Abstract
Background
Chikungunya virus (CHIKV) is an emerging mosquito-borne pathogen circulating in tropical and sub-tropical regions. Although autochthonous transmission has not been reported in Australia, there is a potential risk of local CHIKV outbreaks due to the presence of suitable vectors, global trade, frequent international travel and human adaptation to changes in climate.
Methodology/Principal findings
A time series seasonal decomposition method was used to investigate the seasonality and trend of monthly imported CHIKV cases. This pattern was compared with the seasonality and trend of monthly overseas arrivals. A wavelet coherence analysis was applied to examine the transient relationships between monthly imported CHIKV cases and southern oscillation index (SOI) in time-frequency space. We found that the number and geographical distribution of countries of acquisition for CHIKV in travellers to Australia has increased in recent years. The number of monthly imported CHIKV cases displayed an unstable increased trend compared with a stable linear increased trend in monthly overseas arrivals. Both imported CHIKV cases and overseas arrivals showed substantial seasonality, with the strongest seasonal effects in each January, followed by each October and July. The wavelet coherence analysis identified four significant transient relationships between monthly imported CHIKV cases and 6-month lagged moving average SOI, in the years 2009–2010, 2012, 2014 and 2015–2016.
Conclusion/Significance
High seasonal peaks of imported CHIKV cases were consistent with the high seasonal peaks of overseas arrivals into Australia. Our analysis also indicates that El Niño Southern Oscillation (ENSO) variation may impact CHIKV epidemics in endemic regions, in turn influencing the pattern of imported cases.
Author summary
Chikungunya virus (CHIKV) is mosquito-borne virus circulating in tropical and sub-tropical areas of the globe. Infected travellers from CHIKV-affected areas can initiate outbreaks and epidemics in countries where vector mosquitoes are present. Greater understanding of the pattern of imported cases is required to facilitate risk assessment of CHIKV outbreaks. We investigated the temporal pattern of imported CHIKV cases relative to the pattern of overseas arrivals. We also tested whether variability in El Niño Southern Oscillation (ENSO) can predict the import of CHIKV cases in Australia. We found that the number of monthly imported CHIKV cases displayed an unstable increased trend versus the stable linear increased trend observed in monthly overseas arrivals. Both the numbers of imported CHIKV cases and overseas arrivals showed substantial seasonality. High seasonal peaks of imported CHIKV cases were consistent with the high seasonal peaks of overseas arrivals into Australia. We also identified four significant transient relationships between ENSO variability and CHIKV importation. Our results suggest ENSO may impact the occurrence of CHIKV epidemics in endemic regions, in turn influencing the pattern of imported cases.
Introduction
Chikungunya virus (CHIKV) is a mosquito-borne viral pathogen transmitted by mosquitoes, primarily Aedes aegypti and Aedes albopictus [1]. There are no licenced vaccines or specific antiviral drug treatments for the disease [2, 3]. CHIKV has circulated almost continuously in Asia since 1950’s, with over 1.9 million cases reported since 2005 from India, Indonesia, Maldives, Myanmar and Thailand combined [4, 5]. In the Americas, there have been approximately 2.4 million suspected and confirmed cases since December 2013 [6]. CHIKV outbreaks have been occurring in the Pacific since 2011 [7]. There has been substantial geographic expansion of CHIKV outbreaks in the past decade, at least in part due to increased number of international travellers, global trade and evolutionary adaptation of CHIKV to new vector species [8–10].
Infected travellers from affected areas can initiate new CHIKV epidemics in countries where vector mosquitoes are present [11]. CHIKV epidemics triggered by imported CHIKV cases have occurred in the United States, France and Italy [12–16]. Human behavioural/social/land use adaptations to large scale changes in climate may also influence geographical distribution of the CHIKV vector mosquitoes Ae. aegypti and Ae. albopictus [11, 17–20]. Further, the global potential of CHIKV epidemics could be affected by weather variation because virus transmission, survival and abundance of Ae. aegypti and Ae. albopictus are highly dependent on local climatic conditions [8, 21, 22]. Previous studies have indicated that climate change might cause an increase in the frequency of CHIKV outbreaks [22–25].
Although no locally acquired CHIKV cases have been reported to date in Australia, the increasing number of infected travellers from endemic regions has become an important public health consideration for areas where the vectors are established, particularly in North Queensland, Australia where local transmission of dengue viruses occurs periodically [26, 27]. Indeed, imported dengue cases have led to autochthonous dengue outbreaks in North Queensland [18, 28, 29], vectored by Ae. aegypti. In 2016, Ae. albopictus was responsible for a dengue outbreak on the islands of Erub and Badu in the Australian Torres Strait [30]. This was the first Ae. albopictus mediated dengue outbreak in Australian history. Ae. albopictus is currently only found on these islands but has the potential to extend its range across mainland Australia [31]. Since the National Notifiable Diseases Surveillance System [32] started reporting chikungunya virus infection in 2008, imported cases to Australia have been recorded [27, 33]. Outbreaks of vector-borne and particularly Aedes-borne diseases have been related to the behaviours of El Niño–Southern Oscillation (ENSO) [34–36]. Previous studies have suggested that the occurrence of CHIKV outbreaks might be associated with El Niño events in India and Indonesia [37, 38]. To facilitate CHIKV risk assessment, it is important to understand the pattern of imported cases into Australia. We aimed to investigate the temporal pattern of imported CHIKV cases relative to the pattern of overseas arrivals, as well as identify whether variability in ENSO can predict the import of CHIKV cases in Australia.
Methods
Data collection
CHIKV is a notifiable disease in Australia and suspected cases are laboratory confirmed [32]. Monthly imported CHIKV cases were collected from the National Notifiable Diseases Surveillance System [39] for the period from January 2008 to December 2017. The data can be accessed from the Australian Government Department of Health website (http://www9.health.gov.au/cda/source/cda-index.cfm). Data on yearly imported CHIKV cases by country of acquisition were only available from 2013 to 2017 on the Australia Government Department of Health website (http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-vectorborne-overseas-acquired.htm). We also obtained the monthly imported CHIKV cases from New South Wales (NSW), Queensland (Qld), South Australia (SA), Tasmania (Tas), Victoria (Vic), Western Australia (WA) and Northern Territory (NT), in order to explore the spatiotemporal patterns of monthly imported CHIKV cases among different states and territories across Australia. The Australian Capital Territory (ACT) did not report cases between January 2008 and December 2017. In Australia, the majority of imported CHIKV cases were acquired from the Pacific region, Southeast Asia and South and Central Asia [40]. Thus, we used data on the monthly number of overseas arrivals from those areas between January 2008 and December 2017. The overseas arrivals included all recorded movements across the Australian international border [41]. The overseas arrivals data are available from Australian Bureau of Statistics website (http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3401.0Mar%202018?OpenDocument).
