.0047003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rigaud VO, Ferreira LR, Ayub-Ferreira SM, Avila MS, Brandao SM, Cruz FD, et al. Circulating miR-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget. 2017;8(4):6994–7002. Epub 2017/01/05. 10.18632/oncotarget.14355 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
BC, breast cancer; RT, radiotherapy; miRNA, microRNA.
Mean ± SEM of the expression (log(2-DCt)+3) of selected miRNAs is shown. Statistics: Single factor ANOVA test. p value indicates differences between groups.
BC, breast cancer; RT, radiotherapy; miRNA, microRNA.
miRNA expression was calculated as log(2-DCt)+3. Pearson correlation coefficient (r) is shown. **means significant differences at p<0.01 (bilateral).
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.