The Southern Oscillation Index (SOI) is used to measure the intensity of El Niño and La Niña events, which strongly affect changes in temperature and precipitation [42, 43]. Thus, we used the global climatic factor of SOI to explore the potential impact of ENSO events on the dynamics of imported CHIKV cases in Australia. The monthly SOI is available from the Australian Government Bureau of Meteorology website (www.bom.gov.au/climate/current/soihtm1.shtml).
Statistical analysis
We used a time series seasonal decomposition analysis to detect the seasonality and the trends for monthly imported CHIKV cases and monthly overseas arrivals. The model is given by: Yt = Tt + St + Et, where Yt denotes the original times series for the monthly imported cases and the monthly overseas arrivals; Tt, St and Et denote the trend and cycle component, the seasonal component and the error component, respectively. The seasonal component can capture the seasonal pattern of the data, a pattern that will likely repeat every year. Analyses were undertaken using IBM SPSS Statistics 25.
Wavelet analysis is one of the most efficient approaches for non-stationary time series data. The method can identify the periodical variability by decomposition of a time series into the time-frequency space [44, 45] and can quantify the temporal evolution of imported CHIKV cases with different periodic components. The Morlet wavelet φ(τ) was used to construct the continuous wavelet transform Wx(s,δ) and Wy(s,δ) for the monthly imported CHIKV cases time series x(t) and the monthly overseas arrivals time series y(t) respectively. A continuous wavelet transform analysis was used to explore localized intermittent periodicities in each time series x(t) and y(t). The Morlet wavelet φ(τ) and the continuous wavelet transform W(s,δ) were given by [44, 45]
where τ is dimensionless time; ω is nondimensional frequency; s represents a wavelet scale; δ is the localized time index. The (*) shows the operation of complex conjugate. A cross wavelet transform Wxy(s,δ) was described as , which determines the regions with high common power between x(t) and y(t). Wavelet coherence analysis can determine significant coherence with a consistent phase relationship between x(t) and y(t) in the time and frequency domain. The wavelet coherence Rxy(s,δ) is defined as:
Here, S is a smoothing operator. Following established methods, the number of monthly imported CHIKV cases were converted into a normal distribution by a logarithmic transformation [45]. The 5% significance level was determined using Monte Carlo methods (1000 Monte Carlo randomizations). Wavelet analysis was conducted using the package “biwavelet” in R [46]. The squared wavelet coherence shows as a “heat map”. The phase (lead-lag) relationships between x(t) and y(t) are presented by arrows pointing in the heat map. There is a positive (negative) relationship between x(t) and y(t) when the arrows point to right (left). The arrows pointing up (down) mean that x(t) lags (leads) y(t) by π/2.
In general, SOI fluctuates monthly, while the development of an El Niño or a La Niña event requires negative or positive SOI values for a period of several months [47]. Before starting the wavelet analysis, we examined the correlations between monthly imported CHIKV cases and 2–6 month lagged moving averages of SOI, respectively. We performed linear regression models using SPSS software to examine the relationships between monthly imported CHIKV cases and 2–6 month lagged moving average. The 6-month lagged moving average SOI displayed the highest R2 value for the linear regression model and the highest value of correlation coefficient. Thus, the 6-month lagged moving average SOI was used in the wavelet coherence analysis.
Results
Descriptive analysis
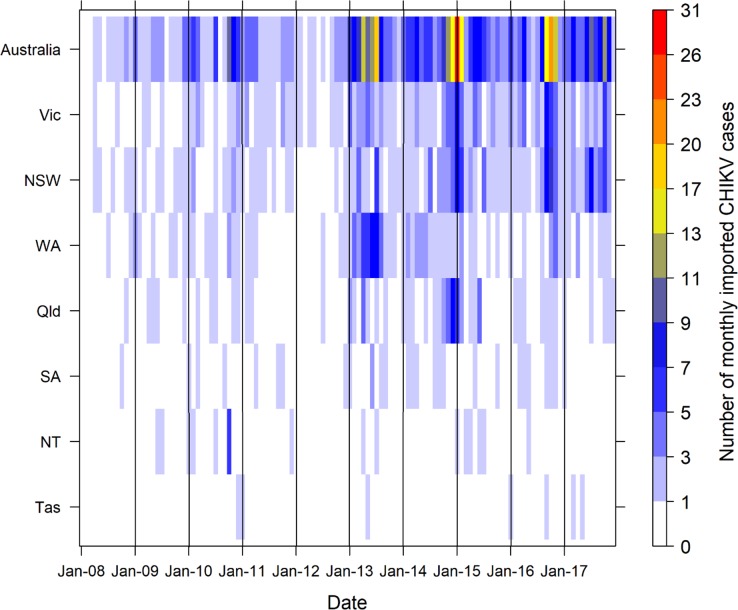
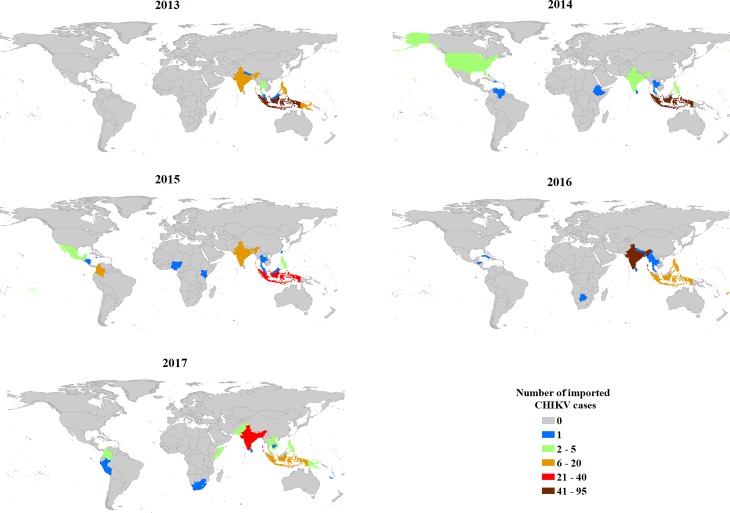
There were 730 imported CHIKV cases from 2008 to 2017 in Australia. The largest number of monthly imported CHIKV cases was 31 cases in January 2015. The largest number of annual imported CHIKV cases occurred in 2013 (134 cases), followed by 2016 (114 cases), 2015 (111 cases) and 2014 (110 cases). The mean of monthly imported CHIKV cases was 6.08 (range: 0–31) during the study period across Australia and varied between states (Table 1). NSW and VIC had the highest means of monthly imported CHIKV cases when compared with the other states (Fig 1). These states also have the largest populations. The frequency of the months with imported CHIKV cases per year has increased since 2013 in NSW (10–11 months), VIC (10–11 months), QLD (5–9 months) and WA (6–12 months) states (Fig 1). Excluding 13 cases with unknown or unclear provenance, the five countries most frequently reported as location of CHIKV acquisition from 2013 to 2017 were Indonesia (33.27% of imported cases), India (20.25%), Samoa (8.27%), Bangladesh (6.34%) and Philippines (4.05%) (Fig 2). There were eight countries of acquisition in 2013, including Indonesia (95 cases), Papua New Guinea (15 cases), India (11 cases), Philippines (6 cases), Thailand (3 cases), Singapore (2 cases), Malaysia (1 case) and Nepal (1 case). However, after 2013, the number of source countries of imported CHIKV cases substantially increased and ranged from 15 to 24 countries per year. Since 2014, the geographical distribution of countries of acquisition has expanded from Southeast Asia, South and Central Asia and Southwest Pacific region to include Eastern Africa, Western Africa, Southern Africa, North America, Central America, South America and the south Pacific regions (Fig 2).
Table 1. Summary statistics of number of monthly imported CHIKV cases from January 2008 to December 2017 across states and territories of Australia.
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Australia | 6.08 | 5.96 | 0 | 31 |
| NSW | 1.78 | 2.39 | 0 | 12 |
| NT | 0.20 | 0.75 | 0 | 7 |
| Qld | 0.70 | 1.42 | 0 | 9 |
| SA | 0.28 | 0.57 | 0 | 3 |
| Tas | 0.08 | 0.34 | 0 | 2 |
| Vic | 1.83 | 1.99 | 0 | 11 |
| WA | 1.23 | 1.91 | 0 | 11 |
Fig 1. Heat map of the monthly imported CHIKV cases by the states, from January 2008 to December 2017 in Australia.
Fig 2. Spatiotemporal variability of annual imported CHIKV cases by countries, from 2013 to 2017 in Australia.
The confirmed number of annual CHIKV cases by the countries were presented in S1 Table. Map was made using ArcMap 10.6.
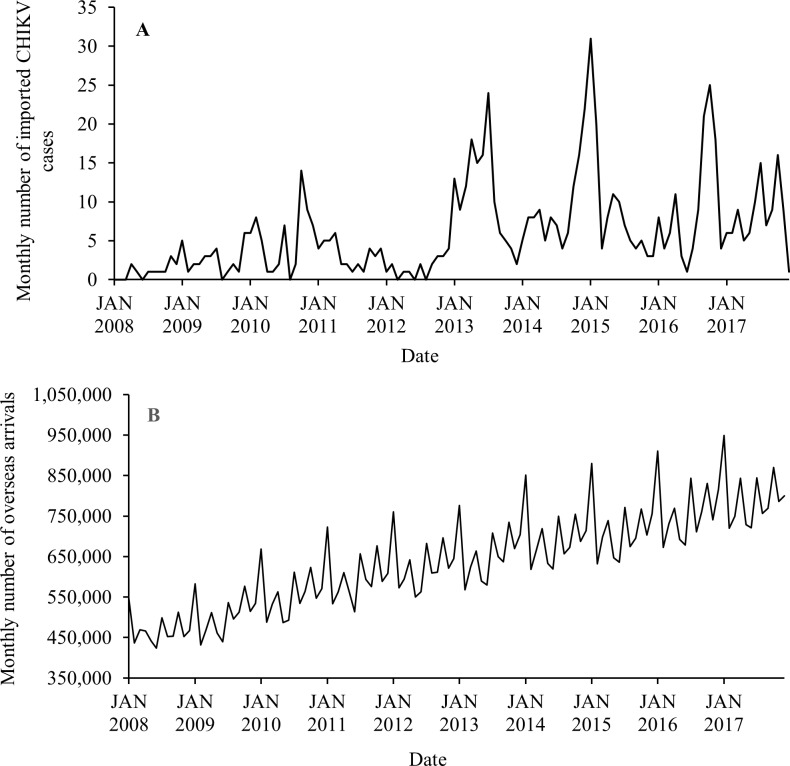
Temporal variabilities of the monthly imported CHIKV cases and the monthly overseas arrivals from the Pacific region, Southeast Asia, South and Central Asia in Australia from January 2008 to December 2017 are displayed in Fig 3. An increase in the number of monthly imported CHIKV cases has occurred from the beginning of 2013 (Fig 3A). Although increased trends for both monthly imported CHIKV cases and monthly overseas arrivals were observed over the study period (Fig 3B), the average of monthly imported CHIKV cases increased by 605.3% from 2012 to 2013, while the average of monthly overseas arrivals only increased by 3.6% from 2012 to 2013. The increase in overseas arrivals was more steady and regular over the whole study period.
Fig 3.
Temporal variation in the numbers of monthly imported CHIKV cases (A) and the monthly overseas arrivals from the Pacific region, Southeast Asia, South and Central Asia (B), from January 2008 to December 2017.
Seasonality and trend
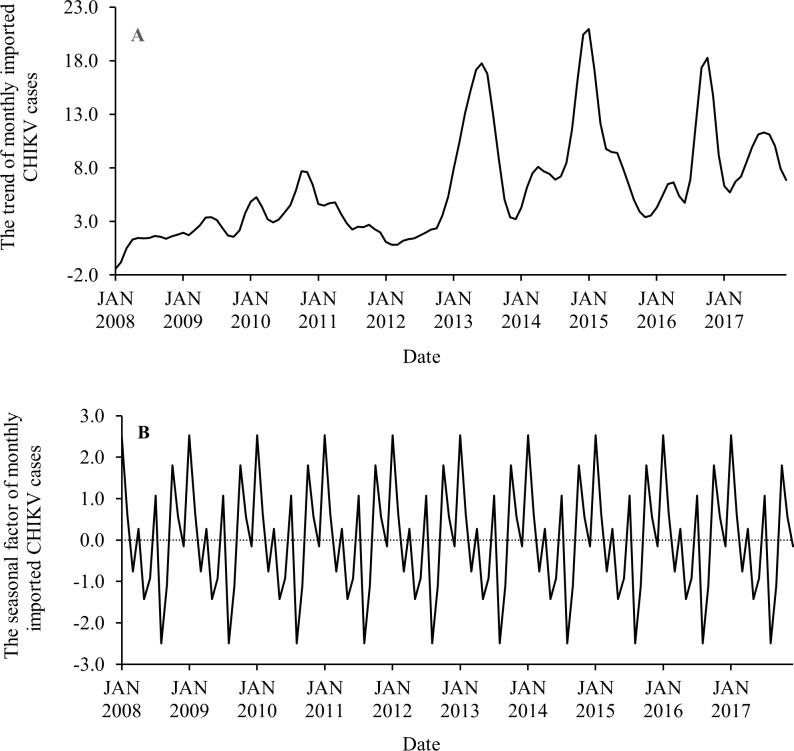
Seasonal decomposition analysis showed that the monthly number of imported CHIKV cases displayed an increased trend from 2008 to 2015, with a slight decreased trend from 2016 to 2017 (Fig 4A). Seasonal decomposition analysis also indicated that there was significant seasonality for monthly imported CHIKV cases (Fig 4B). For the monthly imported CHIKV cases, the strongest positive seasonal effects were found in each January, followed by October, July, February, November and April. The strongest negative seasonal effects appeared in each August, followed by May, September, June, March and December.
Fig 4.
Seasonal decomposition analysis showing the trend (A) and seasonal factor (B) of monthly imported CHIKV cases, from January 2008 to December 2017.
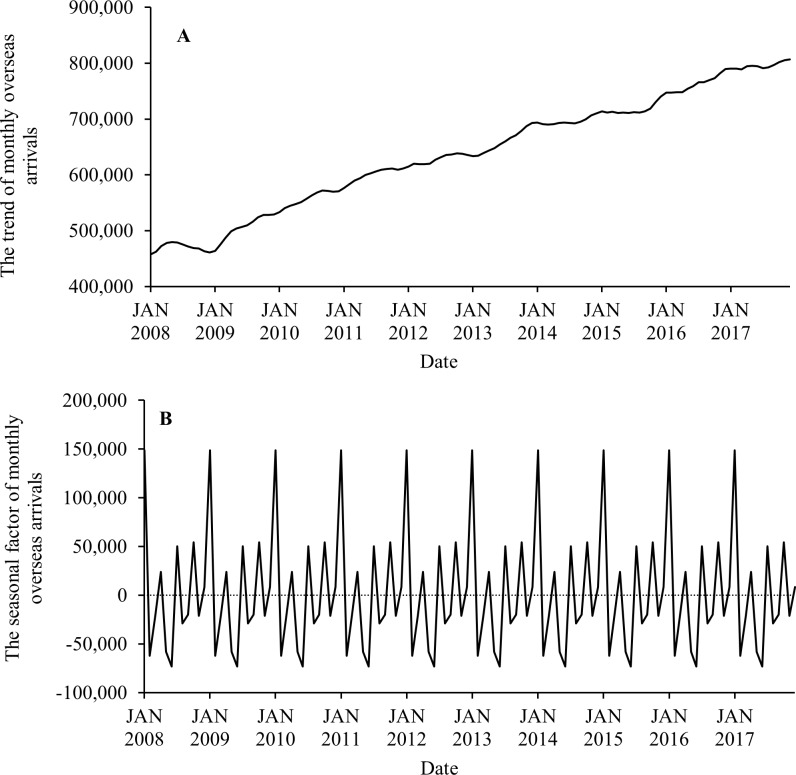
In contrast to imported CHIKV cases, the monthly number of overseas arrivals exhibited a linear increased trend during the study period (Fig 5A). There was also a strong component of seasonality. For the monthly overseas arrivals, the strongest positive seasonal effects occurred in each January, followed by October, July, April and December, while the strongest negative seasonal effects were observed in each June, followed by February, May, August, November, March and September (Fig 5B).
Fig 5.
Seasonal decomposition analysis showing the trend (A) and seasonal factor (B) of monthly overseas arrivals, from January 2008 to December 2017.
Wavelet coherence analysis
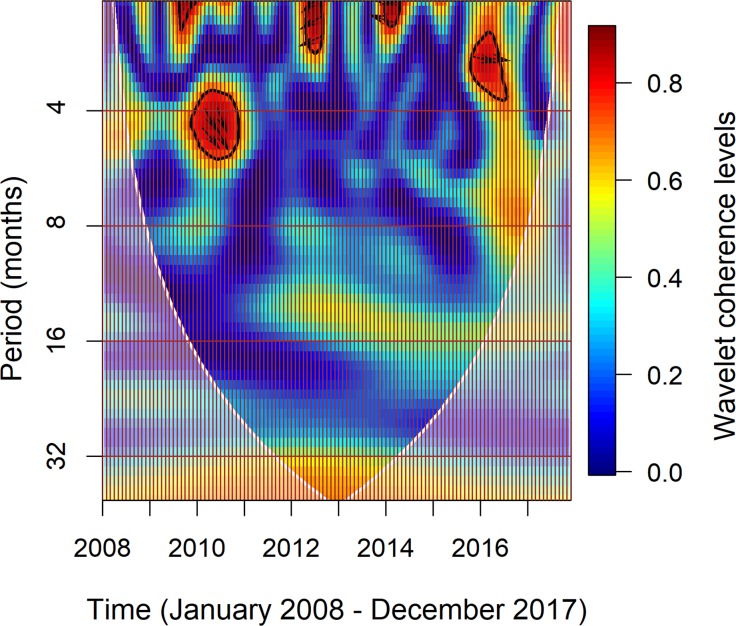
The wavelet coherence analysis captured some significant transient relationships between monthly imported CHIKV cases and the 6-month lagged moving average SOI in the time frequency space. The heat map (Fig 6) shows in which periods there were significant correlations between the two time series at a given point in time. The monthly imported number of CHIKV cases was significantly positively associated with the 6-month lagged moving average SOI with a slight lead phase, with a period of ~ 3.5–6 months from December 2009 to October 2010 (Fig 6). There were significant negative relationships between the monthly imported CHIKV cases and the 6-month lagged moving average SOI, with a period of ~ 2–3 months during May to August 2012 with a slight lag phase, and with a period of ~ 2–2.5 months from January to April 2014 with a slight lead phase. The numbers of monthly imported CHIKV cases were significantly and positively related to the 6-month lagged moving average SOI, with a period of ~ 2.6–3.8 months from November 2015 to June 2016.
Fig 6. Squared wavelet coherence between monthly imported CHIKV cases and 6-month lagged moving average SOI.
The vertical axis shows the frequency of wavelet coherence. The brown vertical grid lines indicate month of the year. The area below the white dashed lines is the ‘cone of influence’, where edge effects restrict the ability to explain the results (shown as a lighter shade). The dark red colour represents the higher correlation between them. The thick black contour designates the 5% statistical significance levels against red noise. The 5% significance levels determined using Monte Carlo methods (1000 Monte Carlo randomizations). Arrow denotes a specific phase relationship between monthly imported CHIKV cases and 6-month lagged moving average SOI.
Discussion
An increased number of countries of acquisition for CHIKV cases imported into Australia was observed during the study period, supporting previous reports of global expansion of CHIKV endemicity [1, 48, 49]. The Pacific region and Southeast/South/Central Asia were the most frequent sources of imported CHIKV cases in Australia, particularly Indonesia and India. From 2014, the geographic range of countries of acquisition expanded to include United States of America, South America, Caribbean Islands, South Africa, and Eastern/Western Africa. Although the number of international arrivals displayed a stable linear increasing trend over the study period, the monthly imported CHIKV cases did not follow the same trend and displayed some instability. It is likely that the trend of monthly imported CHIKV cases reflects the most recent dynamics of CHIKV epidemics in the most frequent countries of acquisition and the number of passenger movements from large epidemic countries.
There were also the peaks in overseas importation from specific countries that coincided with large epidemics observed in those regions. For example, there were massive CHIKV outbreaks in the Americas in 2014 [50], along with the first local chikungunya outbreaks in June 2014 in Florida in the continental United States [51] and in the U.S. Virgin Islands [52]. These outbreaks coincided with CHIKV importation into Australia in 2014 from Barbados, Dominican Republic, El Salvador, Grenada, Jamaica, United States of America and Venezuela. A major CHIKV outbreak was reported in Papua New Guinea in 2013 [7], which resulted in the largest number of imported CHIKV cases into Australia during the period of 2013–2017. Samoa and Tonga experienced large CHIKV outbreaks in 2014 which corresponded to the high numbers of imported CHIKV cases into Australia in 2014 [7]. A substantial CHIKV outbreak was reported in India in 2016 [53], which also led to 54.39% of CHIKV cases into Australia originating in India that year. Bangladesh experienced a significant chikungunya outbreak in 2017 [54], with Australia observing the largest number of imported CHIKV cases in 2017 being from Bangladesh. Moreover, it is notable that there was an increase in the frequency and number of imported CHIKV cases into Australia over the study period, suggesting that the potential risk of autochthonous outbreak will increase if there is sufficient vector mosquito density under suitable weather conditions, in regions with competent vectors in Australia.
From the seasonal decomposition analysis, although the seasonal patterns of monthly imported CHIKV cases and monthly overseas arrivals were not fully synchronized, we observed the seasonal effects on both imported CHIKV cases and overseas arrivals to be strongest each January, followed by October and July. It is likely that the summer holiday period might facilitate the number of imported CHIKV cases due to returning international travellers during the peak Australian vacation season. Additionally, the second and third highest seasonal factor values occurred in each October and July, periods that coincide with Australian school and university holidays.
Interestingly, the timing of the lowest seasonal effects exhibited a noticeable difference. For example, the weakest seasonal effects on monthly imported CHIKV cases occurred each August, followed by May and September, while monthly overseas arrivals were at their lowest in each June, followed by February and May. Almost all countries of acquisition identified in our study experience a tropical and subtropical climate and many experience a dry season from June to September (e.g. Fiji, Kiribati, Indonesia, Malaysia, Samoa, Singapore, Papua New Guinea and the Philippines). Most of India experiences very hot summer weather during April to September [55]. Dry seasons and high temperatures may likely decrease vector abundance in source countries by reducing the production and survival rate of vectors, decreasing the likelihood of CHIKV outbreaks [56]. Overall, the results confirmed that temporal variation in imported CHIKV cases might be driven not only by the behaviour of international travellers but also by the dynamics of CHIKV outbreaks and epidemics in the source countries.
To our knowledge, this is the first study to explore the impact of ENSO on CHIKV importation to non-endemic areas. The wavelet analysis revealed significant positive relationships between the monthly imported CHIKV cases and the 6-month lagged moving average SOI during December 2009 to October 2010 and from November 2015 to June 2016. Historically, there were EI Niño events from 2009 to 2010 and from 2015 to 2016 [57]. During 2009 to 2010, the main acquisition countries included Indonesia, India and Malaysia [26]. Large CHIKV outbreaks were reported across India in 2010 [58], and Indonesia in 2009 (83,756 reported cases) and 2010 (53,899 reported cases) [38]. A large number of CHIKV cases were observed in 2010 in Bali, Indonesia, [38], a popular destination for international travellers, particularly Australians. Moreover, Malaysia reported large outbreaks during 2008 to 2010 [59], which is also a popular destination for international travellers.
The relationship between arboviral transmission and temperature and rainfall is complex and regionally inconsistent [60]. However, up to a threshold, temperature generally has a positive relationship with Aedes spp. abundance [56]. Similarly, moderate rainfall is generally found to favour higher vector abundances [30]. El Niño events are typically related to warm and dry weather conditions [57]. Moderate to high temperatures could facilitate increased CHIKV transmission because increasing temperature leads to a higher biting rate and shorter extrinsic incubation period for vector mosquitoes [34]. Conversely, extremely high temperature could increase the mortality rate of mosquitoes [61]. Increased SOI could lead to a decrease in the intensity of El Niño conditions during such events [62]. Thus, the positive relationship between the monthly imported CHIKV cases and the 6-month lagged moving average SOI suggested that moderate weather conditions under El Niño episodes might enhance the importation of CHIKV.
Conversely, we also observed significant negative relationships between the number of monthly imported CHIKV cases and the 6-month lagged moving average SOI for the period May-August 2012 and the period January-April 2014. For these periods, an increase in SOI was likely to decrease the number of monthly imported CHIKV cases under neutral ENSO conditions (i.e. absence of La Niña or El Niño). Increased SOI could increase the chance of a La Niña event developing during the neutral ENSO period. La Niña typically leads to cold temperature and heavy rain weather conditions. Heavy rainfall could flush out mosquito breeding sites [63], leading to lowered CHIKV transmission risk. Conversely, neutral ENSO conditions that lead to equable weather conditions with low to moderate rainfall may lead to increased CHIKV transmission risk. It is interesting that in the period from 2012–2014 a series of CHIKV epidemics occurred in the Pacific [7, 64], which led to large numbers of imported CHIKV cases into Australia. Our results suggest that ENSO behaviours could drive local environmental conditions, which lead to changes in the patterns of local CHIKV epidemics in different endemic regions.
Limitations
Some limitations of this study should be acknowledged. First, the imported CHIKV cases used in this analysis were only from laboratory confirmed individuals and therefore did not include infected persons who were asymptomatic or suffered only mild symptoms and thus did not seek medical advice. Second, the data by countries of acquisition were only publicly available for annual imported cases from 2013 to 2017. Therefore, we could not provide the spatiotemporal distribution of imported CHIKV cases by countries across the whole study period. We were also unable to calculate monthly incidence rates (numbers of imported cases over overseas arrivals) for each particular country over the study period, as the latter set of data were not publicly available. Third, the impact of ENSO could result in varying climate conditions in different parts of the world. Using aggregated data on monthly imported CHIKV cases from all source countries in the study could not fully reveal the associations between ENSO behaviours and imported CHIKV cases. Future studies may address the problem by using the imported CHIKV cases by countries or by a group of countries where they have the same weather conditions when the data are available. Additionally, future studies should investigate whether there are differences in the effects of different ENSO events, such as El Niño and La Niña, on local CHIKV transmission in endemic countries. The overall trend of increased number of CHIKV imports suggests that Australia remains at risk of a CHIKV outbreak. The patterns of importation we observed for Australia are likely to be mirrored in other countries that are non-endemic for CHIKV and have strong travel links with the countries of acquisition identified here. Australian health authorities have acknowledged the increasing risk of local CHIKV outbreaks, particularly in North Queensland, through the development of the Queensland Chikungunya Management Plan in 2014 [65].
Conclusions
We found that the geographical distribution of countries of acquisition has expanded significantly, with the frequency and number of imported CHIKV cases into Australia increasing in recent years. Although the timing of the highest seasonal peak imported cases was consistent with overseas arrivals, the lowest seasonal peak overseas arrivals did not result in the lowest seasonal peak imported cases in Australia. The present study can provide useful information for understanding the seasonality of CHIKV risk and guide surveillance for health authorities at corresponding times of the year. Messaging might be tailored to particular seasons, importation pathways and epidemics in key countries of acquisition. It is likely that the dynamics of imported cases are related to the behaviours of ENSO. Our results suggest that climate could be a potential important driver of CHIKV epidemics and that ENSO behaviours could be a useful predictor to forecast mosquito-borne disease epidemics in endemic countries.
Supporting information
(DOCX)
Acknowledgments
We thank the National Notifiable Diseases Surveillance System and Australian Bureau of Statistics for providing the data on the imported CHIKV cases and overseas arrivals, respectively.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was funded by the Australian National Health and Medical Research Council (APP1125317). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weaver SC, Lecuit M. Chikungunya Virus and the Global Spread of a Mosquito-Borne Disease. The new england journal of medicine. 2015;372(13):1231–9. 10.1056/NEJMra1406035 [DOI] [PubMed] [Google Scholar]
- 2.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, Lamballerie Xd. Chikungunya in the Americas. The Lancet. 2014;383:514. [DOI] [PubMed] [Google Scholar]
- 3.Powers AM. How Chikungunya Virus Virology Affects Its Epidemiology and Transmission: Implications for Influencing Public Health. The J Infect Dis. 2016;214:449–52. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Chikungunya. 2017 [cited 3 August 2018]. Available from: http://www.who.int/news-room/fact-sheets/detail/chikungunya.
- 5.Randika Wimalasiri Yapa B.M.C, Stassen L, Huang X, Hafner L, Hu W, Devine G, et al. Chikungunya in South East Asia and the Western Pacific region: A systematic review Emerg Microbes Infect. 2019; 10.1080/22221751.2018.1559708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitas ARR, Donalisio MR, Alarcón-Elbal PM. Excess Mortality and Causes Associated with Chikungunya, Puerto Rico, 2014–2015. Emerg Infect Dis. 2018;24 10.3201/eid2412.170639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, et al. Concurrent outbreaks of dengue, chikungunya and Zika virus infections–an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Eurosurveillance. 2014;19(41):20929 10.2807/1560-7917.ES2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 8.Tjaden NB, Suk JE, Fischer D, Thomas SM, Beierkuhnlein C, Semenza JC. Modelling the effects of global climate change on Chikungunya transmission in the 21(st) century. Sci Rep. 2017;7(1):3813 10.1038/s41598-017-03566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Depoortere E, Salmaso S, Pompa MG, Guglielmetti P, Coulombier D. Chikungunya in Europe. The Lancet. 2008;371(9614):723 10.1016/S0140-6736(08)60336-0 [DOI] [PubMed] [Google Scholar]
- 10.Tabachnick WJ. Challenges in predicting climate and environmental effects on vector- borne disease episystems in a changing world. J Exp Biol. 2010;213(6):946–54. 10.1242/jeb.037564 [DOI] [PubMed] [Google Scholar]
- 11.Charrel RN, Lamballerie Xd, Raoult D. Chikungunya Outbreaks—The Globalization of Vectorborne Diseases. N Engl J Med. 2007;356:769–71. 10.1056/NEJMp078013 [DOI] [PubMed] [Google Scholar]
- 12.Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, Minodier P, et al. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12(10):1493–9. 10.3201/eid1210.060610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendrick K, Stanek D, Blackmore C. Transmission of Chikungunya Virus in the Continental United States—Florida, 2014. Centers for Disease Control and Prevention. 2014;63:1137. [PMC free article] [PubMed] [Google Scholar]
- 14.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. The Lancet. 2007;370:1840–6. [DOI] [PubMed] [Google Scholar]
- 15.Gould EA, Gallian P, Lamballerie Xd, NCharrel R. First cases of autochthonous dengue fever and chikungunya fever in France: from bad dream to reality. Clinical Microbiol Infect. 2010;16:1702–4. [DOI] [PubMed] [Google Scholar]
- 16.Ciocchetta S, Prow NA, Darbro JM, Frentiu FD, Savino S, Montarsi F, et al. The new European invader Aedes (Finlaya) koreicus: a potential vector of chikungunya virus. Pathog Glob Health. 2018;112(3):107–14. 10.1080/20477724.2018.1464780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell RC, Currie BJ, Lindsay MD, Mackenzie JS, Ritchie SA, Whelan PI. Dengue and climate change in Australia: predictions for the future should incorporate knowledge from the past. Med J Aust. 2009;190(5):265–8. [DOI] [PubMed] [Google Scholar]
- 19.Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next. Microbes Infect. 2010;12(4):272–9. 10.1016/j.micinf.2009.12.011 [DOI] [PubMed] [Google Scholar]
- 20.Trewin BJ, Darbro JM, Jansen CC, Schellhorn NA, Zalucki MP, Hurst TP, et al. The elimination of the dengue vector, Aedes aegypti, from Brisbane, Australia: The role of surveillance, larval habitat removal and policy. PLoS Negl Trop Dis. 2017;11(8):e0005848 10.1371/journal.pntd.0005848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell LP, Luther C, Moo-Llanes D, Ramsey JM, Danis-Lozano R, Peterson AT. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos Trans R Soc Lond B Biol Sci. 2015;370(1665). 10.1098/rstb.2014.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein PR. Chikungunya fever resurgence and global warming. Am J Trop Med Hyg. 2007;76:404–. [PubMed] [Google Scholar]
- 23.Fischer D, Thomas SM, Suk JE, Sudre B, Hess A, Tjaden NB, et al. Climate change effects on Chikungunya transmission in Europe: geospatial analysis of vector’s climatic suitability and virus’ temperature requirements. Int J Health Geogr. 2013;12:51 10.1186/1476-072X-12-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roiz D, Boussès P, Simard F, Paupy C, Fontenille D. Autochthonous Chikungunya Transmission and Extreme Climate Events in Southern France. PLoS Negl Trop Dis. 2015; 10.1371/journal.pntd.0003854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhiman RC, Pahwa S, Dhillon GPS, Dash AP. Climate change and threat of vector-borne diseases in India: are we prepared? Parasitol Res. 2010;106:763–73. 10.1007/s00436-010-1767-4 [DOI] [PubMed] [Google Scholar]
- 26.Viennet E, Knope K, Faddy HM, Williams CR, Harley D. Assessing the threat of chikungunya virus emergence in Australia. Commun Dis Intell Q Rep. 2013;37(2):E136–43. . [DOI] [PubMed] [Google Scholar]
- 27.Suhrbier A, Devine G. Chikungunya virus, risks and responses for Australia. Aust N Z J Public Health. 2016;40(3):207–9. 10.1111/1753-6405.12515 [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Williams G, Clements AC, Hu W. Imported Dengue Cases, Weather Variation and Autochthonous Dengue Incidence in Cairns, Australia. PloS ONE. 2013;8(12):e81887 10.1371/journal.pone.0081887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu W, Clements A, Williams G, Tong S, Mengersen K. Spatial Patterns and Socioecological Drivers of Dengue Fever Transmission in Queensland, Australia. Environ Health Perspect. 2012;120(2):260–6. 10.1289/ehp.1003270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muzari MO, Devine G, Davis J, Crunkhorn B, van den Hurk A, Whelan P, et al. Holding back the tiger: Successful control program protects Australia from Aedes albopictus expansion. PLoS Negl Trop Dis. 2017;11(2):e0005286 10.1371/journal.pntd.0005286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson J, Ritchie SA, Russell RC, Webb CE, Cook A, Zalucki MP, et al. Effects of Cohabitation on the Population Performance and Survivorship of the Invasive Mosquito Aedes albopictus and the Resident Mosquito Aedes notoscriptus (Diptera: Culicidae) in Australia. J Med Entomol. 2015;52(3):375–85. 10.1093/jme/tjv004 [DOI] [PubMed] [Google Scholar]
- 32.The Department of Health. Australian national notifiable diseases and case definitions. 2018 [cited 30 Novermber 2018]. Available from: http://www.health.gov.au/casedefinitions#c.
- 33.Johnson DF, Druce JD, Chapman S, Swaminathan A, Wolf J, Richards JS, et al. Chikungunya virus infection in travellers to Australia. Med J Aust. 2008;188(1):41–3. [DOI] [PubMed] [Google Scholar]
- 34.Caminade C, Turner J, Metelmann S, Hesson JC, Blagrove MSC, Solomon T, et al. Global risk model for vector-borne transmission of Zika virus reveals the role of El Niño 2015. PNAS. 2017;114(1):119–24. 10.1073/pnas.1614303114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flahault A, Castaneda RRd, Bolon I. Climate change and infectious diseases. Public Health Rev. 2016;37:21 10.1186/s40985-016-0035-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincenti-Gonzalez MF, Tami A, Lizarazo EF, Grillet ME. ENSO-driven climate variability promotes periodic major outbreaks of dengue in Venezuela. Sci Rep. 2018;8(1):5727 10.1038/s41598-018-24003-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakarla SG, Mopuri R, Mutheneni SR, Bhimala KR, Kumaraswamy S, Kadiri MR, et al. Temperature dependent transmission potential model for chikungunya in India. Sci Total Environ. 2019;647:66–74. 10.1016/j.scitotenv.2018.07.461 [DOI] [PubMed] [Google Scholar]
- 38.Yoshikawa MJ, Kusriastuti R. Surge of Dengue Virus Infection and Chikungunya Fever in Bali in 2010: The Burden of Mosquito-Borne Infectious Diseases in a Tourist Destination. Trop Med Health. 2013;41:67–78. 10.2149/tmh.2011-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Department of Health. Introduction to the National Notifiable Diseases Surveillance System. 2015 [cited 30 Novermber 2018]. Available from: http://www.health.gov.au/internet/main/publishing.nsf/content/cda-surveil-nndss-nndssintro.htm.
- 40.Knope KE, Kurucz N, Doggett SL, Muller M, Johansen CA, Feldman R, et al. Arboviral diseases and malaria in Australia, 2012–13: Annual report of the National Arbovirus and Malaria Advisory Committee. Commun Dis Intell Q Rep. 2016;40:E17–E47. [DOI] [PubMed] [Google Scholar]
- 41.Australian Bureau of Statistics. Overseas arrivals and Departure, Australia, Mar 2018. 2018 [cited 30 Novermber 2018]. Available from: http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/3401.0Explanatory%20Notes1Mar%202018?OpenDocument.
- 42.Stone RC, Hammer GL, Marcussen T. Prediction of global rainfall probabilities using phases of the Southern Oscillation Index. Nature. 1996;384:252 10.1038/384252a0 [DOI] [Google Scholar]
- 43.Ranasinghe R, McLoughlin R, Short A, Symonds G. The Southern Oscillation Index, wave climate, and beach rotation. Mar Geol. 2004;204(3):273–87. [Google Scholar]
- 44.Torrence C, Compo GP. A Practical Guide to Wavelet Analysis. Bull Am Meteorol Soc. 1998;79(1):61–78. [Google Scholar]
- 45.Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process Geophys. 2004;11(5/6):561–6. [Google Scholar]
- 46.Gouhier TC, Grinsted A, Simko V. Conduct Univariate and Bivariate Wavelet Analyses. 2018 [cited 25 September 2018] 25/09/2018. Available from: https://cran.r-project.org/web/packages/biwavelet/biwavelet.pdf.
- 47.Australian Government Bureau of Meteorology. El Niño, La Niña and Australia's Climate. 2005 [cited 9 August 2018]. Available from http://www.bom.gov.au/info/leaflets/nino-nina.pdf2005.
- 48.Heitmann A, Jansen S, Lühken R, Helms M, Pluskota B, Becker N, et al. Experimental risk assessment for chikungunya virus transmission based on vector competence, distribution and temperature suitability in Europe, 2018. Eurosurveillance. 2018;23(29):1800033 10.2807/1560-7917.ES.2018.23.29.1800033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan Y, Pickett BE, Shrivastava S, Gresh L, Balmaseda A, Amedeo P, et al. Differing epidemiological dynamics of Chikungunya virus in the Americas during the 2014–2015 epidemic. PLoS Negl Trop Dis. 2018;12(7):e0006670 Epub 2018/07/31. 10.1371/journal.pntd.0006670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer M, Staples JE, Arboviral Diseases Branch NCfE, Zoonotic Infectious Diseases CDC. Notes from the field: chikungunya virus spreads in the Americas—Caribbean and South America, 2013–2014. MMWR Morbidity and mortality weekly report. 2014;63(22):500–1. . [PMC free article] [PubMed] [Google Scholar]
- 51.Kendrick K, Stanek D, Blackmore C. Transmission of chikungunya virus in the continental United States-Florida, 2014. Centers for Disease Control and Prevention. 2014;63:1137. [PMC free article] [PubMed] [Google Scholar]
- 52.Feldstein LR, Ellis EM, Rowhani-Rahbar A, Halloran ME, Ellis BR. The First Reported Outbreak of Chikungunya in the U.S. Virgin Islands, 2014–2015. Am J Trop Med Hyg. 2016;95(4):885–9. 10.4269/ajtmh.16-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur N, Jain J, Kumar A, Narang M, Zakaria MK, Marcello A, et al. Chikungunya outbreak in Delhi, India, 2016: report on coinfection status and comorbid conditions in patients. New Microbes New Infect. 2017;20:39–42. 10.1016/j.nmni.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahman MM, Been Sayed SJ, Moniruzzaman M, Kabir AKMH, Mallik MU, Hasan MR, et al. Clinical and Laboratory Characteristics of an Acute Chikungunya Outbreak in Bangladesh in 2017. Am J Trop Med Hyg. 2019;100(2):405–10. 10.4269/ajtmh.18-0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meteorological Services of WeatherOnline. Climate of the wold. 2018 [cited 30 October 2018]. Available from: https://www.weatheronline.co.uk/reports/climate/Papua-New-Guinea.htm.
- 56.Huber JH, Childs ML, Caldwell JM, Mordecai EA. Seasonal temperature variation influences climate suitability for dengue, chikungunya, and Zika transmission. PLoS Negl Trop Dis. 2018;12(5):e0006451 Epub 2018/05/11. 10.1371/journal.pntd.0006451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Australian Government Bureau of Meteorology. El Niño—Detailed Australian Analysis. 2018 [cited 28 August 2018]. Available from: http://www.bom.gov.au/climate/enso/enlist/index.shtml2018.
- 58.Shrinet J, Jain S, Sharma A, Singh SS, Mathur K, Rana V, et al. Genetic characterization of Chikungunya virus from New Delhi reveal emergence of a new molecular signature in Indian isolates. Virology Journal. 2012;9(1):100 10.1186/1743-422x-9-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohd Zim MA, Sam IC, Omar SFS, Chan YF, AbuBakar S, Kamarulzaman A. Chikungunya infection in Malaysia: Comparison with dengue infection in adults and predictors of persistent arthralgia. J Clinl Virol. 2013;56(2):141–5. 10.1016/j.jcv.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 60.Sun K, Zhang Q, Pastore-Piontti A, Chinazzi M, Mistry D, Dean NE, et al. Quantifying the risk of local Zika virus transmission in the contiguous US during the 2015–2016 ZIKV epidemic. BMC Medicine. 2018;16(1):195 10.1186/s12916-018-1185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang HMY, Macoris MLG, Galvani KC, Andrighetti MTM, Wanderley DMV. Assessing the effects of temperature on the population of Aedes aegypti, the vector of dengue. Epidemiol Infect. 2009;137(8):1188 10.1017/S0950268809002040 [DOI] [PubMed] [Google Scholar]
- 62.Australian Government Bureau of Meteorology. Climate Glossary Southern Oscillation Index. 2014 [cited 22 August 2018]. Available from: http://www.bom.gov.au/climate/glossary/soi.shtml.
- 63.Seidahmed OM, Eltahir EA. A Sequence of Flushing and Drying of Breeding Habitats of Aedes aegypti (L.) Prior to the Low Dengue Season in Singapore. PLoS Negl Trop Dis. 2016;10(7):e0004842 Epub 2016/07/28. 10.1371/journal.pntd.0004842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horwood PF, Reimer LJ, Dagina R, Susapu M, Bande G, Katusele M, et al. Outbreak of Chikungunya Virus Infection, Vanimo, Papua New Guinea. Emerging Infectious Diseases. 2013;19(9):1535–8. 10.3201/eid1909.130130 PMC3810919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Queensland Goverment. Queensland chikungunya management plan 2014–2019. 2014 [cited 4 March 2019]. Available from: https://www.health.qld.gov.au/__data/assets/pdf_file/0027/444672/chikungunya-management-plan.pdf: 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.